The Structural and Thermal Characteristics of Musa paradisiaca L. Lignin for Carbon Footprint Reduction Applications
Abstract
1. Introduction
2. Methodology
2.1. Raw Materials
2.2. Di-ethyl Ether PreTreatment
2.3. Lignin Extraction
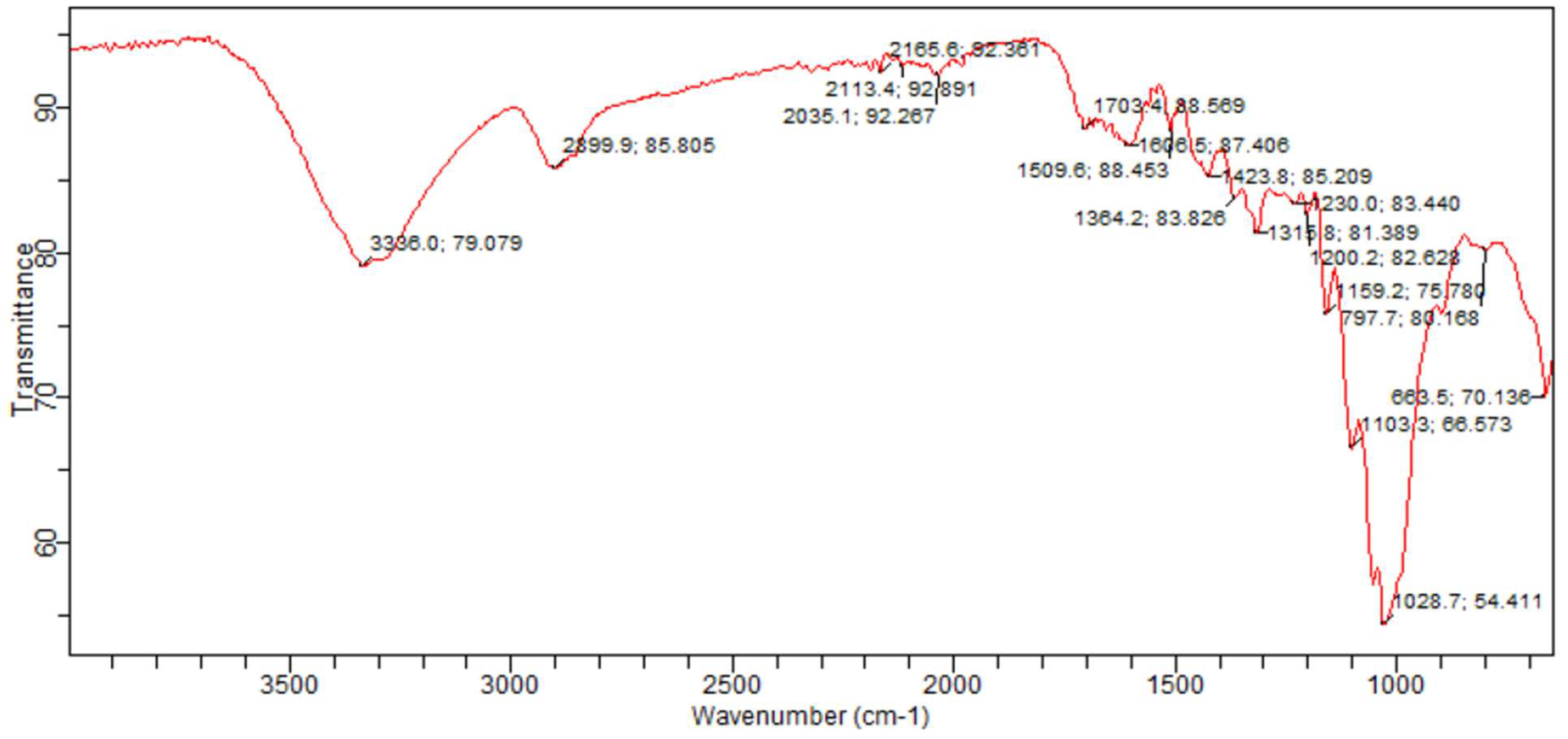
2.4. Functional Group Determination Using Fourier Transform Infrared Spectroscopy (FTIR)
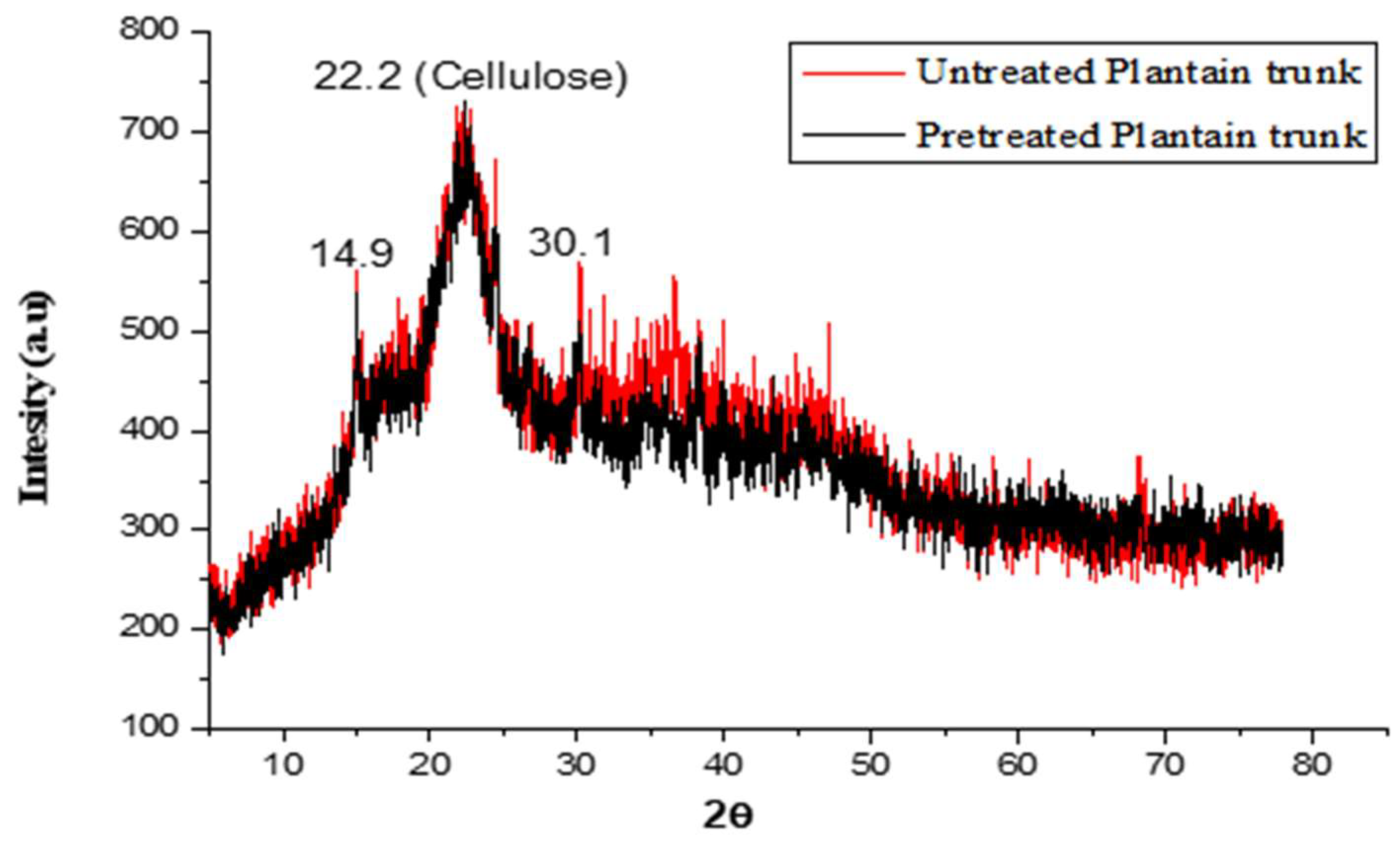
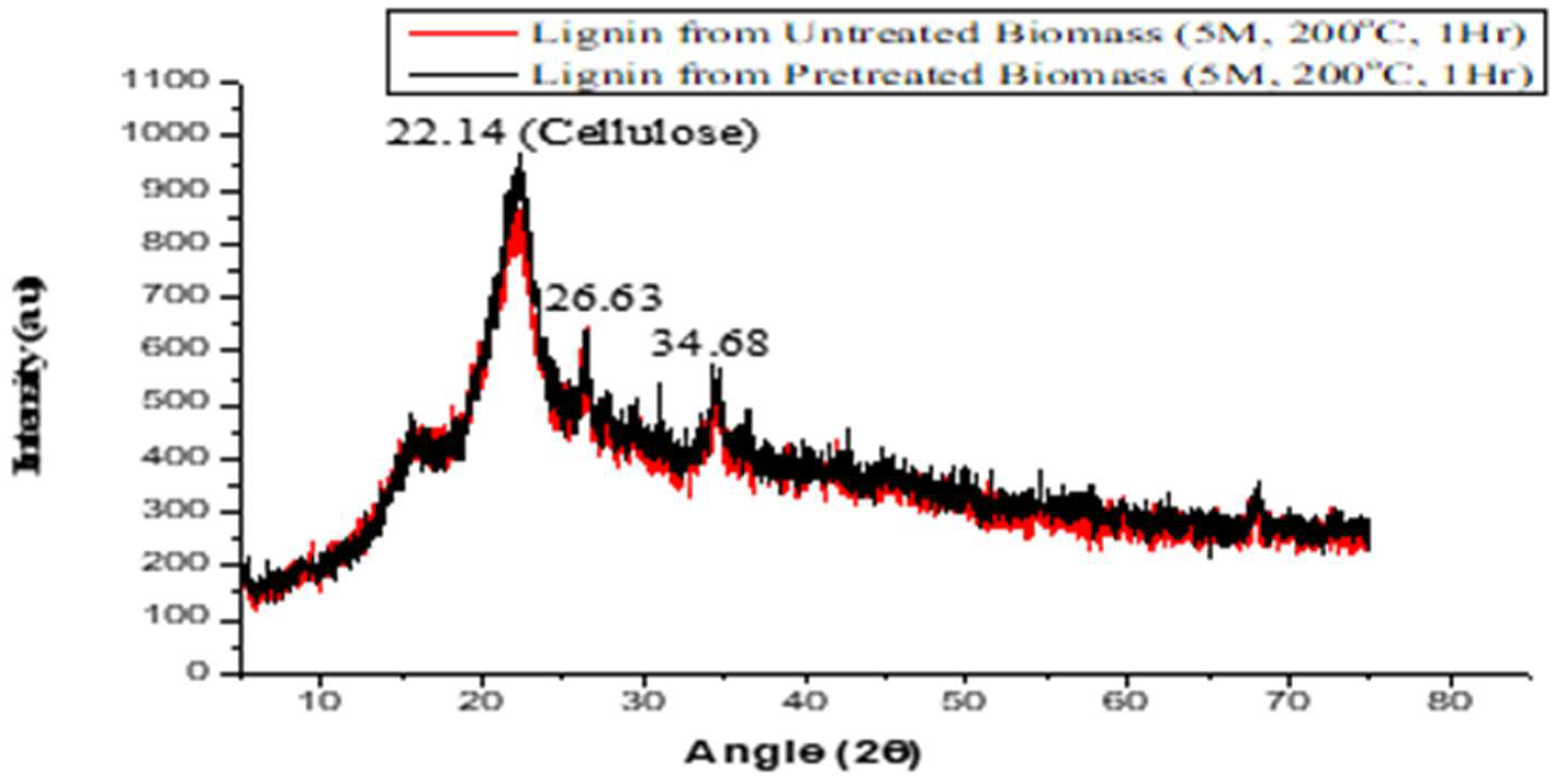
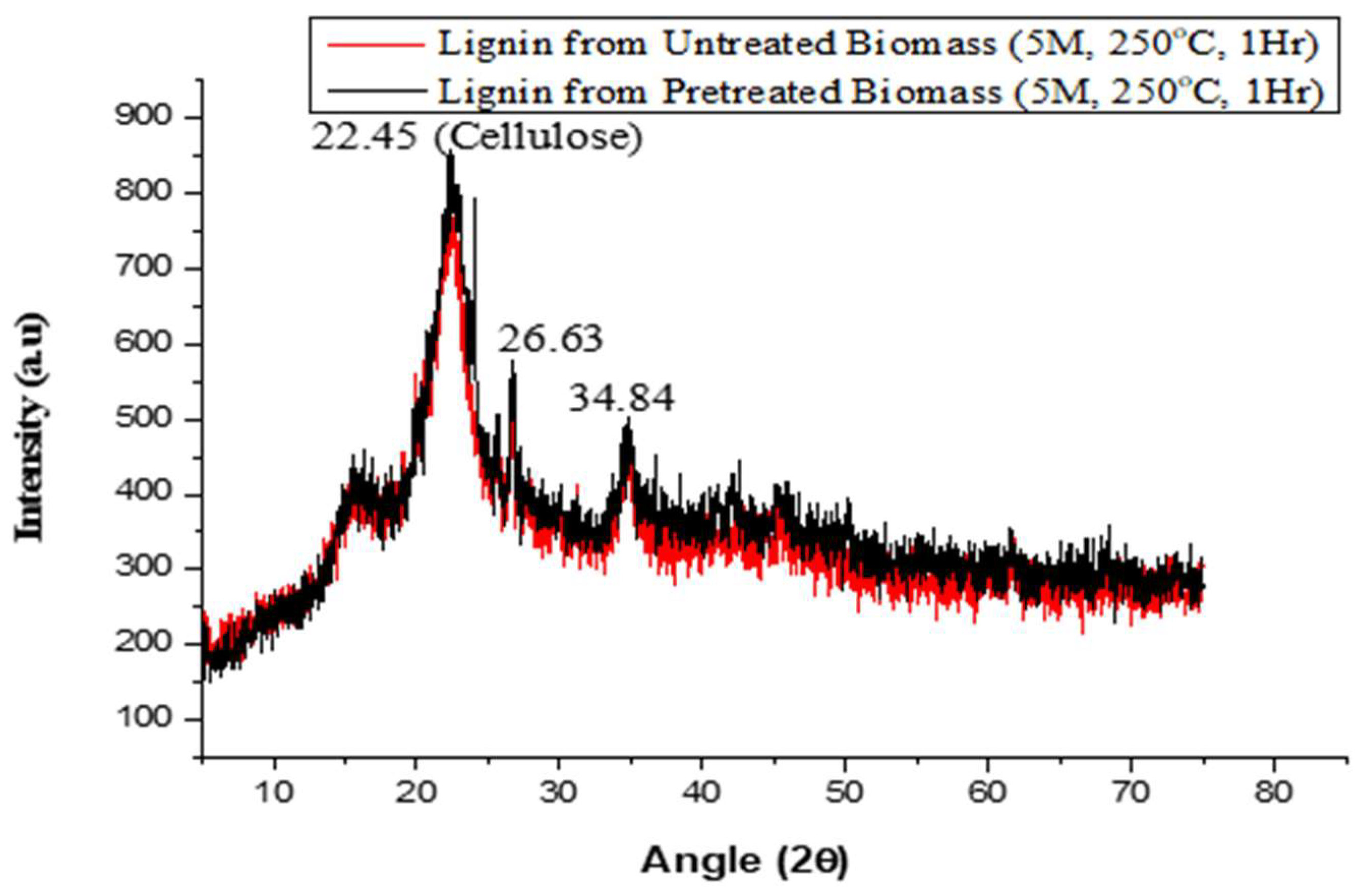
2.5. Analysis of Phases and Crystal Structure Using X-ray Diffraction (XRD)
2.6. Thermal Behaviours of the Samples Using Differential Scanning Calorimetry (DSC)
2.7. Thermal Stability Using Thermogravimetric Analysis (TGA)
2.8. Elemental Composition Determination Using X-ray Fluorescence (XRF)
3. Results and Discussion
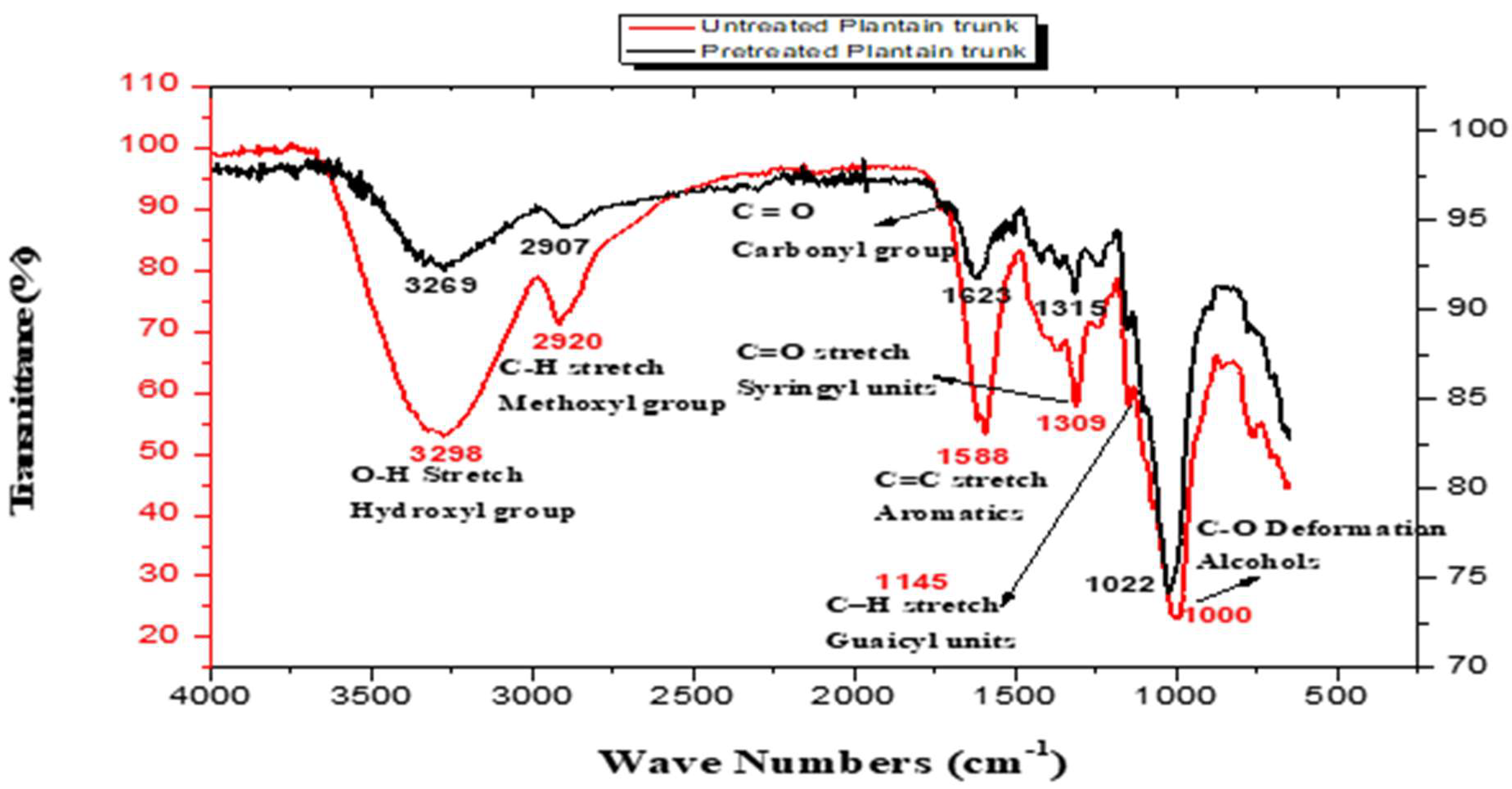
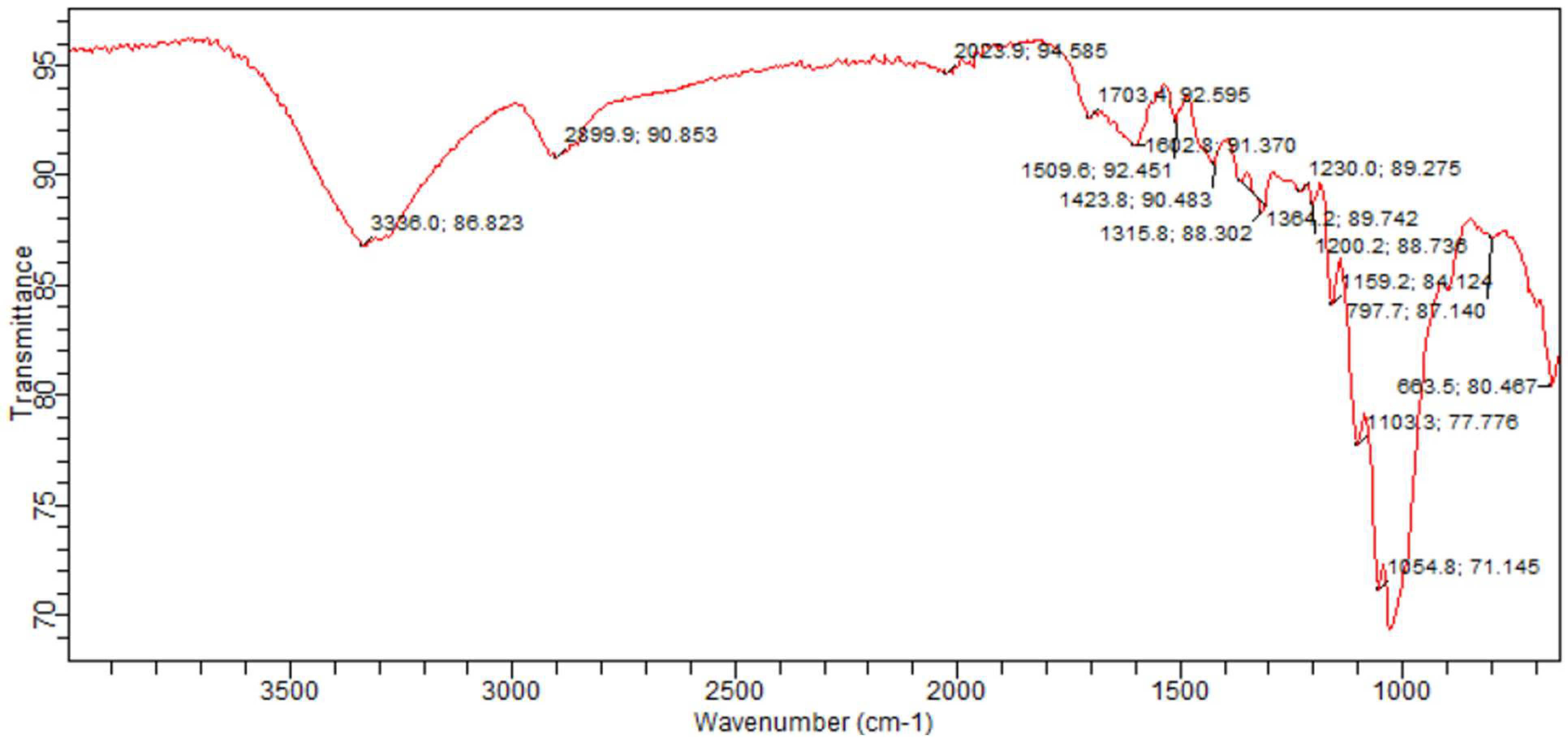
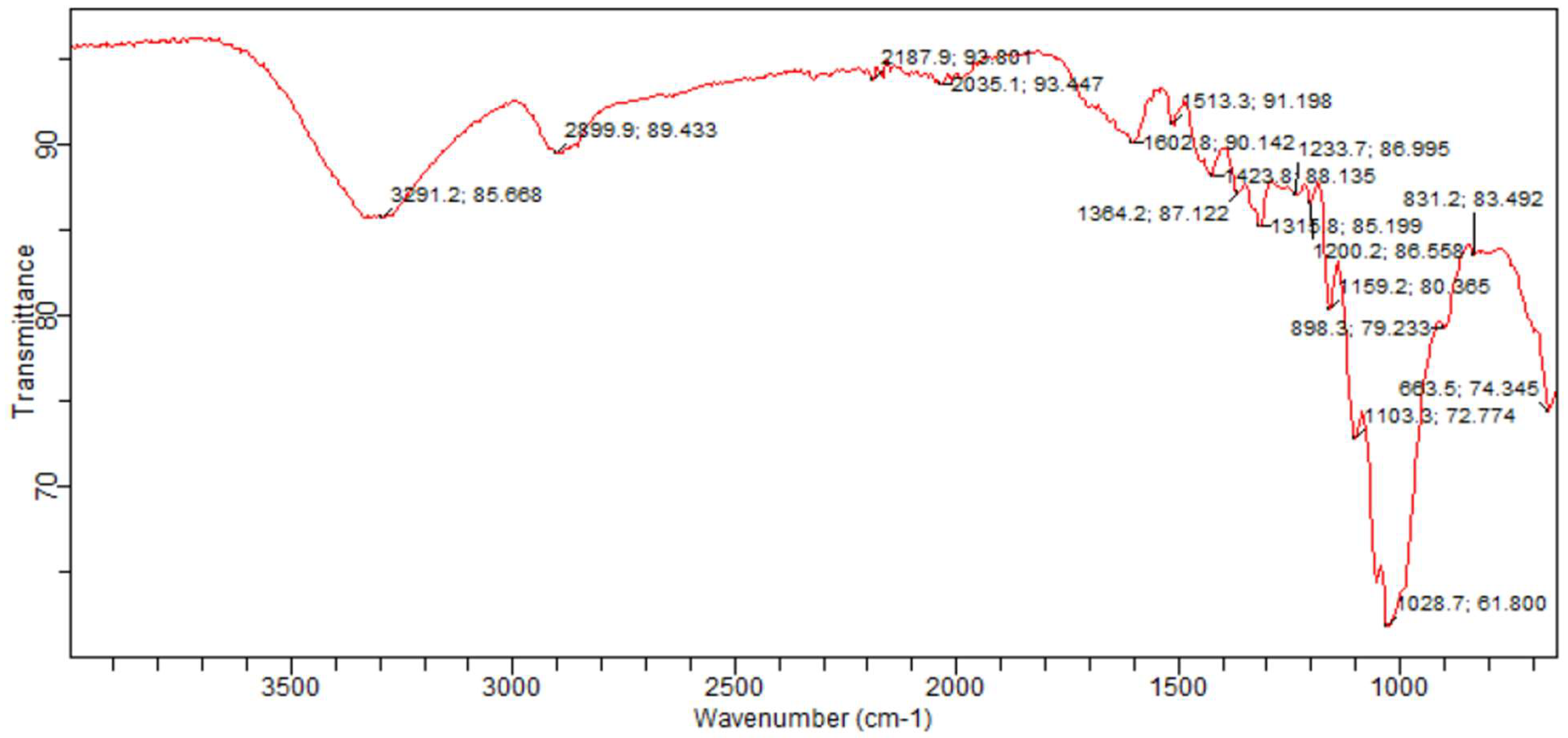
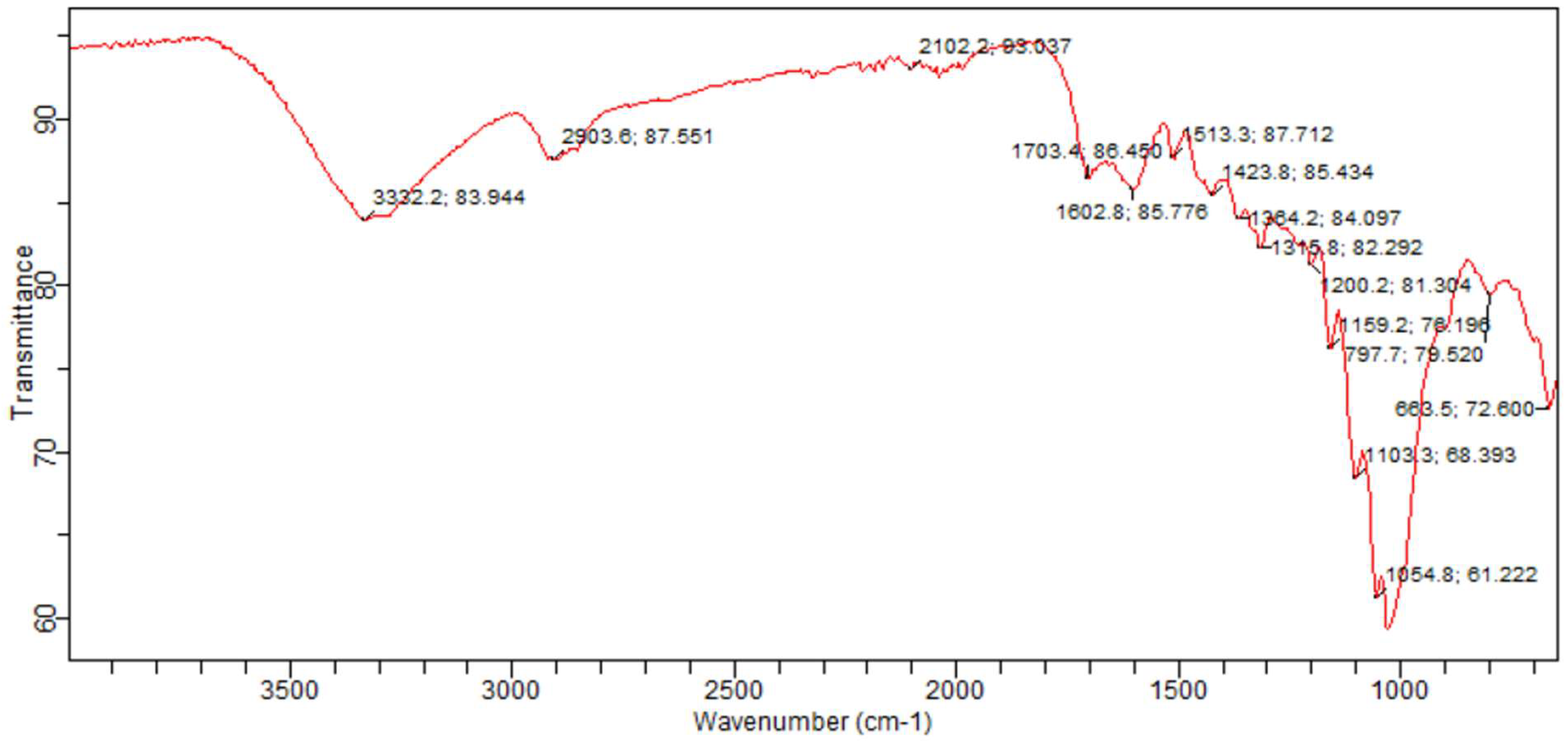
3.1. Sample Functional Group Determination
3.2. Analysis of Phases and Crystal Structures That Were Present in the Study Samples
3.3. Thermal Behaviours of the Sample Using Differential Scanning Calorimetry (DSC)
3.4. Thermal Stability of the Study Samples
3.5. Samples’ Elemental Composition Determination
3.6. Comparison of the Properties of Lignin from Plantain Pseudostem and Fossil-Based Carbon Fibre Precursors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dixit, N.; Jain, P.K. 3D printed carbon fiber reinforced thermoplastic composites: A review. Mater. Today Proc. 2021, 43, 678–681. [Google Scholar] [CrossRef]
