Observation and Simulation of CO2 Fluxes in Rice Paddy Ecosystems Based on the Eddy Covariance Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Observation Instruments
2.3. Data Processing
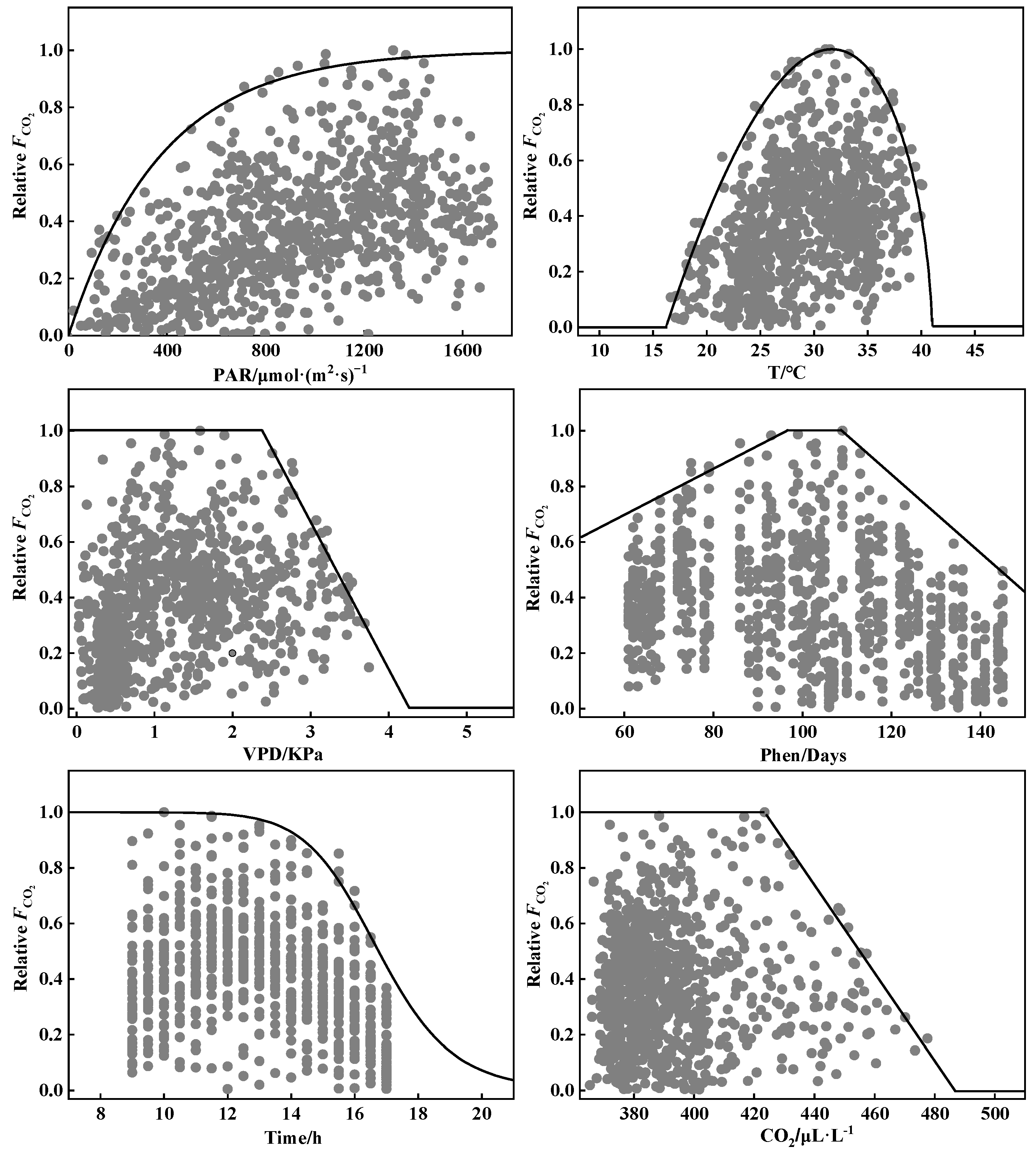
2.4. CO2 Flux Model
- (1)
- Temperature stress coefficient (ftemp)
- (2)
- Vapor pressure deficit stress coefficient (fVPD)
- (3)
- Photosynthetically active radiation stress coefficient (fPAR)
- (4)
- Phenology stress coefficient (fphen)
- (5)
- Time stress coefficient (fTime)
- (6)
- CO2 concentration stress coefficient (fCO2)
3. Results and Discussion
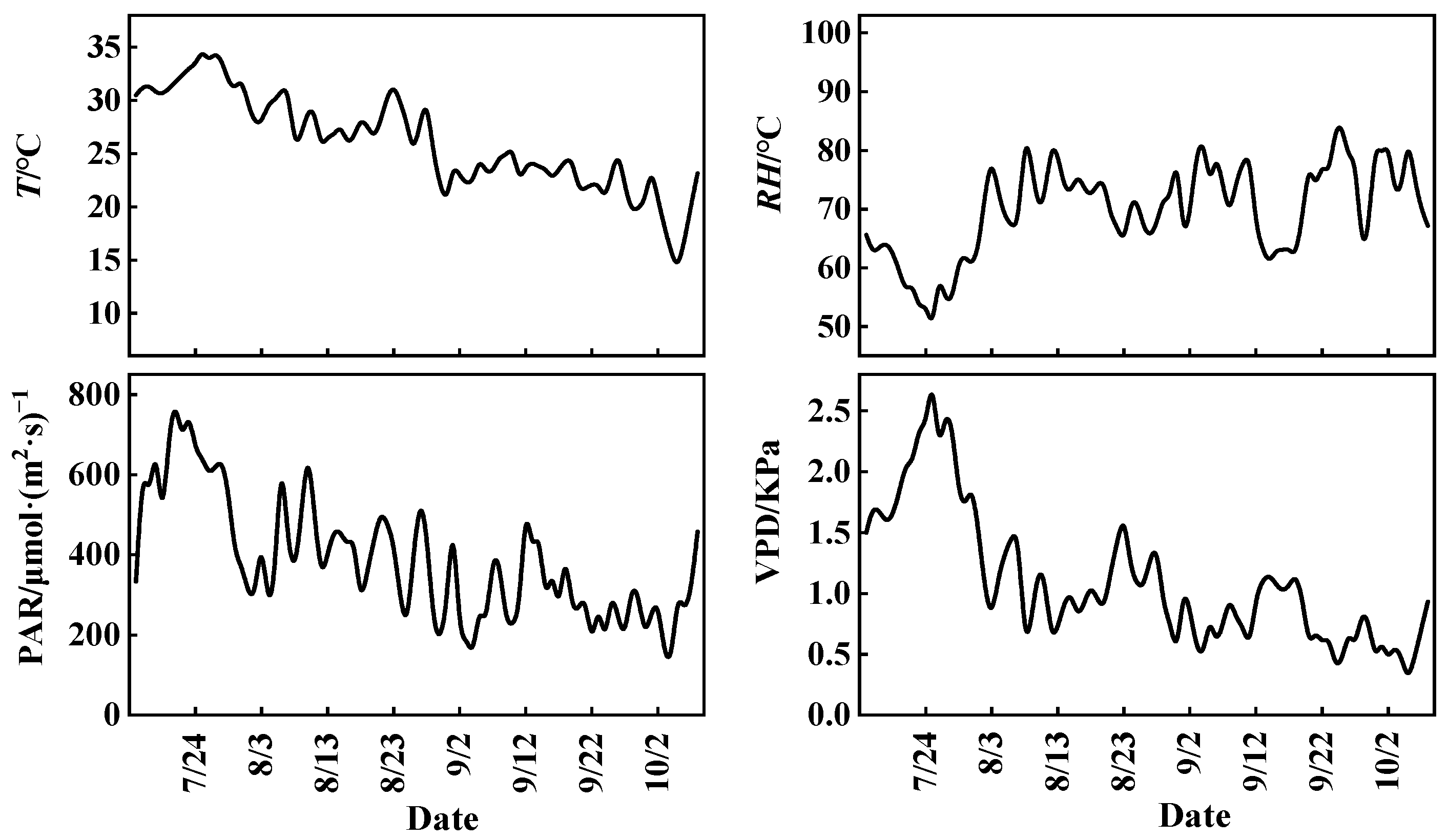
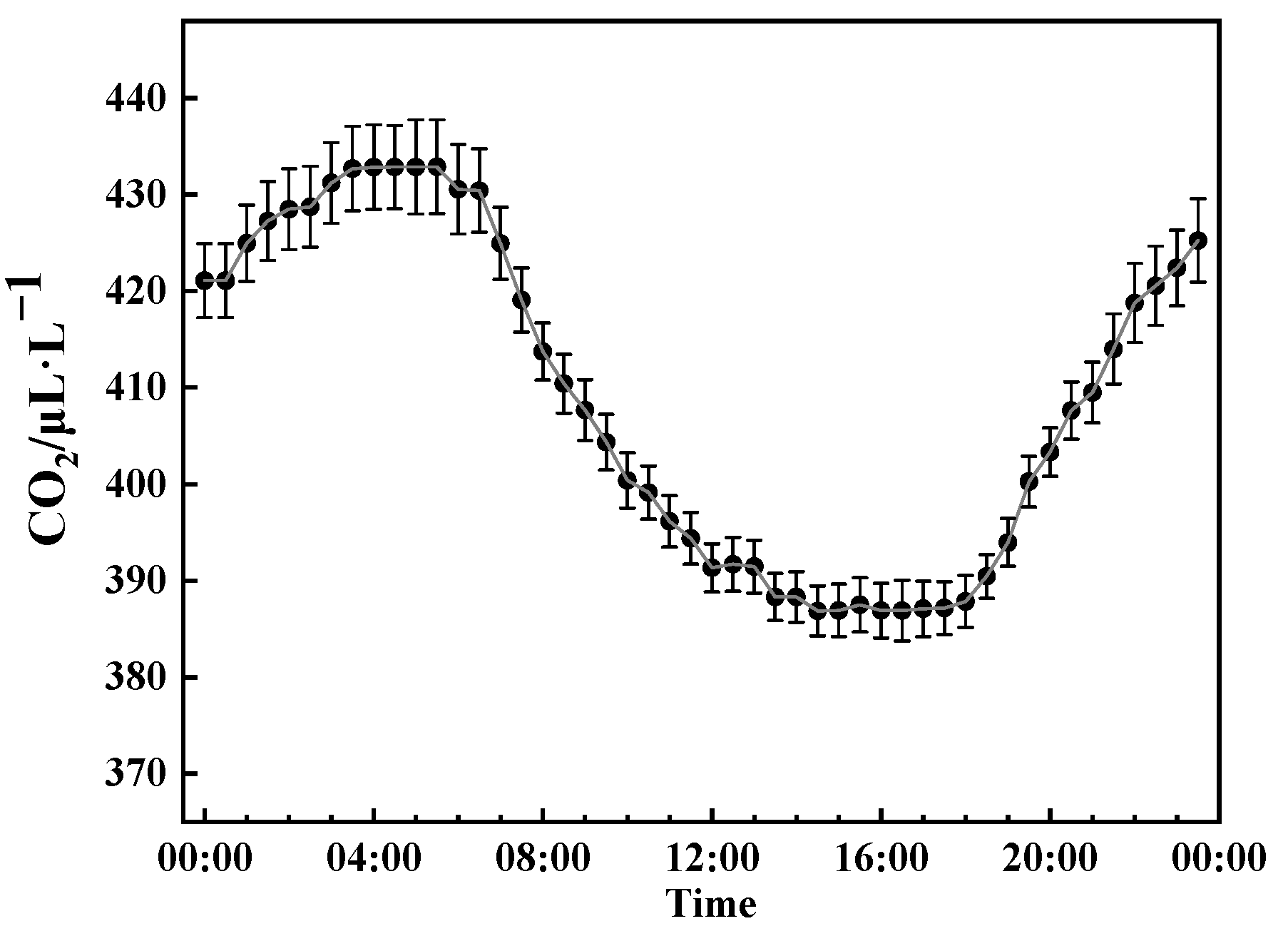
3.1. Variations in Meteorological Factors and CO2 Concentration
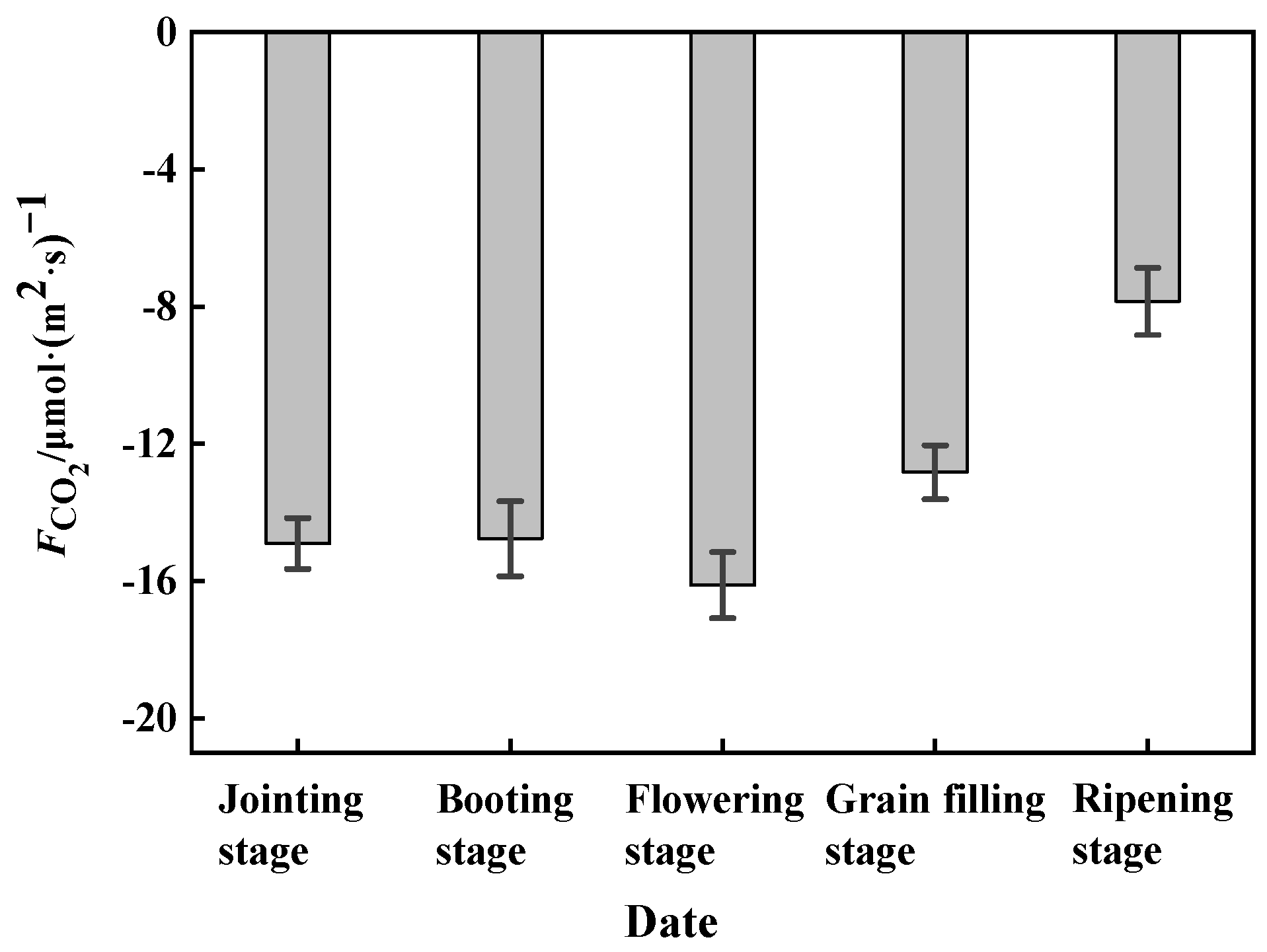
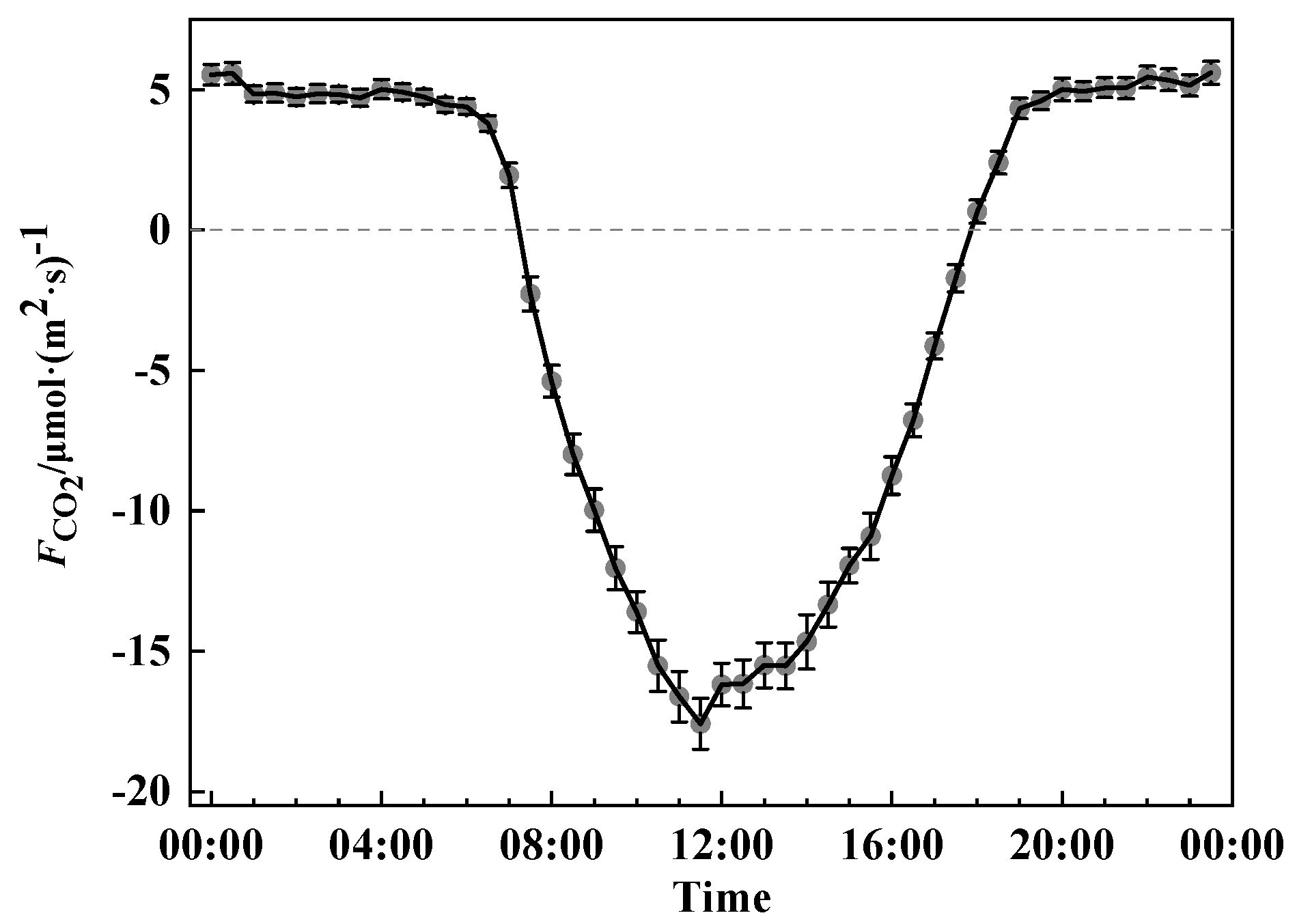
3.2. The Variation in CO2 Flux
3.3. Parameterization of the CO2 Flux Model
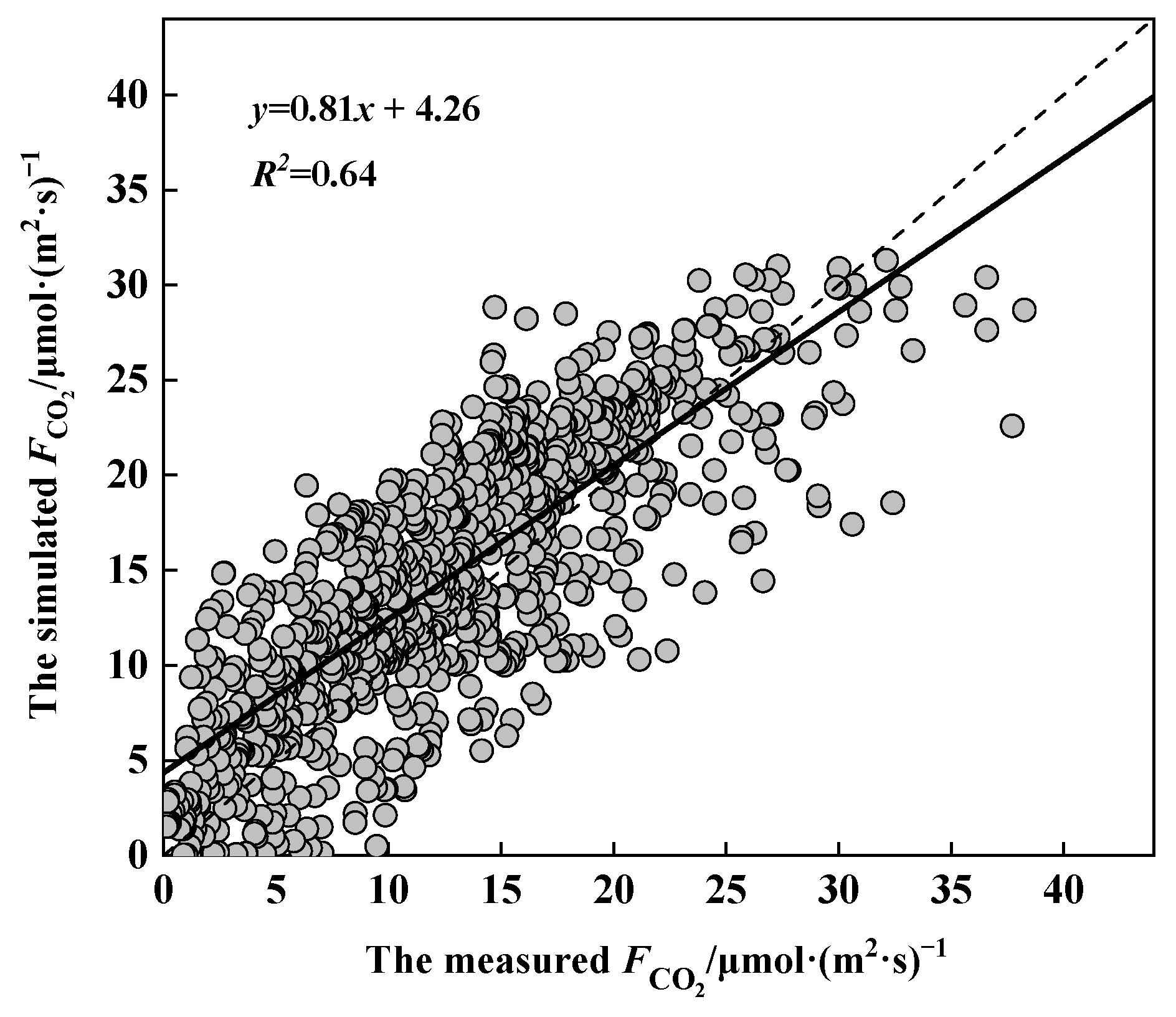
3.4. Validation of the CO2 Flux Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fedorov, V.B.; Trucchi, E.; Goropashnaya, A.V.; Waltari, E.; Whidden, S.E.; Stenseth, N.C. Impact