Genotoxicity and Cytotoxicity Induced In Vitro by Airborne Particulate Matter (PM2.5) from an Open-Cast Coal Mining Area
Abstract
1. Introduction
2. Materials and Methods
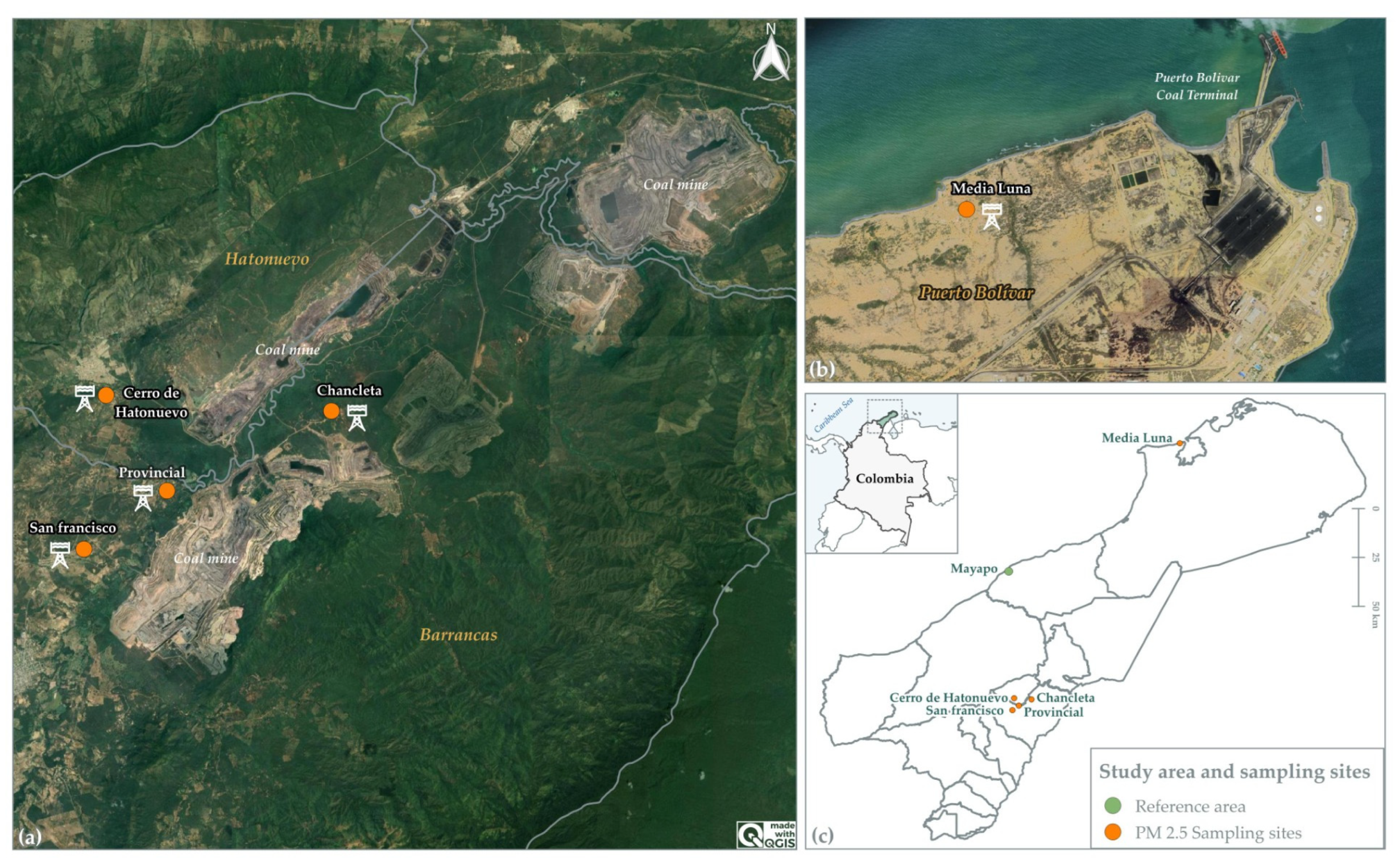
2.1. Location of Sampling Sites and Study Area
2.2. Collection of PM2.5 Samples
2.2.1. Chemicals
2.2.2. Extractable Organic Matter (EOM) Determination
2.2.3. Cleanup
2.2.4. High-Resolution Gas Chromatography-Mass Spectrometry (GC–MS)
2.3. Blood Samples and Lymphocyte Isolation
Primary Human Lymphocyte Cultures and Assessment of Cytotoxicity
2.4. High-Throughput Single-Cell Gel Electrophoresis and Modified Comet Assay
2.5. Cytokinesis-Block Micronucleus (CBMN) Assay
CREST Immunostaining
2.6. Statistical Analysis
3. Results
3.1. Chemical Characteristics of ACE, CH and DCM Extracts
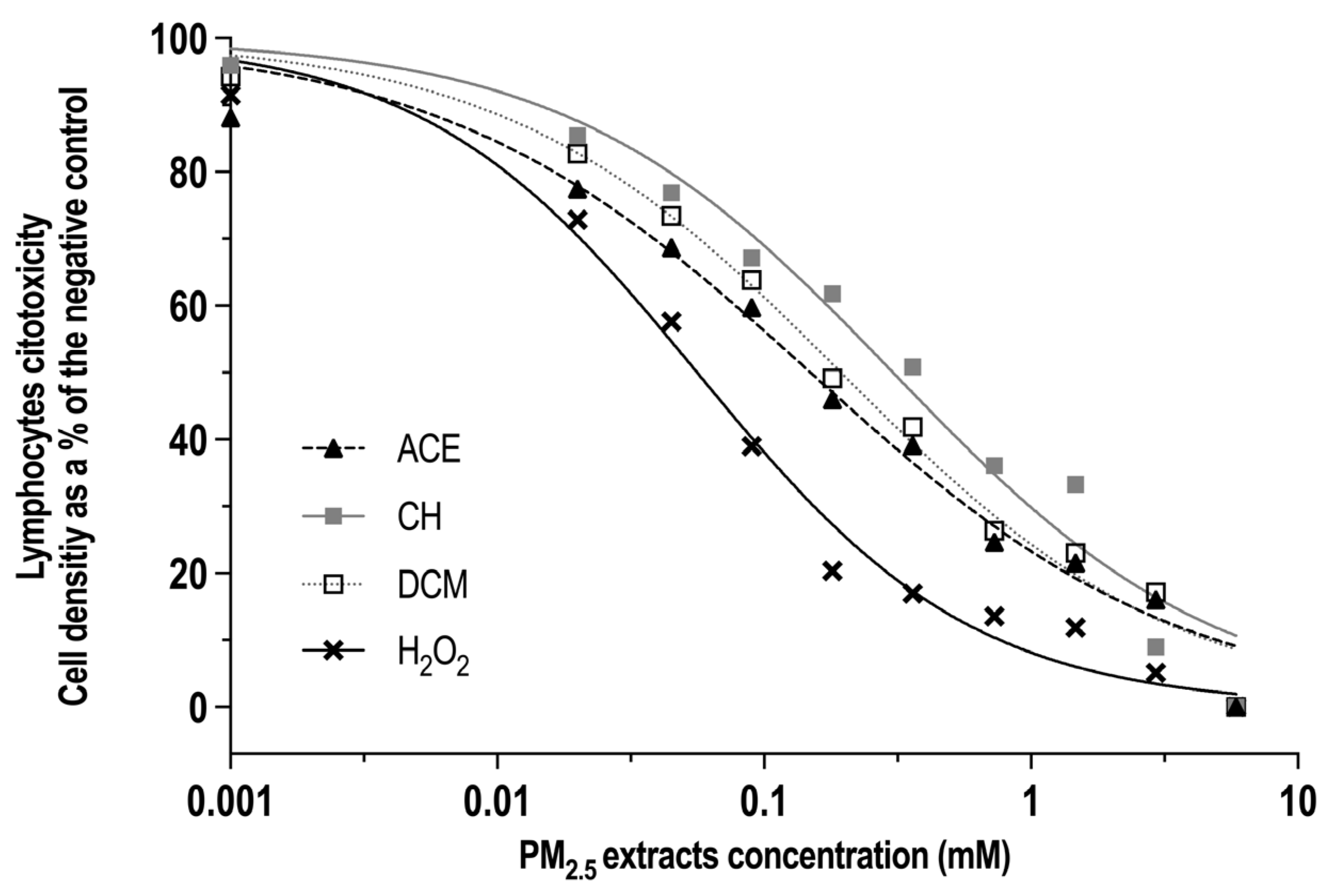
3.2. Cytotoxicity of ACE, CH and DCM Extracts in Human Lymphocyte Cultures
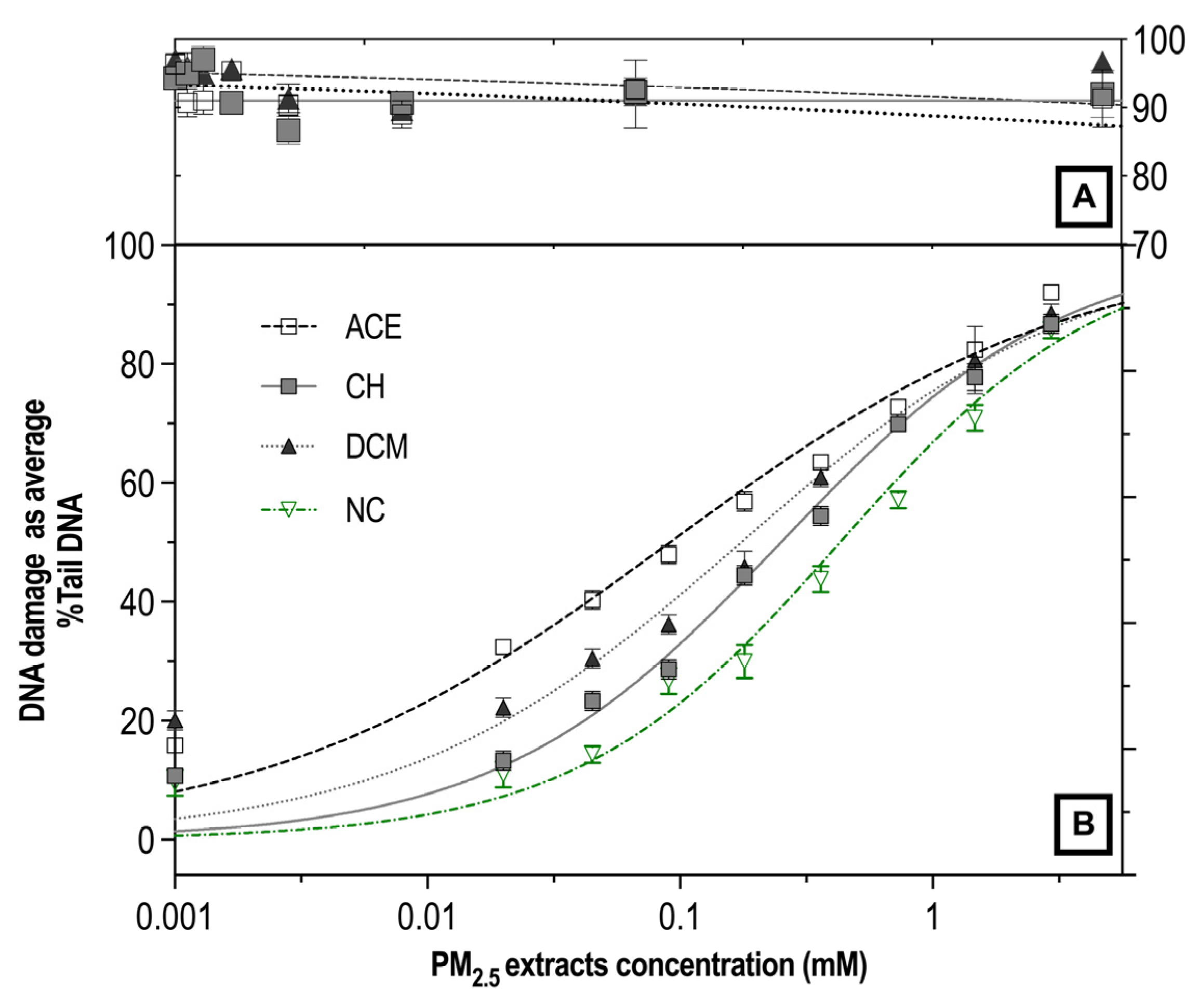
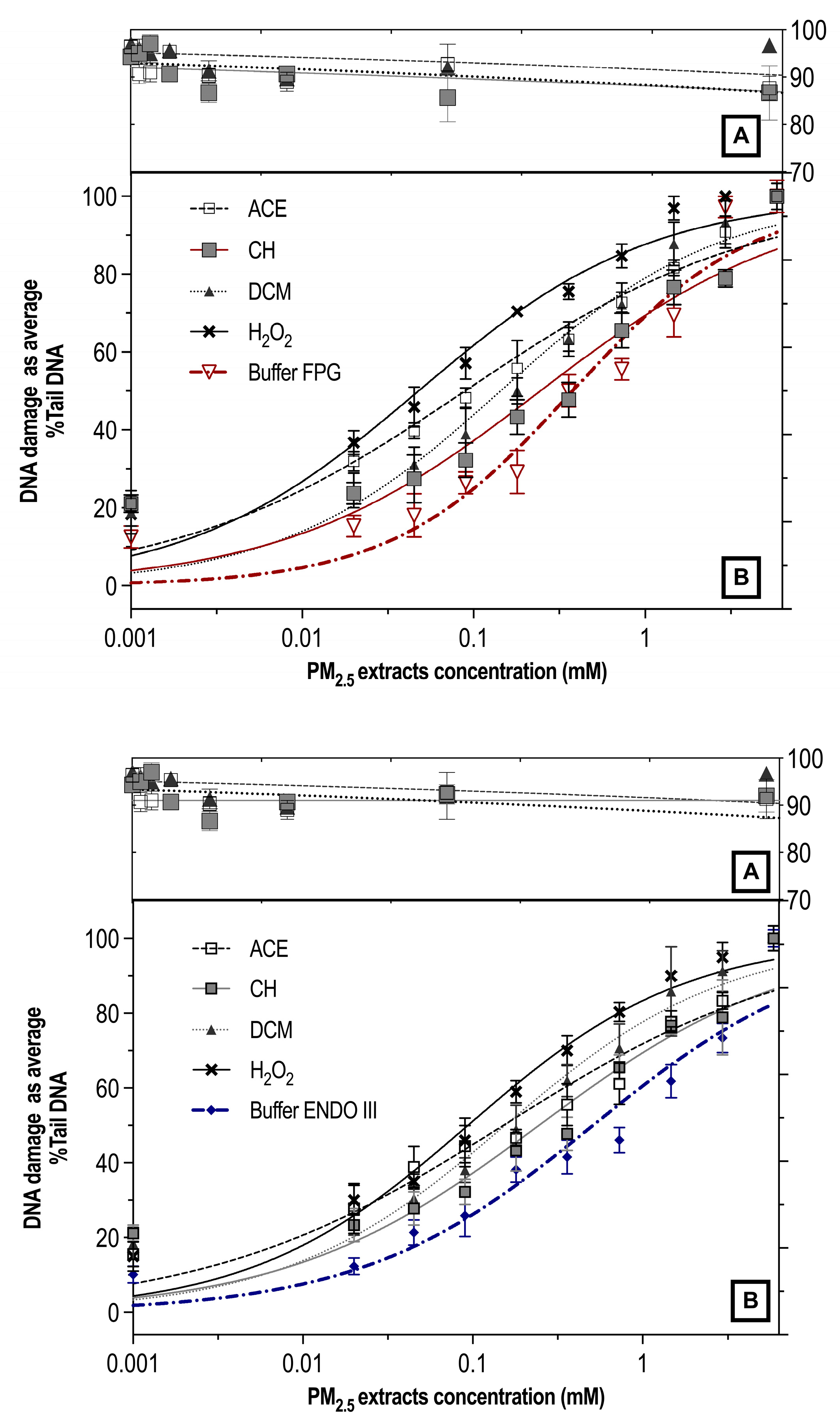
3.3. Genotoxicity and Oxidative Damage Induced by ACE, CH and DCM Extracts in Human Lymphocyte Cultures
3.4. Effect of ACE, CH and DCM Extracts on CBMN-Cyt Assay Parameters and CREST Staining
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., 3rd; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J. Clin. 2020. [CrossRef]
- Espitia-Pérez, L.; da Silva, J.; Brango, H.; Espitia-Pérez, P.; Pastor-Sierra, K.; Salcedo-Arteaga, S.; de Souza, C.T.; Dias, J.F.; Hoyos-Giraldo, L.S.; Gómez-Pérez, M.; et al. Genetic damage in environmentally exposed populations to open-pit coal mining residues: Analysis of buccal micronucleus cytome (BMN-cyt) assay and alkaline, Endo III and FPG high-throughput comet assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 836, 24–35. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Zhang, Y.; Zhao, T.; Wang, J.; Wu, H.; Hu, P. Temporal and spatial distributions of particulate matters around mining areas under two coal mining methods in arid desert region of northwest China. Environ. Technol. Innov. 2020, 19, 101029. [Google Scholar] [CrossRef]