- Warren, C.D. Carbon fiber in future vehicles. SAMPE J. 2001, 37, 7–15. [Google Scholar]
- Leitten, C.F.; Griffith, W.L., Jr.; Compere, A.L.; Shaffer, J.T. Society of Automotive Engineers Paper 2002-01-1907; SAE International: Warrendale, PA, USA, 2002. [Google Scholar]
- Zhang, M.; Liu, W.; Niu, H.; Wu, D. Structure-property relationship of carbon fibers derived from polyimide/polyacrylonitrile blends. High Perform. Polym. 2019, 31, 168–177. [Google Scholar] [CrossRef]
- Hertwich, E.G. Increased carbon footprint of materials production driven by rise in investments. Nat. Geosci. 2021, 14, 151–155. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Lourenço, A.; Pereira, H. Compositional Variability of Lignin in Biomass. In Lignin—Trends and Applications; Poletto, M., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Sajjadi, M.; Ahmadpoor, F.; Nasrollahzadeh, M.; Ghafuri, H. Lignin-derived (nano) materials for environmental pollution remediation: Current challenges and future perspectives. Int. J. Biol. Macromol. 2021, 178, 394–423. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Lawoko, M. A review on lignin based polymeric, micro-and nano- structured materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Jingjing, L. Isolation of Lignin from Wood; Saimaa University of Applied Sciences: Imatra, Finland, 2011; pp. 2–50. Available online: http://www.theseus.fi/handle/10024/37903 (accessed on 6 April 2022.).
