Investigation of the Measurement Uncertainties in the Measurement of BTEX in the Volatile Organic Compound Group
Abstract
1. Introduction
2. Material and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanayankottupoyil, J.; Kuruvilla, J. Characterization and Source Apportionment of Ambient VOC Concentrations: Assessing Ozone Formation Potential in the Barnett Shale Oil and Gas Region. Atmos. Pollut. Resour. 2024, 102327. [Google Scholar] [CrossRef]
- Mai, J.L.; Yang, W.W.; Zeng, Y.; Guan, Y.; Chen, S. Volatile organic compounds (VOCs) in residential indoor air during interior finish period: Sources, variations, and health risks. Hyg. Environ. Health Adv. 2024, 9, 100087. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, B.; Yang, Y.; Kang, S.; Wang, F.; Xu, M.; Wang, W.; Feng, Y.; Hopke, P.K. Primary and oxidative source analyses of consumed VOCs in the atmosphere. J. Hazard. Mater. 2024, 476, 134894. [Google Scholar] [CrossRef] [PubMed]
- Elia, E.A.; Stylianou, M.; Agapiou, A. Investigation of the source of VOCs emission from indoor construction materials using electronic sensors and TD-GC-MS. Environ. Pollut. 2024, 348, 123765. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yang, X.; Gu, H.; Hu, J.; Zhang, T.; Chen, J.; Pan, X.; Xiu, G.; Zhang, W.; Lin, M. Characterization and sources of volatile organic compounds (VOCs) during 2022 summer ozone pollution control in Shanghai, China. Atmos. Environ. 2024, 327, 120464. [Google Scholar] [CrossRef]
- Halios, C.H.; Landeg-Cox, C.; Lowther, S.D.; Middleton, A.; Marczylo, T.; Dimitroulopoulou, S. Chemical in European resides-Part I: A review of emissions, concentrations, and health effects of volatile organic compounds (VOCs). Sci. Total Environ. 2023, 839, 156201. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Hu, W.; Yang, Y.; Zhang, S.; Yuan, R.; Lv, P.; Zhang, W.; Zhang, Y.; Zhang, Y. Improving VOC control strategies in industrial parks based on emission behavior, environmental effects, and health risks: A case study through atmospheric measurement and emission inventory. Sci. Total Environ. 2023, 865, 161235. [Google Scholar] [CrossRef]
- Hussain, M.S.; Gupta, G.; Mishra, R.; Patel, N.; Saurabh, G.; Alzarea, S.I.; Kumbhar, P.; Disouza, J.; Dureja, H.; Kukreti, N.; et al. Unlocking the secrets: Volatile Organic Compounds (VOCs) and their devastating effects on lung cancer. Pathol.-Res. Practice 2024, 255, 155157. [Google Scholar] [CrossRef]
- Hodgson, A.T. A Review and a limited comparison of methods for measuring total volatile organic compounds in indoor air. Indoor Air 1995, 5, 247–257. [Google Scholar] [CrossRef][Green Version]
- Taipale, R.; Ruuskanen, T.M.; Rinne, J.; Kajos, M.K.; Hakola, H.; Pohja, T.; Kulmala, M. Technical Note: Quantitative long-term measurements of VOC concentrations by PTR-MS-measurement, calibration, and volume mixing ratio calculation methods. Atmos. Chem. Phys. 2008, 8, 6681–6698. [Google Scholar] [CrossRef]
- Kaser, L.; Karl, T.; Schnitzhofer, R.; Grauss, M.; Herdlinger-Blatt, I.S.; Digangi, J.P.; Sive, B.; Turnipseed, A.; Hornbrook, R.S.; Zheng, W.; et al. Comparision of different real time VOC measurement techniques in a ponderosa pine forest. Atmos. Chem. Phys. 2013, 13, 2893–2906. [Google Scholar] [CrossRef]
- Posudin, Y. Methods of Analysis of Volatile Organic Compounds. In Methods of Measuring Environmental Parameters; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Chapter 18. [Google Scholar]
- Brown, R.H. Monitoring volatile organic compounds in air-the development of ISO standards and a critical appraisal of the methods. J. Environ. Monit. 2002, 4, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, M.; Szylak-Szydlowski, M. The application of situ methods to monitor VOC concentrations in urban areas—A bibliometric analysis and measuring solution review. Sustainability 2022, 14, 8815. [Google Scholar] [CrossRef]
- Sassi, G.; Demichelis, A.; Lecuna, M.; Sassi, M.P. Preparation of standard VOC mixtures for climate monitoring. Intern. J. Environ. Anal. Chem. 2015, 95, 1195–1207. [Google Scholar] [CrossRef]
- TS EN ISO/IEC 17025 2017; General Requirements for the Competence of Testing and Calibration Laboratories. The Turkish Standards Institute: Ankara, Türkiye, 2017.
