Abstract
One of the key reasons for the success of invasive plants is the functional differences between invasive plants and native plants. However, atmospheric nitrogen deposition may disrupt the level of available nitrogen in soil and the functional differences between invasive plants and native plants, which may alter the colonization of invasive plants. Thus, there is a pressing necessity to examine the effects of atmospheric nitrogen deposition containing different nitrogen components on the functional differences between invasive plants and native plants. However, the progress made thus far in this field is not sufficiently detailed. This study aimed to elucidate the effects of artificially simulated nitrogen deposition containing different nitrogen components (i.e., nitrate, ammonium, urea, and mixed nitrogen) on the functional differences between the Asteraceae invasive plant Bidens pilosa L. and the Asteraceae native plant Pterocypsela laciniata (Houtt.) Shih. The study was conducted over a four-month period using a pot-competitive co-culture experiment. The growth performance of P. laciniata, in particular with regard to the sunlight capture capacity (55.12% lower), plant supporting capacity (45.92% lower), leaf photosynthetic area (51.24% lower), and plant growth competitiveness (79.92% lower), may be significantly inhibited under co-cultivation condition in comparison to monoculture condition. Bidens pilosa exhibited a more pronounced competitive advantage over P. laciniata, particularly in terms of the sunlight capture capacity (129.43% higher), leaf photosynthetic capacity (40.06% higher), and enzymatic defense capacity under stress to oxidative stress (956.44% higher). The application of artificially simulated nitrogen deposition was found to facilitate the growth performance of monocultural P. laciniata, particularly in terms of the sunlight capture capacity and leaf photosynthetic area. Bidens pilosa exhibited a more pronounced competitive advantage (the average value of the relative dominance index of B. pilosa is ≈ 0.8995) than P. laciniata under artificially simulated nitrogen deposition containing different nitrogen components, especially when treated with ammonium (the relative dominance index of B. pilosa is ≈ 0.9363) and mixed nitrogen (the relative dominance index of B. pilosa is ≈ 0.9328). Consequently, atmospheric nitrogen deposition, especially the increased relative proportion of ammonium in atmospheric nitrogen deposition, may facilitate the colonization of B. pilosa via a stronger competitive advantage.
1. Introduction
Invasive plants (IPs) can have a profound impact on environmental health and ecological security. In particular, IPs can affect the structure and ecological function of native ecosystems, which can result in the loss of native biodiversity [1,2,3,4]. At present, there are in excess of 500 IPs distributed throughout China [5,6]. In particular, the Asteraceae family has the highest species number of IPs at the family classification level, with a total of 92 IPs in the family Asteraceae [5,6]. Thus, the investigation of the mechanisms underlying the success of IPs, particularly those belonging to the Asteraceae family, represents a pivotal area of research within the field of invasion ecology in recent years [7,8,9].
One of the key reasons for the success of IPs is the functional differences between IPs and native plants. In particular, both IPs and native plants are subject to similar, if not identical, selection pressures, exerted by the environment [10,11,12,13]. More importantly, IPs generally exhibit higher values for the key functional traits, including plant height, leaf area, photosynthetic capacity, nutrient use efficiency, and environmental tolerance, etc. Consequently, they exhibit higher growth performance compared to native plants, even under stressful environments [14,15,16,17]. It is therefore essential to illuminate the functional differences and differences in growth performance-related functional traits between IPs and native plants to identify the intrinsic mechanisms that determine whether an IP is successfully invaded.
In general, nitrogen (N) is the main nutrient limiting plant growth in several terrestrial ecosystems [18,19,20,21]. Therefore, the capacity of IPs to obtain N is a pivotal element in determining their success in colonizing diverse habitats. More importantly, it is evident that IPs exhibit a greater capacity for N acquisition compared to native plants, due to their high availability and utilization of N [22,23,24,25]. In addition, the invasiveness and invasion intensity of numerous IPs are significantly related to the level of available N in soil [26,27,28,29]. Nevertheless, atmospheric N deposition may significantly disrupt the level of available N in soil and the interactions between IPs and native plants, which may influence the colonization of IPs.
In recent years, there has been a notable increase in atmospheric N deposition, which is largely attributed to the release of N-containing compositions into the atmosphere as a consequence of the excessive combustion of fossil fuels, unreasonable and/or unsuitable production and consumption of N-containing fertilizers, and the fast expansion of animal husbandry and cultivation [30,31,32,33]. Presently, East Asia (predominantly China) has one of the three maximum rates of atmospheric N deposition globally [31,34,35,36]. In addition, other parts of the globe are also experiencing more serious atmospheric N deposition problems, such as Europe and the United States [33,37,38,39]. Nevertheless, it has been demonstrated that atmospheric N deposition may promote the invasiveness of several IPs, which in turn leads to the acceleration of the colonization of IPs by increasing the level of available N in soil [40,41,42,43]. However, atmospheric N deposition encompasses a multitude of different N components, including nitrate (NO3-N), ammonium (NH4-N), urea (CO(NH2)2-N), etc., and that the relative proportions of these N components in atmospheric N deposition may also be subject to change contingent on the alterations in energy policy and the composition of energy sources employed [31,34,35,36]. Nevertheless, atmospheric N deposition containing different N components can result in alterations in the level of available N in soil and the interactions between IPs and native plants. Such variations may result in differences in the functional differences between IPs and native plants. This could have a significant impact on the colonization of IPs. Therefore, there is a compelling rationale for investigating the effects of atmospheric N deposition containing different N components on the functional differences between IPs and native plants, with the aim of elucidating the mechanisms that facilitate the success of IPs in the context of atmospheric N deposition, particularly in the context of different N components. Nevertheless, the current state of knowledge in this field is not sufficiently detailed.
This study aimed to elucidate the effects of artificially simulated N deposition containing different N components (including nitrate (NO3-N), ammonium (NH4-N), urea (CO(NH2)2-N), and mixed N with NO3-N:NH4-N:CO(NH2)2-N = 1:1:1) on the functional differences between the Asteraceae IPs Bidens pilosa L. and the Asteraceae native plant Pterocypsela laciniata (Houtt.) Shih. The study was conducted over a four-month period using a pot-competitive co-culture experiment. Bidens pilosa is a member of the Asteraceae family, and the species number of IPs belonging to this family that have been introduced to China is higher than that of any other family at the family level [5,6]. Bidens pilosa is native to tropical America and was introduced to China in ~1857 with imported crops and vegetables. In particular, the species number of IPs sourced from America is higher than that sourced from other countries and/or districts in China [5,6]. However, B. pilosa has been identified as a significant threat to ecosystem structure and function, particularly in terms of the loss of native biodiversity in China, and B. pilosa has been classified as a harmful IP in China [2,44,45,46]. The two Asteraceae plants occupy similar habitats, including agroecosystems, wasteland, and areas adjacent to the main road in China. Additionally, the two Asteraceae plants also share similar lifestyles, with erect herbs being a common feature. Furthermore, they exhibit comparable plant heights, reaching up to ≈2–3 m. More importantly, the two Asteraceae plants frequently co-occur in the same habitats, such as agroecosystems, wasteland, and areas adjacent to the main road, etc. Furthermore, the distributions of the two Asteraceae plants in China are among the areas most affected by atmospheric N deposition [31,34,35,36].
The following questions were proposed for this study: (1) Does B. pilosa exhibit higher values of the key functional traits (e.g., plant height, leaf area, and leaf nitrogen and chlorophyll contents) compared to P. laciniata? (2) Does artificially simulated N deposition confer a competitive advantage to B. pilosa over P. laciniata? (3) Which component of artificially simulated N deposition exerts the greatest influence on the competitive advantage of B. pilosa?