- Ramirez Franco, J.H.; Enríquez Enríquez, M.K. Remoción de plomo (II) usando lignina obtenida a partir del procesamiento del seudotallo de plátano. Acta Agronómica 2015, 64, 209–213. [Google Scholar] [CrossRef]
- Oliveira, L.; Evtuguin, D.; Cordeiro, N.; Silvestre, A.J.D. Structural characterization of stalk lignin from banana plant. Ind. Crops Prod. 2009, 29, 86–95. [Google Scholar] [CrossRef]
- Geies, A.; Abdelazim, M.; Sayed, A.M.; Ibrahim, S. Thermal, Morphological and Cytotoxicity Characterization of Hardwood Lignins Isolated by In-Situ Sodium Hydroxide-Sodium Bisulfate Method. Nat. Resour. 2020, 11, 427–438. [Google Scholar] [CrossRef]
- Li, Q.; Hu, C.; Li, M.; Truong, P.; Naik, M.T.; Prabhu, D.; Hoffmann, L.; Rooney, W.L.; Yuan, J.S. Discovering Biomass Structural Determinants Defining the Properties of Plant-Derived Renewable Carbon Fiber. IScience 2020, 23, 101405. [Google Scholar] [CrossRef] [PubMed]
- Lallave, M.; Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Otero, J.C.; Marquez, M.; Barrero, A.; Loscertales, I.G. Filled and Hollow Carbon Nanofibers by Coaxial Electrospinning of Alcell Lignin without Binder Polymers. Adv. Mater. 2007, 19, 4292–4296. [Google Scholar] [CrossRef]
- Ihueze, C.C.; Okafor, C.E.; Okoye, C.I. Natural fibre composite design and characterization for limit stress prediction in multi axial stress sate. J. King Saud Univ. Eng. 2013, 27, 193–206. [Google Scholar] [CrossRef]