- Prasad, S.; Gao, C.X.; Borg, B.; Broder, J.; Brown, D.; Ikin, J.F.; Makar, A.; McCrabb, T.; Hoy, R.; Thompson, B.; et al. Chronic Obstructive Pulmonary Disease in Adults Exposed to Fine Particles from a Coal Mine Fire. Ann. Am. Thorac. Soc. 2022, 19, 186–195. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Lu, Y.; Li, M. Probabilistic risk analysis for coal mine gas overrun based on FAHP and BN: A case study. Environ. Sci. Pollut. Res. 2022, 29, 28458–28468. [Google Scholar] [CrossRef]
- Cohen, A.J.; Ross Anderson, H.; Ostro, B.; Pandey, K.D.; Krzyzanowski, M.; Künzli, N.; Gutschmidt, K.; Pope, A.; Romieu, I.; Samet, J.M.; et al. The global burden of disease due to outdoor air pollution. J. Toxicol. Env. Health A 2005, 68, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, G.; Ertürk Arı, P.; Emerce, E.; Arı, A.; Odabaşı, M.; Schins, R.; Burgaz, S.; Gaga, E.O. Investigation of spatial and temporal variation of particulate matter in vitro genotoxicity and cytotoxicity in relation to the elemental composition. Mutat. Res. Genet. Toxicol. Env. Mutagen. 2019, 842, 22–34. [Google Scholar] [CrossRef]
- Ahmad, M.; Chen, J.; Panyametheekul, S.; Yu, Q.; Nawab, A.; Khan, M.T.; Zhang, Y.; Ali, S.W.; Phairuang, W. Fine particulate matter from brick kilns site and roadside in Lahore, Pakistan: Insight into chemical composition, oxidative potential, and health risk assessment. Heliyon 2024, 10, e25884. [Google Scholar] [CrossRef]
- Badran, G.; Ledoux, F.; Verdin, A.; Abbas, I.; Roumie, M.; Genevray, P.; Landkocz, Y.; Lo Guidice, J.-M.; Garçon, G.; Courcot, D. Toxicity of fine and quasi-ultrafine particles: Focus on the effects of organic extractable and non-extractable matter fractions. Chemosphere 2020, 243, 125440. [Google Scholar] [CrossRef]