- Konieczka, P.; Namiesnik, J. Estimating uncertainty in analytical precedures based on chromatographic techniques. J. Chromatogr. A 2010, 1217, 882–891. [Google Scholar] [CrossRef]
- Plaisance, H.; Leonardis, T.; Gerboles, M. Assessment of uncertainty of benzene measurements by Radiello diffusive sampler. Atmos. Environ. 2008, 42, 2555–2568. [Google Scholar] [CrossRef]
- Yang, J.; Fan, Q.; Wang, Q.; Tian, W.; Qiu, P.; Gao, B.; Du, J. Uncertainty evaluation for nine VOC gas certified references materials used for indoor air testing. Microchem. J. 2022, 283, 107935. [Google Scholar] [CrossRef]
- Güzel, B.; Canli, O. Method validation and measurement uncertainty of possible thirty volatile organic compounds (VOCs) presented in the polyethylene present in bottled drinking waters sold in Turkey. J. Anal. Sci. Technol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Weisel, C.P.; Jhang, J.; Turpin, B.J.; Morandi, M.T.; Colome, S.; Stock, T.H.; Spektor, D.M.; Korn, L.; Winer, A.; Alimokhtarı, S.; et al. Relationship of Indoor, Outdoor and Personal Air (RIOPA) study: Study design, methods and quality assurance/control results. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 123–137. [Google Scholar] [CrossRef]
- Sassi, G.; Demichelis, A.; Sassi, M.P. Uncertainty analysis of the diffusion rate in the dynamic generation of volatile organic compounds mixtures. Meas. Sci. Technol. 2011, 22, 105104. [Google Scholar] [CrossRef]
- TS CEN/TS 13649:2014; Stationary Source Emissions—Determination of the Mass Concentration of Individual Gaseous Organic Compounds—Sorptive Sampling Method Followed by Solvent Extraction or Thermal Desorption. Turkish Standards Institute: Ankara, Türkiye, 2014.
- EURACHEM/CITAC CG 4:2012; Guide Quantifying Uncertainty in Analytical Measurement. 3rd ed. Eurachem: Bucharest, Romania, 2012.
- Ballesta, P.P.; Field, R.; Saeger, E.D. Interlaboratory exercises for volatile organic compound determination. Atmos. Environ. 2001, 35, 5729–5740. [Google Scholar] [CrossRef]
- US EPA. Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially-Prepared Canisters and Analyzed by Gas Chromatograpy. In Compendium Method TO-15; Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999; p. 1. [Google Scholar]
- Sidal, H.C. Calculating the Measurement Uncertainty of BTEX VOC Groups in Stack Gas Sampling. Master’s Thesis, Ondokuz Mayis Universitesi Institute of Graduate Studies Department of Environmental Engineering, Samsun, Türkiye, 2023. [Google Scholar]