- Cecci, R.R.R.; Passos, A.A.; de Aguiar Neto, T.C.; Silva, L.A. Banana pseudostem fibers characterization and comparison with reported data on jute and sisal fibers. SN Appl. Sci. 2020, 2, 20. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef]
- Vardhini, K.J.V.; Murugan, R.; Selvi, C.T.; Surjit, R. Optimisation of alkali treatment of banana fibres on lignin removal. Indian J. Fibre Text. Res. 2016, 41, 156–160. [Google Scholar]
- Pereira, A.L.S.; Nascimento, D.M.; Souza Filho, M.d.S.M.; Cassales, A.R.; Morais, J.P.S.; Paula, R.C.M.; Rosa, M.F.; Feitosa, J.P.A. Banana (Musa sp. Cv. Pacovan) Pseudostem Fibers are Composed of Varying Lignocellulosic Composition throughout the Diameter. BioResources 2014, 9, 7749–7763. [Google Scholar] [CrossRef]
- Darmawan, S.; Wistara, N.J.; Pari, G.; Maddu, A.; Syafii, W. Characterization of Lignocellulosic Biomass as Raw Material for the Production of Porous Carbon-based Materials. BioResources 2016, 11, 3561–3574. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef]
- Wang, X.; Cui, X.; Zhang, L. Preparation and Characterization of Lignin-containing Nanofibrillar Cellulose. Procedia Environ. Sci. 2012, 16, 125–130. [Google Scholar] [CrossRef]
- Luo, J. Lignin-Based Carbon Fiber. Ph.D. Thesis, The University of Maine, Orono, ME, USA, 2010; pp. 5–50. Available online: https://digitalcommons.library.umaine.edu/etd/832 (accessed on 20 February 2023).
