Abstract
Volatile organic compounds (VOCs) are one of significant contributors to air pollution and have profound effects on human health and the environment. This study introduces a detailed analysis of VOC emissions from various industries within an industrial complex using a high-resolution measurement instrument. This study aimed to identify the VOC profiles and their concentrations across 12 industries. Sampling was conducted across 99 facilities in an industrial complex in South Korea, and VOC analysis was performed based on measurement data using a Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry (PTR-ToF-MS). The results indicated that the emission of oxygenated VOCs (OVOCs) was dominant in most industries. Aromatic hydrocarbons were also dominant in most industries, except in screen printing (SP), lubricating oil and grease manufacturing (LOG), and industrial laundry services (ILS) industries. Chlorinated VOCs (Cl-VOCs) showed a relatively higher level in the metal plating (MP) industry than those in other industries and nitrogen-containing VOCs (N-VOCs) showed high levels in general paints and similar product manufacturing (PNT), MP, and ILS industries, respectively. The gravure printing industry was identified as the highest emitter of VOCs, with the highest daily emissions reaching 5934 mg day−1, primarily consisting of ethyl acetate, toluene, butyl acetate, and propene. The findings suggest that the VOC emissions from the gravure printing and plastic synthetic leather industries should be primarily reduced, and it would be the most cost-effective approach to improving air quality. This study can provide the fundamental data for developing effective reduction technologies and policies of VOC, ultimately contributing to enhanced atmospheric models and regulatory measures.
1. Introduction
Volatile organic compounds (VOCs) are a diverse group of hydrocarbons, some of which significantly contribute to air pollution, impacting both human health and the environment [1,2,3,4,5]. These compounds are emitted from various sources and play a crucial role in atmospheric chemistry, facilitating the formation of ground-level ozone and secondary organic aerosols (SOAs) [5,6,7,8,9,10].
The industrial sector is a major source of anthropogenic VOCs, with each industry exhibiting a unique VOC emission profile based on their specific processes and materials. Profiling VOC emissions is essential for identifying key pollutants and their sources, developing targeted control strategies, assessing air quality, formulating environmental policies, and improving atmospheric models to predict secondary pollutants like ozone and particulate matter [11,12,13,14].
Historically, the characterization and profiling of VOC emissions have predominantly relied on offline sampling and laboratory analysis using gas chromatography (GC) [15,16,17]. However, this approach has several limitations [18,19,20]. GC struggles to detect very low concentrations of VOCs, making the accurate analysis of trace levels challenging. Furthermore, the separation and quantification of individual components in complex mixtures are more time-consuming, and different columns should be used according to functional VOC group.
Due to these limitations, there has been a growing interest in alternative methods such as proton-transfer-reaction time-of-flight mass spectrometry (PTR-ToF-MS) [21,22]. PTR-ToF-MS offers higher sensitivity and selectivity for a broad range of VOCs, including those present at very low concentrations, which is crucial for accurate environmental monitoring [23,24]. Compared with the GC method, PTR-ToF-MS can detect a wider variety of oxygenated VOCs (OVOCs) due to its soft ionization process, which preserves the integrity of these compounds [25]. This process is highly effective in analyzing complex mixtures of VOCs, allowing for the simultaneous identification and quantification of various OVOCs [11,22,26,27]. For instance, a study by Han et al. suggested that PTR-ToF-MS is more suitable than GC for measuring OVOCs due to its superior detection capabilities [28]. Given these advantages, PTR-ToF-MS is expected to gain increasing attention in future research. Preliminary investigations like this study are necessary to establish source profiles based on measurements from this equipment for comprehensive source apportionment with PTR-ToF-MS.
The aim of this study is to provide a characteristic profile of VOC emissions from several industries in an industrial complex, using a PTR-ToF-MS to quantify targeted VOCs from 12 industries, such as the manufacturing of wood furniture, laundry services, printing, etc. Measurement data were used to understand the patterns of VOC functional groups and the distribution of individual VOC species for each industry. To utilize VOC mitigation policy, the daily VOC emissions from each industry tested in this study were estimated based on the measured concentrations and exhaust air flow rates.
2. Materials and Methods
2.1. Sampling Site and Methods
Sampling of VOCs was conducted within an industrial complex in South Korea. This industrial complex included over 1400 facilities. Excluding those that were not operational or lacked emission facilities, a total of 99 facilities were selected for sampling. Some of these facilities had multiple emission points, and in such cases, samples were collected from all emission points, resulting in a total of 143 samples.
The samples were collected using a 5 L Tedlar bag in a vacuum chamber with an indirect suction method (negative pressure sampling) at the stack of each facility (Figure 1). The valves and tubing lines of the chamber are constructed of Teflon, ensuring that the loss of VOCs by adsorption is minimized during sampling. In this method, all parts except for the sampling line are completely sealed. When a vacuum is made inside the chamber and outside the bag by an air pump, the sample fills the bag inside the chamber due to negative pressure (evacuated lung approach). Before sampling, the stack flue gas was flushed for 3–5 min to clean the lines in the sampling system. When sample collection is completed, the vacuum was quickly released, and the valve was sealed to prevent the leakage or ingress of any extraneous other gases.
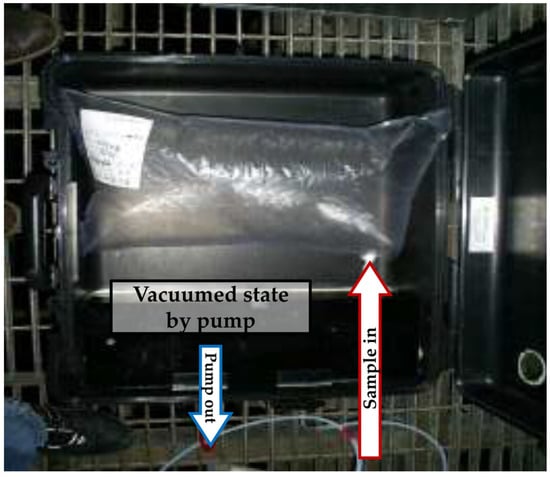
Figure 1.
Negative pressure sampling with a vacuum chamber. The red-bordered and blue-bordered arrows indicate the direction of sample inflow and the direction of air outflow from the chamber by the pump, respectively.
To prevent photochemical reactions of reactive gas-phase compounds such as NOx and/or VOCs within the sample, all processes were shielded from light until the measurements in the laboratory, as sunlight exposure could trigger such reactions. Additionally, the temperature and flow rate at the stacks were simultaneously measured to obtain background information on the emission gases.
2.2. Measurement Conditions and VOC Analysis Using PTR-ToF-MS
The measurement of VOCs was conducted using PTR-ToF-MS 1000 (Ionicon Analytik GmbH, Innsbruck, Austria). To prevent sample degradation, the analyses were performed on the same day when the samples were collected. The samples were stored in Tedlar bags and transported to the PTR-ToF-MS for measurement.
The target VOC species were selected from 41 species designated as hazardous air pollutants (HAPs) recommended by the EPA for analysis, and their general characteristics and their groups were summarized as shown in Table 1. OVOCs are VOC species that contain oxygen atoms, such as aldehydes, acetates, carboxylic acids, ketones, esters, ethers, etc. Although alcohols also contain oxygen atoms, they are excluded from the OVOCs group and categorized separately in this study to highlight their distinct characteristics, i.e., the role as a precursor before oxidation.

Table 1.
The target VOC species measured by PTR-ToF-MS.
Two software programs developed by IONICON Analytik GmbH were used for data acquisition, i.e., ToF version 4.0 software for operating the equipment (PTR-ToF-MS) and Viewer version 3.3.17 (IONICON Analytik GmbH, Innsbruck, Austria) for processing the raw data detected by ToF software, respectively [29,30]. The ToF version 4.0 software sets the measurement conditions, such as the measurement mode with H3O+, O2+, NO+ as the primary ions, mass range, analysis time. Additionally, it provides real-time measurement data and trends in counts per second (cps) or concentration (ppbv). Viewer 3.3.17 is the post-processing software that enables the calibration of mass data, calculates the actual concentration of analyte, and saves the results in a text file. These software programs are regularly updated to improve the convenience and usability of PTR-ToF-MS for users [31].