- Romano, S.; Perrone, M.R.; Becagli, S.; Pietrogrande, M.C.; Russo, M.; Caricato, R.; Lionetto, M.G. Ecotoxicity, genotoxicity, and oxidative potential tests of atmospheric PM10 particles. Atmos. Environ. 2020, 221, 117085. [Google Scholar] [CrossRef]
- Arregocés, H.A.; Bonivento, G.J.; Ladino, L.A.; Beristain-Montiel, E.; Restrepo, G.; Miranda, J.; Alvarez-Ospina, H.; Rojano, R. Human health risk assessment of PM10-bound heavy metals and PAHs around the Latin America’s Largest opencast coal mine. Environ. Sci. Pollut. Res. 2023, 30, 125915–125930. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, I.W.H.; Enlo-Scott, Z.; Nagy, E.; Mudway, I.S.; Tetley, T.D.; Arlt, V.M.; Phillips, D.H. Genotoxicity of fine and coarse fraction ambient particulate matter in immortalised normal (TT1) and cancer-derived (A549) alveolar epithelial cells. Environ. Mol. Mutagen. 2018, 59, 290–301. [Google Scholar] [CrossRef]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Gao, D.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization of water-insoluble oxidative potential of PM2.5 using the dithiothreitol assay. Atmos. Environ. 2020, 224, 117327. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Sidwell, A.; Smith, S.C.; Roper, C. A comparison of fine particulate matter (PM2.5) in vivo exposure studies incorporating chemical analysis. J. Toxicol. Env. Health B Crit. Rev. 2022, 25, 422–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, S.; Chen, B.; Bao, L.; Ma, J.; Han, W.; Xu, A.; Yu, K.N.; Wu, L.; Chen, S. Ambient PM2.5 exposure causes cellular senescence via DNA damage, micronuclei formation, and cGAS activation. Nanotoxicology 2022, 16, 757–775. [Google Scholar] [CrossRef]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef]