- Amit, T.A.; Roy, R.; Raynie, D.E. Thermal and structural characterization of two commercially available technical lignins for potential depolymerization via hydrothermal liquefaction. Curr. Res. Green Sustain. Chem. 2021, 4, 100106. [Google Scholar] [CrossRef]
- Abdullah, N.; Sulaiman, F.; Miskam, M.A.; Taib, R.M. Characterization of Banana (Musa spp.) Pseudo-Stem and Fruit-Bunch-Stem as a Potential Renewable Energy Resource. Int. J. Energy Power Eng. 2014, 8, 815–819. [Google Scholar]
- Sun, R.; Tomkinson, J.; Lloyd Jones, G. Fractional characterization of ash-AQ lignin by successive extraction with organic solvents from oil palm EFB fibre. Polym. Degrad. Stab. 2000, 68, 111–119. [Google Scholar] [CrossRef]
- Chen, G.; Leung, D.Y.C. Experimental Investigation of Biomass Waste, (Rice Straw, Cotton Stalk, and Pine Sawdust), Pyrolysis Characteristics. Energy Sources 2003, 25, 331–337. [Google Scholar] [CrossRef]
- Chen, K. Bio-Renewable Fibres Extracted from Lignin/Polylactide (PLA) Blend. Master’s Thesis, Iowa State University, Ames, IA, USA, 2012. [Google Scholar] [CrossRef][Green Version]
- Karbownik, I.; Rac-Rumijowska, O.; Fiedot-Toboła, M.; Rybicki, T.; Teterycz, H. The Preparation and Characterization of Polyacrylonitrile-Polyaniline (PAN/PANI) Fibers. Materials 2019, 12, 664. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Osipova, N.I.; Plutalova, A.V.; Toms, R.V.; Gervald, A.Y.; Prokopov, N.I.; Kulichikhin, V.G. Melt-Spinnable Polyacrylonitrile—An Alternative Carbon Fiber Precursor. Polymers 2022, 14, 5222. [Google Scholar] [CrossRef]
- Terra, B.M.; de Andrade, D.A.; de Mesquita, R.N. Characterization of polyacrylonitrile thermal stabilization process for carbon fiber production using intelligent algorithms. Polym. Test. 2021, 100, 107238. [Google Scholar] [CrossRef]
- American Chemical Society National Historical Chemical Landmark. High Performance Carbon Fibre. 2003. Available online: http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/carbonfibres.html (accessed on 3 June 2023).
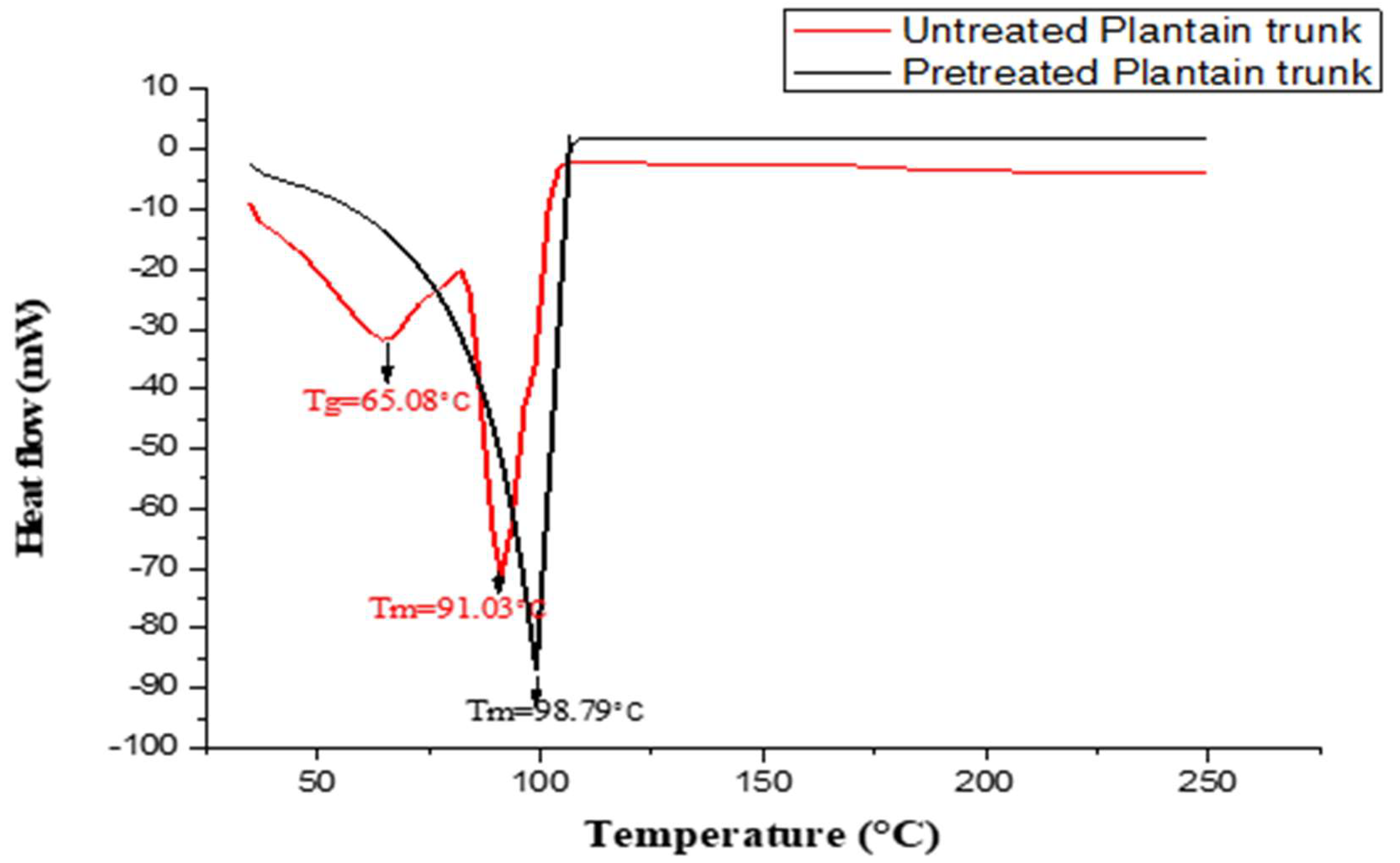

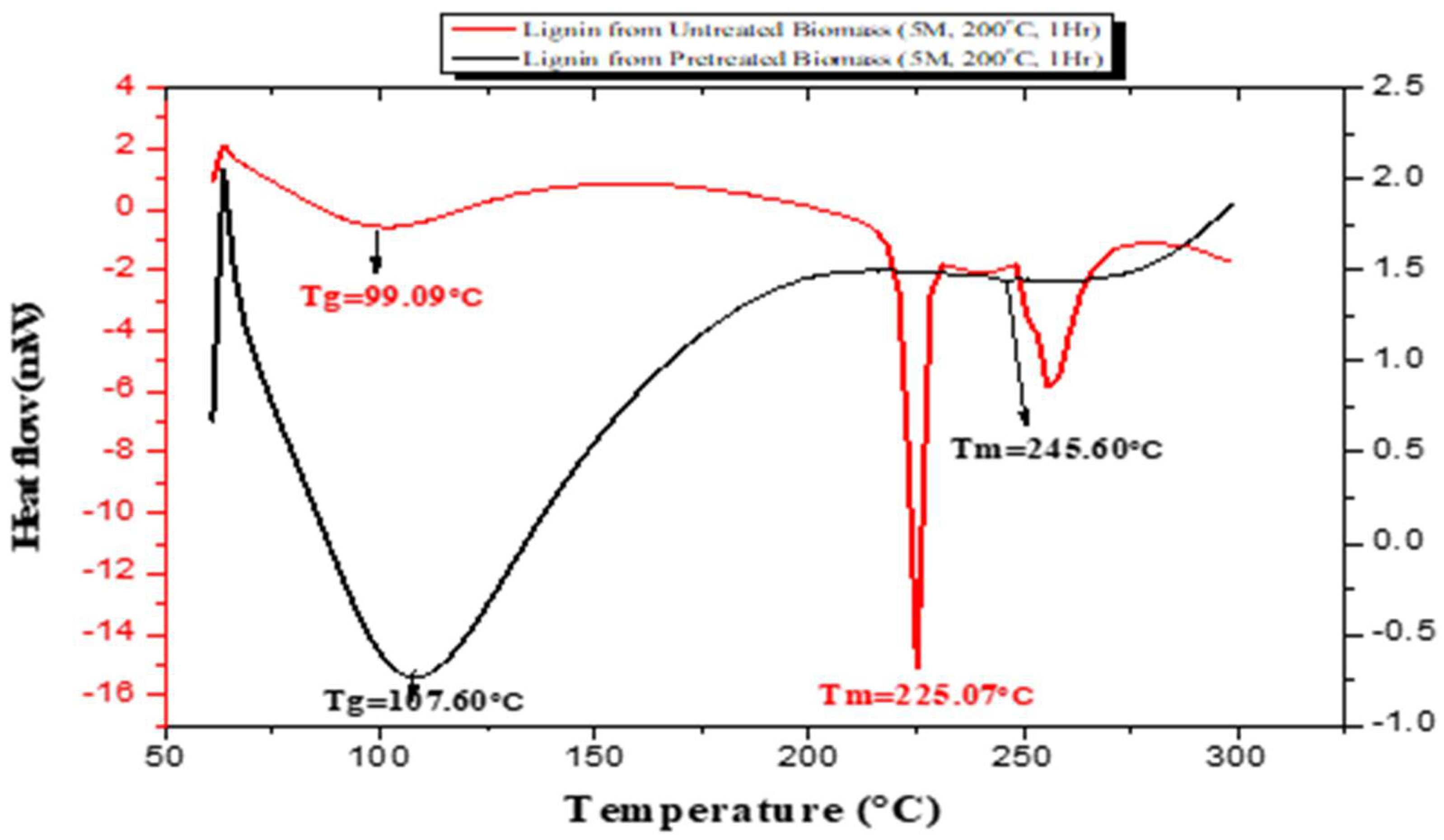