ToF 4.0 can monitor and express real-time concentrations, but it requires time for repeating measurements and data processing to derive the final concentration. For this reason, the automated measurement and evaluation (AME) mode developed by IONICON is being used these days and this software can monitor only those pre-selected (targeted) by the user [32,33]. It allows the user to set analysis conditions for each targeted compound and provides the advantage of obtaining the final concentration in ppbv. The energy (E/N) in the ionization process can be adjusted to either suppress or enhance the fragmentation of molecules. This unique feature of PTR-MS is particularly useful to separate isomers such as xylene and ethylbenzene. This technique provides highly specific information about compounds with different product ion ratios. The AME software automates this process and can compile data from over 8 different modes. Analysis conditions are shown in Table 2 [34,35].

Table 2.
The analytical conditions for PTR-ToF-MS.
There are two methods for quantifying analytes in PTR-ToF-MS, the methods using k-rates and utilizing compound-specific calibration, respectively [36]. TO-14A standard gas (Restek Corp., Bellefonte, PA, USA) to quantify VOCs was used by employing a theoretical estimation method based on ion transmission efficiency. Aromatics in the TO-14A standard gas were used as transmission calibration standards, while the theoretical concentrations of other compounds were calculated using k-rate constants from previous studies [37,38,39,40].
2.3. Calculation for VOC Emissions
The daily VOC emissions from each facility were calculated based on the measured concentrations of VOC species and flow rates of stack flue gas. To achieve this, we simultaneously measured the inner diameter of the outlet, and the temperature and velocity of the flue gas. The daily emissions from a single company can be calculated through Equation (1):
where Ei represents the daily total VOC emissions (mg day−1) from individual facilities. Cj denotes the measured concentration (ppbv) of individual VOC compounds, while MWj represents the molecular weight (g mol−1) of the respective compounds. TE and QE express the temperature (°C) and volumetric flow rate (m3 min−1) derived from the diameter of the emission outlet and the velocity of the emission gas (m s−1), respectively. WH (hour) denotes the daily operational hours, which in this study is assumed to be 8 h per day. Based on the industry groups classified in Section 2.4, the VOC emissions from each facility were summated by industry classification for comparative analysis of their characteristics.
2.4. Classification of Business Types
In Korea, the Korean standard industrial classification (KSIC) is commonly used in industrial statistics [41]. KSIC classifies industries into five levels, from major categories to subclassifications, totaling 1196 sectors. However, the clean air policy support system (CAPSS) in Korea, which calculates emissions of air pollutants such as VOCs from industrial facilities, utilizes a different classification system from KSIC. It is based on the European coordination of information on air emissions (CORINAIR) and the emission source classification system (SNAP 97), which has been revised several times and currently applies 13 major classification criteria [42,43].
In this study, the classification of industries was conducted from the perspective of air pollution, focusing on industries that have a number of annual VOC emissions. Therefore, the selected industries were classified as a second-level category based on the 2021 data from CAPSS (Table 3).

Table 3.
Annual VOC emissions reported by CAPSS in 2021 [41].
Industries related to the use of organic solvents have a large number of VOC emissions, such as painting, cleaning, and the petroleum products industry. In 2021, annual VOC emissions from painting facilities accounted for approximately 40% (330,445 ton yr−1) of the total. The next highest sector was other organic solvent uses, which includes printing, household and commercial organic solvent uses, and asphalt road paving, with annual emissions of 167,568 ton yr−1.
One of the major contributors among various industrial sources using organic solvents is the petrochemical industry [44,45]. However, this industry is typically located in petroleum-related industrial complexes, separated from general industrial complexes. Therefore, petrochemical industries were excluded in this study to focus on general industrial complexes [44,46,47,48].
According to the KSIC classification system, the reclassification of industries was conducted for the 99 sampled facilities. Finally, 12 industries were selected to investigate quantitative VOC source profiles based on emissions proportion with notable characteristics of each sample. In cases where multiple facilities and samples were included within an industry group, concentrations and emissions were averaged based on compounds, emission point temperature, and flow rate.
3. Results and Discussion
3.1. Characteristics for Functinal Group of VOCs
Proportional variations in VOC functional groups, which reflect the distinct characteristics of industrial activities, are shown in Figure 2. Alkane emissions generally indicate the use of fossil fuel materials. Alkanes showed the highest level in the CP industry (19.1%), but an extremely low level in the SP industry. Alkenes ranged from 1.7% to 11.8% in the PSL and GP industries, respectively. Aromatic VOCs constituted a substantial proportion in several industries such as the PNT, CP, PCB, and PSL industries, representing at 60.3%, 52.5%, 51.5%, and 37.6% respectively. The ILS and SP industries showed the highest levels and similar proportions in the alcohol group, at 32.0% and 31.3%, respectively. Additionally, the OP and CP industries also showed relatively high alcohol emissions at 17.3% and 13.6%, respectively. OP had the highest contribution from the aromatic group at 37.1% among printing industries, and SP showed a split contribution with OVOCs and alcohols each accounting for 55.6% and 31.3%, respectively.
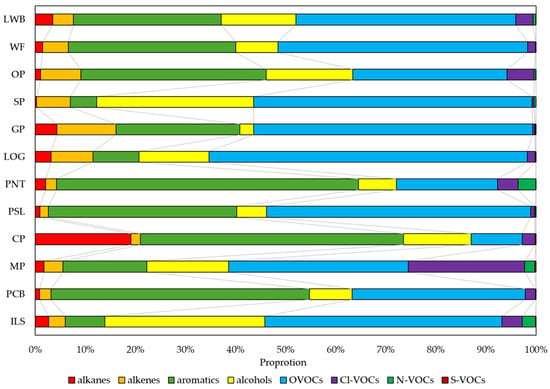
Figure 2.
Source profiles of VOC groups by industries.
OVOCs dominated the source profile in most industries, with the highest proportions observed in the LOG industry at 63.5%, and represented similar proportions among GP, SP, and PSL at 55.7%, 55.6%, and 52.8% respectively. Chlorinated VOCs (Cl-VOCs) exhibited low levels in most industries but showed the highest proportion (23.2%) in the MP industry. This result might be due to the extensive use of chlorinated solvents and chemical materials in the cleaning and degreasing stages of metal plating [49].
Figure 3 shows the patterns of VOC groups emitted by different industries. Most industries exhibited a dominant contribution of the OVOCs and aromatic groups. Overall, groups of nitrogen-containing VOCs (N-VOCs), sulfur-containing VOCs (S-VOCs), alkanes, and alkenes showed extremely low fractions in most industries. This is the reason why those groups were integrated with one or two species, compared with other VOC groups, as shown in Table 1. Although the OP, SP, and GP industries are printing-related, the patterns were not similar due to the different materials and processes used in each industry. However, the patterns of wood-related industries (LWB and WF) were similar, because the same materials are used in both industries and they are in a related business field. The LOG industry showed a mostly unique pattern towards OVOCs, indicating the highest contribution of OVOCs (63.5%) in the VOC groups. The MP industry also showed a unique pattern with the high emission of Cl-VOCs. On the other hand, some similar patterns among different industries were observed, such as WF-GP-PSL industries, LWB-OP industries, PNT-PCB industries, and SP-ILS industries, respectively. To identify these similar patterns among different industries, further analysis for individual VOC compounds (see Section 3.2) should be needed.