2. Materials and Methods
2.1. Experimental Design
Bidens pilosa (Figure S1) was designated as the target IP. Pterocypsela laciniata (Figure S2) was proposed as the native species. Seeds of both plants were collected in October 2022 from Zhenjiang, Jiangsu, China (32.15–32.16° N; 119.52–119.53° E). The selected ecosystems were classified as wastelands. Bidens pilosa was the only invasive plant species in the sampled communities. It is likely that the selected B. pilosa individuals were naturally dispersed in the sampled communities. The native plant species in the sampled communities are dominated by herbaceous plants, such as Setaria viridis (L.) P. Beauv., Echinochloa crus-galli (L.) P. Beauv., Arthraxon hispidus (Trin.) Makino, and Artemisia argyi H. Lév. and Vaniot. The geographical location of the sampling area is provided in Figure S3. Zhenjiang has a humid subtropical monsoon climate, and in 2022 the average annual temperature in Zhenjiang was ~17.1 °C, and an average monthly temperature reached a maximum of ~28.1 °C in July and a minimum of ~3.7 °C in January [47]. In 2022, the annual sunshine hours in Zhenjiang were ~1909.0 h, and the monthly average sunshine hours reached a maximum value of ~208.2 h in December, and a minimum value of ~125.9 h in August [47]. The annual precipitation in Zhenjiang in 2022 was ~1164.1 mm, and the average monthly precipitation reached a maximum value of ~432.1 mm in July, and a minimum value of ~2.7 mm in December [47].
A pot competitive co-culture experiment was conducted to examine the growth of B. pilosa and P. laciniata (Figure S4). Pasture yellow soil (manufacturer: Shenzhibei Sci. & Technol. Co., Ltd., Baishan, China; pH value: ~6.3; soil electrical conductivity: ≤3 ms/cm; organic content: ≥30%; ~3 kg/planting basin) was used as culture substrate. The reason for using pasture yellow soil as a culture substrate was to minimize the potential for previous introduction of IPs, as well as to reduce the risk of contamination from atmospheric N deposition in natural soils. The seeds of both plants were placed in garden pots (top diameter 25 cm; height 16.5 cm). Six uniformly sized, vigorous of B. pilosa and/or P. laciniata seedlings were cultivated in each garden pot. The following treatments were employed: (1) six B. pilosa seedlings were planted in each garden pot, representing a monoculture of B. pilosa; (2) three B. pilosa seedlings and three P. laciniata seedlings were planted in each garden pot, representing a co-culture of B. pilosa and P. laciniata; (3) six P. laciniata seedlings were planted in each garden pot, representing a monoculture of P. laciniata. All garden pots were treated with artificially simulated N deposition, specifically (1) nitrate (potassium nitrate (KNO3, AR, ≥99%; Aladdin®, Shanghai, China); inorganic nitrogen); (2) ammonium (ammonium chloride (NH4Cl, GR, ≥99.8%; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China); inorganic nitrogen); (3) urea (CO(NH2)2, BC, ≥99.5%; Sangon Biotech Co., Ltd., Shanghai, China; organic nitrogen); (4) mixed N (nitrate:ammonium:urea = 1:1:1), at 5 g N m−2 yr−1. Sterile distilled water was used as the control (0 g N L−1). The content of artificially simulated N deposition, which contained different N components, replicated the actual content of natural atmospheric N deposition (i.e., 5 g N m−2 yr−1) in the southern Jiangsu, China [34,35,48,49]. The proportions of the three monomers in the N mixture were designed to simulate the actual proportions of natural atmospheric N deposition (i.e., equal mixing) in the southern Jiangsu, China [50,51,52]. The present study tested a range of planting type combinations (i.e., monocultural B. pilosa, co-cultivated B. pilosa and P. laciniata, and monocultural P. laciniata) and N component combinations (i.e., nitrate, ammonium, urea, and mixed N). Three replicates were arranged for each treatment. Seedlings of both plants were cultivated in the greenhouse at Jiangsu University, Zhenjiang, Jiangsu, China (32.2061° N, 119.5128° E) under natural light from April to July 2023 for ~4 months. The design of this experiment is shown in Figure 1.
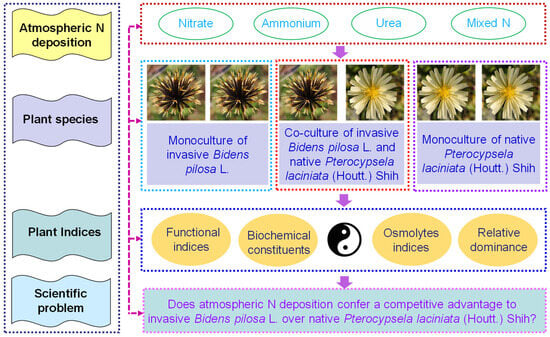
Figure 1.
The chart of the experimental design in this study.
Following ~4 months of pot competitive co-culture experimentation, all individuals of B. pilosa and P. laciniata were collected to determine their functional indices, biochemical constituents, and osmolytes indices of B. pilosa and P. laciniata, as well as the relative dominance index of B. pilosa.
2.2. Determination of Plant Indices
The functional traits closely related to the growth performance of B. pilosa and P. laciniata, including plant height, ground diameter, leaf dimensions, green leaf area, specific leaf area, leaf chlorophyll and N contents, and biomass, were determined. The biomass stability index of both plants and the relative dominance index of B. pilosa were also quantified. Similarly, biochemical constituents, and osmolytes indices of both plants were determined. The ecological significance, measuring method, and the corresponding references of the analyzed indices in this study are presented in Table S1.
2.3. Statistical Analysis
Shapiro–Wilk’s test and Bartlett’s test were employed to determine the extent of departure from the normality and the homogeneity of the examined variances, respectively. The statistical analysis of the differences in the values of the functional indices, biochemical constituents, and osmolytes indices of B. pilosa and P. laciniata, as well as the relative dominance index of B. pilosa among different treatments was conducted using the one-way analysis of variance (ANOVA) with the Duncan’s test. Two-way ANOVA was employed to evaluate the effects of plant species and N component on the functional indices, biochemical constituents, and osmolytes indices of B. pilosa and P. laciniata. The effect size of each factor was also evaluated using Partial Eta Squared (η2), which were calculated to be used in a two-way ANOVA. p ≤ 0.05 was considered to represent a statistically significant difference. Statistical analyses were conducted using IBM SPSS Statistics 26.0 (IBM, Inc., Armonk, NY, USA).
3. Results and Discussion
Plant height, ground diameter, leaf width, green leaf area, and biomass of co-cultivated P. laciniata were significantly lower than those of monocultural P. laciniata (p < 0.05; Figure 2, Figure 3 and Figure 4). Thus, the sunlight capture capacity, plant supporting capacity, leaf photosynthetic area, and plant growth competitiveness of co-cultivated P. laciniata were found to be significantly lower than those of monocultural P. laciniata. Hence, the growth performance of P. laciniata may be significantly reduced under co-cultivation conditions compared to monoculture condition. The diminished growth performance of P. laciniata under co-cultivation conditions may be attributed to the decreased availability of nutrients (especially N) resulting from the intensified interspecific competition under co-cultivation conditions. Our previous studies have also provided evidence to support this conclusion [53,54,55,56]. More importantly, no significant differences were detected in the growth performance of B. pilosa between the monoculture and co-cultivation conditions in the majority of cases (p > 0.05; Figure 2, Figure 3, Figure 4 and Figure 5). Accordingly, the competitive advantage of B. pilosa is not affected by cultivation type. Consequently, B. pilosa exhibited a more pronounced competitive advantage compared to P. laciniata, especially under co-cultivation conditions.
