Future Implications of Climate Change on Arum palaestinum Boiss: Drought Tolerance, Growth and Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Climate Change Projections
| No. | Model Name | The Institution | Horizontal Resolution | References |
|---|---|---|---|---|
| 1 | ACCESS-CM2 | Australian Bureau of Meteorology and CSIRO | 1.9° × 1.3° | [30] |
| 2 | ACCESS-ESM1-5 | 1.9° × 1.2° | [31] | |
| 3 | BCC-CSM2-MR | Beijing Climate Center, China | 1.1° × 1.1° | [32] |
| 4 | CAMS-CSM1-0 | Chinese Academy of Meteorological Sciences | 1.1° × 1.1° | [33] |
| 5 | CanESM5 | Canadian Centre for Climate Modelling and Analysis | 2.8° × 2.8° | [34] |
| 6 | CESM2 | National Center for Atmospheric Research (Boulder, CO, USA) | 1.3° × 0.9° | [35] |
| 7 | CESM2-WACCM | 1.3° × 0.9° | [36] | |
| 8 | CNRM-CM6-1 | Centre National de Recherches Météorologiques, France | 1.4° × 1.4° | [37] |
| 9 | CNRM-CM6-1-HR | 0.5° × 0.5° | ||
| 10 | CNRM-ESM2-1 | 1.4° × 1.4° | [38] | |
| 11 | EC-Earth3 | European Centre for Medium-Range Weather Forecasts | 0.7° × 0.7° | [39] |
| 12 | EC-Earth3-Veg | 0.7° × 0.7° | [40] | |
| 13 | FGOALS-f3-L | State Key Laboratory of Numerical Modeling for Atmospheric Sciences and Geophysical Fluid Dynamics, USA | 1.3° × 1° | [41] |
| 14 | FGOALS-g3 | 2° × 2.3° | [42] | |
| 15 | FIO-ESM-2-0 | Institute of Oceanography, Ministry of Natural Resources, Fujian, China | 1.3° × 0.9° | [43] |
| 16 | GFDL-ESM4 | Geophysical Fluid Dynamics Laboratory, (NOAA, USA) | 1.3° × 1° | [44] |
| 17 | INM-CM4-8 | Institute for Numerical Mathematics, Moscow, Russia | 2° × 1.5° | [45] |
| 18 | INM-CM5-0 | 2° × 1.5° | ||
| 19 | IPSL-CM6A-LR | Institut Pierre-Simon Laplace, Guyancourt, France | 2.5° × 1.3° | [46] |
| 20 | MIROC6 | University of Tokyo, the National Institute for Environmental Studies, and the Japan Agency for Marine-Earth Science and Technology | 1.4° × 1.4° | [47] |
| 21 | MIROC-ES2L | 2.8° × 2.8° | [48] | |
| 22 | MPI-ESM1-2-HR | Max Planck Institute for Meteorology, Hamburg, Germany | 0.9° × 0.9° | [49] |
| 23 | MPI-ESM1-2-LR | 1.9° × 1.9° | [50] | |
| 24 | MRI-ESM2-0 | Meteorological Research Institute, Ibaraki, Japan | 1.1° × 1.1° | [51] |
| 25 | NESM3 | Nanjing University of Information Science and Technology, Nanking, China | 1.9° × 1.9° | [52] |
| 26 | NorESM2-LM | Norwegian Climate Prediction Model | 2.5° × 1.9° | [53] |
| 27 | UKESM1-0-LL | UK Met Office Hadley Centre | 1.9° × 1.3° | [54] |
2.2. Testing for Drought Tolerance of A. palaestinum
2.2.1. Cultural Practices and Therapies
2.2.2. Testing for Drought Tolerance
2.3. Data Collection
2.4. Data Analysis
3. Results
3.1. The Projected Climate Change
3.2. Agronomic Traits of the Black Calla Lily under Drought Conditions
3.3. Osmotic Adjustment
3.3.1. Shoot Total Nonstructural Carbohydrates and Total Reducing Sugar Content
3.3.2. Shoot Proline Content
3.4. Water Use Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allan, R.P.; Hawkins, E.; Bellouin, N.; Collins, B. IPCC, 2021: Summary for Policymakers. 2021. Available online: https://www.ipcc.ch/report/ar6/wg1/chapter/summary-for-policymakers/ (accessed on 30 May 2022).