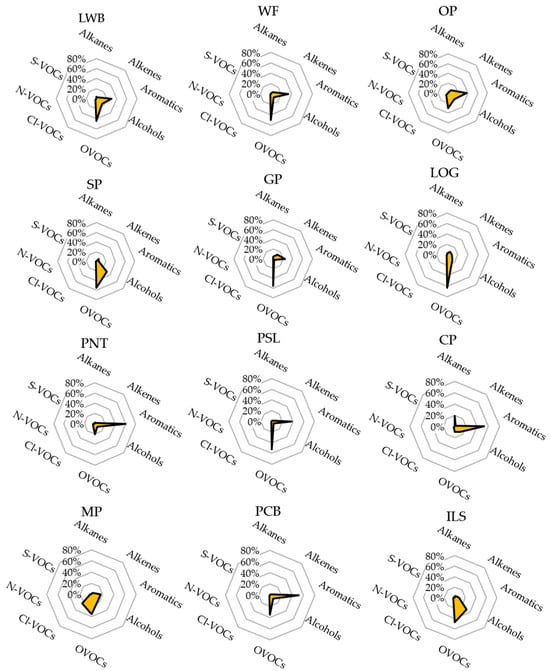
Figure 3.
Radial charts for patterns of VOC groups by industries.
3.2. Characteristics of Individual VOC Species
Figure 4 illustrates the emission profiles of individual VOC compounds in various industries and is classified by each VOC group. In the GP industry, ethyl acetate (32.9%), toluene (24.3%), butyl acetate (15.5%), and propene (11.8%) were detected in high proportions, collectively constituting over 80% of the total. For the SP industry, acetone (49.2%), ethanol (30.2%), and propene (6.8%) were observed as the primary components. In the case of the OP industry, the most abundant species were toluene (24.4%), followed by methanol (12.9%) and acetone (11.4%). The high content of ethyl acetate in the GP industry is likely influenced by the use of water-based inks in the printing process. Earlier studies reported that more than 60% of ethyl acetate is emitted when using water-based inks instead of solvent-based inks [50]. Oh et al. (2022) investigated VOC species at the stacks of GP and SP industries in Korea, and suggested that ethyl acetate, toluene, and acetic acid were the main emitted species in the GP industry, while acetone and ethanol were predominant in SP [51]. In the OP industry, lithography and other printing techniques were used in the offset printing process, where the presence of OVOCs was most significant, as noted in earlier studies [50,52,53,54]. Compared with that of these studies, the high detection rates of methanol and acetone in this study can be reasonably level. The high proportion of toluene is likely due to the use of an organic cleaning agent in the washing process of ink residues after printing [55]. In addition, isopropyl alcohol is widely used in printing processes as a solvent for offset printing inks and is effective in removing ink residues on the printing machines and machine parts. Although this compound is a VOC indicator in the printing industry, no detection of this compound was observed in this study [53,56,57].
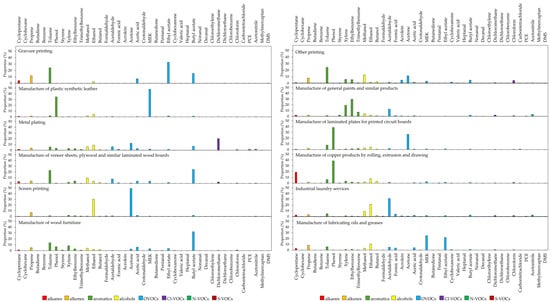
Figure 4.
Characteristics of VOC species emitted from various industries.
In the PCB industry, phenol, acetone, and toluene were abundant, constituting 38.6%, 26.6%, and 7.8%, respectively. Similarly, in the study by Shen et al. (2018), acetone accounted for approximately 60% of the stack flue gas, and toluene was approximately 12% [16]. This result suggested that phenol generally is emitted during the ink curing process due to the use of epoxy and phenolic compounds to reach complete curing. Lv et al. (2021) also conducted a study to develop VOC source profiles for this industry, and they identified that acetone along with isopropanol and ethanol was the main pollutant measured from PCB manufacturing industry [58]. They compared their results with those of the experiments conducted in Shanghai, where acetone and isopropanol were also detected with high levels [59]. These differences are likely due to the sampling spots (fugitive emissions and stack emissions), emphasizing the importance of the measurement method in comparing the results.
In the LOG industry, MEK (25.1%), ethyl acetate (22.1%), and ethanol (10.6%) were observed as the dominant VOC species. It was found that the investigated facility in this study used organic solvents based on MEK and ethanol, which likely contributed to the high levels of these compounds in flue gas. Dinh et al. (2015) investigated VOC emissions from spray lubricants and found high levels of n-butane, iso-butane, and propene [60]. A study on VOC emissions in the lubricant industry is limited because lubricants are a complicated material and mostly made up of large molecules with more than 20 carbon [61,62,63]. Therefore, studies in this industry have often focused on Semi-VOCs (SVOCs) [64,65,66].
In the PSL industry, MEK (47.8%) and phenol (34.5%) were observed as the most significant VOC species. Dimethyl formamide (DMF) and MEK are used as organic solvents in the synthetic resin manufacturing industry. Additionally, the lack of DMF detection in this study is likely due to a recovery system of the facility for the wastewater containing DMF. Similar results were observed in a study by Chang and Lin. (2006), which investigated VOC emissions from producing synthetic fiber leather using DMF and MEK as solvents [67]. Generally, phenolic monomers are used in the tanning process of synthetic leather, and it causes a high level of phenol emission [68]. Yang et al. (2020) reported that MEK and toluene from synthetic leather manufacturing facilities using water-based solvent resins accounted for 60% and 24%, respectively [69]. A study by Shen et al. (1999) highlighted that DMF, toluene, and MEK were major VOC species emitted from the polyurethane (PU) synthetic leather surface coating industry [70]. These findings agree with our results, confirming that these compounds are predominant in the MPL industry and chemical processes in productions such as coating, dyeing, and finishing processes due to the use of solvents.
In the PNT industry, the analysis identified ethylbenzene (29.8%), xylene (19.3%), and acetaldehyde (12.6%) as the highest-level species. A study by Jiménez-López and Hincapié-Llanos (2022) suggested that solvents such as xylene and toluene are major species detected during the production of paints due to the use of synthetic raw materials, such as synthetic plastics, synthetic fibers, and synthetic rubber, and their evaporation during the drying process [71]. Song et al. (2012) and Kim et al. (2022) investigated VOC speciation from paint manufacturing facilities in Korea. They also found significant emissions of xylene, toluene, and ethylbenzene from paint manufacturing processes [72,73]. Ghobakhloo et al. (2023) measured VOCs to evaluate the toxicity by exposure to workers in paint factories [74]. Their results similarly indicated that xylene, toluene, and ethylbenzene are predominant compounds. However, a study by Song et al. (2021) showed slightly different results [75]. While they reported high levels of xylene and ethylbenzene, consistent with earlier studies and our results, butyl acetate was the most abundant compound, which accounted for only 0.7% in our study. This difference can be explained by the type of process and which adsorption systems were applied in the study by Song et al. (2021).
In the WF industry, the emissions of butyl acetate were highest at 32.2%, followed by toluene at 13.5% and xylene at 8.4%. Liu et al. (2023) studied VOC emissions in the furniture manufacturing industry using various coating materials [76]. They found that butyl acetate (14.6%) and toluene (11.5%) were significantly emitted in processes using UV coating solvents. However, they reported that m/p-xylene was identified as the predominant species (approximately 17%), which differs from our results. Their study highlighted that VOC emissions varied depending on the type of coating process, including solvent-based coatings, water-based coatings, drying workshops, and UV radiation coatings. Adamová et al. (2020) reported that terpenes, aldehydes, and ketones were responsible for the high emissions of VOC groups emitted from wood and wood-based panel manufacturing [77]. Specifically, terpenes were largely emitted from wood chips produced during manufacturing, while aldehydes such as formaldehyde were predominant due to the use of adhesives, solvents, and coatings. Similarly, Teng et al. (2023) found that butyl acetate, toluene, and m-xylene were the main VOCs emitted from solvent-based coating processes, with portions of 18.5%, 17.5%, and 12.4%, respectively [78]. Xiong et al. (2019) investigated VOC emissions from wood production facilities and identified ethyl acetate, butyl acetate, and xylene as the major emission species [79]. These findings suggest that the major VOC emitted species in our study were caused by the MWF industry employing solvent-based coating processes, in agreement with reports in earlier studies.