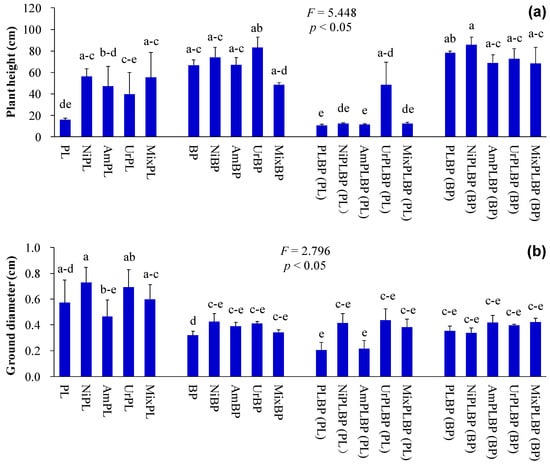
Figure 2.
Plant height and ground diameter of B. pilosa and P. laciniata under monoculture and co-cultivation conditions, respectively ((a), plant height; (b), ground diameter). Bars (mean and standard error, n = 3) with different lowercase letters representing statistically significant differences (p ≤ 0.05). Abbreviations: PL, monocultural P. laciniata; NiPL, monocultural P. laciniata treated with nitrate; AmPL, monocultural P. laciniata treated with ammonium; UrPL, monocultural P. laciniata treated with urea; MixPL, monocultural P. laciniata treated with mixed N; BP, monocultural B. pilosa; NiBP, monocultural B. pilosa treated with nitrate; AmBP, monocultural B. pilosa treated with ammonium; UrBP, monocultural B. pilosa treated with urea; MixBP, monocultural B. pilosa treated with mixed N; PLBP(PL), co-cultivated P. laciniata; NiPLBP(PL), co-cultivated P. laciniata treated with nitrate; AmPLBP(PL), co-cultivated P. laciniata treated with ammonium; UrPLBP(PL), co-cultivated P. laciniata treated with urea; MixPLBP(PL), co-cultivated P. laciniata treated with mixed N; PLBP(BP), co-cultivated B. pilosa; NiPLBP(BP), co-cultivated B. pilosa treated with nitrate; AmPLBP(BP), co-cultivated B. pilosa treated with ammonium; UrPLBP(BP), co-cultivated B. pilosa treated with urea; Mix AmPLBP(BP), co-cultivated B. pilosa treated with mixed N.
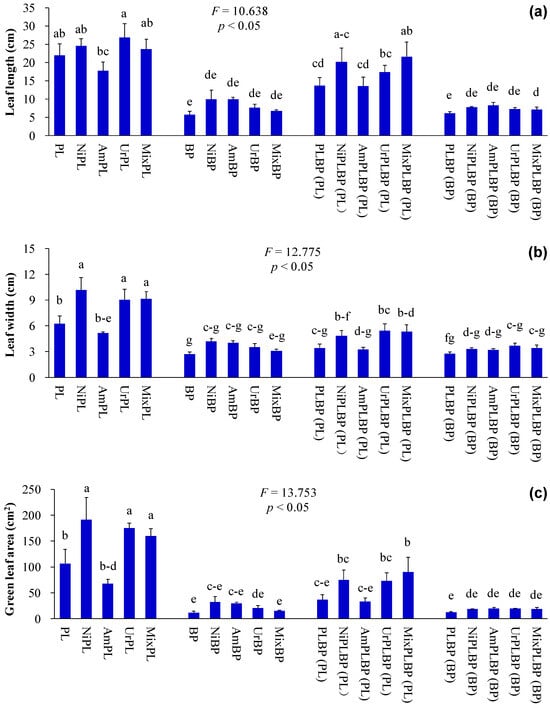
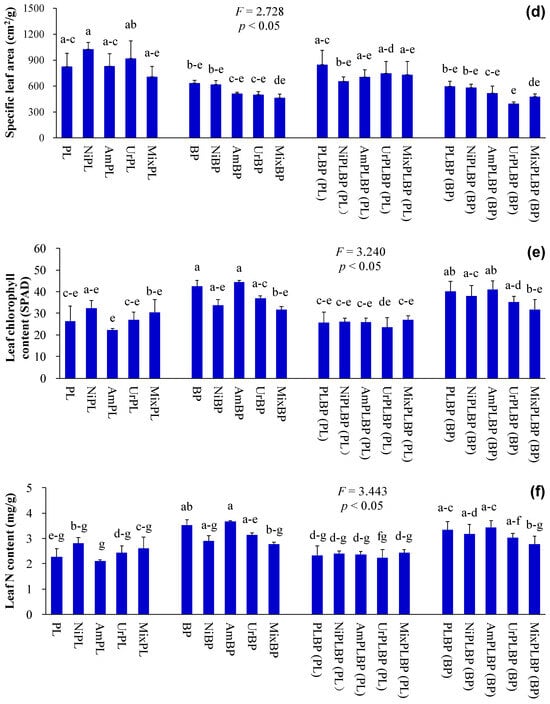
Figure 3.
Leaf functional traits of B. pilosa and P. laciniata under monoculture and co-cultivation conditions, respectively ((a), leaf length; (b), leaf width; (c), green leaf area, (d), specific leaf area, (e), leaf chlorophyll content; (f), leaf N content). Bars (mean and standard error, n = 3) with different lowercase letters representing statistically significant differences (p ≤ 0.05). Abbreviations have the same meanings as described in Figure 2.
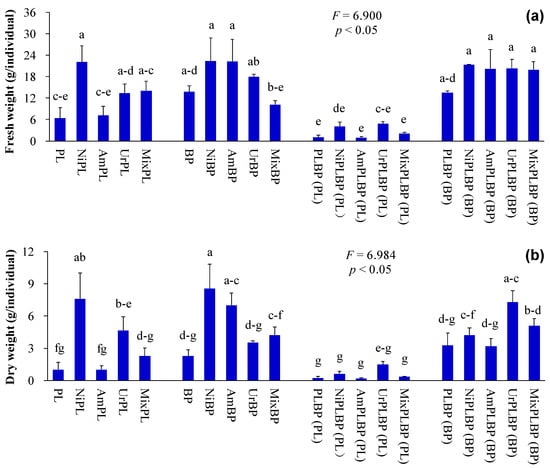
Figure 4.
Biomass of B. pilosa and P. laciniata under monoculture and co-cultivation conditions, respectively ((a), fresh weight; (b), dry weight). Bars (mean and standard error, n = 3) with different lowercase letters representing statistically significant differences (p ≤ 0.05). Abbreviations have the same meanings as described in Figure 2.
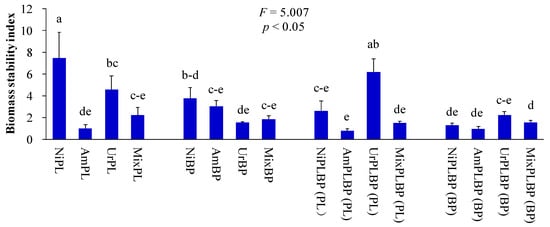
Figure 5.
The biomass stability index of B. pilosa and P. laciniata under monoculture and co-cultivation conditions, respectively. Bars (mean and standard error, n = 3) with different lowercase letters representing statistically significant differences (p ≤ 0.05). Abbreviations have the same meanings as described in Figure 2.
