Indoor Air Quality in a Museum Storage Room: Conservation Issues Induced in Plastic Objects
Abstract
1. Introduction
- Classify objects from a compositional point of view, organising their storage into homogeneous groups according to the molecular structure of their primary polymeric main component.
- Identify the decay patterns present on the objects and develop a specialised illustrated glossary containing the terminology related to the decay of plastic materials.
- Provide museum conservators with the best strategy for preserving the objects. These guidelines aim to prevent the release of pollutants caused by the decay mechanisms of the objects, which could negatively impact their proper conservation in the storage cabinets.
- Identification of constituent materials and revision of the museum’s existing inventory;
- Environmental assessment of the storage condition;
- Detection of actively degrading objects, using indicators such as odour (e.g., strong acetic or acidic smell), observation of plasticisers separation and crystallisation, signs of mechanical damages, and the placement of self-prepared bromocresol green strips indicators prepared in-house near the objects.
2. Aims of the Paper
- Does the emission of pollutants from the objects affect the overall air quality of the storage rooms?
- Based on the measurements of indoor air quality in both the storage room and the metal cabinets, can these conditions be deemed optimal for conserving plastic objects?
- Can the findings guide museum conservators in isolating high-risk malignant objects in separate cases? Would this approach represent the most effective preservation strategy [18]?
3. Materials and Methods
4. Results and Discussion
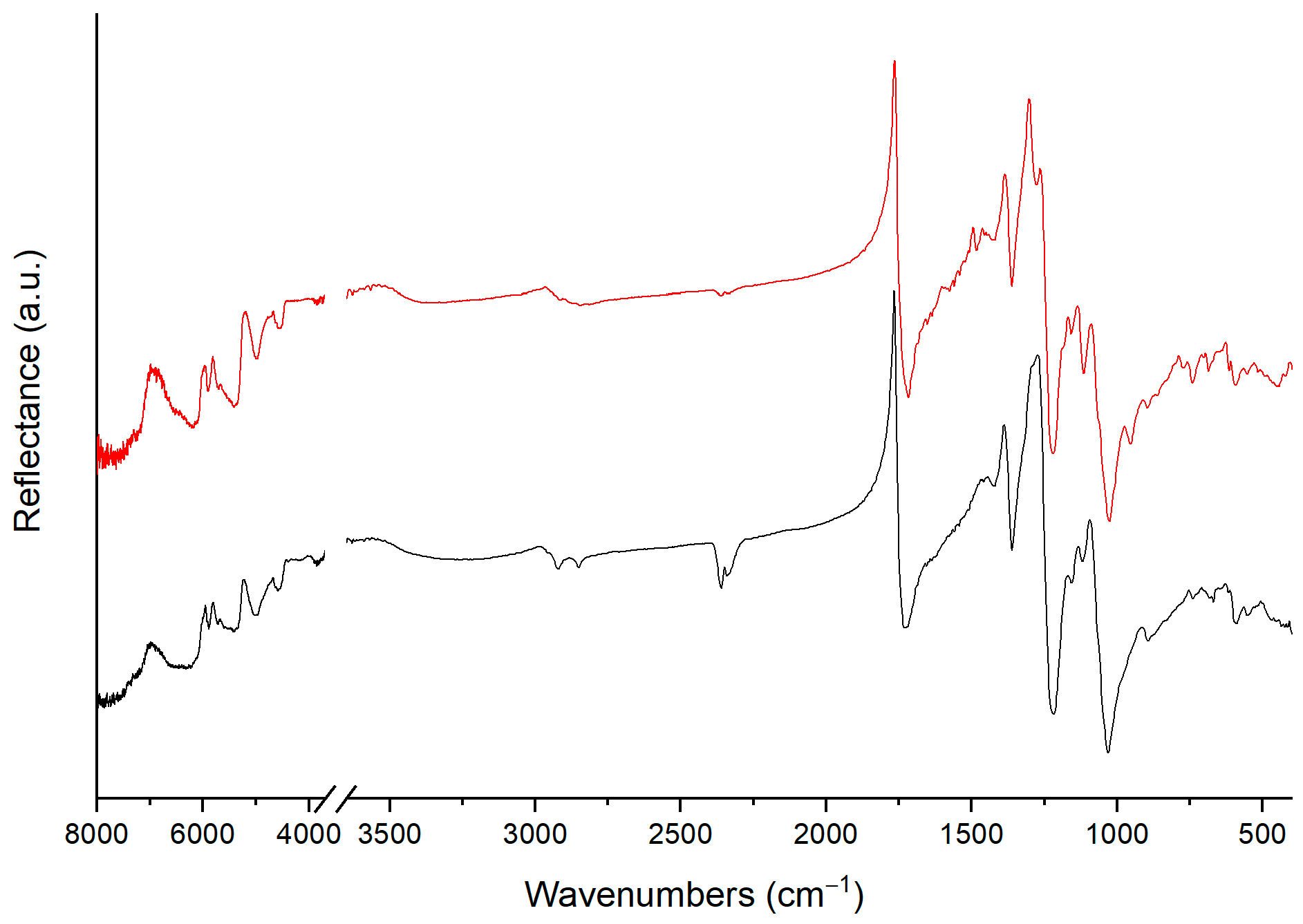
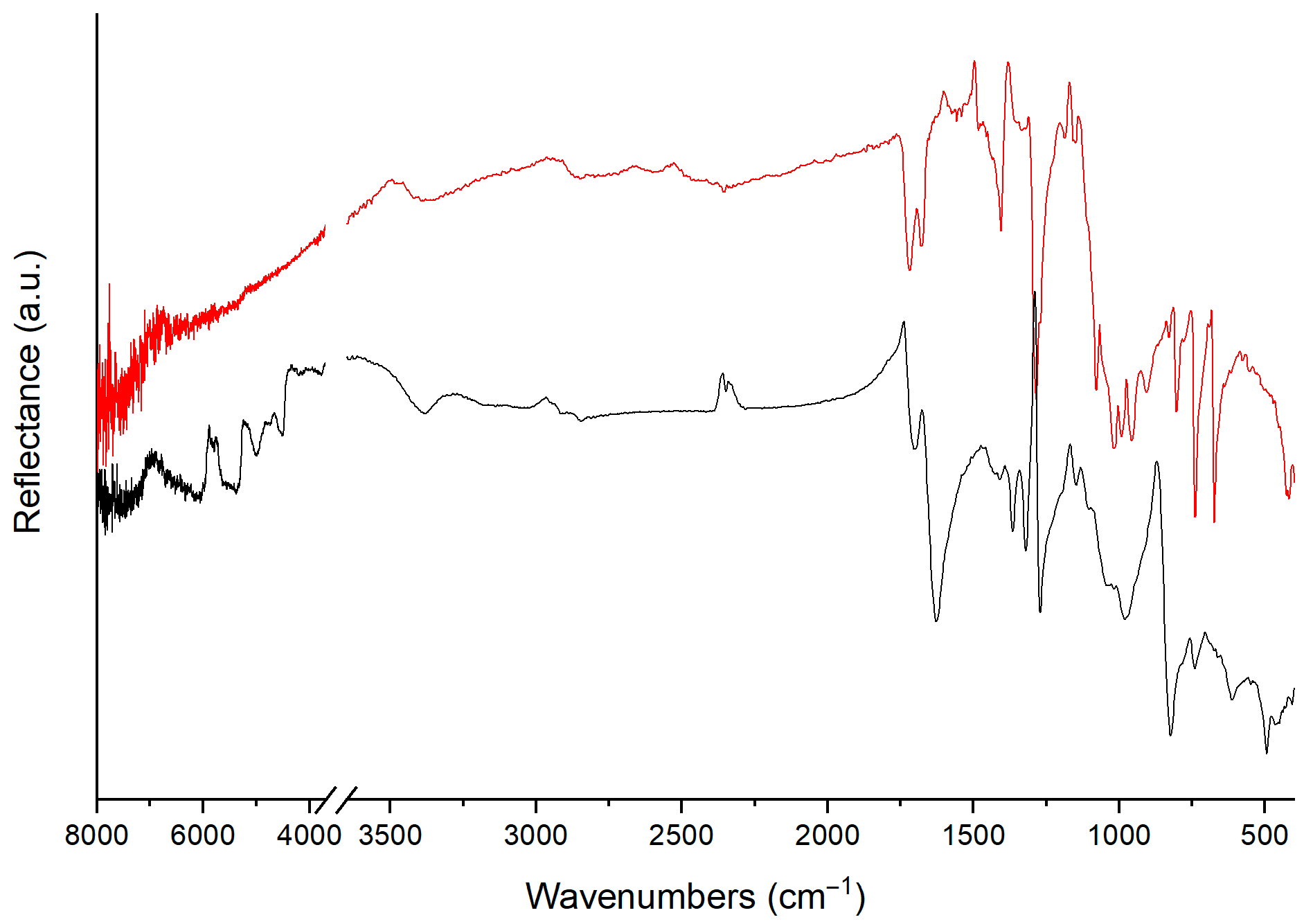
4.1. Classifying Plastic Materials Using FTIR Spectra
- Typology;
- Representativeness;
- State of conservation.