In the MP industry, dichloromethane (20.3%) was the most predominant species, followed by OVOCs such as acetone (12.2%), ethanol (8.9%), and ethyl acetate (7.1%). Dichloromethane is commonly used to clean and degrease metal surfaces, dissolving contaminants, and evaporating quickly due to its high volatility. Abbaspour (2022) reported that chlorinated hydrocarbons are widely used in the cleaning processes of the metal plating industry [80]. Zheng et al. (2013) found that the major VOC groups emitted from metal surface coating facilities were aromatics (such as styrene and toluene) and OVOCs (such as isobutyl ketone, 1,2-dichloroethane, and ethyl acetate) [81]. However, our results were different from those results, possibly due to different solvents being used in metal coating paints at various processes. A study of Zheng also stated that the small sample size may not represent the general characteristics of the MP industry. Zhong et al. (2017) further reported that OVOCs and aromatics, such as toluene, ethylbenzene, and xylene, are primarily emitted during the metal surface coating process [82]. The differences observed in our study likely arise from various processes such as cleaning and curing. Although several studies investigated VOC emission ratios based on solvents used in metal coating, further study covering the entire process in the MP industry is still needed [53].
In the LWB industry, butyl acetate (24.4%) and toluene (22.6%) were identified as the major VOC species in emission gas, followed by methanol (9.6%) and acetaldehyde (7.6%). Butyl acetate and toluene, two of the most dominant compounds, showed similar proportions to the major contributors in the WF industry. This similarity is likely because the WF industry is based on the LWB industry. However, higher levels of alcohols and aldehydes were detected in the LWB industry, likely due to the release and volatilization of various compounds from adhesives and resins. The processes contributing to VOC emissions in this industry include adhesive manufacturing and coating, hot pressing, drying, and curing [77]. Cao et al. (2019) investigated the types of VOCs emitted from the production of various types of plywood and reported that aromatic hydrocarbons accounted for more than 65% of the total emissions in all plywood types [83]. Esters such as ethyl acetate are primarily used in adhesive manufacturing and coating processes, which likely explains the high detection of butyl acetate [84]. Park et al. (2013) found that toluene and formaldehyde, used as solvents in adhesives and coatings, were the major components emitted from the hot-pressing process, which is consistent with our results showing the high proportion of toluene [85]. The most important adhesives used in plywood manufacturing are urea- (UF) and phenol-formaldehyde (PF), which emit significant levels from the hot-pressing process [86]. However, UF and PF were not measured in this study because the investigated facility in this study employed an air pollution control system. Liu et al. (2020) highlighted 1,2-dichloropropane as a major VOC emitted during the production of wood-based panels, along with esters and aromatic hydrocarbons [87]. They emphasized that the results could vary depending on the raw materials and additives used in the plywood manufacturing process and explained the differences observed in earlier studies.
In the CP industry, phenol (38.6%) and cyclopentane (19.1%) were identified as the major compounds, followed by ethanol (7.8%) and toluene (6.2%). The facilities studied in this research primarily performed casting and rolling processes using copper. During the rolling process, oils (such as lubricants) are used to reduce friction and enhance the surface quality of copper products when they pass through rollers. Copper products are exposed to high temperatures during casting and rolling, leading to the volatilization of oils and the significant emission of VOCs. There is lack of recent studies measuring and characterizing VOC emissions from the copper product manufacturing industry. However, there are a few studies on VOC emissions from non-ferrous metal-manufacturing industries. The US EPA studied methods to control VOC emissions from the evaporation of lubricants during the rolling process of non-ferrous metals, but they did not identify the main VOC species from the process [88]. Yang et al. (2020) investigated VOC emissions from the non-ferrous metal industry and found that compounds from the OVOCs and aromatics groups were predominantly emitted, with ethyl acetate, acetone, and toluene as the major individual compounds [69]. The high levels of phenol and cyclopentane detected in our study are likely attributed to the use of lubricants in the manufacturing processes [89,90,91].
In the ILS industry, acetaldehyde was identified as the most abundant species (31.3%), followed by ethanol at 21.2% and methanol at 8.9%, respectively. Typically, laundry processes use chlorinated-based solvents, such as tetrachloroethylene and trichloroethane, and fluorinated and/or petroleum-based organic solvents. These solvents evaporate or are released as residual solvents during the washing process. Park et al. (2016) measured limited VOC species from small-scale laundries and reported that toluene and chlorobenzene were the highest emission species due to the use of petroleum-based solvents in the ILS facilities [92]. Several earlier studies also investigated laundry industries using hydrocarbon solvents, identifying C9-C12 hydrocarbons such as n-undecane, decane, nonane, and dodecane as the main compounds [93,94]. However, Lee et al. (2021) quantified emissions from the dry cleaning process and found hexane, methylene chloride, and acetone as the predominant VOC species [95]. Along with those in earlier research, disagreements about which major species were emitted from the ILS industry are shown in our study. These differences could be attributed to factors such as the type and nature of laundry, the weight and volume of the items being cleaned, operating times and temperatures, and the types of solvents used [94].
3.3. Calculation for Emissions of VOC Species
The daily VOC emissions from the concentrations were calculated using Equation (1) and are shown in Figure 5. Among all industries, GP showed the highest emissions, releasing 5934 mg day−1 of VOCs. This emission was more than twice as high compared with the emissions from the PSL industry, which had the second-highest VOC emissions at 2445 mg day−1. WF, OP, and PNT represented very similar daily emissions, calculated at 364 mg day−1, 363 mg day−1, and 363 mg day−1, respectively. The total daily VOC emissions from all industries (12 facilities) were 13,295 mg day−1. In the GP industry, the highest emissions were represented for ethyl acetate (1967 mg day−1), followed by toluene (1447 mg day−1), butyl acetate (898 mg day−1), and propene (707 mg day−1). Ethyl acetate showed the highest emission at 2081 mg day−1 (15.7%) and most of that originated from GP. Toluene showed the second highest emission at 1950 mg day−1 (14.7%), significantly influenced by GP and LWB. Butyl acetate was the third highest compound at 1419 mg day−1 (10.7%), particularly influenced by GP, LWB, and WF.
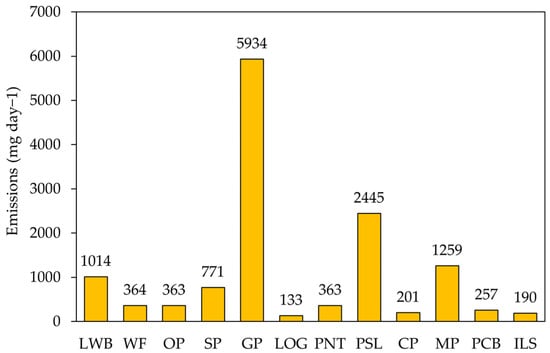
Figure 5.
Emissions of VOCs by industries. Numbers above each box represents the emissions of VOCs for each industry (mg day−1).
This study aimed to identify VOC source profiles by measuring VOC emissions from various industries and figuring out predominant species in each industry. One of the expected findings of this study is to provide fundamental data for developing effective technologies or policies to control VOC emissions in industrial complexes. Given that the development of VOC reduction technologies requires substantial budget and time, it is essential to estimate the emission levels for controlling VOC pollutant from various facilities within industrial complexes. This study suggested that even if a species was measured at a high proportion, its emission could be estimated at a low level. For example, in the PCB industry, phenol and acetone accounted for nearly 40% and 27% of the component ratio, respectively. However, their total daily emissions were only 201 mg day−1, which is quite negligible compared with the total emissions (13,295 mg day−1). Conclusively, controlling VOC emissions of the GP and PSL industries should be targeted and focused for effective VOC mitigation in industrial complexes in Korea.