The functional differences between IPs and native plants may be of critical importance in determining the success of IPs. More importantly, the results demonstrated that IPs exhibited a more pronounced competitive advantage over native plants, which were recruited by the higher values of key functional traits, such as plant height, leaf area, photosynthetic capacity, nutrient use efficiency, and environmental tolerance, etc. Consequently, Ips demonstrated superior growth performance than native plants, even under stressful environments [11,14,15,16]. Similarly, the plant height, leaf chlorophyll and N contents, and plant peroxidase activity of B. pilosa were significantly higher than those of P. laciniata under both monoculture and co-cultivation conditions (p < 0.05; Figure 2, Figure 3 and Figure 6). More importantly, plant species significantly affected all functional indices (except ground diameter) (p < 0.00001; Table S2). Thus, B. pilosa exhibited a more pronounced competitive advantage in comparison to P. laciniata. The pronounced competitive advantage of B. pilosa is likely attributable to its stronger sunlight capture capacity, leaf photosynthetic capacity, and enzymatic defense capacity under stress to oxidative stress compared to P. laciniata. However, leaf length of B. pilosa was found to be significantly shorter than that of P. laciniata under both monoculture and co-cultivation conditions (p < 0.05; Figure 3). Thus, the leaf photosynthetic area of B. pilosa was found to be significantly smaller than that of P. laciniata under both monoculture and co-cultivation conditions. Accordingly, the leaf photosynthetic area does not appear to be a determining factor in the strong competitive advantage exhibited by B. pilosa. In other words, B. pilosa can obtain a strong competitive advantage mainly by means of partial key functional traits, e.g., stronger sunlight capture capacity, leaf photosynthetic capacity, and enzymatic defense capacity under stress to oxidative stress. The significantly functional differences between B. pilosa and P. laciniata permit B. pilosa to gain a stronger competitive advantage and to occupy more ecological niches in the habitats, which support the niche differentiation hypothesis (i.e., invasive and native species tend to exhibit functional divergence, resulting in invasive species exhibiting distinct functional traits compared to native species, thereby enabling the former to successfully invade new habitats via the higher growth competitiveness) [57,58,59,60] and the Darwin’s naturalization hypothesis (i.e., invasive species that are phylogenetically unrelated to native species should be more successful, as they can exploit the unoccupied ecological niches in the invaded communities) [61,62,63,64]. Accordingly, the “Master-of-some” strategy (i.e., invasive species are more competitive in favorable habitat, such as the increased resource availability), in contrast to the “Jack-of-all” strategy (i.e., invasive species are more competitive in stressful habitats, such as the decreased resource availability) or “Jack and master” strategy (i.e., invasive species are more competitive in both unfavorable and favorable habitats) [65,66,67], serves to enhance the competitive advantage of B. pilosa, especially under atmospheric nitrogen deposition.
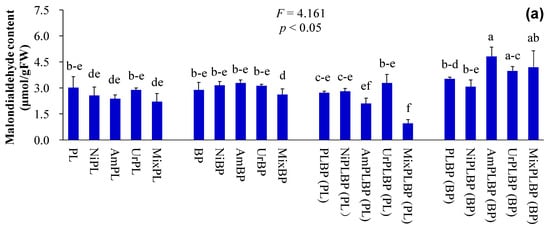
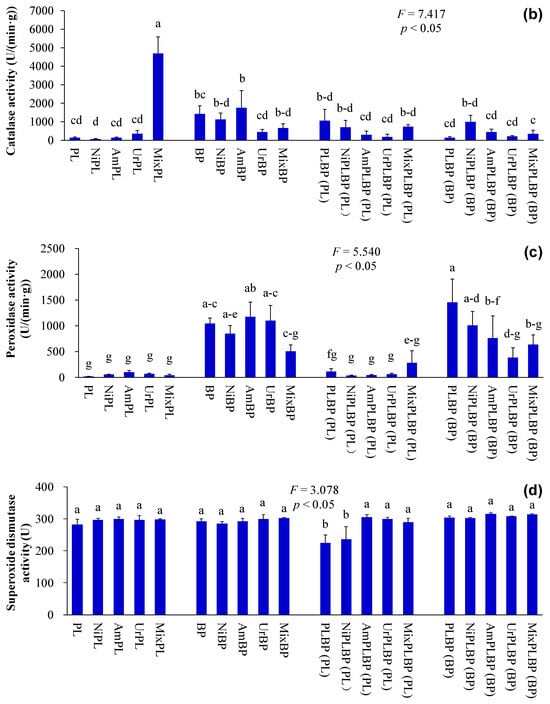
Figure 6.
Biochemical constituents and osmolytes indices of B. pilosa and P. laciniata under monoculture and co-cultivation conditions, respectively ((a), malondialdehyde content; (b), catalase activity; (c), peroxidase activity; (d), superoxide dismutase activity). Bars (mean and standard error, n = 3) with different lowercase letters represent statistically significant differences (p ≤ 0.05). Abbreviations have the same meanings as described in Figure 2.
As one of the essential nutrients required by plants, the application of exogenous N generally results in the enhanced growth performance of plants, attributed to the increased level of available N in soil. This is evidenced by numerous studies [68,69,70,71]. Similarly, the application of artificially simulated N deposition led to a significant increase in plant height, leaf width, and green leaf area of monocultural P. laciniata in the majority of cases (p < 0.05; Figure 2 and Figure 3). Thus, the application of artificially simulated N deposition may be beneficial to the growth performance of monocultural P. laciniata, particularly in terms of the sunlight capture capacity and leaf photosynthetic area.
It can be generally observed that N acquisition and utilization capacity is a crucial factor in the success of IPs [22,23,24,25]. Hence, the application of exogenous N can facilitate the invasiveness of IPs. In this study, the values of the relative dominance index of B. pilosa (average value is ≈0.8995) was found to be obviously greater than 0.5 when exposed to artificially simulated N deposition containing different N components, especially when exposed to ammonium (the relative dominance index of B. pilosa is ≈0.9363) and mixed nitrogen (the relative dominance index of B. pilosa is ≈0.9328) (Figure 7). Consequently, B. pilosa demonstrated a more pronounced competitive advantage than P. laciniata under the application of artificially simulated N deposition containing different N components, especially when treated with ammonium and mixed N. Accordingly, artificially simulated N deposition, regardless of N component, may be conducive to the success of P. laciniata, especially under the deposition of ammonium and mixed N. This finding may be attributed to the fact that B. pilosa exhibits a proclivity for ammonium uptake and utilization. In particular, previous studies have demonstrated that other IPs also displays a preference for ammonium uptake and utilization over other N components [42,72,73,74]. It is noteworthy that the relative proportion of ammonium in atmospheric N deposition is increasing in certain countries and regions, including China [75,76,77] and the United States of America [78,79,80]. Accordingly, the augmented relative proportion of ammonium in atmospheric N deposition may further facilitate the colonization of B. pilosa via a more pronounced competitive advantage.
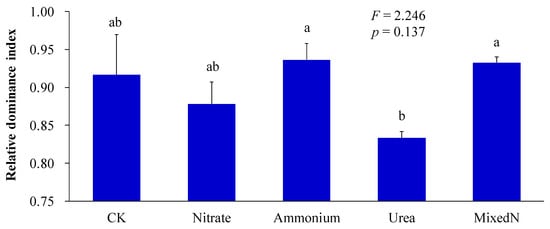
Figure 7.
The relative dominance index of B. pilosa under co-cultivation condition. Bars (mean and standard error, n = 3) with different lowercase letters represent statistically significant differences (p ≤ 0.05). Abbreviations have the same meanings as described in Figure 2.
In essence, there is a pressing need to impede or even halt the colonization of B. pilosa, especially under co-cultivation conditions and when exposed to atmospheric N deposition, particularly when there is an increase in the relative proportion of ammonium in atmospheric N deposition. The findings of this study also provide a substantial practical basis for the environmental management of IPs, including effective early warning prevention and control of IPs, especially when exposed to atmospheric N deposition. In particular, it is of great importance to reduce the level of atmospheric N deposition, in particular the proportion of ammonium, via the alterations in energy policy and the composition of energy sources employed. This is to minimize the competitive advantage of B. pilosa under atmospheric N deposition, especially with an increase in the relative proportion of ammonium in atmospheric N deposition.