- Espitia-Pérez, L.; da Silva, J.; Espitia-Pérez, P.; Brango, H.; Salcedo-Arteaga, S.; Hoyos-Giraldo, L.S.; de Souza, C.T.; Dias, J.F.; Agudelo-Castañeda, D.; Valdés Toscano, A.; et al. Cytogenetic instability in populations with residential proximity to open-pit coal mine in Northern Colombia in relation to PM10 and PM2.5 levels. Ecotoxicol. Environ. Saf. 2018, 148, 453–466. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Wang, Z.; Yang, L.; Wang, J.; Wang, W. Trace elements in PM2.5 in Shandong Province: Source identification and health risk assessment. Sci. Total Environ. 2018, 621, 558–577. [Google Scholar] [CrossRef]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, Q.; Wang, M.; Chang, T. Oxidation potential and coupling effects of the fractionated components in airborne fine particulate matter. Environ. Res. 2022, 213, 113652. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lin, Z.; Liao, K.; Xi, Z.; Wang, D. Adverse effects of coal combustion related fine particulate matter (PM2.5) on nematode Caenorhabditis elegans. Sci. Total Environ. 2015, 512–513, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Aneja, V.P.; Isherwood, A.; Morgan, P. Characterization of particulate matter (PM10) related to surface coal mining operations in Appalachia. Atmos. Environ. 2012, 54, 496–501. [Google Scholar] [CrossRef]
- Longhin, E.; Holme, J.A.; Gutzkow, K.B.; Arlt, V.M.; Kucab, J.E.; Camatini, M.; Gualtieri, M. Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: Characterization of the process and possible mechanisms involved. Part. Fibre Toxicol. 2013, 10, 63. [Google Scholar] [CrossRef]
- MINAMBIENTE. Resolución 2254. 2017. Available online: https://www.minambiente.gov.co/documento-normativa/resolucion-2554-de-2017/ (accessed on 22 June 2024).