of past climate warming on genomic diversity and demographic history of collared lemmings across the Eurasian Arctic. Proc. Natl. Acad. Sci. USA 2020, 117, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Zickfeld, K.; Eby, M.; Matthews, H.D.; Weaver, A.J. Setting cumulative emissions targets to reduce the risk of dangerous climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 16129–16134. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McConkey, B.G.; Liang, B.C.; Angers, D.A.; Janzen, H.H.; Kröbel, R.; Cerkowniak, D.D.; Smith, W.N. Increasing crop yields and root input make Canadian farmland a large carbon sink. Geoderma 2019, 336, 49–58. [Google Scholar] [CrossRef]
- Lei, H.; Yang, D. Seasonal and interannual variations in carbon dioxide exchange over a cropland in the North China Plain. Glob. Change Biol. 2010, 16, 2944–2957. [Google Scholar] [CrossRef]
- Yue, Y.; Ni, J.; Ciais, P.; Piao, S.; Wang, T.; Huang, M.; Borthwick, A.G.L.; Li, T.; Wang, Y.; Chappell, A.; et al. Lateral transport of soil carbon and land-atmosphere CO2 flux induced by water erosion in China. Proc. Natl. Acad. Sci. USA 2016, 113, 6617–6622. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Q.; Li, J.; Li, L.-H.; Li, X.-G.; Yu, G.-R.; Sun, X.-M. Simulation of diurnal variations of CO2, water and heat fluxes over winter wheat with a model coupled photosynthesis and transpiration. Agric. For. Meteorol. 2006, 137, 194–219. [Google Scholar] [CrossRef]
- Mei, B.; Zheng, X.; Xie, B.; Dong, H.; Zhou, Z.; Wang, R.; Deng, J.; Cui, F.; Tong, H.; Zhu, J. Nitric oxide emissions from conventional vegetable fields in southeastern China. Atmos. Environ. 2009, 43, 2762–2769. [Google Scholar] [CrossRef]
- Chen, H.; Li, H.; Wei, Y.; McBean, E.; Liang, H.; Wang, W.; Huang, J.J. Partitioning eddy covariance CO2 fluxes into ecosystem respiration and gross primary productivity through a new hybrid four sub-deep neural network. Agric. Ecosyst. Environ. 2024, 361, 108810. [Google Scholar] [CrossRef]
- Baldocchi, D.D. Assessing the eddy covariance technique for evaluating carbon dioxide exchange rates of ecosystems: Past, present and