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 72, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.P.R.A.D.; Rambal, S.; Soares, A.M.; Mouillot, F.; Nogueira, J.M.P.; Martins, G.A. Plant Physiological Ecology and the Global Changes. Cienc. Agrotecnol. 2012, 36, 253–269. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. (Eds.) IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; De Siqueira, M.F.; Gringer, A.; Hannah, L.; et al. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Hauser, M. Regional climate sensitivity of climate extremes in CMIP6 versus CMIP5 multimodel ensembles. Earth’s Future 2020, 8, e2019EF001474. [Google Scholar] [CrossRef]
- Zamani, Y.; Monfared, S.A.H.; Hamidianpour, M. A comparison of CMIP6 and CMIP5 projections for precipitation to observational data: The case of northeastern Iran. Theor. Appl. Climatol. 2020, 142, 1613–1623. [Google Scholar] [CrossRef]
- Zhu, H.H.; Jiang, Z.H.; Li, J.; Li, W.; Sun, C.X.; Li, L. Does CMIP6 inspire more confidence in simulating climate extremes over China? Adv. Atmos. Sci. 2020, 37, 1119–1132. [Google Scholar] [CrossRef]
- Wobus, C.; Kunkel, K.E.; Easterling, D.R. Precipitation extremes projected to increase in the western US but uncertain due to model choice. Environ. Res. Lett. 2020, 207–230. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. (Eds.) IPCC (2021) Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Chang. 2017, 42, 169–180. [Google Scholar] [CrossRef]
- Tack, J.; Barkley, A.; Lanier Nalley, L. Estimating yield gaps with limited data: An application to United States wheat. Am. J. Agric. Econ. 2015, 97, 1464–1477. [Google Scholar] [CrossRef]
- Soeder, D.J.; Kappel, W.M. Water Resources and Natural Gas Production from the Marcellus Shale; US Department of the Interior, US Geological Survey: Reston, VA, USA, 2009; pp. 1–6. [Google Scholar]
- El-Desouky, S.K.; Kim, K.H.; Ryu, S.Y.; Eweas, A.F.; Gamal-Eldeen, A.M.; Kim, Y.K. A new pyrrole alkaloid isolated from Arum palaestinum Boiss. And its biological activities. Arch. Pharmacal Res. 2007, 30, 927–931. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Said, O. Arab herbal medicines. In Botanical Medicine in Clinical Practice; Preedy, V.R., Watson, R.R., Eds.; CAB International: Wallingford, UK, 2008; pp. 31–39. [Google Scholar]
- Lindzen, R.S. Some coolness concerning global warming. Bull. Am. Meteorol. Soc. 1990, 71, 288–299. [Google Scholar] [CrossRef]
- Das, M. Performance of Asalio (Lepidium sativum L.) genotypes under semi-arid condition of middle Gujarat. Indian J. Plant Physiol. 2010, 15, 85–89. [Google Scholar]
- El-Desouky, S.K.; Hawas, U.W.; Kim, Y.K. Two new diketopiperazines from Arum palaestinum. Chem. Nat. Compd. 2014, 50, 1075–1078. [Google Scholar] [CrossRef]
- Farid, M.M.; Hussein, S.R.; Ibrahim, L.F.; El-Desouky, M.A.; Elsayed, A.M.; Saker, M.M. Shoot regeneration, biochemical, molecular and phytochemical investigation of Arum palaestinum Boiss. Afr. J. Biotechnol. 2014, 13, 3522–3530. [Google Scholar] [CrossRef]
- Jaradat, N.; Eid, M.; Mohyeddin, A.; Naser, A. Variations of exhaustive extraction yields and methods of preparations for (Arum palaestinum) Solomon’s Lily plant in all regions of West Bank/Palestine. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 356–360. [Google Scholar]
- Naseef, H.; Qadadha, H.; Abu Asfour, Y.; Sabri, I.; Al-Rimawi, F.; Abu-Qatouseh, L.; Farraj, M. Anticancer, antibacterial, and antifungal activities of Arum palaestinum plant extracts. World J. Pharm. Res. 2017, 6, 31–43. [Google Scholar]
- Makhadmeh, I.; Al-Lozi, S.; Duwayri, M.; Shibli, R.A.; Migdadi, H. Assessment of genetic variation in wild Arum species from Jordan using amplified fragment length polymorphism (AFLP) markers. Jordan J. Agric. Sci. 2010, 6, 224–239. [Google Scholar]