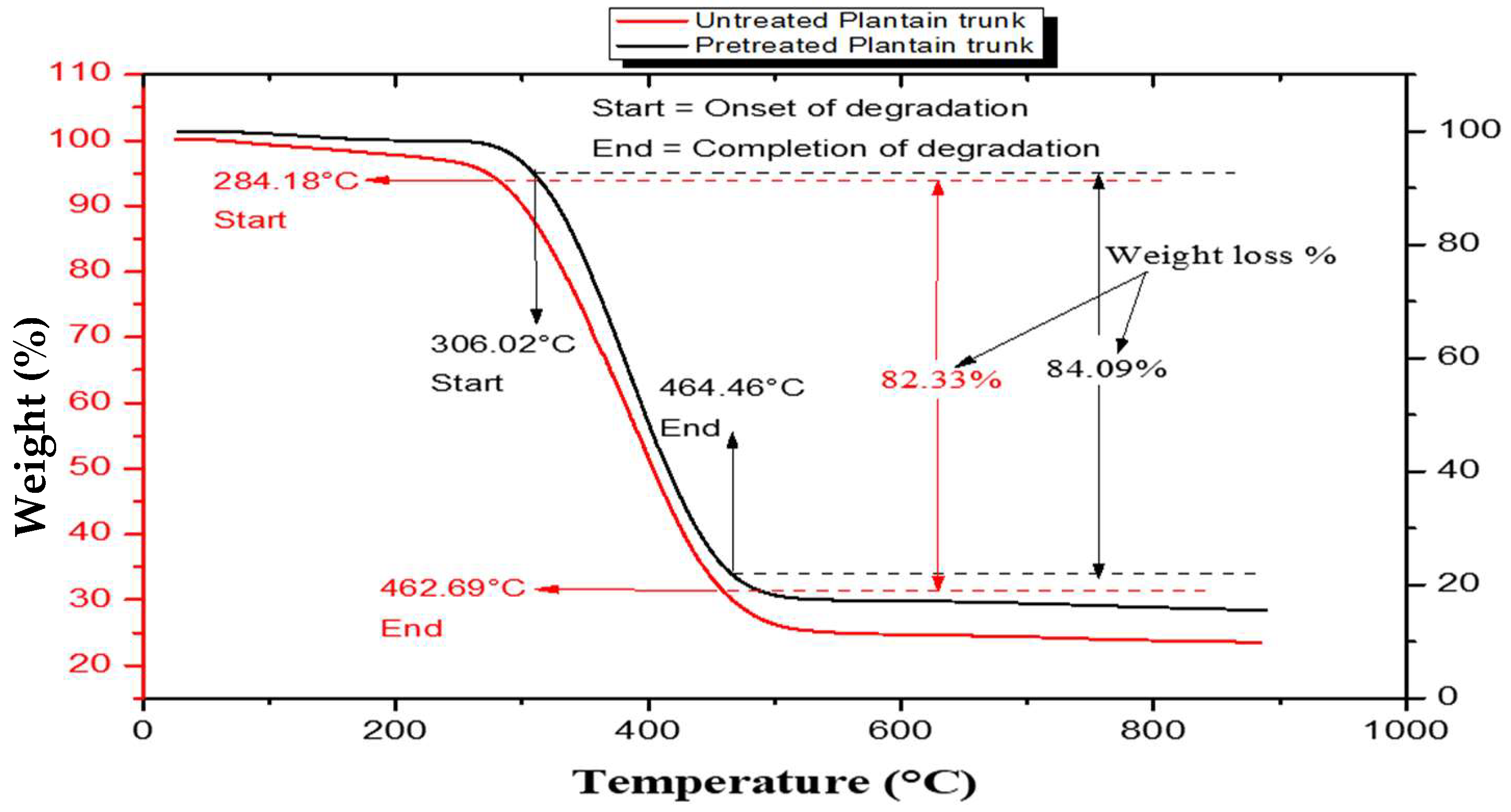
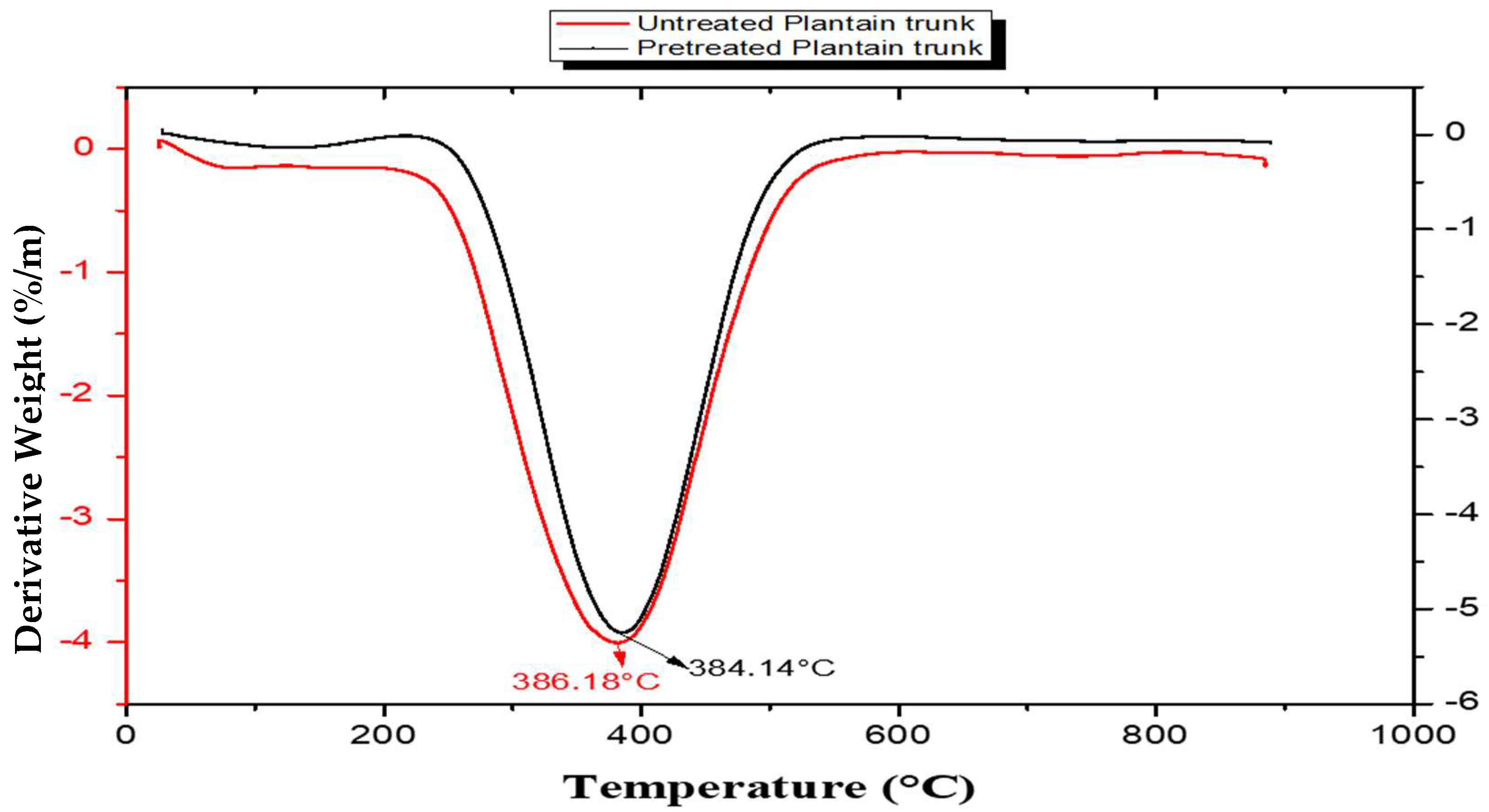
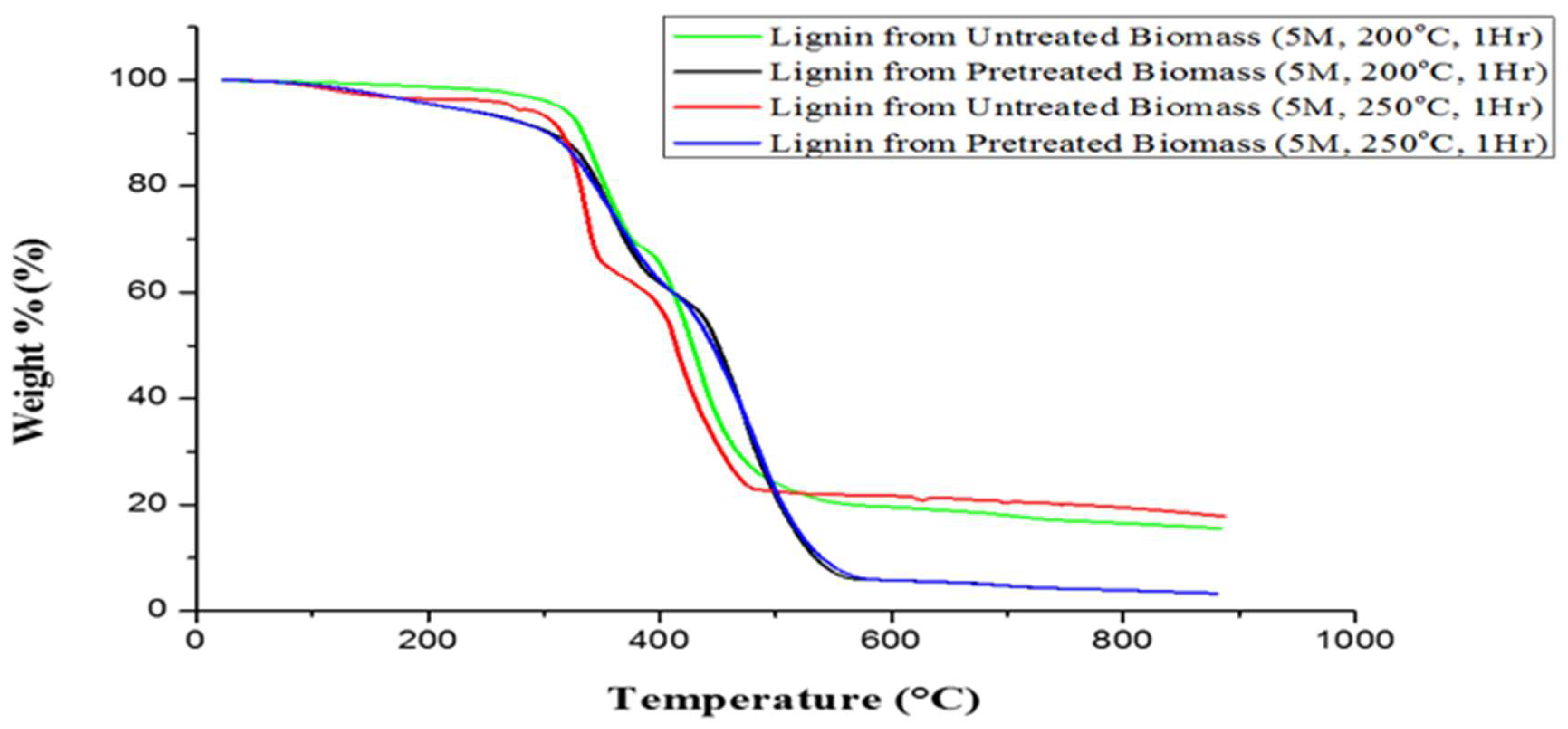
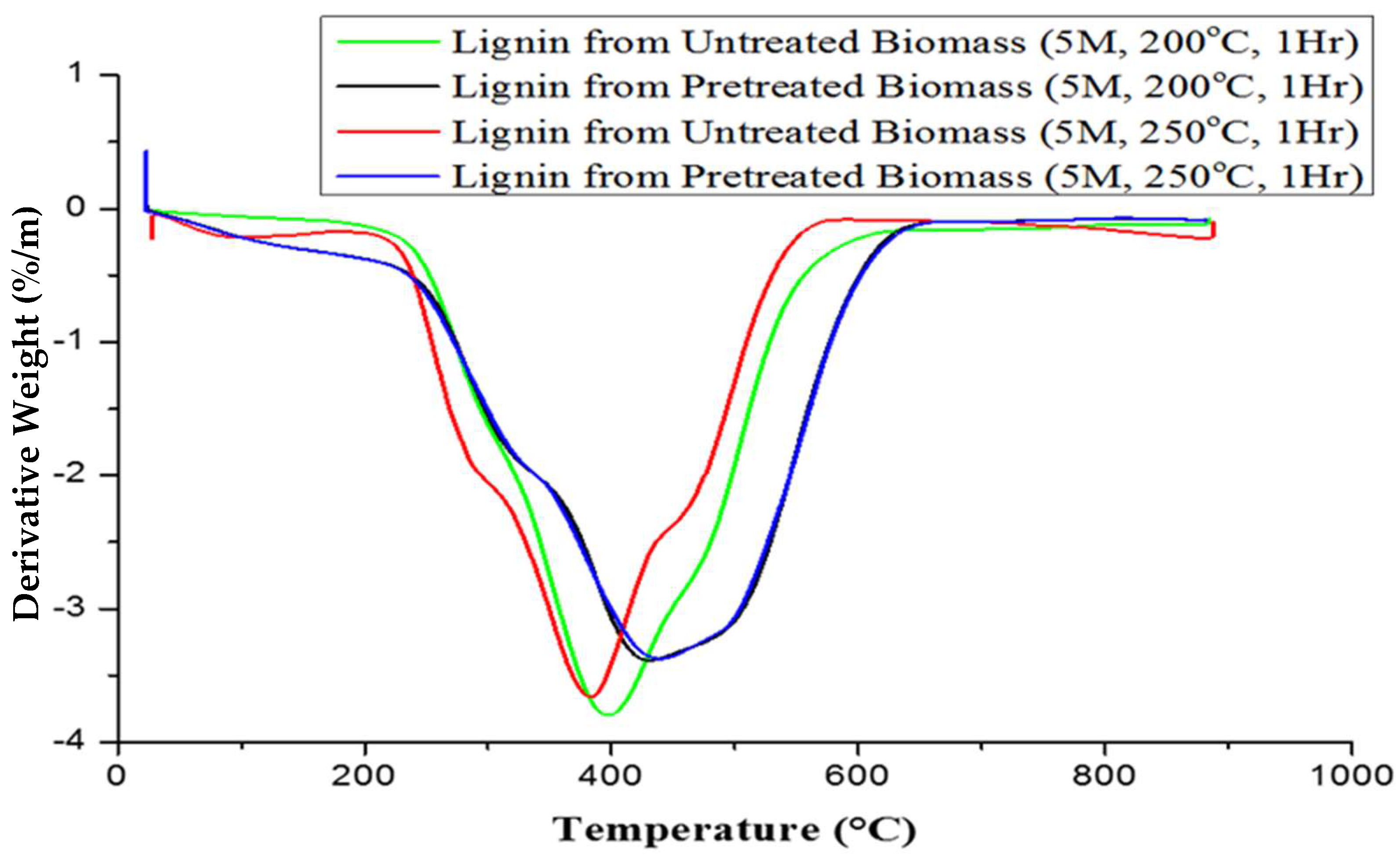
| S/N | Lignin Sample | 1st Stage (°C) | 2nd Stage (°C) | 3rd Stage (°C) | DTGmax (°C) |
|---|---|---|---|---|---|
| 1. | Untreated plantain pseudostem | 64.96–284.18 | 284.18–462.69 | 462.69–800 | 386.18 |
| 2. | Pretreated plantain pseudostem | 59.54–306.02 | 306.02–464.86 | 464.86–800 | 384.14 |
| 3. | Lignin from untreated pseudostem (5M, 200 °C, 1 h) | 65.53–318.77 | 318.77–477.32 | 477.32–800 | 397.22 |
| 4. | Lignin from pretreated pseudostem (5M, 200 °C, 1 h) | 60.10–320.65 | 320.65–533.75 | 533.75–800 | 429.97 |
| 5. | Lignin from untreated pseudostem (5M, 250 °C, 1 h) | 58.31–304.24 | 304.24–477.32 | 477.32–800 | 382.53 |
| 6. | Lignin from pretreated pseudostem (5M, 250 °C, 1 h) | 58.31–313.34 | 313.34–535.54 | 535.54–800 | 442.62 |
| S/N | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Element | Fe | Cu | Ni | Zn | Al | Mg | Na | S | P | Ca | K | Mn | Rb | Sr | |
| 2 | Concentration (%) | 0.079 | 0.003 | 0.009 | 0.009 | 0.030 | 0.015 | 0.011 | 0.066 | 0.080 | 0.676 | 4.435 | 0.131 | 0.004 | 0.003 | |
| Br | Cl | Cr | V | Mo | W | Bi | Ba | Pb | Sn | Si | As | Nb | Ta | Ag | ||
| 0.001 | 0.324 | 0.001 | 0.00 | 0.00 | 0.038 | 0.100 | 0.001 | 0.674 | 0.010 | [0.18] | 0.00 | 0.056 | 0.007 | 0.00 |
| S/N | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Element | Fe | Cu | Ni | Zn | Al | Mg | Na | S | P | Ca | K | Mn | Rb | Sr | |
| 2 | Concentration (%) | 0.206 | 0.002 | 0.036 | 0.010 | 0.079 | 0.023 | 0.206 | 0.125 | 0.069 | 2.577 | 0.150 | 0.168 | 0.002 | 0.006 | |
| Br | Cl | Cr | V | Mo | W | Bi | Ba | Pb | Sn | Si | As | Nb | Ta | Ag | ||
| 0.005 | 0.502 | 0.002 | −0.007 | 0.00 | 0.058 | 0.143 | 1.318 | 0.748 | 0.00 | 0.00 | −0.002 | 0.117 | 0.020 | 0.00 |
| S/N | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Element | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | ||
| 2 | Concentration (%) | 0.396 | 11.507 | 1.987 | 0.88 | 0.254 | 0.357 | 0.107 | 0.107 | 1.180 | 0.785 | 0.006 | 0.017 | 0.017 | 0.003 | ||