- Sun, J.; Fang, R.; Wang, H.; Xu, D.-X.; Yang, J.; Huang, X.; Cozzolino, D.; Fang, M.; Huang, Y. A review of environmental metabolism disrupting chemicals and effect biomarkers associating disease risks: Where exposomics meets metabolomics. Environ. Int. 2022, 158, 106941. [Google Scholar] [CrossRef]
- Kumbıçak, Ü.; Çavaş, T.; Çinkılıç, N.; Kumbıçak, Z.; Vatan, Ö.; Yılmaz, D. Evaluation of in vitro cytotoxicity and genotoxicity of copper–zinc alloy nanoparticles in human lung epithelial cells. Food Chem. Toxicol. 2014, 73, 105–112. [Google Scholar] [CrossRef]
- Ulloa, A. The rights of the Wayúu people and water in the context of mining in La Guajira, Colombia: Demands of relational water justice. Hum. Geogr. 2020, 13, 6–15. [Google Scholar] [CrossRef]
- Espitia-Pérez, L.; Arteaga–Pertuz, M.; Soto, J.S.; Espitia-Pérez, P.; Salcedo-Arteaga, S.; Pastor–Sierra, K.; Galeano–Páez, C.; Brango, H.; da Silva, J.; Henriques, J.A.P. Geospatial analysis of residential proximity to open-pit coal mining areas in relation to micronuclei frequency, particulate matter concentration, and elemental enrichment factors. Chemosphere 2018, 206, 203–216. [Google Scholar] [CrossRef]
- Deluquez, E.P. Cooperación Internacional y derechos humanos frente a la minería en Colombia. Rev. Int. Coop. Y Desarro. 2015, 2, 125–152. [Google Scholar]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 127–152. [Google Scholar]
- Azqueta, A.; Langie, S.A.S.; Boutet-Robinet, E.; Duthie, S.; Ladeira, C.; Møller, P.; Collins, A.R.; Godschalk, R.W.L. DNA repair as a human biomonitoring tool: Comet assay approaches. Mutat. Res. Rev. Mutat. Res. 2019, 781, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Velasco, E.; Martínez-Perafán, F.; Carvajal-Varona, S.; García-Vallejo, F.; Hoyos-Giraldo, L.S. Assessment of DNA damage in car spray painters exposed to organic solvents by the high-throughput comet assay. Toxicol. Mech. Methods 2016, 26, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Wolfreys, A.; Fedyk, J.; Bourner, C.; Windebank, S. The ability of the Comet assay to discriminate between genotoxins and cytotoxins. Mutagenesis 1998, 13, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kryston, T.B.; Georgiev, A.B.; Pissis, P.; Georgakilas, A.G. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 711, 193–201. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef]
- Muruzabal, D.; Collins, A.; Azqueta, A. The enzyme-modified comet assay: Past, present and future. Food Chem. Toxicol. 2021, 147, 111865. [Google Scholar] [CrossRef]
- Fenech, M. Cytokinesis-block micronucleus cytome assay. Nat. Protoc. 2007, 2, 1084–1104. [Google Scholar] [CrossRef]
- Pastor-Sierra, K.; Espitia-Pérez, L.; Espitia-Pérez, P.; Peñata-Taborda, A.; Brango, H.; Galeano-Páez, C.; Bru-Cordero, O.E.; Palma-Parra, M.; Díaz, S.M.; Trillos, C.; et al. Micronuclei frequency and exposure to chemical mixtures in three Colombian mining populations. Sci. Total Environ. 2023, 901, 165789. [Google Scholar] [CrossRef]
- Fenech, M.; Chang, W.P.; Kirsch-Volders, M.; Holland, N.; Bonassi, S.; Zeiger, E. HUMN project: Detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 65–75. [Google Scholar] [CrossRef]
- Daisey, J.M. ORGANIC COMPOUNDS IN URBAN AEROSOLS. Ann. New York Acad. Sci. 1980, 338, 50–69. [Google Scholar] [CrossRef]
- Picot, A. Exploration and Exploitation of Shale Gas and Shale Oil (Parent Rock Hydrocarbon) by Fracking; Toxicology–Chemistry Association: Brussels Belgium, 2012. [Google Scholar]
- Wang, X. Fine Particle and Mercury Formation and Control During Coal Combustion; Washington University: St. Louis, MO, USA, 2014. [Google Scholar]
- Wu, Q.; Su, Q.; Simpson, S.L.; Tan, Q.G.; Chen, R.; Xie, M. Isotopically Modified Bioassay Bridges the Bioavailability and Toxicity Risk Assessment of Metals in Bedded Sediments. Env. Sci. Technol. 2022, 56, 16919–16928. [Google Scholar] [CrossRef] [PubMed]
- Meepage, J.N.; Welker, J.K.; Meyer, C.M.; Mohammadi, S.; Stanier, C.O.; Stone, E.A. Advances in the Separation and Detection of Secondary Organic Aerosol Produced by Decamethylcyclopentasiloxane (D(5)) in Laboratory-Generated and Ambient Aerosol. ACS EST Air 2024, 1, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Zimmermann, R.; Van Langenhove, H. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos. Environ. 2010, 44, 1831–1846. [Google Scholar] [CrossRef]