- Huxham, M.; Thomas, C.I.P. Sampling precedures for instrinsically valid volatile organic compounds measurements. Analyst 2000, 125, 825–832. [Google Scholar] [CrossRef]


| Components of Uncertainty | Reference Standard Flow (q)(L/min) | Value (X) | Distribution Type | Factor | u(x)1 |
|---|---|---|---|---|---|
| Sampling Device Flowmeter (Main Line) | 1.090 | 0.124 | Normal | 2 | 0.062 |
| Sampling Device Flowmeter (Dilution Line) | 1.095 | 0.124 | Normal | 2 | 0.062 |
| Sampling Device Temperature Meter (Sample) t = (30.09 °C) | - | 0.42 | Normal | 2 | 0.210 |
| Sampling Device Temperature Meter (Dilution) t = (30.09 °C) | - | 0.42 | Normal | 2 | 0.210 |
| Sampling Device Temperature Meter (Probe) t = (30.09 °C) | - | 0.42 | Normal | 2 | 0.210 |
| From the Sampling Device Combined Uncertainty | Ub(1) | 0.0813 | |||
| Components of Uncertainty | VOC Mix (For 2000 mg.L−1) | Value (X) | Distribution Type | Factor | u(x)1 | u(x)b |
|---|---|---|---|---|---|---|
| Benzene (Analysis) | 1997 | 110 | Normal | 2 | 55.00 | 0.028 |
| Benzene (Calibration) | 1997 | 29.23 | Normal | 2 | 14.615 | |
| Toluene (Analysis) | 2000 | 110 | Normal | 2 | 55.00 | 0.028 |
| Toluene (Calibration) | 2000 | 29.34 | Normal | 2 | 14.670 | |
| Ethylbenzene (Analysis) | 1998 | 110 | Normal | 2 | 55.00 | 0.028 |
| Ethylbenzene (Calibration) | 1998 | 29.31 | Normal | 2 | 14.655 | |
| m,p-Xylene (Analysis) | 1999 | 110 | Normal | 2 | 55.00 | 0.027 |
| m,p-Xylene (Calibration) | 1999 | 29.32 | Normal | 2 | 14.660 | |
| o-Xylene (Analysis) | 1998 | 110 | Normal | 2 | 55.00 | 0.027 |
| o-Xylene (Calibration) | 1998 | 35.41 | Normal | 2 | 17.705 |
| Components of Internal Uncertainty | Internal (For 2500 mg/L) | Value (X) | Distribution Type | Factor | u(x)1 | u(x)3 |
|---|---|---|---|---|---|---|
| Chlorobenzene D5 | 2507.4 | 24.9 | Normal | 2 | 12.45 | |
| 1,4-Dichlorobenzene D4 | 2506.2 | 26.4 | Normal | 2 | 13.20 | 0.0088 |
| Fluorobenzene | 2509.5 | 24.7 | Normal | 2 | 12.35 |
| Components of Internal Uncertainty | Value (X) | Distribution Type | Factor | u(x)1 | u(x)4 |
|---|---|---|---|---|---|
| Micropipette (1 mL) | 0.99 | Normal | 2 | 0.495 | |
| Temperature Effect Uncertainty | 0.002 | Rectangle | 1.73 | 0.001 | 0.002 |
| Micro pipette (50 µL) | 0.19 | Normal | 2 | 0.095 | |
| Temperature Effect Uncertainty | 0.0001 | Rectangle | 1.73 | 0.000 |
