Seasonal Patterns and Allergenicity of Casuarina Pollen in Sydney, Australia: Insights from 10 Years of Monitoring and Skin Testing
Abstract
1. Introduction
2. Materials and Methods
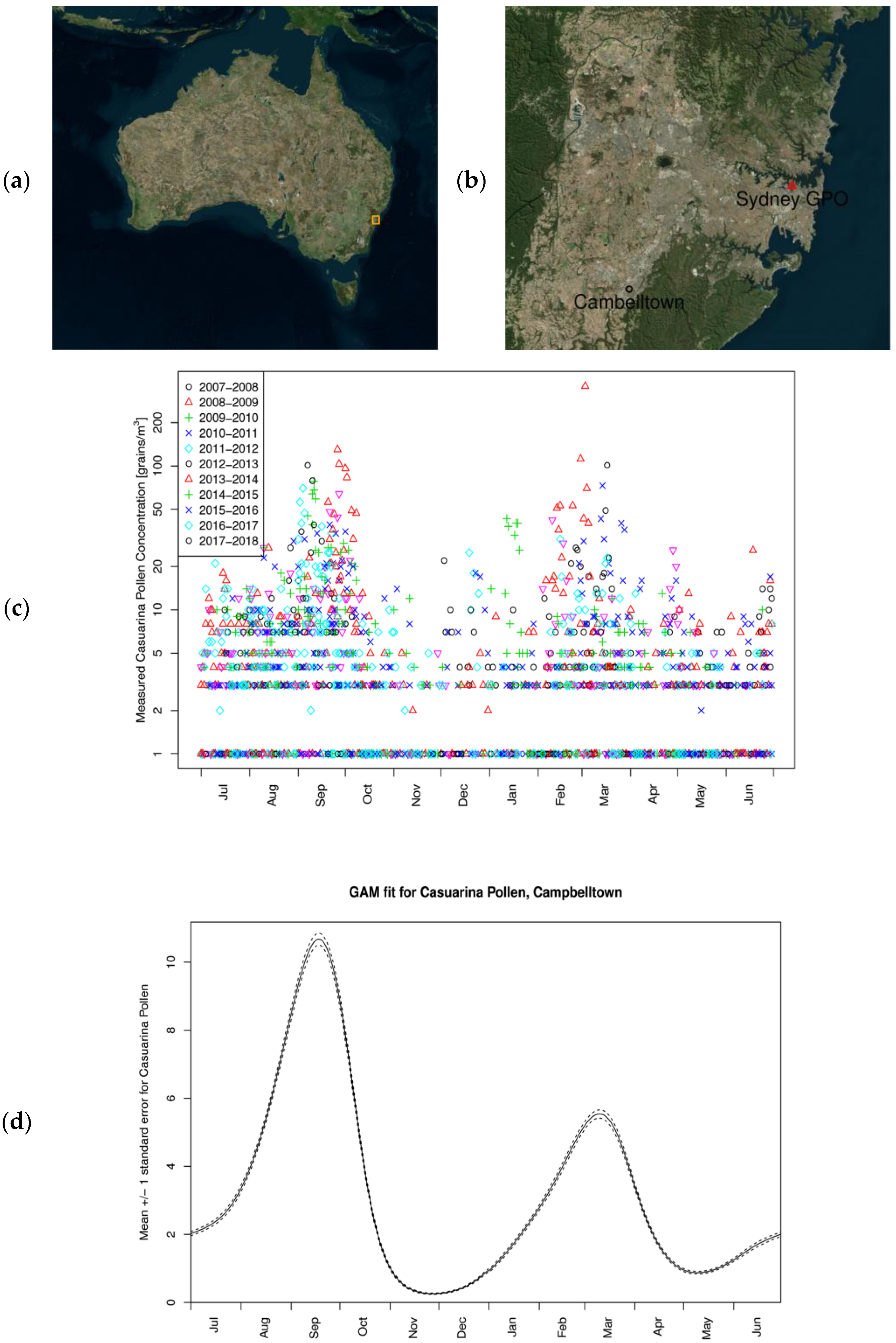
2.1. Study Sites and Airborne Pollen Collection
2.2. Environmental Variables
2.3. Skin Prick Test Data
2.4. Statistical Analysis
3. Results
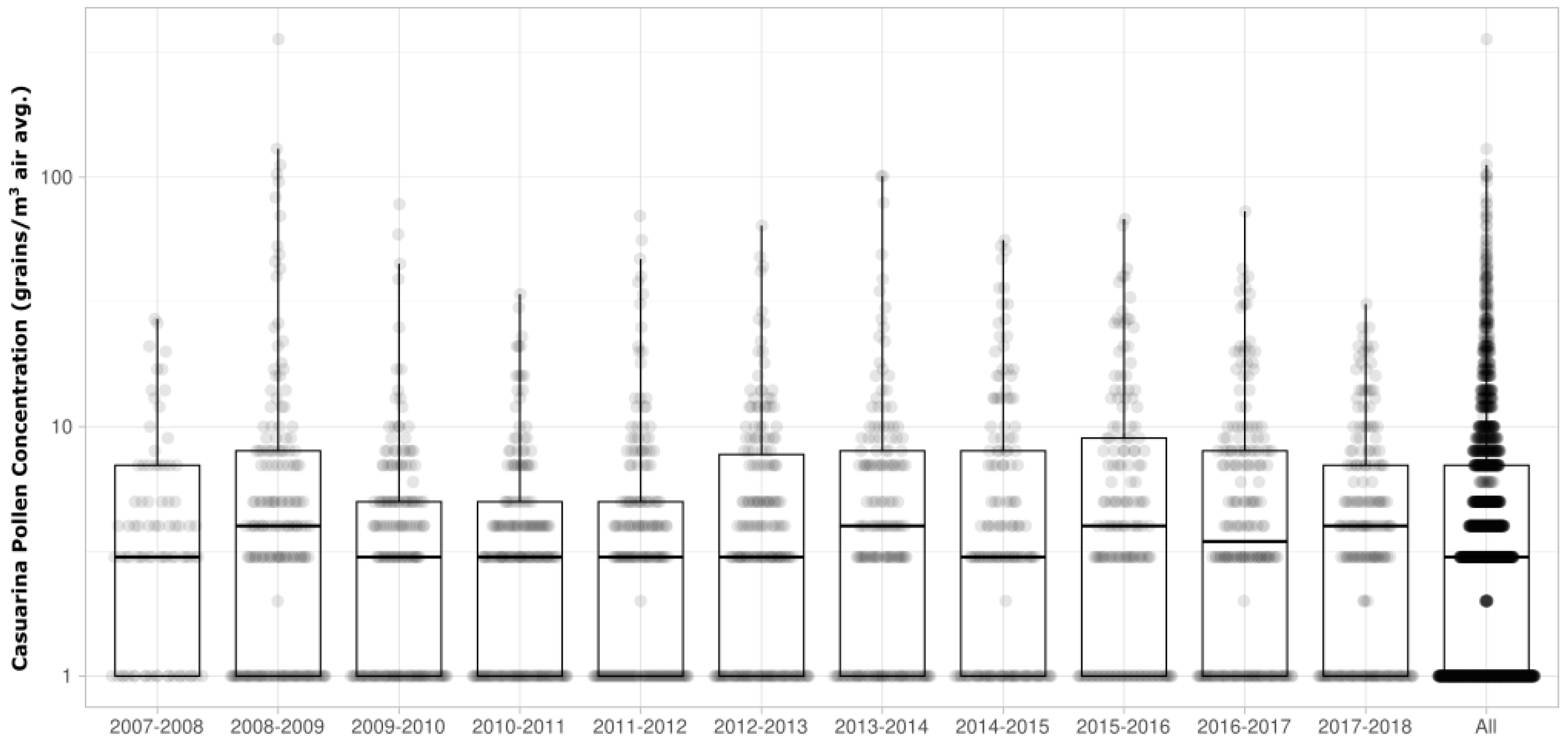
3.1. Casuarina Pollen Monitoring in Campbelltown, Sydney Australia
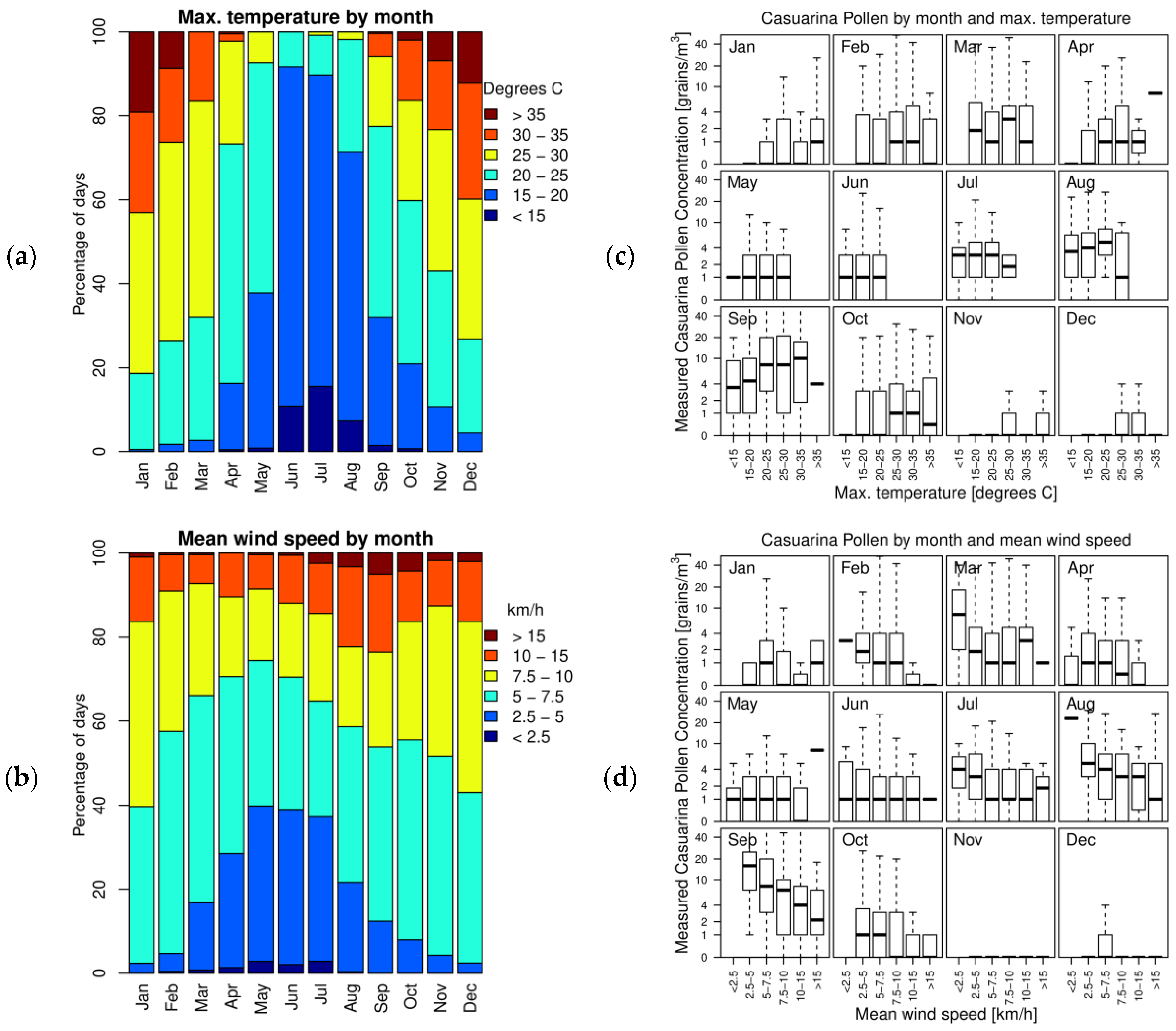
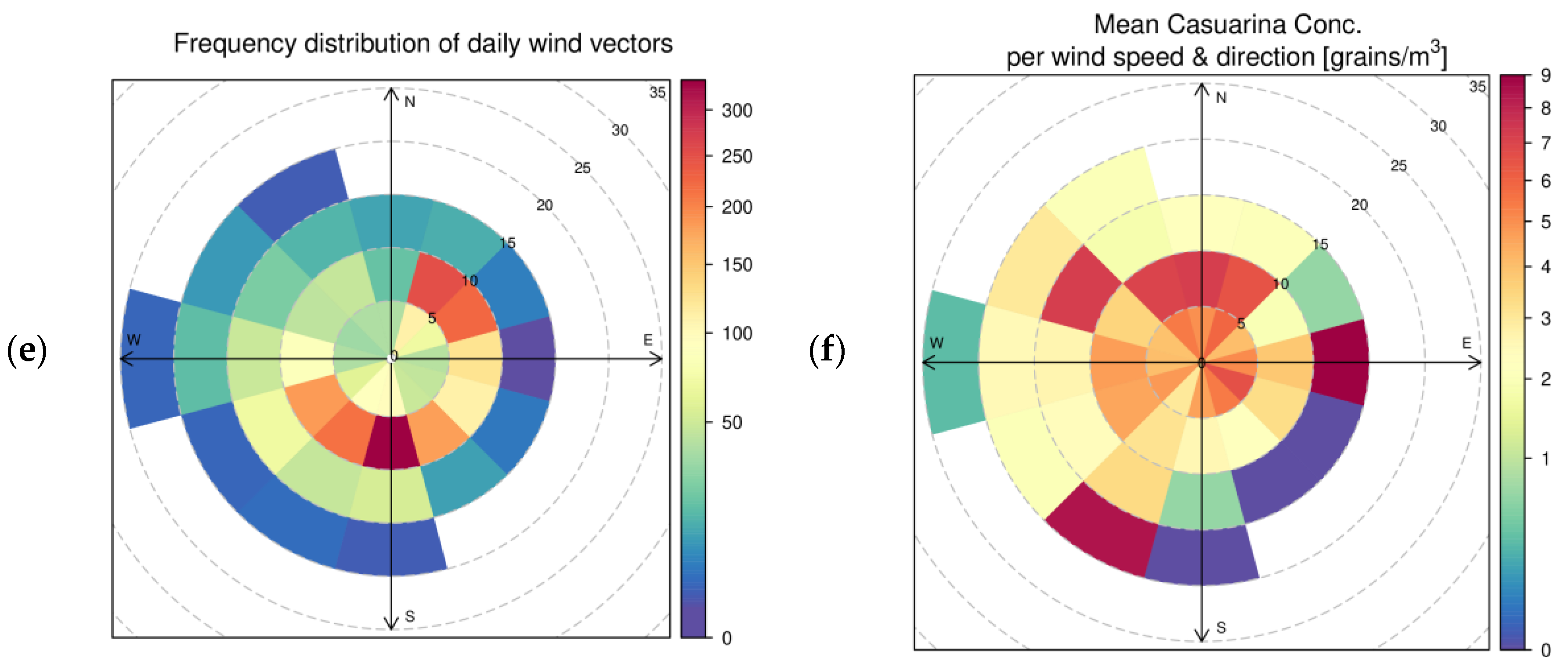
3.2. Factors Which Modulate Casuarina Pollen Concentration in the Air
3.3. Skin Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines; 2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, L.J.; Richardson, D.M.; Wilson, J.R.U. Casuarina: Biogeography and ecology of an important tree genus in a changing world. Biol. Invasions 2014, 16, 609–633. [Google Scholar] [CrossRef]
- Taylor, E.L.; Taylor, T.N.; Krings, M. Flowering Plants. In Paleobotany, 2nd ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Coetzee, J.A.; Praglowski, J. Pollen evidence for the occurrence of Casuarina and Myrica in the Tertiary of South Africa. Grana 1984, 23, 23–41. [Google Scholar] [CrossRef]