4. Conclusions
This study explored a comprehensive analysis of VOC emissions from various industries within industrial complexes using PTR-ToF-MS. The findings highlighted the unique VOC profiles of different industrial sectors, emphasizing the considerable contribution of OVOCs in most industries, which play a crucial role in secondary organic aerosol formation and the gas phase reaction of ozone. The metal plating industry exhibited a high proportion of Cl-VOCs, primarily due to the extensive use of chlorinated solvents in cleaning and degreasing processes. Aromatic hydrocarbons also dominated in most industries, except in SP, LOG, and ILS industries. Cl-VOCs showed a relatively higher level in the MP industry than those in other industries and N-VOCs showed high levels in PNT, MP, and ILS industries, respectively.
The results identified substantial variability in VOC emissions, with the GP industry emerging as the highest source, releasing 5934 mg day−1 of VOCs. Ethyl acetate, toluene, butyl acetate, and propene were identified as the primary VOCs in the GP industry, collectively accounting for over 80% of its total emissions. Overall, the total daily VOC emissions from all investigated industries amounted to 13,295 mg day−1, with ethyl acetate and toluene being the most emitted compounds. This study highlighted the importance of targeting VOC sources for controlling and reducing the VOC pollutant in industrial complexes. Therefore, focusing on and targeting the GP and PSL industries would be the most effective approach to controlling VOC emissions in an industrial complex Table S1.
The detailed characterization of VOC source profiles in this study would be helpful to interpret the result of atmospheric models such as the Positive Matrix Factorization (PMF) model and to develop targeted mitigation strategies that are more effective in reducing overall VOC emissions.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/atmos15101156/s1, Table S1. VOC emissions (mg day−1) from the industrial exhaust gas by each species.
Author Contributions
Conceptualization, J.-S.H.; methodology, B.-H.O.; validation, H.-S.J. and J.-S.H.; formal analysis, K.-C.K. and C.-S.L.; investigation, K.-C.K. and J.-D.B.; data curation, H.-S.J. and J.-S.H.; writing—original draft preparation, K.-C.K. and Y.-J.L.; writing—review and editing, K.-C.K., C.-S.L., and B.-H.O.; supervision, H.-S.J. and J.-S.H.; funding acquisition, H.-S.J. and J.-S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Korea Environment Industry & Technology Institute (KEITI) through R&D Project for Management of Atmosphere environment, funded by the Korea Ministry of Environment (MOE) (2021003410002) and the Experts Training Graduate Program for Particulate Matter Management from the Ministry of Environment, Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Byeong-Hun Oh was employed by the company APM Engineering Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Rumchev, K.; Brown, H.; Spickett, J. Volatile Organic Compounds: Do They Present a Risk to Our Health? Rev. Environ. Health 2007, 22, 39–56. [Google Scholar] [CrossRef]
- Pandey, P.; Yadav, R. A Review on Volatile Organic Compounds (VOCs) as Environmental Pollutants: Fate and Distribution. IJPE 2018, 4, 14–26. [Google Scholar] [CrossRef]
- Soni, V.; Singh, P.; Shree, V.; Goel, V. Effects of VOCs on Human Health. In Air Pollution and Control; Sharma, N., Agarwal, A.K., Eastwood, P., Gupta, T., Singh, A.P., Eds.; Springer: Singapore, 2018; pp. 119–142. ISBN 978-981-10-7185-0. [Google Scholar]
- Zhou, X.; Zhou, X.; Wang, C.; Zhou, H. Environmental and Human Health Impacts of Volatile Organic Compounds: A Perspective Review. Chemosphere 2023, 313, 137489. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Kumar, A.; Singh, V.; Chakraborty, B.; Kumar, R.; Min, L. Recent Advancement in Organic Aerosol Understanding: A Review of Their Sources, Formation, and Health Impacts. Water Air Soil Pollut. 2023, 234, 750. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric Chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Ziemann, P.J.; Atkinson, R. Kinetics, Products, and Mechanisms of Secondary Organic Aerosol Formation. Chem. Soc. Rev. 2012, 41, 6582–6605. [Google Scholar] [CrossRef]
- Tsigaridis, K.; Kanakidou, M. Secondary Organic Aerosol Importance in the Future Atmosphere. Atmos. Environ. 2007, 41, 4682–4692. [Google Scholar] [CrossRef]
- Borbon, A.; Gilman, J.B.; Kuster, W.C.; Grand, N.; Chevaillier, S.; Colomb, A.; Dolgorouky, C.; Gros, V.; Lopez, M.; Sarda-Esteve, R.; et al. Emission Ratios of Anthropogenic Volatile Organic Compounds in Northern Mid-latitude Megacities: Observations versus Emission Inventories in Los Angeles and Paris. JGR Atmos. 2013, 118, 2041–2057. [Google Scholar] [CrossRef]
- Ehn, M.; Thornton, J.A.; Kleist, E.; Sipilä, M.; Junninen, H.; Pullinen, I.; Springer, M.; Rubach, F.; Tillmann, R.; Lee, B.; et al. A Large Source of Low-Volatility Secondary Organic Aerosol. Nature 2014, 506, 476–479. [Google Scholar] [CrossRef]
- Louie, P.K.K.; Ho, J.W.K.; Tsang, R.C.W.; Blake, D.R.; Lau, A.K.H.; Yu, J.Z.; Yuan, Z.; Wang, X.; Shao, M.; Zhong, L. VOCs and OVOCs Distribution and Control Policy Implications in Pearl River Delta Region, China. Atmos. Environ. 2013, 76, 125–135. [Google Scholar] [CrossRef]
- Ling, Z.H.; Guo, H. Contribution of VOC Sources to Photochemical Ozone Formation and Its Control Policy Implication in Hong Kong. Environ. Sci. Policy 2014, 38, 180–191. [Google Scholar] [CrossRef]
- He, Z.; Wang, X.; Ling, Z.; Zhao, J.; Guo, H.; Shao, M.; Wang, Z. Contributions of Different Anthropogenic Volatile Organic Compound Sources to Ozone Formation at a Receptor Site in the Pearl River Delta Region and Its Policy Implications. Atmos. Chem. Phys. 2019, 19, 8801–8816. [Google Scholar] [CrossRef]
- Cui, L.; Wu, D.; Wang, S.; Xu, Q.; Hu, R.; Hao, J. Measurement Report: Ambient Volatile Organic Compound (VOC) Pollution in Urban Beijing: Characteristics, Sources, and Implications for Pollution Control. Atmos. Chem. Phys. 2022, 22, 11931–11944. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.; Lu, S.; Zeng, L.; Tang, D. Source Profiles of Volatile Organic Compounds (VOCs) Measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Shen, L.; Xiang, P.; Liang, S.; Chen, W.; Wang, M.; Lu, S.; Wang, Z. Sources Profiles of Volatile Organic Compounds (VOCs) Measured in a Typical Industrial Process in Wuhan, Central China. Atmosphere 2018, 9, 297. [Google Scholar] [CrossRef]