| Chemicals | Uncertainty | Purity (%) | Value (X) | Distribution Type | Factor | u(x)1 | u(x)5 |
|---|---|---|---|---|---|---|---|
| Methanol (CH3OH) | 2507.4 | - | 0.10 | Rectangle | 1.73 | 0.000691 | |
| Methanol (CH3OH) | 2506.2 | 99.8 | Rectangle | 0.060 | 0.00067 | ||
| Carbondisulfide (CS2) | 2509.5 | - | 0.05 | Rectangle | 1.73 | 0.009817 | |
| Carbondisulfide (CS2) | 99.9 | Rectangle | 0.02887 |
| BTEX | Sample (n) | Calibration Equation (Y) | Coefficient of Determination (R2) | p Value | Residual (Error) Standard Deviation (So) | Uncertainty Value U(Co) |
|---|---|---|---|---|---|---|
| Benzene | 12 | 0.99938 | <0.05 | 23.5044 | 0.795 | |
| Toluene | 12 | 0.99967 | <0.05 | 21.748 | 0.730 | |
| Ethylbenzene | 12 | 0.99967 | <0.05 | 26.347 | 0.740 | |
| m,p-Xylene | 12 | 0.99968 | <0.05 | 42.113 | 0.720 | |
| o-Xylene | 12 | 0.99969 | <0.05 | 23.183 | 0.778 |
| BTEX | Std. Dev. (SD) (%) | Rort,A,B | U(Rec) (%) | p Value | Uncertainty Value (UR) |
|---|---|---|---|---|---|
| Benzene | 12.978 8.497 | 0.956 0.928 | 4.104 2.687 | <0.05 <0.05 | 0.0245 |
| Toluene | 13.139 22.926 | 0.986 1.107 | 4.155 7.250 | <0.05 <0.05 | 0.0418 |
| Ethylbenzene | 14.655 3.340 | 1.094 1.032 | 4.634 1.056 | <0.05 <0.05 | 0.0238 |
| m,p-Xylene | 15.475 3.774 | 1.073 0.998 | 4.894 1.193 | <0.05 <0.05 | 0.0252 |
| o-Xylene | 17.334 4.205 | 1.060 0.970 | 5.481 1.330 | <0.05 <0.05 | 0.0282 |
| BTEX | Std. Dev. (S) | Relative Standard Deviation (%RSD) | p Value | Uncertainty Value (UR) |
|---|---|---|---|---|
| Benzene | 0.181 0.425 | 2.077 5.080 | >0.05 <0.05 | 3.8807 |
| Toluene | 0.200 0.371 | 2.347 4.491 | <0.05 <0.05 | 3.5828 |
| Ethylbenzene | 0.152 0.327 | 1.701 3.715 | <0.05 <0.05 | 2.8894 |
| m,p-Xylene | 0.162 0.323 | 1.804 3.651 | <0.05 <0.05 | 2.8797 |
| o-Xylene | 0.153 0.303 | 1.704 3.391 | <0.05 <0.05 | 2.6834 |
| BTEX | Std. Dev. (S) | Relative Standard Deviation (%RSD) | p Value | Uncertainty Value (UR) |
|---|---|---|---|---|
| Benzene | 0.237 0.460 | 5.586 10.635 | <0.05 <0.05 | 8.4940 |
| Toluene | 0.221 0.420 | 5.218 9.799 | <0.05 <0.05 | 7.8499 |
| Ethylbenzene | 0.284 0.396 | 6.176 8.761 | <0.05 <0.05 | 7.5796 |
| m,p-xylene | 0.290 0.403 | 6.265 8.872 | <0.05 <0.05 | 7.6797 |
| o-Xylene | 0.287 0.395 | 6.207 8.650 | <0.05 <0.05 | 7.5281 |
| BTEX | Relative Compound Uncertainty (Ub) | k Factors (for 95%) | Extended Uncertainty (Ug) |