| Plastic Material Type | Number of Items | |
|---|---|---|
| Cellulose nitrate | CN | 9 |
| Cellulose acetate | CA | 8 |
| Casein | Protein | 5 |
| Polystyrene | PS | 4 |
| Acrylics | PMMA | 3 |
| Phenolics | PF | 2 |
| Urea–Formaldehyde | UF | 2 |
| Polyethylene | PE | 1 |
| Polyethylene terephthalate | PET | 1 |
4.2. Indoor Air Quality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferdyn-Grygierek, J. Indoor environment quality in the museum building and its effect on heating and cooling demand. Energy Build. 2014, 85, 32–44. [Google Scholar] [CrossRef]
- Verticchio, E.; Frasca, F.; Cavalieri, P.; Teodonio, L.; Fugaro, D.; Siani, A.M. Conservation risks for paper collections induced by the microclimate in the repository of the Alessandrina Library in Rome (Italy). Heri. Sci. 2022, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Chiantore, O.; Poli, T. Indoor air quality in museum display cases: Volatile emissions, materials contributions, impacts. Atmosphere 2021, 12, 364. [Google Scholar] [CrossRef]
- Motta, O.; Pironti, C.; Ricciardi, M.; Rostagno, C.; Bolzacchini, E.; Ferrero, L.; Cucciniello, R.; Proto, A. Leonardo da Vinci’s “Last Supper”: A case study to evaluate the influence of visitors on the Museum preservation systems. Environ. Sci. Pollut. Res. 2021, 29, 29391–29398. [Google Scholar] [CrossRef]
- Ault, A.P.; Grassian, V.H.; Carslaw, N.; Collins, D.B.; Destaillats, H.; Donaldson, D.J.; Farmer, D.K.; Jimenez, J.L.; McNeill, V.F.; Morrison, G.C.; et al. Indoor surface chemistry: Developing a molecular picture of reactions on indoor interfaces. Chem 2020, 6, 3203–3218. [Google Scholar] [CrossRef]
- Lee, D.S.; Holland, M.R.; Falla, N. The potential impact of ozone on materials in the U.K. Atmos. Environ. 1996, 30, 1053–1065. [Google Scholar] [CrossRef]
- Nunes, S.; Ramacciotti, F.; Neves, A.; Angelin, E.M.; Ramos, A.M.; Roldão, É.; Wallaszkovits, N.; Armijo, A.A.; Melo, M.J. A diagnostic tool for assessing the conservation condition of cellulose nitrate and acetate in heritage collections: Quantifying the degree of substitution by infrared spectroscopy. Herit. Sci. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Geyer, R. Production, Use, and Fate of Synthetic Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–32. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A review. Bull. Environ. Contam. Toxicol. 2020, 107, 577–584. [Google Scholar] [CrossRef]
- Kearney, M. From Samples to Complex Objects: Detecting Material Degradation in Plastic Artworks. Ph.D. Thesis, University College London, London, UK, 2021. Available online: https://discovery.ucl.ac.uk/id/eprint/10126241/ (accessed on 3 May 2023).