- Sun, J.; Shen, Z.; Zhang, Y.; Zhang, Z.; Zhang, Q.; Zhang, T.; Niu, X.; Huang, Y.; Cui, L.; Xu, H.; et al. Urban VOC Profiles, Possible Sources, and Its Role in Ozone Formation for a Summer Campaign over Xi’an, China. Environ. Sci. Pollut. Res. 2019, 26, 27769–27782. [Google Scholar] [CrossRef]
- Hegazi, A.H.; Andersson, J.T. Limitations to GC-MS Determination of Sulfur-Containing Polycyclic Aromatic Compounds in Geochemical, Petroleum, and Environmental Investigations. Energy Fuels 2007, 21, 3375–3384. [Google Scholar] [CrossRef]
- Nevigato, T.; Masci, M.; Orban, E.; Di Lena, G.; Casini, I.; Caproni, R. Analysis of Fatty Acids in 12 Mediterranean Fish Species: Advantages and Limitations of a New GC-FID/GC–MS Based Technique. Lipids 2012, 47, 741–753. [Google Scholar] [CrossRef]
- Gin, J.; Imwinkelried, E.J. Gas Chromatography-Mass Spectrometer (GC/MS): In Scientific Evidence, Even “Gold Standard” Techniques Have Limitations; SSRN Scholarly Paper: Rochester, NY, USA, 2018. [Google Scholar]
- Zhou, X.; Li, Z.; Zhang, T.; Wang, F.; Wang, F.; Tao, Y.; Zhang, X.; Wang, F.; Huang, J. Volatile Organic Compounds in a Typical Petrochemical Industrialized Valley City of Northwest China Based on High-Resolution PTR-MS Measurements: Characterization, Sources and Chemical Effects. Sci. Total Environ. 2019, 671, 883–896. [Google Scholar] [CrossRef]
- Maji, S.; Yadav, R.; Beig, G.; Gunthe, S.S.; Ojha, N. On the Processes Governing the Variability of PTR-MS Based VOCs and OVOCs in Different Seasons of a Year over Hillocky Mega City of India. Atmos. Res. 2021, 261, 105736. [Google Scholar] [CrossRef]
- Krechmer, J.; Lopez-Hilfiker, F.; Koss, A.; Hutterli, M.; Stoermer, C.; Deming, B.; Kimmel, J.; Warneke, C.; Holzinger, R.; Jayne, J.; et al. Evaluation of a New Reagent-Ion Source and Focusing Ion–Molecule Reactor for Use in Proton-Transfer-Reaction Mass Spectrometry. Anal. Chem. 2018, 90, 12011–12018. [Google Scholar] [CrossRef] [PubMed]
- Coggon, M.M.; Stockwell, C.E.; Claflin, M.S.; Pfannerstill, E.Y.; Xu, L.; Gilman, J.B.; Marcantonio, J.; Cao, C.; Bates, K.; Gkatzelis, G.I.; et al. Identifying and Correcting Interferences to PTR-ToF-MS Measurements of Isoprene and Other Urban Volatile Organic Compounds. Atmos. Meas. Technol. 2024, 17, 801–825. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Lubinska-Szczygeł, M.; Różańska, A.; Namieśnik, J.; Dymerski, T. PTR-MS and GC-MS as Complementary Techniques for Analysis of Volatiles: A Tutorial Review. Anal. Chim. Acta 2018, 1035, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Derstroff, B. Investigation of Oxygenated and Intermediate Volatility Organic Compounds (OVOCs/IVOCs) with a Proton Transfer Reaction—Time Of Flight—Mass Spectrometer (PTR-TOF-MS); Johannes Gutenberg-Universität Mainz: Mainz, Germany, 2017. [Google Scholar]
- Huang, X.-F.; Wang, C.; Zhu, B.; Lin, L.-L.; He, L.-Y. Exploration of Sources of OVOCs in Various Atmospheres in Southern China. Environ. Pollut. 2019, 249, 831–842. [Google Scholar] [CrossRef]
- Han, C.; Liu, R.; Luo, H.; Li, G.; Ma, S.; Chen, J.; An, T. Pollution Profiles of Volatile Organic Compounds from Different Urban Functional Areas in Guangzhou China Based on GC/MS and PTR-TOF-MS: Atmospheric Environmental Implications. Atmos. Environ. 2019, 214, 116843. [Google Scholar] [CrossRef]
- Reinecke, T.; Leiminger, M.; Jordan, A.; Wisthaler, A.; Müller, M. Ultrahigh Sensitivity PTR-MS Instrument with a Well-Defined Ion Chemistry. Anal. Chem. 2023, 95, 11879–11884. [Google Scholar] [CrossRef]
- Di Girolamo, A.; Pedrotti, M.; Koot, A.; Verstappen, F.; van Houwelingen, A.; Vossen, C.; Bouwmeester, H.; de Ridder, D.; Beekwilder, J. The Use of Proton Transfer Reaction Mass Spectrometry for High Throughput Screening of Terpene Synthases. J. Mass Spectrom. 2023, 58, e4951. [Google Scholar] [CrossRef]
- Cappellin, L.; Biasioli, F.; Granitto, P.M.; Schuhfried, E.; Soukoulis, C.; Costa, F.; Märk, T.D.; Gasperi, F. On Data Analysis in PTR-TOF-MS: From Raw Spectra to Data Mining. Sens. Actuators B Chem. 2011, 155, 183–190. [Google Scholar] [CrossRef]
- Automated Measurement and Evaluation (AME). Available online: https://www.ionicon.com/accessories/details/automated-measurement-and-evaluation-ame (accessed on 5 August 2024).
- Park, J.; Kim, K.; Choi, G.; Kim, T.; Yoon, C.; Lee, S. Verification for Real-time Analysis (AME Method) of VOCs Concentration using PTR-TOF/MS. Proc. 62th Korean Soc. Atmos. Environ. Conf. 2019, 129. [Google Scholar]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Märk, L.; Seehauser, H.; Schottkowsky, R.; Sulzer, P.; Märk, T.D. A High Resolution and High Sensitivity Proton-Transfer-Reaction Time-of-Flight Mass Spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Piel, F.; Müller, M.; Winkler, K.; Skytte af Sätra, J.; Wisthaler, A. Introducing the Extended Volatility Range Proton-Transfer-Reaction Mass Spectrometer (EVR PTR-MS). Atmos. Meas. Technol. 2021, 14, 1355–1363. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Min, C.; Kim, S.; Yoon, G.; Kim, S. A Study on the Trimethylsilanol Analysis Method of Semiconductor Processing using a Proton Transfer Reaction—Time of Flight Mass Spectrometer. J. Korean Soc. Urban Environ. 2017, 17, 85–95. [Google Scholar]
- Koss, A.R.; Sekimoto, K.; Gilman, J.B.; Selimovic, V.; Coggon, M.M.; Zarzana, K.J.; Yuan, B.; Lerner, B.M.; Brown, S.S.; Jimenez, J.L.; et al. Non-Methane Organic Gas Emissions from Biomass Burning: Identification, Quantification, and Emission Factors from PTR-ToF during the FIREX 2016 Laboratory Experiment. Atmos. Chem. Phys. 2018, 18, 3299–3319. [Google Scholar] [CrossRef]
- Stockwell, C.E.; Veres, P.R.; Williams, J.; Yokelson, R.J. Characterization of Biomass Burning Emissions from Cooking Fires, Peat, Crop Residue, and Other Fuels with High-Resolution Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Atmos. Chem. Phys. 2015, 15, 845–865. [Google Scholar] [CrossRef]
- Yuan, B.; Koss, A.; Warneke, C.; Gilman, J.B.; Lerner, B.M.; Stark, H.; de Gouw, J.A. A High-Resolution Time-of-Flight Chemical Ionization Mass Spectrometer Utilizing Hydronium Ions (H3O+ ToF-CIMS) for Measurements of Volatile Organic Compounds in the Atmosphere. Atmos. Meas. Technol. 2016, 9, 2735–2752. [Google Scholar] [CrossRef]
- Cappellin, L.; Karl, T.; Probst, M.; Ismailova, O.; Winkler, P.M.; Soukoulis, C.; Aprea, E.; Märk, T.D.; Gasperi, F.; Biasioli, F. On Quantitative Determination of Volatile Organic Compound Concentrations Using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Environ. Sci. Technol. 2012, 46, 2283–2290. [Google Scholar] [CrossRef]
- Statistics Korea Korean Standard Industrial Classification. Available online: https://kssc.kostat.go.kr:8443/ksscNew_web/ekssc/main/main.do# (accessed on 9 July 2024).