|---|---|---|---|
| Benzene | 0.15231 | 2 | 0.3046 |
| Toluene | 0.14779 | 2 | 0.2955 |
| Ethylbenzene | 0.14156 | 2 | 0.2831 |
| m,p-xylene | 0.14117 | 2 | 0.2823 |
| o-Xylene | 0.14368 | 2 | 0.2873 |
| Reporting | Benzene | Toluene | Ethylbenzene | m, p-Xylene | o-Xylene |
|---|---|---|---|---|---|
| Relative Compound Uncertainty | 0.15231 | 0.14779 | 0.14156 | 0.14117 | 0.14368 |
| Measurement Result (ppm) | 10.000 | 10.000 | 10.000 | 10.000 | 10.000 |
| Standard Compound Uncertainty | 1.5231 | 1.4779 | 1.4156 | 1.4117 | 1.4387 |
| Extended Uncertainty (k = 2) | 3.0462 | 2.9558 | 2.8312 | 2.8234 | 2.8736 |
| Relative Uncertainty (%) | 30.462 | 29.558 | 28.312 | 28.234 | 28.736 |
| Reporting k = 2 at 95% confidence interval | 10 ± 3.046 | 10 ± 2.955 | 10 ± 2.831 | 10 ± 2.823 | 10 ± 2.873 |
| Uncertainty calculation formula | Y ± 0.304Y | Y ± 0.295Y | Y ± 0.283Y | Y ± 0.282Y | Y ± 0.287Y |
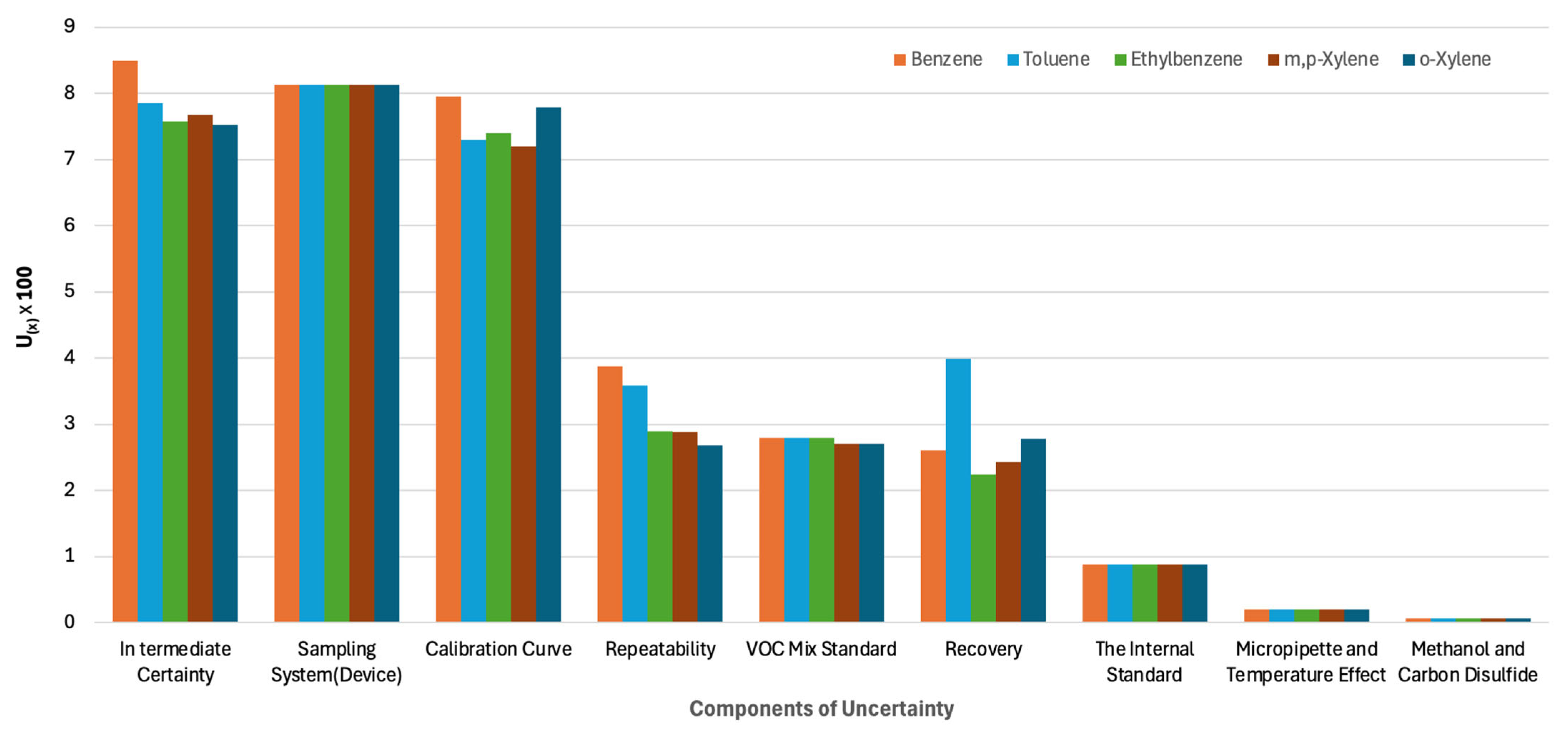
| Components of Uncertainty Percentages (%) | Benzene | Toluene | Ethylbenzene | m,p-Xylene | o-Xylene |
|---|---|---|---|---|---|
| Intermediate Certainty | 24 | 23 | 23 | 24 | 23 |
| Sampling System (Device) | 23 | 23 | 25 | 25 | 25 |
| Calibration Curve | 23 | 21 | 23 | 22 | 24 |
| Repeatability | 11 | 10 | 9 | 9 | 8 |
| VOC Mix Standard | 8 | 8 | 9 | 8 | 8 |
| Recovery | 7 | 11 | 7 | 8 | 8 |
| The Internal Standard | 3 | 3 | 3 | 3 | 3 |
| Micropipette and Temperature Effect | 1 | 1 | 1 | 1 | 1 |
| Methanol and Carbon Disulfide | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sıdal, H.C.; Akdemir, A. Investigation of the Measurement Uncertainties in the Measurement of BTEX in the Volatile Organic Compound Group. Atmosphere 2024, 15, 1416. https://doi.org/10.3390/atmos15121416
Sıdal HC, Akdemir A. Investigation of the Measurement Uncertainties in the Measurement of BTEX in the Volatile Organic Compound Group. Atmosphere. 2024; 15(12):1416. https://doi.org/10.3390/atmos15121416
Chicago/Turabian StyleSıdal, Hayri Cihan, and Andaç Akdemir. 2024. "Investigation of the Measurement Uncertainties in the Measurement of BTEX in the Volatile Organic Compound Group" Atmosphere 15, no. 12: 1416. https://doi.org/10.3390/atmos15121416
APA StyleSıdal, H. C., & Akdemir, A. (2024). Investigation of the Measurement Uncertainties in the Measurement of BTEX in the Volatile Organic Compound Group. Atmosphere, 15(12), 1416. https://doi.org/10.3390/atmos15121416