4. Conclusions
In conclusion, this study aims to elucidate the functional differences between B. pilosa and P. laciniata in the context of atmospheric N deposition containing different N components. The principal findings are as follows: (1) The sunlight capture capacity, plant supporting capacity, leaf photosynthetic area, and plant growth competitiveness of co-cultivated P. laciniata were found to be significantly lower than those of monocultural P. laciniata. (2) The sunlight capture capacity, leaf photosynthetic capacity, and enzymatic defense capacity under stress to oxidative stress of B. pilosa were meaningfully greater than those of P. laciniata under both monoculture and co-cultivation conditions. (3) The results of the artificially simulated N deposition demonstrated a significant increase in plant height, leaf width, and green leaf area of monocultural P. laciniata in the majority of cases. (4) The values of the relative dominance index of B. pilosa were found to be significantly greater than 0.5 in response to artificially simulated N deposition containing different N components, especially when exposed to ammonium and mixed N. In summary, atmospheric N deposition, especially the increased relative proportion of ammonium in atmospheric N deposition, may facilitate the colonization of B. pilosa via a stronger competitive advantage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos15070825/s1, Table S1 [81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]: The ecological significances, determination methods, and the corresponding references for the determined indices; Table S2: Two-way ANOVA on the effects of plant species and nitrogen component on the functional indices, biochemical constituents, and osmolytes indices of B. pilosa and P. laciniata. p values equal to or less than 0.05 are shown in bold; Figure S1: Bidens pilosa L.; Figure S2: Pterocypsela laciniata (Houtt.) Shih; Figure S3: The geographical location (Zhenjiang, Jiangsu, China) of the sampling area (square with red) in this study (Map number: GS(2022)4317; produced by the Ministry of Natural Resources of China; http://bzdt.ch.mnr.gov.cn/ (accessed on 6 June 2024)); Figure S4: The picture of some of the garden pots used in this study.
Author Contributions
C.L.: data curation; investigation; methodology; writing—review and editing; Y.L. (Yue Li): data curation; investigation; methodology; writing—review and editing; Y.L. (Yingsheng Liu): data curation; investigation; methodology; writing—review and editing; S.Z.: data curation; investigation; methodology; writing—review and editing; H.Z.: funding acquisition; project administration; writing—review and editing; Z.X. (Zhelun Xu): data curation; investigation; methodology; writing—review and editing; Z.X. (Zhongyi Xu): data curation; formal analysis; writing—review and editing; D.D.: funding acquisition; project administration; writing—review and editing; C.W.: conceptualization; formal analysis; funding acquisition; project administration; supervision; writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Scientific Research Start-up Fund for High-level Talents of Jinling Institute of Technology (jit-rcyj-202302), Open Science Research Fund of Key Laboratory of Forest Plant Ecology, Ministry of Education (Northeast Forestry University), China (K2020B02), Special Research Project of School of Emergency Management, Jiangsu University (KY-C-01), National Natural Science Foundation of China (32071521), Research project on the application of invasive plants in soil ecological restoration in Jiangsu (20240110), Carbon Peak and Carbon Neutrality Technology Innovation Foundation of Jiangsu Province (BK20220030), and Jiangsu Collaborative Innovation Center of Technology and Material of Water Treatment (no grant number).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
Acknowledgments
We greatly appreciate the anonymous reviewers for the insightful comments that greatly improved this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| IPs | invasive plants |
| N | nitrogen |
| PL | monocultural P. laciniata |
| NiPL | monocultural P. laciniata treated with nitrate |
| AmPL | monocultural P. laciniata treated with ammonium |
| UrPL | monocultural P. laciniata treated with urea |
| MixPL | monocultural P. laciniata treated with mixed N |
| BP | monocultural B. pilosa |
| NiBP | monocultural B. pilosa treated with nitrate |
| AmBP | monocultural B. pilosa treated with ammonium |
| UrBP | monocultural B. pilosa treated with urea |
| MixBP | monocultural B. pilosa treated with mixed N |
| PLBP(PL) | co-cultivated P. laciniata |
| NiPLBP(PL) | co-cultivated P. laciniata treated with nitrate |
| AmPLBP(PL) | co-cultivated P. laciniata treated with ammonium |
| UrPLBP(PL) | co-cultivated P. laciniata treated with urea |
| MixPLBP(PL) | co-cultivated P. laciniata treated with mixed N |
| PLBP(BP) | co-cultivated B. pilosa |
| NiPLBP(BP) | co-cultivated B. pilosa treated with nitrate |
| AmPLBP(BP) | co-cultivated B. pilosa treated with ammonium |
| UrPLBP(BP) | co-cultivated B. pilosa treated with urea |
| Mix AmPLBP(BP) | co-cultivated B. pilosa treated with mixed N |
References
- Beshai, R.A.; Truong, D.A.; Henry, A.K.; Sorte, C.J.B. Biotic resistance or invasional meltdown? Diversity reduces invasibility but not exotic dominance in southern California epibenthic communities. Biol. Invasions 2023, 25, 533–549. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, A.; Kohli, R.K.; Singh, H.P.; Batish, D.R. Bidens pilosa (Asteraceae) invasion reshapes the pattern of plant communities and edaphic properties across the north-western Himalayan landscape. Ecol. Inform. 2023, 77, 102281. [Google Scholar] [CrossRef]
- Savage, C.; Savage, K.; Keller, K.R. Effect of Carpobrotus edulis invasion history on plant communities. West. N. Am. Nat. 2023, 83, 484–497. [Google Scholar] [CrossRef]
- Ettinger, C.L.; LaForgia, M.L. Invasive plant species interact with drought to shift key functions and families in the native rhizosphere. Plant Soil 2024, 494, 567–588. [Google Scholar] [CrossRef]
- Yan, X.L.; Liu, Q.R.; Shou, H.Y.; Zeng, X.F.; Zhang, Y.; Chen, L.; Liu, Y.; Ma, H.Y.; Qi, S.Y.; Ma, J.S. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodivers. Sci. 2014, 22, 667–676. [Google Scholar]
- Wang, C.Y.; Liu, J.; Xiao, H.G.; Zhou, J.W.; Du, D.L. Floristic characteristics of alien invasive seed plant species in China. An. Acad. Bras. Ciênc. 2016, 88, 1791–1797. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.J.; Png, G.K.; Jo, I.; McGrannachan, C.; Allen, K.; Peltzer, D.A.; D’Antonio, C.; Dickie, I.A.; French, K.; Leishman, M.R.; et al. Managing multi-species plant invasions when interactions influence their impact. Front. Ecol. Environ. 2023, 21, 370–379. [Google Scholar] [CrossRef]