| As2O3 | PbO | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | WO3 | Br | CeO2 | ThO2 | Y2O3 | Nb2O5 | I | BaO | ||
| 0.006 | 0.062 | 0.003 | 0.002 | 0.002 | 0.272 | −0.020 | 0.005 | 0.011 | 0.006 | 0 | 0 | 0.002 | 0.001 | 0 | 0.020 | ||
| Ag2O | SnO2 | U3O8 | Bi2O3 | GeO2 | Cs2O | Sb2O3 | La2O3 | CdO | Eu2O3 | Gd2O3 | Lu2O3 | Co3O4 | |||||
| 0.001 | 0 | 0 | 0 | 0.001 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S/N | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Element | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | ||
| 2 | Concentration (%) | 0.243 | 10.132 | 2.122 | 0 | 0.361 | 0.414 | 0.092 | 0.056 | 1.022 | 0.941 | 0.004 | 0.006 | 0.014 | 0.002 | ||
| As2O3 | PbO | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | WO3 | Br | CeO2 | ThO2 | Y2O3 | Nb2O5 | I | BaO | ||
| 0.001 | 0.010 | 0.002 | 0.001 | 0.001 | 0.259 | −0.03 | 0.002 | 0.001 | 0.002 | 0.274 | 0 | 0 | −0.030 | 0.001 | 0.020 | ||
| Ag2O | SnO2 | U3O8 | Bi2O3 | GeO2 | Cs2O | Sb2O3 | La2O3 | CdO | Eu2O3 | Gd2O3 | Lu2O3 | Co3O4 | |||||
| 0.001 | 0.222 | 0 | 0.035 | 0.001 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S/N | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Element | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | ||
| 2 | Concentration (%) | 0.409 | 12.072 | 2.205 | 0.37 | 0.106 | 0.281 | 0.095 | 0.077 | 0.682 | 0.694 | 0.003 | 0.008 | 0.032 | 0.001 | ||
| As2O3 | PbO | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | WO3 | Br | CeO2 | ThO2 | Y2O3 | Nb2O5 | I | BaO | ||
| 0.002 | 0.010 | 0.002 | 0.001 | 0.001 | 0.488 | −0.03 | 0.001 | 0.001 | 0.004 | 0 | 0 | 0 | −0.030 | 0.001 | 0.020 | ||
| Ag2O | SnO2 | U3O8 | Bi2O3 | GeO2 | Cs2O | Sb2O3 | La2O3 | CdO | Eu2O3 | Gd2O3 | Lu2O3 | Co3O4 | |||||
| 0.001 | 0 | 0 | 0.035 | 0.001 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S/N | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Element | Fe2O3 | SiO2 | Al2O3 | MgO | P2O5 | SO3 | TiO2 | MnO | CaO | K2O | CuO | ZnO | Cr2O3 | V2O5 | ||
| 2 | Concentration (%) | 0.597 | 11.069 | 3.005 | 0 | 1.276 | 1.052 | 0.120 | 0.131 | 0.673 | 2.078 | 0.007 | 0.025 | 0.020 | 0.004 | ||