future. Glob. Change Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.; Zhang, W.; Sun, W.; Liu, S.; Jiang, J.; Wu, J.; Yu, W.; Wang, Y.; Yang, Z. Agro-C: A biogeophysical model for simulating the carbon budget of agroecosystems. Agric. For. Meteorol. 2009, 149, 106–129. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Zheng, Y. Observation of ozone deposition flux and its contribution to stomatal uptake over a winter wheat field in eastern China. Atmos. Environ. 2024, 326, 120472. [Google Scholar] [CrossRef]
- Massman, W.J.; Lee, X. Eddy covariance flux corrections and uncertainties in long-term studies of carbon and energy exchanges. Agric. For. Meteorol. 2002, 113, 121–144. [Google Scholar] [CrossRef]
- Falge, E.; Baldocchi, D.; Olson, R.; Anthoni, P.; Aubinet, M.; Bernhofer, C.; Burba, G.; Ceulemans, R.; Clement, R.; Dolman, H.; et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 2001, 107, 43–69. [Google Scholar] [CrossRef]
- Tong, L.; Wang, X.; Geng, C.; Wang, W.; Lu, F.; Song, W.; Liu, H.; Yin, B.; Sui, L.; Wang, Q. Diurnal and phenological variations of O3 and CO2 fluxes of rice canopy exposed to different O3 concentrations. Atmos. Environ. 2011, 45, 5621–5631. [Google Scholar] [CrossRef]