- Lazzari, M.; Reggio, D. What fate for plastics in artworks? An overview of their identification and degradative behaviour. Polymers 2021, 13, 883. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Hamrang, A. Degradation and Stabilzation of Cellulose Based Plastics e Artifacts. Ph.D. Thesis, The Manchester Metropolitan University, Manchester, UK, 1994. [Google Scholar]
- Littlejohn, D.; Pethrick, R.A.; Quye, A.; Ballany, J.M. Investigation of the degradation of cellulose acetate museum artefacts. Polym. Degrad. Stab. 2013, 98, 416–424. [Google Scholar] [CrossRef]
- Puls, J.; Wilson, S.A.; Hölter, D. Degradation of Cellulose Acetate-Based Materials: A review. J. Polym. Environ. 2010, 19, 152–165. [Google Scholar] [CrossRef]
- Blank, S. An introduction to plastics and rubbers in collections. Stud. Conserv. 1990, 35, 53–63. [Google Scholar] [CrossRef]
- Shashoua, Y. Conservation of Plastics—Materials Science, Degradation and Preservation; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Katz, S. Plastics: Designs and Materials; Studio Vista: London, UK, 1978. [Google Scholar]
- Katz, S. Plastics: Common Objects, Classic Designs; with a Collector’s Guide; ABRAMS: New York, USA, 1984. [Google Scholar]
- Mossman, S. TI Early Plastics: Perspectives, 1850–1950; Burns & Oates: Leicester, UK, 1997. [Google Scholar]
- MoDiP Museum of Design in Plastics. Available online: https://www.modip.ac.uk/ (accessed on 3 January 2023).
- PHS The Plastic Historical Society. Available online: https://plastiquarian.com/ (accessed on 3 January 2023).
- Del Fiore, A.; Reverberi, M.; Ricelli, A.; Pinzari, F.; Serranti, S.; Fabbri, A.A.; Bonifazi, G.; Fanelli, C. Early detection of toxigenic fungi on maize by hyperspectral imaging analysis. Int. J. Food Microbiol. 2010, 144, 64–71. [Google Scholar] [CrossRef]