- Guo, X.; Ma, J.Y.; Liu, L.L.; Li, M.Y.; Wang, H.; Sun, Y.K.; Wang, T.; Wang, K.L.; Meyerson, L.A. Effects of salt stress on interspecific competition between an invasive alien plant Oenothera biennis and three native species. Front. Plant Sci. 2023, 14, 1144511. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Dar, M.; Ahmad, M.; Singh, R.; Kumar Kohli, R.; Singh, H.P.; Batish, D.R. Invasive plants alter soil properties and nutrient dynamics: A case study of Anthemis cotula invasion in Kashmir Himalaya. Catena 2023, 226, 107069. [Google Scholar] [CrossRef]
- Minden, V.; Verhoeven, K.; Olde Venterink, H. Adaptive plasticity and fitness costs of endangered, nonendangered, and invasive plants in response to variation in nitrogen and phosphorus availabilities. Ecol. Evol. 2023, 13, e10075. [Google Scholar] [CrossRef]
- Khatri, K.; Negi, B.; Bargali, K.; Bargali, S.S. Trait variability in co-occurring invasive and native plant species in road side population of Kumaun Himalaya. Braz. J. Bot. 2022, 45, 1099–1110. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Tordoni, E.; Tomasella, M.; Savi, T.; Tonet, V.; Palandrani, C.; Castello, M.; Nardini, A.; Bacaro, G. Functional differentiation of invasive and native plants along a leaf efficiency/safety trade-off. Environ. Exp. Bot. 2021, 188, 104518. [Google Scholar] [CrossRef]
- Kumar, M.; Garkoti, S.C. Functional traits, growth patterns, and litter dynamics of invasive alien and co-occurring native shrub species of chir pine forest in the central Himalaya, India. Plant Ecol. 2021, 222, 723–735. [Google Scholar] [CrossRef]
- Hibit, J.; Daehler, C.C. Plant functional, biogeographical and phylogenetic diversity are related to native and non-native plant abundance in invaded Hawaiian forests. Biol. Invasions 2024, 26, 705–717. [Google Scholar] [CrossRef]
- Grotkopp, E.; Erskine-Ogden, J.; Rejmánek, M. Assessing potential invasiveness of woody horticultural plant species using seedling growth rate traits. J. Appl. Ecol. 2010, 47, 1320–1328. [Google Scholar] [CrossRef]
- Yu, Y.L.; Cheng, H.Y.; Wang, S.; Wei, M.; Wang, C.Y.; Du, D.L. Drought may be beneficial to the competitive advantage of Amaranthus spinosus. J. Plant Ecol. 2022, 15, 494–508. [Google Scholar] [CrossRef]
- Lenda, M.; Steudel, B.; Skórka, P.; Zagrodzka, Z.B.; Moroń, D.; Bączek-Kwinta, R.; Janowiak, F.; Baran, A.; Possingham, H.P.; Knops, J.M.H. Multiple invasive species affect germination, growth, and photosynthesis of native weeds and crops in experiments. Sci. Rep. 2023, 13, 22146. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.X.; Le Roux, J.J.; Jiang, Z.Y.; Sun, F.; Peng, C.L.; Li, W.H. Soil nitrogen dynamics and competition during plant invasion: Insights from Mikania micrantha invasions in China. New Phytol. 2021, 229, 3440–3452. [Google Scholar] [CrossRef]
- Francis, C.A.; Beman, J.M.; Kuypers, M.M.M. New processes and players in the nitrogen cycle: The microbial ecology of anaerobic and archaeal ammonia oxidation. ISME J. 2007, 1, 19–27. [Google Scholar] [CrossRef]
- van den Elzen, E.; van den Berg, L.J.L.; van der Weijden, B.; Fritz, C.; Sheppard, L.J.; Lamers, L.P.M. Effects of airborne ammonium and nitrate pollution strongly differ in peat bogs, but symbiotic nitrogen fixation remains unaffected. Sci. Total Environ. 2018, 610–611, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.Y.; Jiang, S.; Li, B.; Pan, X.Y. Higher resource capture ability and utilization efficiency facilitate the successful invasion of exotic plant? A case study of Alternanthera philoxeroides. Am. J. Plant Sci. 2013, 4, 1839–1845. [Google Scholar] [CrossRef]
- Osunkoya, O.O.; Bayliss, D.; Panetta, F.D.; Vivian-Smith, G. Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann. Bot. 2010, 106, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Lei, Y.B.; Wang, R.F.; Callaway, R.M.; Alfonso, V.B.; Inderjit; Li, Y.P.; Zheng, Y.L. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc. Natl. Acad. Sci. USA 2009, 106, 1853–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.W.; Zhang, X.Y.; Liang, J.F.; Gao, J.Q.; Xu, X.L.; Yu, F.H. High nitrogen uptake and utilization contribute to the dominance of invasive Spartina alterniflora over native Phragmites australis. Biol. Fertil. Soils 2021, 57, 1007–1013. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Kamutando, C.N.; Vikram, S.; Kamgan-Nkuekam, G.; Makhalanyane, T.P.; Greve, M.; Le Roux, J.J.; Richardson, D.M.; Cowan, D.; Valverde, A. Soil nutritional status and biogeography influence rhizosphere microbial communities associated with the invasive tree Acacia dealbata. Sci. Rep. 2017, 7, 6472. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.W.; Yang, M.Z.; Chen, Y.J.; Chen, L.M.; Zhang, D.Z.; Mei, L.; Shi, Y.T.; Zhang, H.B. Changes in non-symbiotic nitrogen-fixing bacteria inhabiting rhizosphere soils of an invasive plant Ageratina adenophora. Appl. Soil Ecol. 2012, 54, 32–38. [Google Scholar] [CrossRef]
- Li, J.; He, J.Z.; Liu, M.; Yan, Z.Q.; Xu, X.L.; Kuzyakov, Y. Invasive plant competitivity is mediated by nitrogen use strategies and rhizosphere microbiome. Soil Biol. Biochem. 2024, 192, 109361. [Google Scholar] [CrossRef]
- Chen, H.Y.; Huang, S.Z. Composition and supply of inorganic and organic nitrogen species in dry and wet atmospheric deposition: Use of organic nitrogen composition to calculate the Ocean’s external nitrogen flux from the atmosphere. Cont. Shelf Res. 2021, 213, 104316. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Zhang, F.; Zhang, X.; Xu, W.; Liu, X.; Li, Y.; Wang, Z.; Xie, Y. Enhanced nitrous oxide emissions caused by atmospheric nitrogen deposition in agroecosystems over China. Environ. Sci. Pollut. Res. 2021, 28, 15350–15360. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Zhang, F.; Zhang, X.; Xu, W.; Liu, X.; Wang, Z.; Xie, Y. Soil Nitrous Oxide Emissions by Atmospheric Nitrogen Deposition over Global Agricultural Systems. Environ. Sci. Technol. 2021, 55, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Dentener, F.; Drevet, J.; Lamarque, J.F.; Bey, I.; Eickhout, B.; Fiore, A.M.; Hauglustaine, D.; Horowitz, L.W.; Krol, M.; Kulshrestha, U.C.; et al. Nitrogen and sulfur deposition on regional and global scales: A multimodel evaluation. Glob. Biogeochem. Cycles 2006, 20, GB4003. [Google Scholar] [CrossRef]
- Fu, Y.D.; Xu, W.; Wen, Z.; Han, M.J.; Sun, J.H.; Tang, A.H.; Liu, X. Enhanced atmospheric nitrogen deposition at a rural site in northwest China from 2011 to 2018. Atmos. Res. 2020, 245, 105071. [Google Scholar] [CrossRef]