- Lambert, K.A.; Katelaris, C.; Burton, P.; Cowie, C.; Lodge, C.; Garden, F.L.; Prendergast, L.A.; Toelle, B.G.; Erbas, B. Tree pollen exposure is associated with reduced lung function in children. Clin. Exp. Allergy 2020, 50, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Katelaris, C.H.; Burke, T.V. A 7 year pollen profile of major Olympic Games venues in Sydney, Australia. Aerobiologia 2003, 19, 121–124. [Google Scholar] [CrossRef]
- Watanabe, M.; Igishi, T.; Burioka, N.; Yamasaki, A.; Kurai, J.; Takeuchi, H.; Sako, T.; Yoshida, A.; Yoneda, K.; Fukuoka, Y.; et al. Pollen Augments the Influence of Desert Dust on Symptoms of Adult Asthma Patients. Allergol. Int. 2011, 60, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.K.; Singh, M.B.; Knox, R.B. Grass pollen in the atmosphere of Melbourne: Seasonal distribution over nine years. Grana 1995, 34, 58–63. [Google Scholar] [CrossRef]
- Wood, S. Generalized Additive Models; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Postma, M.; Goedhart, J. PlotsOfData—A web app for visualizing data together with their summaries. PLoS Biol. 2019, 17, e3000202. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.; Haberle, S.G.; Johnston, F.H.; Bowman, D.M. Seasonal distribution of pollen in the atmosphere of Darwin, tropical Australia: Preliminary results. Grana 2007, 46, 34–42. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Publisher Correction: Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2020, 7, 274. [Google Scholar] [CrossRef]
- Bass, D.J.; Delpech, V.; Beard, J.; Bass, P.; Walls, R.S. Late summer and fall (March–May) pollen allergy and respiratory disease in Northern New South Wales, Australia. Ann. Allergy Asthma Immunol. 2000, 85, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Green, B.J.; Dettmann, M.E.; Rutherford, S.; Simpson, R.W. Airborne pollen of Brisbane, Australia: A five-year record, 1994–1999. Grana 2002, 41, 242–250. [Google Scholar] [CrossRef]
- Nagarajan, B.; Nicodemus, A.; Sivakumar, V.; Mandal, A.K.; Kumaravelu, G.; Jayaraj, R.S.C.; Bai, V.N.; Kamalakannan, R. Phenology and Control Pollination Studies in Casuarina equisetifolia Forst. Silvae Genet. 2006, 55, 149–155. [Google Scholar] [CrossRef]
- Green, B.J.; Dettmann, M.E.; Yli-Panula, E.; Rutherford, S.; Simpson, R. Aeropalynology of Australian native arboreal species in Brisbane, Australia. Aerobiologia 2004, 20, 43–52. [Google Scholar] [CrossRef]
- Zivitz, N. Allergy to Australian pine: A report of three cases. J. Allergy 1942, 13, 314–316. [Google Scholar] [CrossRef]
- Bucholtz, G.A.; Hensel, A.E.; Lockey, R.F.; Serbousek, D.; Wunderlin, R.P. Australian pine (Casuarina equisetifolia) pollen as an aeroallergen. Ann. Allergy 1987, 59, 52–56. [Google Scholar] [PubMed]
- Agashe, S.N.; Bapat, B.N.; Bapat, H.N.; Philip, E. Aerobiology of Casuarina pollen and its significance as a potential aeroallergen. Aerobiologia 1994, 10, 123–128. [Google Scholar] [CrossRef]
- Sercombe, J.K.; Green, B.J.; Rimmer, J.; Burton, P.K.; Katelaris, C.H.; Tovey, E.R. London Plane Tree bioaerosol exposure and allergic sensitization in Sydney, Australia. Ann. Allergy Asthma Immunol. 2011, 107, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E. Eucalyptus pollen allergy and asthma in children: A cross-sectional study in South-East Queensland, Australia. PLoS ONE 2015, 10, e0126506. [Google Scholar] [CrossRef]
- Erbas, B.; Akram, M.; Dharmage, S.C.; Tham, R.; Dennekamp, M.; Newbigin, E.; Taylor, P.; Tang, M.L.K.; Abramson, M.J. The role of seasonal grass pollen on childhood asthma emergency department presentations. Clin. Exp. Allergy 2012, 42, 799–805. [Google Scholar] [CrossRef]