- Allen, J.O.; Dookeran, N.M.; Smith, K.A.; Sarofim, A.F.; Taghizadeh, K.; Lafleur, A.L. Measurement of Polycyclic Aromatic Hydrocarbons Associated with Size-Segregated Atmospheric Aerosols in Massachusetts. Environ. Sci. Technol. 1996, 30, 1023–1031. [Google Scholar] [CrossRef]
- Lammel, G. Polycyclic Aromatic Compounds in the Atmosphere—A Review Identifying Research Needs. Polycycl. Aromat. Compd. 2015, 35, 316–329. [Google Scholar] [CrossRef]
- Dasgupta, S.; Cao, A.; Mauer, B.; Yan, B.; Uno, S.; McElroy, A. Genotoxicity of oxy-PAHs to Japanese medaka (Oryzias latipes) embryos assessed using the comet assay. Environ. Sci. Pollut. Res. 2014, 21, 13867–13876. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, C.; Yan, B.; Feng, C.; Zhao, G.; Lin, C.; Yuan, M.; Wu, C.; Ren, Y.; Hu, Y. Identification and removal of polycyclic aromatic hydrocarbons in wastewater treatment processes from coke production plants. Environ. Sci. Pollut. Res. 2013, 20, 6418–6432. [Google Scholar] [CrossRef]
- Guntupalli, S.; Thunuguntla, V.; Reddy, K.S.; Newton, M.I.; Rao, C.; Bondili, J. Enhanced degradation of carcinogenic PAHs benzo (a) pyrene and benzo (k) fluoranthene by a microbial consortia. Indian. J. Sci. Technol. 2016, 9, 35. [Google Scholar] [CrossRef]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Li, X.; Xin, J.; Liu, Z.; Sun, Y.; Liu, J.; Zhang, Y.; Du, W.; Jin, X.; et al. Emission characteristics of size distribution, chemical composition and light absorption of particles from field-scale crop residue burning in Northeast China. Sci. Total Environ. 2020, 710, 136304. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Y.; Feng, Y.; Song, W.; Cao, F.; Zhang, Y.; Li, Q.; Yang, X.; Chen, J. Different formation mechanisms of PAH during wood and coal combustion under different temperatures. Atmos. Environ. 2020, 222, 117084. [Google Scholar] [CrossRef]
- Hill, S.C.; Douglas Smoot, L. Modeling of nitrogen oxides formation and destruction in combustion systems. Prog. Energy Combust. Sci. 2000, 26, 417–458. [Google Scholar] [CrossRef]
- Han, M.; Kong, J.; Yuan, J.; He, H.; Hu, J.; Yang, S.; Li, S.; Zhang, L.; Sun, C. Method development for simultaneous analyses of polycyclic aromatic hydrocarbons and their nitro-, oxy-, hydroxy- derivatives in sediments. Talanta 2019, 205, 120128. [Google Scholar] [CrossRef] [PubMed]
- Lara, S.; Villanueva, F.; Cabañas, B.; Sagrario, S.; Aranda, I.; Soriano, J.; Martin, P. Determination of policyclic aromatic compounds,(PAH, nitro-PAH and oxy-PAH) in soot collected from a diesel engine operating with different fuels. Sci. Total Environ. 2023, 900, 165755. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.A.; Asimakopoulos, A.G.; Barbosa Jr, F.; Kannan, K. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ. 2017, 586, 152–162. [Google Scholar] [CrossRef]
- Gurdemir, G.; Erkekoglu, P.; Balci, A.; Sur, U.; Ozkemahli, G.; Tutkun, E.; Yilmaz, H.; Asci, A.; Kocer-Gumusel, B. Oxidative stress parameters, selenium levels, DNA damage, and phthalate levels in plastic workers. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 253–270. [Google Scholar] [CrossRef]
- Sedha, S.; Lee, H.; Singh, S.; Kumar, S.; Jain, S.; Ahmad, A.; Bin Jardan, Y.A.; Sonwal, S.; Shukla, S.; Simal-Gandara, J.; et al. Reproductive toxic potential of phthalate compounds—State of art review. Pharmacol. Res. 2021, 167, 105536. [Google Scholar] [CrossRef]
- Velali, E.; Papachristou, E.; Pantazaki, A.; Besis, A.; Samara, C.; Labrianidis, C.; Lialiaris, T. In vitro cellular toxicity induced by extractable organic fractions of particles exhausted from urban combustion sources—Role of PAHs. Environ. Pollut. 2018, 243, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Thepnuan, D.; Yabueng, N.; Chantara, S.; Prapamontol, T.; Tsai, Y.I. Simultaneous determination of carcinogenic PAHs and levoglucosan bound to PM2.5 for assessment of health risk and pollution sources during a smoke haze period. Chemosphere 2020, 257, 127154. [Google Scholar] [CrossRef]
- Krug, J.D.; Riedel, T.P.; Lewandowski, M.; Lonneman, W.A.; Turlington, J.M.; Zavala, J.; Warren, S.H.; Kleindienst, T.E.; DeMarini, D.M. Mutagenic atmospheres generated from the photooxidation of NOx with selected VOCs and a complex mixture: Apportionment of aromatic mutagenicity for reacted gasoline vapor. Atmos. Environ. 2024, 334, 120668. [Google Scholar] [CrossRef]