| As2O3 | PbO | Rb2O | Ga2O3 | NiO | Cl | ZrO2 | Ta2O5 | WO3 | Br | CeO2 | ThO2 | Y2O3 | Nb2O5 | I | BaO | ||
| 0.007 | 0.099 | 0.002 | 0.003 | 0.001 | 0.762 | −0.02 | 0.003 | 0.011 | 0.001 | 0.277 | 0 | 0.002 | −0.030 | 0 | 0.024 | ||
| Ag2O | SnO2 | U3O8 | Bi2O3 | GeO2 | Cs2O | Sb2O3 | La2O3 | CdO | Eu2O3 | Gd2O3 | Lu2O3 | Co3O4 | |||||
| 0.001 | 0.209 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Scheme | Property | Polyacrylonitrile (Pan) | Pseudostem Lignin |
|---|---|---|---|
| 1. | Availability | Fossil-based [29] | Agrowaste-derived |
| 2. | Thermal Stability | Decomposes between 296 and 434 °C. [30] | Decomposes between 313.34 and 535.54 °C |
| 3. | Melting Temperature | Above 300 °C [31] | Around 225–267 °C |
| 4. | Crystal Structure | Interconnected carbon chain with a rigid structure [32] | Mostly amorphous |
| 5. | Chemical Structure | Synthetic polymer with a linear chain [33] | Natural polymer with a complex structure-based source |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odili, C.C.; Olanrewaju, O.A.; Ofordile, C.O.; Adeosun, S.O. The Structural and Thermal Characteristics of Musa paradisiaca L. Lignin for Carbon Footprint Reduction Applications. Atmosphere 2024, 15, 55. https://doi.org/10.3390/atmos15010055
Odili CC, Olanrewaju OA, Ofordile CO, Adeosun SO. The Structural and Thermal Characteristics of Musa paradisiaca L. Lignin for Carbon Footprint Reduction Applications. Atmosphere. 2024; 15(1):55. https://doi.org/10.3390/atmos15010055
Chicago/Turabian StyleOdili, Chiosa Cletus, Oludolapo Akanni Olanrewaju, Cyprian Onyedikachi Ofordile, and Samson Oluropo Adeosun. 2024. "The Structural and Thermal Characteristics of Musa paradisiaca L. Lignin for Carbon Footprint Reduction Applications" Atmosphere 15, no. 1: 55. https://doi.org/10.3390/atmos15010055
APA StyleOdili, C. C., Olanrewaju, O. A., Ofordile, C. O., & Adeosun, S. O. (2024). The Structural and Thermal Characteristics of Musa paradisiaca L. Lignin for Carbon Footprint Reduction Applications. Atmosphere, 15(1), 55. https://doi.org/10.3390/atmos15010055