- Silver, J.D.; Sutherland, M.F.; Johnston, F.H.; Lampugnani, E.R.; McCarthy, M.A.; Jacobs, S.J.; Pezza, A.B.; Newbigin, E.J. Seasonal asthma in Melbourne, Australia, and some observations on the occurrence of thunderstorm asthma and its predictability. PLoS ONE 2018, 13, e0194929. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, I.C.; Johnston, F.H. Respiratory hospital admissions were associated with ambient airborne pollen in Darwin, Australia, 2004–2005. Clin. Exp. Allergy 2007, 37, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.H.; Hanigan, I.C.; Bowman, D.M. Pollen loads and allergic rhinitis in Darwin, Australia: A potential health outcome of the grass-fire cycle. Ecohealth 2009, 6, 99–108. [Google Scholar] [CrossRef]
- Shrestha, S.K.; Katelaris, C.; Dharmage, S.C.; Burton, P.; Vicendese, D.; Tham, R.; Abramson, M.J.; Erbas, B. High ambient levels of grass, weed and other pollen are associated with asthma admissions in children and adolescents: A large 5-year case-crossover study. Clin. Exp. Allergy 2018, 48, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- ABARES, Department of Agriculture, Fisheries and Forestry. Casuarina Forest. 2019. Available online: http://www.agriculture.gov.au/abares/forestsaustralia/profiles/casuarina-forest (accessed on 30 May 2019).