- National Air Emission Inventory and Research Center Emission Source Classification. Available online: https://www.air.go.kr/eng/contents/view.do?contentsId=35&menuId=91 (accessed on 8 July 2024).
- European Environment Agency Index to Methodology Chapters Ordered by SNAP97 Activity. Available online: https://www.eea.europa.eu/publications/EMEPCORINAIR4/page009-a.html (accessed on 8 July 2024).
- Pinthong, N.; Thepanondh, S.; Kultan, V.; Keawboonchu, J. Characteristics and Impact of VOCs on Ozone Formation Potential in a Petrochemical Industrial Area, Thailand. Atmosphere 2022, 13, 732. [Google Scholar] [CrossRef]
- McDonald, B.C.; De Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile Chemical Products Emerging as Largest Petrochemical Source of Urban Organic Emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef]
- Yen, C.-H.; Horng, J.-J. Volatile Organic Compounds (VOCs) Emission Characteristics and Control Strategies for a Petrochemical Industrial Area in Middle Taiwan. J. Environ. Sci. Health Part A 2009, 44, 1424–1429. [Google Scholar] [CrossRef]
- Tiwari, V.; Hanai, Y.; Masunaga, S. Ambient Levels of Volatile Organic Compounds in the Vicinity of Petrochemical Industrial Area of Yokohama, Japan. Air Qual. Atmos Health 2010, 3, 65–75. [Google Scholar] [CrossRef]
- Mo, Z.; Shao, M.; Lu, S.; Qu, H.; Zhou, M.; Sun, J.; Gou, B. Process-Specific Emission Characteristics of Volatile Organic Compounds (VOCs) from Petrochemical Facilities in the Yangtze River Delta, China. Sci. Total Environ. 2015, 533, 422–431. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Case Studies on Safer Alternatives for Solvent Degreasing Applications. Available online: https://www.epa.gov/p2/case-studies-safer-alternatives-solvent-degreasing-applications (accessed on 8 July 2024).
- Liu, Z.; Cao, Z.; Zhao, J.; Fang, Y.; Wei, W. Characteristics of VOCs Emission Components in Typical Solvents Source Industries in Tianjin. IOP Conf. Ser. Earth Environ. Sci. 2021, 781, 032010. [Google Scholar] [CrossRef]
- Oh, B.; Kim, H.; Lee, H.; Lee, C.; Kim, K.; Choi, D.; Joo, H.; Han, J. Source characterization of volatile organic compounds of industrial complex measured by proton-transfer-reaction time-of-flight mass spectrometers in Korea. J. Odor Indoor Environ. 2023, 22, 139–152. [Google Scholar] [CrossRef]
- Liang, X.; Chen, L.; Liu, M.; Lu, H.; Lu, Q.; Gao, B.; Zhao, W.; Sun, X.; Ye, D. Improved Emission Factors and Speciation to Characterize VOC Emissions in the Printing Industry in China. Sci. Total Environ. 2023, 866, 161295. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Yan, Q.; Han, S.; Zhao, Q.; Yang, L.; Liu, Y.; Zhang, R. Typical Industrial Sector-Based Volatile Organic Compounds Source Profiles and Ozone Formation Potentials in Zhengzhou, China. Atmos. Pollut. Res. 2020, 11, 841–850. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Zhang, Y.; Wang, L.; Sun, Z.; Wang, H. Review on Source Profiles of Volatile Organic Compounds (VOCs) in Typical Industries in China. Atmosphere 2023, 14, 878. [Google Scholar] [CrossRef]
- Kiurski, J.S.; Marić, B.B.; Aksentijević, S.M.; Oros, I.B.; Kecić, V.S.; Kovacević, I.M. Indoor Air Quality Investigation from Screen Printing Industry. Renew. Sustain. Energy Rev. 2013, 28, 224–231. [Google Scholar] [CrossRef]
- Aydemir, C.; Özsoy, S.A. Environmental Impact of Printing Inks and Printing Process. J. Graph. Eng. Des. 2020, 11, 11–17. [Google Scholar] [CrossRef]
- Rossitza, S. Offset Printing without Isopropyl Alcohol in Damping Solution. Energy Procedia 2015, 74, 690–698. [Google Scholar] [CrossRef][Green Version]
- Lv, Z.; Liu, X.; Wang, G.; Shao, X.; Li, Z.; Nie, L.; Li, G. Sector-Based Volatile Organic Compounds Emission Characteristics from the Electronics Manufacturing Industry in China. Atmos. Pollut. Res. 2021, 12, 101097. [Google Scholar] [CrossRef]
- Ma, Y.-G. Composition and characteristics of volatile organic chemicals emission from printed circuit board factories. Huan Jing Ke Xue 2012, 33, 2967–2972. [Google Scholar] [PubMed]
- Dinh, T.-V.; Kim, S.-Y.; Son, Y.-S.; Choi, I.-Y.; Park, S.-R.; Sunwoo, Y.; Kim, J.-C. Emission Characteristics of VOCs Emitted from Consumer and Commercial Products and Their Ozone Formation Potential. Environ. Sci. Pollut. Res. 2015, 22, 9345–9355. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y. Evaluation of Lubricating-Oil Performance and Emissions with Lubricant Formulations Using ZDDP as the Selected Additive in GDI Engines: A Simultaneous Study of VOCs and Soot in Oil. J. Mech. Sci. Technol. 2021, 35, 5197–5212. [Google Scholar] [CrossRef]
- Canagaratna, M.R.; Jayne, J.T.; Ghertner, D.A.; Herndon, S.; Shi, Q.; Jimenez, J.L.; Silva, P.J.; Williams, P.; Lanni, T.; Drewnick, F.; et al. Chase Studies of Particulate Emissions from In-Use New York City Vehicles. Aerosol. Sci. Technol. 2004, 38, 555–573. [Google Scholar] [CrossRef]
- Gentner, D.R.; Jathar, S.H.; Gordon, T.D.; Bahreini, R.; Day, D.A.; El Haddad, I.; Hayes, P.L.; Pieber, S.M.; Platt, S.M.; De Gouw, J.; et al. Review of Urban Secondary Organic Aerosol Formation from Gasoline and Diesel Motor Vehicle Emissions. Environ. Sci. Technol. 2017, 51, 1074–1093. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, Z.; Chen, L. Quantifying the Contributions of Diesel Fuel and Lubricating Oil to the SVOC Emissions from a Diesel Engine Using GC × GC-ToFMS. Fuel 2022, 310, 122409. [Google Scholar] [CrossRef]
- Drozd, G.T.; Zhao, Y.; Saliba, G.; Frodin, B.; Maddox, C.; Oliver Chang, M.-C.; Maldonado, H.; Sardar, S.; Weber, R.J.; Robinson, A.L.; et al. Detailed Speciation of Intermediate Volatility and Semivolatile Organic Compound Emissions from Gasoline Vehicles: Effects of Cold-Starts and Implications for Secondary Organic Aerosol Formation. Environ. Sci. Technol. 2019, 53, 1706–1714. [Google Scholar] [CrossRef]
- Shankar, R.; Jung, J.-H.; Loh, A.; An, J.G.; Ha, S.Y.; Yim, U.H. Environmental Significance of Lubricant Oil: A Systematic Study of Photooxidation and Its Consequences. Water Res. 2020, 168, 115183. [Google Scholar] [CrossRef]
- Chang, C.-T.; Lin, K.-L. Assessment of the Strategies for Reducing VOCs Emission from Polyurea-Formaldehyde Resin Synthetic Fiber Leather Industry in Taiwan. Resour. Conserv. Recycl. 2006, 46, 321–334. [Google Scholar] [CrossRef]
- Azhar Zakir, M.J.; Ramalingam, S.; Balasubramanian, P.; Rathinam, A.; Sreeram, K.J.; Rao, J.R.; Nair, B.U. Innovative Material from Paper and Pulp Industry for Leather Processing. J. Clean. Prod. 2015, 104, 436–444. [Google Scholar] [CrossRef]