- Zhu, J.X.; Chen, Z.; Wang, Q.F.; Xu, L.; He, N.P.; Jia, Y.L.; Zhang, Q.Y.; Yu, G.R. Potential transition in the effects of atmospheric nitrogen deposition in China. Environ. Pollut. 2020, 258, 113739. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, L.; Liu, X.J. A database of atmospheric nitrogen concentration and deposition from the nationwide monitoring network in China. Sci. Data 2019, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.A.; Braswell, B.H.; Sulzman, J.; Lamarque, J.F. Nitrogen deposition onto the United States and Western Europe: Synthesis of observations and models. Ecol. Appl. 2005, 15, 38–57. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Zhang, X.; Dobermann, A.; Heffer, P.; Zhou, F. Global nitrogen deposition inputs to cropland at national scale from 1961 to 2020. Sci. Data 2023, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Ussher, S.J.; Fitzsimons, M.F.; Atkinson, S.; Woodward, E.M.S.; Yang, M.; Bell, T.G. Inorganic nitrogen and phosphorus in Western European aerosol and the significance of dry deposition flux into stratified shelf waters. Atmos. Environ. 2021, 261, 118391. [Google Scholar] [CrossRef]
- Ren, G.Q.; Yang, B.; Cui, M.M.; Dai, Z.C.; Xiang, Y.; Zhang, H.Y.; Li, G.L.; Li, J.; Javed, Q.; Du, D.L. Warming and elevated nitrogen deposition accelerate the invasion process of Solidago canadensis L. Ecol. Process. 2022, 11, 62. [Google Scholar] [CrossRef]
- Ding, W.L.; Xu, W.Z.; Gao, Z.J.; Xu, B.C. Effects of water and nitrogen on growth and relative competitive ability of introduced versus native C-4 grass species in the semi-arid Loess Plateau of China. J. Arid Land 2021, 13, 730–743. [Google Scholar] [CrossRef]
- Zhong, S.S.; Xu, Z.L.; Yu, Y.L.; Liu, J.; Wang, Y.Y.; Guo, E.; Wang, C.Y. Rhus typhina decreased soil nitrogen contents and peroxidase activity following the addition of nitrogen. Int. J. Environ. Sci. Technol. 2023, 111, 17–22. [Google Scholar] [CrossRef]
- Sparrius, L.B.; Kooijman, A.M. Invasiveness of Campylopus introflexus in drift sands depends on nitrogen deposition and soil organic matter. Appl. Veg. Sci. 2011, 14, 221–229. [Google Scholar] [CrossRef]
- Mircea, D.M.; Calone, R.; Estrelles, E.; Soriano, P.; Sestras, R.E.; Boscaiu, M.; Sestras, A.F.; Vicente, O. Responses of different invasive and non-invasive ornamental plants to water stress during seed germination and vegetative growth. Sci. Rep. 2023, 13, 13281. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.L.; He, Q.S.; Xie, R.Q.; Hou, J.H.; Shi, C.L.; Li, J.M.; Yu, F.H. Interactive effects of nutrient availability, fluctuating supply, and plant parasitism on the post-invasion success of Bidens pilosa. Biol. Invasions 2021, 23, 3035–3046. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Zhong, S.S.; Xu, Z.L.; Xu, Z.Y.; Zhu, M.W.; Wei, Y.Q.; Wang, C.Y.; Du, D.L. Do the leaves of multiple invasive plants decompose more easily than a native plant’s under nitrogen deposition with different forms? Nitrogen 2024, 5, 202–218. [Google Scholar] [CrossRef]
- Zhejiang Provincial Bureau of Statistics. Zhenjiang Statistical Yearbook 2022; China Statistics Press: Beijing, China, 2022.
- Luo, X.S.; Liu, X.J.; Pan, Y.P.; Wen, Z.; Xu, W.; Zhang, L.; Kou, C.L.; Lv, J.L.; Goulding, K. Atmospheric reactive nitrogen concentration and deposition trends from 2011 to 2018 at an urban site in north China. Atmos. Environ. 2020, 224, 117298. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, L.; Liu, X.J.; Li, W.Q.; Lu, S.H.; Zheng, L.X.; Bai, Z.C.; Cai, G.Y.; Zhang, F.S. Atmospheric organic nitrogen deposition in China. Atmos. Environ. 2012, 49, 422. [Google Scholar] [CrossRef]
- Cornell, S.E. Atmospheric nitrogen deposition: Revisiting the question of the importance of the organic component. Environ. Pollut. 2011, 159, 2214–2222. [Google Scholar] [CrossRef] [PubMed]
- Cornell, S.E.; Jickells, T.D.; Cape, J.N.; Rowland, A.P.; Duce, R.A. Organic nitrogen deposition on land and coastal environments: A review of methods and data. Atmos. Environ. 2003, 37, 2173–2191. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.D.; Zhang, H.S.; Jiang, K.; Zhou, J.W.; Wang, C.Y. Erigeron canadensis affects the taxonomic and functional diversity of plant communities in two climate zones in the North of China. Ecol. Res. 2019, 34, 535–547. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cheng, H.Y.; Wu, B.D.; Jiang, K.; Wang, S.; Wei, M.; Du, D.L. The functional diversity of native ecosystems increases during the major invasion by the invasive alien species, Conyza canadensis. Ecol. Eng. 2021, 159, 106093. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, B.D.; Jiang, K.; Zhou, J.W. Differences in functional traits between invasive and native Amaranthus species under simulated acid deposition with a gradient of pH levels. Acta Oecol. 2018, 89, 32–37. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Zhong, S.S.; Xu, Z.L.; Liu, J.; Xu, Z.Y.; Zhu, M.W.; Wang, C.Y.; Du, D.L. Is the invasive plant Amaranthus spinosus L. more competitive than the native Plant A. tricolor L. when exposed to acid deposition with different sulfur-nitrogen ratios? Atmosphere 2024, 15, 29. [Google Scholar] [CrossRef]
- Ordonez, A.; Wright, I.J.; Olff, H. Functional differences between native and alien species: A global-scale comparison. Funct. Ecol. 2010, 24, 1353–1361. [Google Scholar] [CrossRef]
- Maire, V.; Gross, N.; Boerger, L.; Proulx, R.; Wirth, C.; Pontes, L.d.S.; Soussana, J.-F.; Louault, F. Habitat filtering and niche differentiation jointly explain species relative abundance within grassland communities along fertility and disturbance gradients. New Phytol. 2012, 196, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Boerger, L.; Duncan, R.P.; Hulme, P.E. Functional differences between alien and native species: Do biotic interactions determine the functional structure of highly invaded grasslands? Funct. Ecol. 2013, 27, 1262–1272. [Google Scholar] [CrossRef]
- Silveira, S.D.; Guimaraes, M. The enemy within: Consequences of the invasive bullfrog on native anuran populations. Biol. Invasions 2021, 23, 373–378. [Google Scholar] [CrossRef]
- Pinto-Ledezma, J.N.; Villalobos, F.; Reich, P.B.; Catford, J.A.; Larkin, D.J.; Cavender-Bares, J. Testing Darwin’s naturalization conundrum based on taxonomic, phylogenetic, and functional dimensions of vascular plants. Ecol. Monogr. 2020, 90, e01420. [Google Scholar] [CrossRef]
- Thuiller, W.; Gallien, L.; Boulangeat, I.; De Bello, F.; Münkemüller, T.; Roquet, C.; Lavergne, S. Resolving Darwin’s naturalization conundrum: A quest for evidence. Divers. Distrib. 2010, 16, 461–475. [Google Scholar] [CrossRef]
- Fan, S.Y.; Yang, Q.; Li, S.P.; Fristoe, T.S.; Cadotte, M.W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; et al. A latitudinal gradient in Darwin’s naturalization conundrum at the global scale for flowering plants. Nat. Commun. 2023, 14, 6244. [Google Scholar] [CrossRef]
- Omer, A.; Fristoe, T.; Yang, Q.; Razanajatovo, M.; Weigelt, P.; Kreft, H.; Dawson, W.; Dullinger, S.; Essl, F.; Pergl, J.; et al. The role of phylogenetic relatedness on alien plant success depends on the stage of invasion. Nat. Plants 2022, 8, 906–914. [Google Scholar] [CrossRef]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef]