- Wu, R.J.; Zheng, Y.F.; Hu, C.D. Evaluation of the chronic effects of ozone on biomass loss of winter wheat based on ozone flux-response relationship with dynamical flux thresholds. Atmos. Environ. 2016, 142, 93–103. [Google Scholar] [CrossRef]
- Hu, S.; Li, T.; Wang, Y.; Gao, B.; Jing, L.; Zhu, J.; Wang, Y.; Huang, J.; Yang, L. Effects of free air CO2 enrichment (FACE) on grain yield and quality of hybrid rice. Field Crops Res. 2024, 306, 109237. [Google Scholar] [CrossRef]
- Bonilla-Cordova, M.; Cruz-Villacorta, L.; Echegaray-Cabrera, I.; Ramos-Fernández, L.; Flores del Pino, L. Design of a Portable Analyzer to Determine the Net Exchange of CO2 in Rice Field Ecosystems. Sensors 2024, 24, 402. [Google Scholar] [CrossRef] [PubMed]
- Demyan, M.S.; Ingwersen, J.; Funkuin, Y.N.; Ali, R.S.; Mirzaeitalarposhti, R.; Rasche, F.; Poll, C.; Müller, T.; Streck, T.; Kandeler, E.; et al. Partitioning of ecosystem respiration in winter wheat and silage maize—Modeling seasonal temperature effects. Agric. Ecosyst. Environ. 