- Libalova, H.; Zavodna, T.; Elzeinova, F.; Barosova, H.; Cervena, T.; Milcova, A.; Vankova, J.; Paradeisi, F.; Vojtisek-Lom, M.; Sikorova, J. The Genotoxicity of Organic Extracts from Particulate Emissions Produced by Neat Gasoline (E0) and a Gasoline–Ethanol Blend (E15) in BEAS-2B Cells. J. Xenobiotics 2023, 14, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kenneth, S.K.; Nneka, O.F.; Kalu, A.K.; Joseph, A.O. Radioprotective Potencies of Allium Cepa Extract (ACE) against Radiation-Induced Hepatoxicity in Wistar Rats. Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2023, 12, 59–83. [Google Scholar] [CrossRef]
- Molinelli, A.R.; Santacana, G.E.; Madden, M.C.; Jiménez, B.D. Toxicity and metal content of organic solvent extracts from airborne particulate matter in Puerto Rico. Environ. Res. 2006, 102, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Zhai, J.; Zhong, C.; Zeng, H.; Feng, L.; Yang, Y.; Li, X.; Ma, M.; Luan, T. Effect of oxidative stress induced by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin on DNA damage. J. Hazard. Mater. 2024, 472, 134485. [Google Scholar] [CrossRef]
- Collins, A.R. Measuring oxidative damage to DNA and its repair with the comet assay. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 794–800. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Zhai, Q.; Qiu, Y.; Niu, Y.; Dai, Y.; Zheng, Y.-x.; Duan, H. [Endonuclease modified comet assay for oxidative DNA damage induced by detection of genetic toxicants]. Zhonghua yu fang yi xue za zhi [Chin. J. Prev. Med.] 2014, 48, 208–212. [Google Scholar]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Duan, H.; Jia, X.; Zhai, Q.; Ma, L.; Wang, S.; Huang, C.; Wang, H.; Niu, Y.; Li, X.; Dai, Y.; et al. Long-term exposure to diesel engine exhaust induces primary DNA damage: A population-based study. Occup. Environ. Med. 2016, 73, 83–90. [Google Scholar] [CrossRef]
- ESCODD; Gedik, C.M.; Collins, A. Establishing the background level of base oxidation in human lymphocyte DNA: Results of an interlaboratory validation study. FASEB J. 2005, 19, 82–84. [Google Scholar] [CrossRef]
- Espitia-Pérez, L.; Jiménez-Vidal, L.; Espitia-Pérez, P. Particulate matter exposure: Genomic instability, disease, and cancer risk. In Environmental Health: Management and Prevention Practices; Makan, A., Ed.; IntechOpen: London, UK, 2020; Volume 13, p. 126. [Google Scholar]
- Cao, X.; Padoan, S.; Binder, S.; Bauer, S.; Orasche, J.; Rus, C.-M.; Mudan, A.; Huber, A.; Kuhn, E.; Oeder, S. A comparative study of persistent DNA oxidation and chromosomal instability induced in vitro by oxidizers and reference airborne particles. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2022, 874, 503446. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Pal, V.K.; Kannan, K. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol. 2021, 3, 91–116. [Google Scholar] [CrossRef]
- Sisto, R.; Cavallo, D.; Ursini, C.L.; Fresegna, A.M.; Ciervo, A.; Maiello, R.; Paci, E.; Pigini, D.; Gherardi, M.; Gordiani, A. Direct and oxidative DNA damage in a group of painters exposed to VOCs: Dose–response relationship. Front. Public Health 2020, 8, 445. [Google Scholar] [CrossRef]
- Garcia, A.L.; Picinini, J.; Silveira, M.D.; Camassola, M.; Visentim, A.P.; Salvador, M.; da Silva, J. Environmental Mutagenesis. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2021, 861, 503297. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Araújo, R.; Ramalhete, L.; Teixeira, H.; Calado, C.R. The use of effect biomarkers in chemical mixtures risk assessment—Are they still important? Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 896, 503768. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.D.; Alves, C.; Oliveira, H.; Duarte, I.F. Biological Impact of Organic Extracts from Urban-Air Particulate Matter: An In Vitro Study of Cytotoxic and Metabolic Effects in Lung Cells. Int. J. Mol. Sci. 2023, 24, 16896. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on human health. In Air Pollution and Control; Sharma, N., Ed.; Springer: Singapore, 2018; pp. 119–142. [Google Scholar]
- Luzhna, L.; Kathiria, P.; Kovalchuk, O. Micronuclei in genotoxicity assessment: From genetics to epigenetics and beyond. Front. Genet. 2013, 4, 131. [Google Scholar] [CrossRef]