- Funk, J.L. Differences in plasticity between invasive and native plants from a low resource environment. J. Ecol. 2008, 96, 1162–1173. [Google Scholar] [CrossRef]
- Matzek, V. Trait values, not trait plasticity, best explain invasive species’ performance in a changing environment. PLoS ONE 2012, 7, e48821. [Google Scholar] [CrossRef] [PubMed]
- Amanullah; Marwat, K.B.; Shah, P.; Maula, N.; Arifullah, S. Nitrogen levels and its time of application influence leaf area, height and biomass of maize planted at low and high density. Pak. J. Bot. 2009, 41, 761–768. [Google Scholar]
- Cheng, H.Y.; Wei, M.; Wang, S.; Wu, B.D.; Wang, C.Y. Atmospheric N deposition alleviates the unfavorable effects of drought on wheat growth. Braz. J. Bot. 2020, 43, 229–238. [Google Scholar] [CrossRef]
- Bai, T.; Liu, Y.Y.; Muhammad, I.; Yang, X.; Yin, X.J.; Bai, L.; Wang, Y.J. Mixed nitrogen form addition facilitates the growth adaptation of legume plant to heavy metal contamination in degraded mining areas. Glob. Ecol. Conserv. 2020, 24, e01387. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Peñuelas, J.L.; Nicolás-Peragón, J.L.; Benito, L.F.; Domínguez-Lerena, S. Is nitrogen fertilization in the nursery a suitable tool for enhancing the performance of Mediterranean oak plantations? New For. 2013, 44, 733–751. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Xu, H.; Fan, Z.W.; Hou, Y.P. Effects of Rhus typhina invasion into young Pinus thunbergii forests on soil chemical properties. Ecol. Environ. Sci. 2013, 22, 1119–1123. [Google Scholar]
- Hou, Y.P.; Liu, L.; Chu, H.; Ma, S.J.; Zhao, D.; Liang, R.R. Effects of exotic plant Rhus typhina invasion on soil properties in different forest types. Acta Ecol. Sin. 2015, 35, 5324–5330. [Google Scholar]
- Huangfu, C.H.; Li, H.Y.; Chen, X.W.; Liu, H.M.; Wang, H.; Yang, D.L. Response of an invasive plant, Flaveria bidentis, to nitrogen addition: A test of form-preference uptake. Biol. Invasions 2016, 18, 3365–3380. [Google Scholar] [CrossRef]
- Chen, S.L.; Chen, B.; Wang, S.Q.; Sun, L.G.; Shi, H.; Liu, Z.H.; Wang, Q.Y.; Li, H.; Zhu, T.T.; Li, D.H.; et al. Spatiotemporal variations of atmospheric nitrogen deposition in China during 2008–2020. Atmos. Environ. 2023, 315, 120120. [Google Scholar] [CrossRef]
- Dong, J.; Li, B.; Li, Y.; Zhou, R.; Gan, C.; Zhao, Y.; Liu, R.; Yang, Y.; Wang, T.; Liao, H. Atmospheric ammonia in China: Long-term spatiotemporal variation, urban-rural gradient, and influencing factors. Sci. Total Environ. 2023, 883, 163733. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xing, C.; Li, Q.; Wang, S.; Hu, Q.; Zhu, Y.; Liu, T.; Zhang, C.; Liu, C. Long-term spatiotemporal variations of ammonia in the Yangtze River Delta region of China and its driving factors. J. Environ. Sci. 2025, 150, 202–217. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Burns, D.A.; Driscoll, C.T.; Frey, S.D.; Lovett, G.M.; Watmough, S.A. Decreased atmospheric nitrogen deposition in eastern North America: Predicted responses of forest ecosystems. Environ. Pollut. 2019, 244, 560–574. [Google Scholar] [CrossRef]
- Conrad-Rooney, E.; Gewirtzman, J.; Pappas, Y.; Pasquarella, V.J.; Hutyra, L.R.; Templer, P.H. Atmospheric wet deposition in urban and suburban sites across the United States. Atmos. Environ. 2023, 305, 119783. [Google Scholar] [CrossRef]
- Johansen, A.M.; Duncan, C.; Reddy, A.; Swain, N.; Sorey, M.; Nieber, A.; Agren, J.; Lenington, M.; Bolstad, D.; Samora, B.; et al. Precipitation chemistry and deposition at a high-elevation site in the Pacific Northwest United States (1989–2015). Atmos. Environ. 2019, 212, 221–230. [Google Scholar] [CrossRef]
- Wang, S.; Wei, M.; Cheng, H.Y.; Wu, B.D.; Du, D.L.; Wang, C.Y. Indigenous plant species and invasive alien species tend to diverge functionally under heavy metal pollution and drought stress. Ecotox. Environ. Safe. 2020, 205, 111160. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, B.D.; Wang, C.Y.; Ran, Q. Ecotoxicological effects of metals with different concentrations and types on the morphological and physiological performance of wheat. Ecotox. Environ. Safe. 2019, 167, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rybiński, W.; Garczyński, S. Influence of laser light on leaf area and parameters of photosynthetic activity in DH lines of spring barley (Hordeum vulgare L.). Int. Agrophys. 2004, 18, 261–267. [Google Scholar]
- Xia, T.T.; Miao, Y.X.; Wu, D.L.; Shao, H.; Khosla, R.; Mi, G.H. Active optical sensing of spring maize for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef]
- Huang, S.S.; Sun, L.Q.; Hu, X.; Wang, Y.H.; Zhang, Y.J.; Nevo, E.; Peng, J.H.; Sun, D.F. Associations of canopy leaf traits with SNP markers in durum wheat (Triticum turgidum L. durum (Desf.)). PLoS ONE 2018, 13, e0206226. [Google Scholar] [CrossRef] [PubMed]
- Anwar, J.; Subhani, G.M.; Hussain, M.; Ahmad, J.; Hussain, M.; Munir, M. Drought tolerance indices and their correlation with yield in exotic wheat genotypes. Pak. J. Bot. 2011, 43, 1527–1530. [Google Scholar]
- Farshadfar, E.; Elyasi, P. Screening quantitative indicators of drought tolerance in bread wheat (Triticum aestivum L.) landraces. Eur. J. Exp. Biol. 2012, 2, 577–584. [Google Scholar]
- Sánchez-Reinoso, A.D.; Ligarreto-Moreno, G.; Restrepo-Díaz, H. Evaluation of drought indices to identify tolerant genotypes in common bean bush (Phaseolus vulgaris L.). J. Integr. Agric. 2020, 19, 99–107. [Google Scholar] [CrossRef]
- Darkwa, K.; Ambachew, D.; Mohammed, H.; Asfaw, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop J. 2016, 4, 367–376. [Google Scholar] [CrossRef]
- Niu, H.B.; Liu, W.X.; Wan, F.H.; Liu, B. An invasive aster (Ageratina adenophora) invades and dominates forest understories in China: Altered soil microbial communities facilitate the invader and inhibit natives. Plant Soil 2007, 294, 73–85. [Google Scholar] [CrossRef]
- Ding, W.; Wang, R.; Yuan, Y.; Liang, X.; Liu, J. Effects of nitrogen deposition on growth and relationship of Robinia pseudoacacia and Quercus acutissima seedlings. Dendrobiology 2012, 67, 3–13. [Google Scholar]
- Yuan, Y.F.; Guo, W.H.; Ding, W.J.; Du, N.; Luo, Y.J.; Liu, J.; Xu, F.; Wang, R.Q. Competitive interaction between the exotic plant Rhus typhina L. and the native tree Quercus acutissima Carr. in Northern China under different soil N:P ratios. Plant Soil 2013, 372, 389–400. [Google Scholar] [CrossRef]
- Li, L. Experimental Guidance of Plant Physiology Module, 1st ed.; Science Press: Beijing, China, 2009. [Google Scholar]
- Zhang, J.E. Experimental Methods and Techniques Commonly Used in Ecology; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Cao, Y.; Luo, Q.X.; Tian, Y.; Meng, F.J. Physiological and proteomic analyses of the drought stress response in Amygdalus Mira (Koehne) Yü et Lu roots. BMC Plant Biol. 2017, 17, 53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).