2016, 224, 131–144. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Mai, B.; Zhao, H.; Chu, Z.; Huang, J.; Yuan, Y. Simulating and partitioning ozone flux in winter wheat field: The Surfatm-O3 model. China Environ. Sci. 2018, 38, 455–470. (In Chinese) [Google Scholar]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta BBA Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Pérez, I.A.; Sánchez, M.L.; García M, Á.; Pardo, N.; Fernández-Duque, B. The influence of meteorological variables on CO2 and CH4 trends recorded at a semi-natural station. J. Environ. Manag. 2018, 209, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Xiao, H.; Qian, F.; Huang, Z.; Feng, J.; Wang, X. Daytime and phenological characteristics of O3 and CO2 fluxes of winter wheat canopy under short-term O3 exposure. Water Air Soil Pollut. 2016, 227, 1–14. [Google Scholar] [CrossRef]
- Fares, S.; McKay, M.; Holzinger, R.; Goldstein, A.H. Ozone fluxes in a Pinus ponderosa ecosystem are dominated by non-stomatalprocesses: Evidence from long-term continuous measurements. Agric. For. Meteorol. 2010, 150, 420–430. [Google Scholar] [CrossRef]
- Stella, P.; Personne, E.; Loubet, B.; Lamaud, E.; Ceschia, E.; Béziat, P.; Bonnefond, J.M.; Irvine, M.; Keravec, P.; Mascher, N.; et al. Predicting and partitioning ozone fluxes to maize crops from sowing to harvest: The Surfatm-O3 model. Biogeosciences 2011, 8, 2869–2886. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, Z.; Wang, X.; Liu, X.; Hu, E. Quantification of ozone exposure- and stomatal uptake-yield response relationships for soybean in Northeast China. Sci. Total Environ. 2017, 599–600, 710–720. [Google Scholar] [CrossRef]

| Stress Coefficient | Parameter | Unit | Parameter Value | |
|---|---|---|---|---|
| Before Revision | After Revision | |||
| — | μmol·(m2·s)−1 | 41.4 | 38.4 | |
| fmin | — | — | 0.01 | 0.01 |
| fPAR | L | — | 0.0026 | 0.0027 |
| ftemp | tmin | °C | 20.2 | 16.2 |
| topt | °C | 31.6 | 31.7 | |
| tmax | °C | 40.5 | 40.9 | |
| fVPD | VPDmax | KPa | 1.8 | 2.38 |
| VPDmin | KPa | 3.0 | 4.22 | |
| fphen | Dayc | D | 93 | 99 |
| Dayd | D | 102 | 109 | |
| c1 | — | 0.0043 | 0.0084 | |
| d1 | — | 0.60 | 0.20 | |