- Perrino, C.; Catrambone, M. Development of a variable-path-length diffusive sampler for ammonia and evaluation of ammonia pollution in the urban area of Rome, Italy. Atmos. Environ. 2004, 38, 6667–6672. [Google Scholar] [CrossRef]
- De Santis, F.; Dogeroglu, T.; Fino, A.; Menichelli, S.; Vazzana, C.; Allegrini, I. Laboratory development and field evaluation of a new diffusive sampler to collect nitrogen oxides in the ambient air. Anal. Bioanal. Chem. 2002, 373, 901–907. [Google Scholar] [CrossRef]
- De Santis, F.; Allegrini, I.; Fazio, M.C.; Pasella, D.; Piredda, R. Development of a passive sampling technique for the determination of nitrogen dioxide and sulphur dioxide in ambient air. Anal. Chim. Acta 1997, 346, 127–134. [Google Scholar] [CrossRef]
- Gibson, L.T.; Cooksey, B.G.; Littlejohn, D.; Tennent, N.H. Determination of experimental diffusion coefficients of acetic acid and formic acid vapours in air using a passive sampler. Anal. Chim. Acta 1997, 341, 1–10. [Google Scholar] [CrossRef]
- Roadman, M.J.; Scudlark, J.R.; Meisinger, J.J.; Ullman, W.J. Validation of Ogawa passive samplers for the determination of gaseous ammonia concentrations in agricultural settings. Atmos. Environ. 2003, 37, 2317–2325. [Google Scholar] [CrossRef]
- Scheeren, B.A.; De Santis, F.; Allegrini, I.; Heeres, P. Monitoring SO2 With Passive Samplers: A laboratory evaluation of Na2CO3 and triethanolamine as absorbing media. J. Environ. Anal. Chem. 1994, 56, 73–85. [Google Scholar] [CrossRef]
- Quye, A.; Williamson, C. Plastics: Collecting and Conserving. Williamson; NMS Publishing Limited: Edinburgh, UK, 1999. [Google Scholar]
- Weschler, C.J.; Shields, H.C.; Naik, D.V. Indoor ozone exposures. JAPCA 1989, 39, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, P. The composition of museum atmospheres. Atmos. Environ. 1990, 24, 1–8. [Google Scholar] [CrossRef]