| Onset | Duration | Magnitude (Mean) | Magnitude (Sum over 20) | |||||
|---|---|---|---|---|---|---|---|---|
| Subseason | PS1 | PS2 | PS1 | PS2 | PS1 | PS2 | PS1 | PS2 |
| Tmean | 0.186 | 0.04 | −0.45 | −0.34 | −0.35 | 0.477 | −0.373 | 0.273 |
| T5 | 0.186 | −0.034 | −0.45 | −0.329 | −0.35 | 0.464 | −0.373 | 0.284 |
| T10 | 0.19 | −0.012 | −0.495 | −0.333 | −0.254 | 0.469 | −0.345 | 0.281 |
| T20 | −0.177 | 0.015 | 0.211 | −0.295 | 0.605 | 0.423 | 0.601 | 0.203 |
| Rtot | 0.618 | −0.237 | −0.085 | 0.676 | −0.168 | −0.317 | −0.012 | −0.414 |
| Allergen Tested | % Positive |
|---|---|
| Histamine dichloride (positive control) | 98.5 |
| Glycerosaline (negative control) | 0.7 |
| Cat hair | 28.9 |
| Dog epithelium | 14.5 |
| Dermatophagoides pteronyssinus | 72.5 |
| American cockroach | 45.5 |
| Penicillium notatum | 10.7 |
| Aspergillus fumigatus | 12.6 |
| Alternaria alternata | 19.3 |
| Cladosporium sp. | 15.0 |
| White Birch (Betula) | 20.2 |
| Acacia longifolia | 20.7 |
| Casuarina | 19.7 |
| Privet (Ligustrum) | 23.7 |
| Plane Tree (Platanus) | 24.3 |
| Perennial rye grass (Lolium perenne) | 51.7 |
| Timothy (Phleum pratense) | 51.7 |
| Bermuda (Cynodon dactylon) | 45.9 |
| Bent (Agrostis) | 44.2 |
| Orchard (Dactylis) | 49.1 |
| Bahia (Paspalum notatum) | 45.8 |
| English Plantain (Plantago lanceolata) | 38.6 |
| Dock/Sorrell (Rumex) | 29.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampugnani, E.R.; Silver, J.D.; Burton, P.; Nattala, U.; Katelaris, C.H. Seasonal Patterns and Allergenicity of Casuarina Pollen in Sydney, Australia: Insights from 10 Years of Monitoring and Skin Testing. Atmosphere 2024, 15, 719. https://doi.org/10.3390/atmos15060719
Lampugnani ER, Silver JD, Burton P, Nattala U, Katelaris CH. Seasonal Patterns and Allergenicity of Casuarina Pollen in Sydney, Australia: Insights from 10 Years of Monitoring and Skin Testing. Atmosphere. 2024; 15(6):719. https://doi.org/10.3390/atmos15060719
Chicago/Turabian StyleLampugnani, Edwin R., Jeremy D. Silver, Pamela Burton, Usha Nattala, and Constance H. Katelaris. 2024. "Seasonal Patterns and Allergenicity of Casuarina Pollen in Sydney, Australia: Insights from 10 Years of Monitoring and Skin Testing" Atmosphere 15, no. 6: 719. https://doi.org/10.3390/atmos15060719
APA StyleLampugnani, E. R., Silver, J. D., Burton, P., Nattala, U., & Katelaris, C. H. (2024). Seasonal Patterns and Allergenicity of Casuarina Pollen in Sydney, Australia: Insights from 10 Years of Monitoring and Skin Testing. Atmosphere, 15(6), 719. https://doi.org/10.3390/atmos15060719