| c2 | — | −0.022 | −0.0143 | |
| d2 | — | 3.28 | 2.54 | |
| fTime | e | — | 16.6 | 16.7 |
| f | — | 13.8 | 14.4 | |
| μL·L−1 | — | 486.2 | ||
| μL·L−1 | — | 423.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, J.; Zhao, H.; Zheng, Y. Observation and Simulation of CO2 Fluxes in Rice Paddy Ecosystems Based on the Eddy Covariance Technique. Atmosphere 2024, 15, 517. https://doi.org/10.3390/atmos15050517
Wang J, Wang J, Zhao H, Zheng Y. Observation and Simulation of CO2 Fluxes in Rice Paddy Ecosystems Based on the Eddy Covariance Technique. Atmosphere. 2024; 15(5):517. https://doi.org/10.3390/atmos15050517
Chicago/Turabian StyleWang, Jinghan, Jiayan Wang, Hui Zhao, and Youfei Zheng. 2024. "Observation and Simulation of CO2 Fluxes in Rice Paddy Ecosystems Based on the Eddy Covariance Technique" Atmosphere 15, no. 5: 517. https://doi.org/10.3390/atmos15050517
APA StyleWang, J., Wang, J., Zhao, H., & Zheng, Y. (2024). Observation and Simulation of CO2 Fluxes in Rice Paddy Ecosystems Based on the Eddy Covariance Technique. Atmosphere, 15(5), 517. https://doi.org/10.3390/atmos15050517