- Yang, H.-H.; Gupta, S.K.; Dhital, N.B. Emission Factor, Relative Ozone Formation Potential and Relative Carcinogenic Risk Assessment of VOCs Emitted from Manufacturing Industries. Sustain. Environ. Res 2020, 30, 28. [Google Scholar] [CrossRef]
- Shen, K.P.; Lai, C.C.; Lin, S.S.; Wu, H.H.; Huang, J.J.; Wang, Y.M.; Chen, H.W. Best Available Control Technology (BACT) of VOC for PU (Polyurethane) Synthetic Leather Surface Coating Industry in Taiwan; Air and Waste Management Association: Pittsburgh, PA, USA, 1999. [Google Scholar]
- Jiménez-López, A.M.; Hincapié-Llanos, G.A. Identification of Factors Affecting the Reduction of VOC Emissions in the Paint Industry: Systematic Literature Review—SLR. Prog. Org. Coat. 2022, 170, 106945. [Google Scholar] [CrossRef]
- Song, B.; Lee, S.; Cho, G.; Cho, J.; You, P.; Kim, G. VOC/HAPs Emission Characteristics & Adsorption Evaluation for Paint Products in Busan Area. J. Korean Soc. Environ. Eng. 2012, 34, 316–325. [Google Scholar]
- Kim, M.; Lee, J.; Kim, J.; Lee, H.; Cho, S.; Yu, J.; Kang, C.; Moon, K. Study of Chemical Substances Emitted during Paint Manufacturing through VOC Speciation. Atmosphere 2022, 13, 1245. [Google Scholar] [CrossRef]
- Ghobakhloo, S.; Khoshakhlagh, A.H.; Morais, S.; Mazaheri Tehrani, A. Exposure to Volatile Organic Compounds in Paint Production Plants: Levels and Potential Human Health Risks. Toxics 2023, 11, 111. [Google Scholar] [CrossRef]
- Song, M.; Chun, H. Species and Characteristics of Volatile Organic Compounds Emitted from an Auto-Repair Painting Workshop. Sci. Rep. 2021, 11, 16586. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, C.; Wang, M.; Shao, X.; Yao, Y.; Wang, G.; Li, Y.; Hou, M.; Fan, L.; Ye, D. Characteristics and Environmental and Health Impacts of Volatile Organic Compounds in Furniture Manufacturing with Different Coating Types in the Pearl River Delta. J. Clean. Prod. 2023, 397, 136599. [Google Scholar] [CrossRef]
- Adamová, T.; Hradecký, J.; Pánek, M. Volatile Organic Compounds (VOCs) from Wood and Wood-Based Panels: Methods for Evaluation, Potential Health Risks, and Mitigation. Polymers 2020, 12, 2289. [Google Scholar] [CrossRef]
- Teng, W.; Liu, W.; Shao, X.; Wu, Q. Emission Characteristics, Environmental Impact Assessment and Priority Control Strategies Derived from VOCs Speciation Sourcely through Measurement for Wooden Furniture-Manufacturing Industry in China. Sci. Total Environ. 2023, 877, 162287. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, F.; Sun, L.; Yu, X.; Zhao, J.; Hu, Y.; Wang, Y. Characterization of VOC Emissions from Composite Wood Furniture: Parameter Determination and Simplified Model. Build. Environ. 2019, 161, 106237. [Google Scholar] [CrossRef]
- Abbaspour, P. Metal Plating Industry and Environmental Pollution. Asian Res. J. Curr. Sci. 2022, 4, 1–6. [Google Scholar]
- Zheng, J.; Yu, Y.; Mo, Z.; Zhang, Z.; Wang, X.; Yin, S.; Peng, K.; Yang, Y.; Feng, X.; Cai, H. Industrial Sector-Based Volatile Organic Compound (VOC) Source Profiles Measured in Manufacturing Facilities in the Pearl River Delta, China. Sci. Total Environ. 2013, 456–457, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Sha, Q.; Zheng, J.; Yuan, Z.; Gao, Z.; Ou, J.; Zheng, Z.; Li, C.; Huang, Z. Sector-Based VOCs Emission Factors and Source Profiles for the Surface Coating Industry in the Pearl River Delta Region of China. Sci. Total Environ. 2017, 583, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Shen, J.; Wang, Q.; Li, H.; Xu, C.; Dong, H. Characteristics of VOCs Released from Plywood in Airtight Environments. Forests 2019, 10, 709. [Google Scholar] [CrossRef]
- Qifan, W.; Jun, S.; Yang, Z.; Wanjun, L. Influence of Environmental Factors on Volatile Organic Compound Emissions from Plywood Tested by a Rapid Detection Method. For. Prod. J. 2017, 67, 120–125. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.; Park, B.; Lim, J.; Jang, S.; Kim, S. Effect of Surface Laminate Type on the Emission of Volatile Organic Compounds from Wood-Based Composite Panels. J. Adhes. Sci. Technol. 2013, 27, 620–631. [Google Scholar] [CrossRef]
- Kim, K.-W.; Oh, J.-K.; Lee, B.-H.; Kim, H.-J.; Lee, Y.-K.; Kim, S.-H.; Kim, G.-E. Influence of Surface Finishing Material Types to Formaldehyde and Volatile Organic Compounds Emission from Plywood. J. Korean Wood Sci. Technol. 2008, 36, 39–45. [Google Scholar]
- Liu, Y.; Zhu, X.; Qin, X.; Wang, W.; Hu, Y.; Yuan, D. Identification and Characterization of Odorous Volatile Organic Compounds Emitted from Wood-Based Panels. Environ. Monit. Assess. 2020, 192, 348. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Control of VOC Emissions from Non-Ferrous Metal Rolling Processes. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000HHPU.TXT (accessed on 8 July 2024).
- Peterangelo, S.C.; Gschwender, L.; Snyder, C.E., Jr.; Jones, W.R., Jr.; Nguyen, Q.; Jansen, M.J. Improved Additives for Multiply Alkylated Cyclopentane-Based Lubricants. J. Synth. Lubr. 2008, 25, 31–41. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, L.; Xue, Q. Comparative Studies on Tribological Behavior of Multiply-Alkylated Cyclopentane with Other Liquid Lubricants for Various Frictional Materials. Tribol. Trans. 2009, 52, 602–610. [Google Scholar] [CrossRef]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic Compounds in Plant Oils: A Review of Composition, Analytical Methods, and Effect on Oxidative Stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Park, O.; Lee, K.; Min, K.; Cho, G.; Yoon, K.; Jeung, W.; Cho, Y.; Kim, E.; Yang, J. Generating Characteristics of VOCs in a Commercial Laundry Shop and the Effects on the Health of Workers. J. Korean Soc. Occup. Environ. Hyg. 2016, 26, 159–169. [Google Scholar] [CrossRef][Green Version]
- Song, M.; Kim, K.; Cho, C.; Kim, D. Reduction of Volatile Organic Compounds (VOCs) Emissions from Laundry Dry-Cleaning by an Integrated Treatment Process of Condensation and Adsorption. Processes 2021, 9, 1658. [Google Scholar] [CrossRef]
- Eun, D.; Han, Y.; Park, S.; Yoo, H.; Le, Y.T.-H.; Jeong, S.; Jeon, K.; Youn, J. Analysis of VOCs Emitted from Small Laundry Facilities: Contributions to Ozone and Secondary Aerosol Formation and Human Risk Assessment. Int. J. Environ. Res. Public Health 2022, 19, 15130. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, K.; Choi, Y.; Kim, D. Emissions of Volatile Organic Compounds (VOCs) from an Open-Circuit Dry Cleaning Machine Using a Petroleum-Based Organic Solvent: Implications for Impacts on Air Quality. Atmosphere 2021, 12, 637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).