- Tétreault, J. Polluants Dans Les Musées Et Les Archives: Évaluation Des Risques, Stratégies De Contrôle et Gestion De La Preservation. Canadian Conservation Institute: Ottawa, ON, Canada, 2003. [Google Scholar]
- Spicer, C.W.; Coutant, R.W.; Ward, G.F.; Joseph, D.W.; Gaynor, A.J.; Billick, I.H. Rates and mechanisms of NO2 removal from indoor air by residential materials. Environ. Int. 1989, 15, 643–654. [Google Scholar] [CrossRef]
- Hashim, A.; Rahman, S.A.; Laili, Z.; Elias, M.S.; Ashifa, N.; Salim, A.; Shukor, A.; Azman, A.; Omar, S.A.; Zakaria, Z.A. Measurement of Indoor Air Pollutants in National Museum, Kuala Lumpur. In Proceedings of the Research and Development Seminar Nuklear, Kajang, Malaysia, 30 October–1 November 2018. [Google Scholar]
- Godoi, R.H.M.; Kontozova, V.; Godoi, A.F.L.; Grieken, R.V. Investigation of individual particles and gaseous air pollutants in showcases. In Air pollution and cultural heritage. In Proceedings of the International Workshop on Air Pollution and Cultural Heritage, Seville, Spain, 1–3 December 2003. [Google Scholar]
- Pitts, J.N.; Sanhueza, E.; Atkinson, R.; Carter, W.P.L.; Winer, A.M.; Harris, G.W.; Plum, C.N. An investigation of the dark formation of nitrous acid in environmental chambers. Int. J. Chem. Kinet. 1984, 16, 919–939. [Google Scholar] [CrossRef]
- Schwartz, S.E.; White, W.H.J. Trace Atmospheric Constituents. Properties, Transformation and Fates; Wiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- De Santis, F.; Di Palo, V.; Allegrini, I. Determination of some atmospheric pollutants inside a museum: Relationship with the concentration outside. Sci. Total Environ. 1992, 127, 211–223. [Google Scholar] [CrossRef]
- Myles, W.J.; Reiss, H. Sorption of nitrogen dioxide by cellulose acetate. J. Polym. Sci. 1955, 15, 243–261. [Google Scholar] [CrossRef]