- Kirsch-Volders, M.; Bonassi, S.; Knasmueller, S.; Holland, N.; Bolognesi, C.; Fenech, M.F. Commentary: Critical questions, misconceptions and a road map for improving the use of the lymphocyte cytokinesis-block micronucleus assay for in vivo biomonitoring of human exposure to genotoxic chemicals—A HUMN project perspective. Mutat. Res. Rev. Mutat. Res. 2014, 759, 49–58. [Google Scholar] [CrossRef]
- García-Rodríguez, M.D.C.; Santiago-Moreno, Y.; Molina-Alvarez, B.; Altamirano-Lozano, M. Evaluation of clastogenic/aneugenic damage using the fish micronucleus assay in mice exposed to chromium (VI). In Vivo 2023, 37, 1666–1671. [Google Scholar] [CrossRef]
| Extract | Formula | Name | CAS Number | Chemical Structure |
|---|---|---|---|---|
| ACE | C16H32 | 1-Hexadecene | 629-73-2 |  |
| C17H32O | E-15-Heptadecenal | 700381-35-7 |  | |
| C9H18 | 1-Nonene | 124-11-8 |  | |
| C13H26 | 3—Tridecene (Z) | 41446-53-1 |  | |
| C12H24 | 1-Dodecene | 112-41-4 |  | |
| C13H10O | Benzophenone | 119-61-9 |  | |
| C18H36 | 1-Octadecene | 112-88-9 |  | |
| DCM | C15H24 | 2,4,6-triisopropylbenzene | 717-74-8 |  |
| C16H48O10Si9 | Cyclopentasiloxane | 145344-72-5 |  | |
| C17H36O | 1-Heptadecanol | 1454-85-9 |  | |
| CH | C23H46 | 1-Tricosene | 18835-32-0 |  |
| C24H38O4 | Di-n-octyl phthalate | 117-84-0 |  | |
| C22H44O | 1-Docosene | 1599-67-3 |  |
| Extract | Concentrations | MNBN | MNMONO | NBUD | NPB | NDI |
|---|---|---|---|---|---|---|
| (mM) | ||||||
| ACE | 0 | 2.52 ± 2.12 | 0.05 ± 0.24 | 0.80 ± 1.60 | 0.10 ± 0.31 | 2.13 ± 0.14 |
| 0.05 | 4.34 ± 1.67 | 0.86 ± 0.09 | 0.55 ± 0.33 | 0.28 ± 0.62 | 2.33 ± 0.11 | |
| 0.08 | 4.45 ± 3.67 | 0.98 ± 0.16 | 0.35 ± 0.78 | 0.31 ± 0.21 | 2.05 ± 0.08 | |
| 0.17 | 6.30 ± 6.23 | 0.33 ± 0.71 | 0.43 ± 0.94 | 0.53 ± 1.54 | 1.63 ± 0.28 | |
| 0.34 | 7.53 ± 8.25 * | 0.96 ± 1.95 * | 0.68 ± 1.11 | 0.56 ± 1.98 | 1.61 ± 0.45 | |
| CH | 0 | 1.41 ± 0.28 | 0.15 ± 0.36 | 0.43 ± 0.14 | 0.17 ± 0.89 | 2.16 ± 0.11 |
| 0.05 | 2.13 ± 0.84 | 0.19 ± 0.61 | 0.51 ± 0.40 | 0.19 ± 0.51 | 2.0 ± 0.31 | |
| 0.23 | 3.16 ± 0.52 | 0.18 ± 0.59 | 0.48 ± 0.95 | 0.22 ± 0.54 | 1.68 ± 0.18 | |
| 0.47 | 4.28 ± 2.56 | 0.21 ± 0.61 | 0.60 ± 1.06 | 0.33 ± 0.66 | 1.62 ± 0.28 | |
| 0.94 | 6.59 ± 4.63 * | 0.76 ± 1.69 * | 0.83 ± 0.38 | 0.65 ± 0.17 | 1.52 ± 0.15 | |
| DCM | 0 | 2.08 ± 0.38 | 0.50 ± 0.40 | 0.61 ± 0.17 | 0.20 ± 0.56 | 2.0 ± 0.09 |
| 0.05 | 2.68 ± 0.30 | 0.49 ± 0.23 | 0.55 ± 0.65 | 0.33 ± 0.62 | 1.87 ± 0.13 | |
| 0.13 | 4.75 ± 3.72 | 0.47 ± 1.07 | 0.67 ± 0.78 | 0.09 ± 0.30 | 1.96 ± 0.78 | |
| 0.26 | 5.54 ± 3.98 | 0.55 ± 1.65 | 0.70 ± 0.13 | 0.95 ± 0.22 | 1.70 ± 0.19 | |
| 0.52 | 7.15 ± 7.67 * | 0.68 ± 1.28 * | 0.90 ± 0.35 | 0.40 ± 1.02 * | 1.69 ± 0.20 | |
| Positive control MMS | 1 | 8.15 ± 1.67 * | 1.15 ± 0.96 * | 1.35 ± 1.78 * | 1.23 ± 0.80 * | 1.30 ± 0.11* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeano-Páez, C.; Brango, H.; Pastor-Sierra, K.; Coneo-Pretelt, A.; Arteaga-Arroyo, G.; Peñata-Taborda, A.; Espitia-Pérez, P.; Ricardo-Caldera, D.; Humanez-Álvarez, A.; Londoño-Velasco, E.; et al. Genotoxicity and Cytotoxicity Induced In Vitro by Airborne Particulate Matter (PM2.5) from an Open-Cast Coal Mining Area. Atmosphere 2024, 15, 1420. https://doi.org/10.3390/atmos15121420
Galeano-Páez C, Brango H, Pastor-Sierra K, Coneo-Pretelt A, Arteaga-Arroyo G, Peñata-Taborda A, Espitia-Pérez P, Ricardo-Caldera D, Humanez-Álvarez A, Londoño-Velasco E, et al. Genotoxicity and Cytotoxicity Induced In Vitro by Airborne Particulate Matter (PM2.5) from an Open-Cast Coal Mining Area. Atmosphere. 2024; 15(12):1420. https://doi.org/10.3390/atmos15121420
Chicago/Turabian StyleGaleano-Páez, Claudia, Hugo Brango, Karina Pastor-Sierra, Andrés Coneo-Pretelt, Gean Arteaga-Arroyo, Ana Peñata-Taborda, Pedro Espitia-Pérez, Dina Ricardo-Caldera, Alicia Humanez-Álvarez, Elizabeth Londoño-Velasco, and et al. 2024. "Genotoxicity and Cytotoxicity Induced In Vitro by Airborne Particulate Matter (PM2.5) from an Open-Cast Coal Mining Area" Atmosphere 15, no. 12: 1420. https://doi.org/10.3390/atmos15121420
APA StyleGaleano-Páez, C., Brango, H., Pastor-Sierra, K., Coneo-Pretelt, A., Arteaga-Arroyo, G., Peñata-Taborda, A., Espitia-Pérez, P., Ricardo-Caldera, D., Humanez-Álvarez, A., Londoño-Velasco, E., Espinosa-Sáez, R., Diaz-Ponguta, B., da Silva, J., Silva Corrêa, D., & Espitia-Pérez, L. (2024). Genotoxicity and Cytotoxicity Induced In Vitro by Airborne Particulate Matter (PM2.5) from an Open-Cast Coal Mining Area. Atmosphere, 15(12), 1420. https://doi.org/10.3390/atmos15121420