- Wang, Z.; Shaw, D.; Kahan, T.; Schoemaecker, C.; Carslaw, N. A modelling study of the impact of photolysis on indoor air quality. Indoor Air 2022, 32, e13054. [Google Scholar] [CrossRef]
- Lunden, M.M.; Revzan, K.L.; Fischer, M.L.; Thatcher, T.L.; Littlejohn, D.; Hering, S.V.; Brown, N.J. The transformation of outdoor ammonium nitrate aerosols in the indoor environment. Atmos. Environ. 2003, 37, 5633–5644. [Google Scholar] [CrossRef]
- Febo, A.; Perrino, C. Prediction and experimental evidence for high air concentration of nitrous acid in indoor environments. Atmos. Environ. Part A Gen. Top. 1991, 25, 1055–1061. [Google Scholar] [CrossRef]
- Spicer, C.W.; Kenny, D.V.; Ward, G.F.; Billick, I.H. Transformations, lifetimes, and sources of NO2, HONO, and HNO3 in indoor environments. Air Waste 1993, 43, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; Jones, L. Chemistry-Molecules, Matter, and Change, 3rd ed.; W.H. Freeman & Company: New York, NY, USA, 1997; p. 26. [Google Scholar]
- Saviello, D.; Toniolo, L.; Goidanich, S.; Casadio, F. Non-invasive identification of plastic materials in museum collections with portable FTIR reflectance spectroscopy: Reference database and practical applications. Microchem. J. 2016, 124, 868–877. [Google Scholar] [CrossRef]
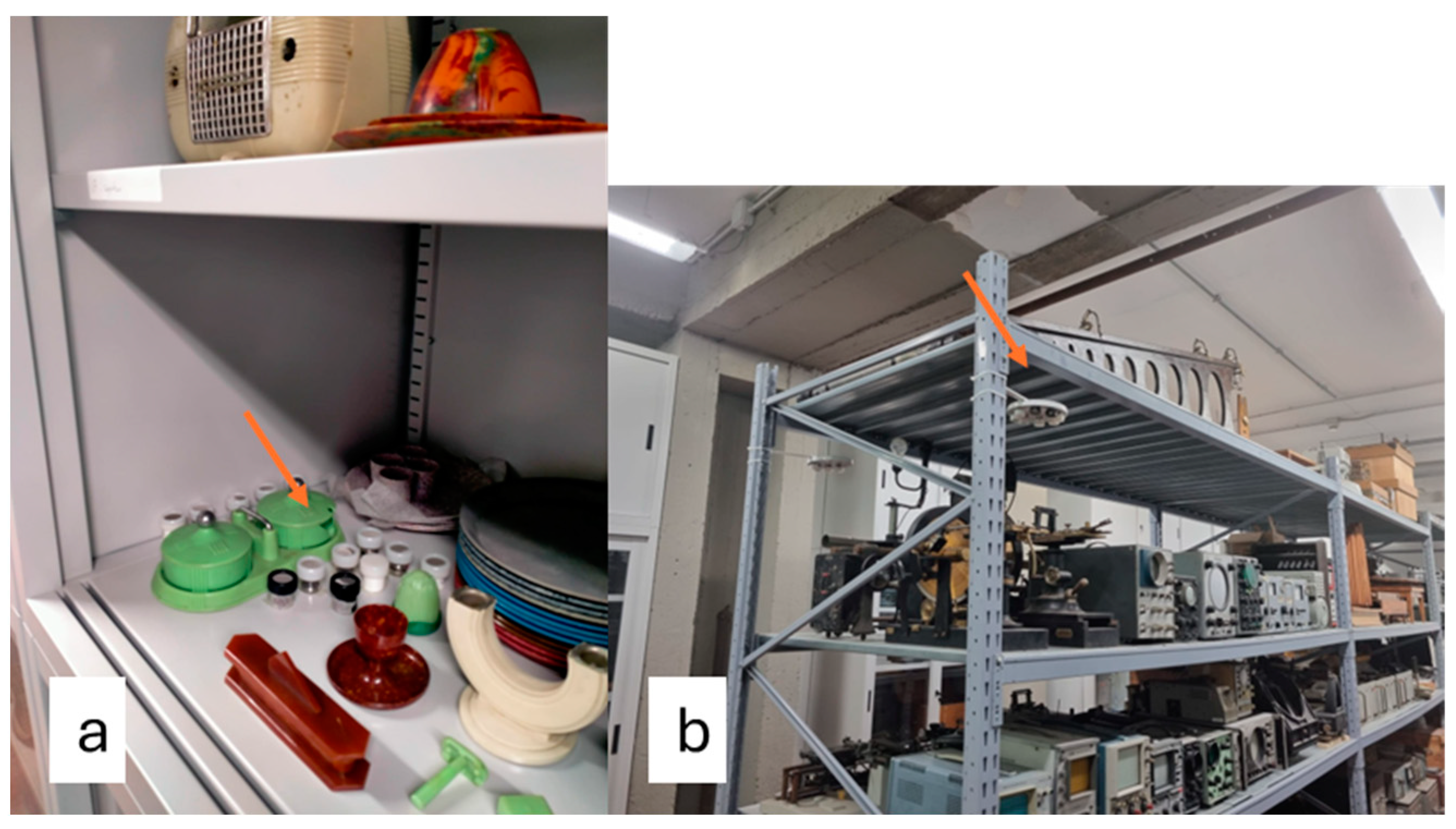
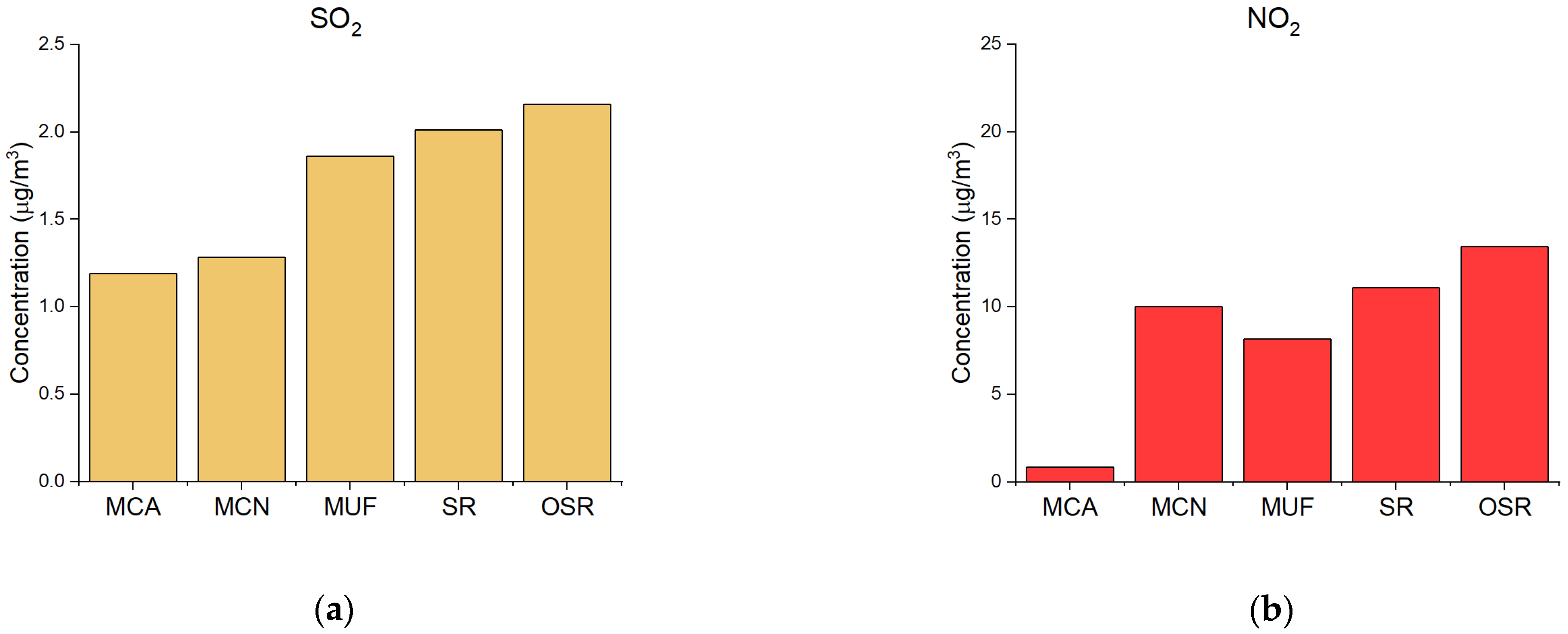

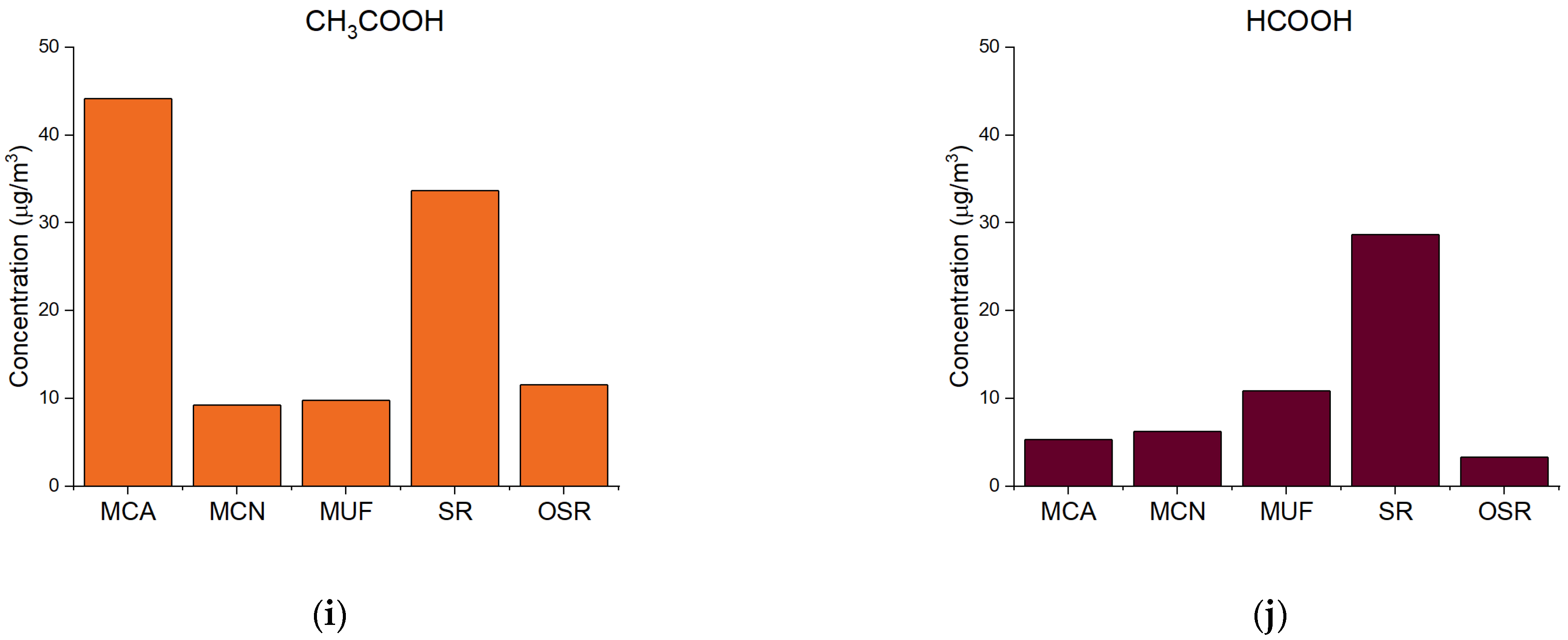
| Site | Description |
|---|---|
| MCA | Metal cabinet containing objects made of cellulose acetate |
| MCN | Metal cabinet containing objects made of cellulose nitrate |
| MUF | Metal cabinet containing objects made of urea–formaldehyde |
| SR | Storage room (hosting the metal cabinet) |
| OSR | A room connecting the storage room with the rest of the museum’s exhibition on the ground floor, through a door |
| Monitoring Campaign—July 2022 | Whole Year—2022 | |||
|---|---|---|---|---|
| T °C | RH% | T °C | RH% | |
| Min | 20.7 | 45 | 15.6 | 37 |
| Max | 21.3 | 56 | 25.8 | 66 |
| Average | 21.0 | 52 | 20.4 | 55 |
| SO2 | NOX | NO2 | NO | HNO2 | HNO3 | O3 | CH3COOH | HCOOH | NH3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| CA/SR | 0.59 | 1.0 | 0.056 | 3.4 | 2.2 | 0.19 | 0.62 | 1.3 | 0.18 | 0.44 |
| CN/SR | 0.64 | 0.73 | 0.90 | 0.36 | 2.1 | 1.8 | 0.63 | 0.27 | 0.22 | 1.0 |
| UF/SR | 0.93 | 0.9 | 0.74 | 1.2 | 1.5 | 0.42 | 0.55 | 0.29 | 0.38 | 1.7 |
| Pollutant | Average Concentration (µg/m3) | Maximum (µg/m3) | Minimum (µg/m3) |
|---|---|---|---|
| NOx | 16 | 21 (OSR) | 12 (MCN) |
| NO2 | 8.7 | 13 (OSR) | 0.85 (MCA) |
| NO | 7.4 | 17 (MCA) | 1.8 (MCN) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catrambone, M.; Cappellina, M.; Olivini, F.; Possenti, E.; Saccani, I.; Sansonetti, A. Indoor Air Quality in a Museum Storage Room: Conservation Issues Induced in Plastic Objects. Atmosphere 2024, 15, 1409. https://doi.org/10.3390/atmos15121409
Catrambone M, Cappellina M, Olivini F, Possenti E, Saccani I, Sansonetti A. Indoor Air Quality in a Museum Storage Room: Conservation Issues Induced in Plastic Objects. Atmosphere. 2024; 15(12):1409. https://doi.org/10.3390/atmos15121409
Chicago/Turabian StyleCatrambone, Maria, Marianna Cappellina, Francesca Olivini, Elena Possenti, Ilaria Saccani, and Antonio Sansonetti. 2024. "Indoor Air Quality in a Museum Storage Room: Conservation Issues Induced in Plastic Objects" Atmosphere 15, no. 12: 1409. https://doi.org/10.3390/atmos15121409
APA StyleCatrambone, M., Cappellina, M., Olivini, F., Possenti, E., Saccani, I., & Sansonetti, A. (2024). Indoor Air Quality in a Museum Storage Room: Conservation Issues Induced in Plastic Objects. Atmosphere, 15(12), 1409. https://doi.org/10.3390/atmos15121409