- Duncan, R.R.; Carrow, R.N. Turfgrass molecular genetic improvement for biotic/edaphic stress resistance. Adv. Agron. 1999, 67, 233–305. [Google Scholar]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Arve, L.E.; Torre, S.; Olsen, J.E.; Tanino, K.K. Stomatal responses to drought stress and air humidity. In Abiotic Stress in Plants-Mechanisms and Adaptations; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Passioura, J.B. The drought environment: Physical, biological and agricultural perspectives. J. Exp. Bot. 2007, 58, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.; Osborn, T.J.; Jones, P.; Lister, D. Version 4 of the CRU TS monthly high-resolution gridded multivariate climate dataset. Sci. Data 2020, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Iturbide, M.; Gutiérrez, J.M.; Alves, L.M.; Bedia, J.; Cerezo-Mota, R.; Cimadevilla, E.; Cofino, A.S.; Di Luca, A.; Faria, S.H.; Gorpdetskaya, I.V.; et al. An update of IPCC climate reference regions for subcontinental analysis of climate model data: Definition and aggregated datasets. Earth Syst. Sci. Data 2020, 12, 2959–2970. [Google Scholar] [CrossRef]
- Bi, D.; Dix, M.; Marsland, S.; O’Farrell, S.; Rashid, H.; Uotila, P.; Hirst, A.; Kowalczyk, E.; Golebiewski, M.; Sullivan, A.; et al. The ACCESS coupled model: Description, control climate and evaluation. Aust. Meteorol. Ocean. J. 2012, 63, 41–64. [Google Scholar] [CrossRef]
- Law, R.M.; Ziehn, T.; Matear, R.J.; Lenton, A.; Chamberlain, M.A.; Stevens, L.E.; Wang, Y.-P.; Srbinovsky, J.; Bi, D.; Yan, H.; et al. The carbon cycle in the Australian Community Climate and Earth System Simulator (ACCESS-ESM1)—Part 1: Model description and pre-industrial simulation. Geosci. Model Dev. 2017, 10, 2567–2590. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing climate center climate system model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Rong, X.Y.; Li, J.; Chen, H.M.; Xin, Y.-F.; Su, J.-Z.; Hua, L.-J.; Zhang, Z.-Q. Introduction of CAMS-CSM model and its participation in CMIP6. Clim. Chang. Res. 2019, 15, 540–544. [Google Scholar] [CrossRef]
- Swart, N.C.; Cole, J.N.S.; Kharin, V.V.; Lazare, M.; Scinocca, J.F.; Gillett, N.P.; Anstey, J.; Arora, V.; Christian, J.R.; Hanna, S.; et al. The Canadian Earth System Model version 5 (CanESM5.0.3). Geosci. Model Dev. 2019, 12, 4823–4873. [Google Scholar] [CrossRef]
- Lauritzen, P.H.; Nair, R.D.; Herrington, A.R.; Callaghan, P.; Goldhaber, S.; Dennis, J.M.; Bacmeister, J.T.; Eaton, B.E.; Zarzycki, C.M.; Taylor, M.A.; et al. NCAR release of CAM-SE in CESM2.0: A reformulation of the spectral element dynamical core in dry-mass vertical coordinates with comprehensive treatment of condensates and energy. J. Adv. Model Earth Syst. 2018, 10, 1537–1570. [Google Scholar] [CrossRef]
- Li, L. CAS FGOALS-g3 model output prepared for CMIP6 ScenarioMIP ssp370 (Version 20191026) [Data set]. Earth Syst. Grid Fed. 2019. [Google Scholar] [CrossRef]
- Voldoire, A.; Saint-Martin, D.; Sénési, S.; Decharme, B.; Alias, A.; Chevallier, M.; Colin, J.; Guérémy, J.; Michou, M.; Moine, M.; et al. Evaluation of CMIP6 DECK experiments with CNRM-CM6-1. J. Adv. Model Earth Syst. 2019, 11, 2177–2213. [Google Scholar] [CrossRef]
- Séférian, R.; Nabat, P.; Michou, M.; Saint-Martin, D.; Voldoire, A.; Colin, J.; Decharme, B.; Delire, C.; Berthet, S.; Chevallier, M.; et al. Evaluation of CNRM Earth-System model, CNRM-ESM2-1: Role of Earth system processes in present-day and future climate. J. Adv. Model Earth Syst. 2019, 11, 4182–4227. [Google Scholar] [CrossRef]
- Massonnet, F.; Ménégoz, M.; Acosta, M.; Yepes-Arbós, X.; Exarchou, E.; Doblas-Reyes, F.J. Replicability of the EC-Earth3 Earth system model under a change in computing environment. Geosci. Model Dev. 2020, 13, 1165–1178. [Google Scholar] [CrossRef]
- Wyser, K.; van Noije, T.; Yang, S.; von Hardenberg, J.; O’Donnell, D.; Döscher, R. On the increased climate sensitivity in the EC-Earth model from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 13, 3465–3474. [Google Scholar] [CrossRef]
- He, B.; Bao, Q.; Wang, X.; Zhou, L.; Wu, X.; Liu, Y.; Wu, G.; Chen, K.; He, S.; HU, W.; et al. CAS FGOALS-f3-L model datasets for CMIP6 historical atmospheric model intercomparison project simulation. Adv. Atmos. Sci. 2019, 36, 771–778. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Tang, Y.; Lin, P.; Xie, J.; Song, M.; Dong, L.; Zhou, T.; Liu, L.; Wang, L.; et al. The flexible global ocean-atmosphere-land system model grid-point version 3 (FGOALS-g3): Description and evaluation. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002012. [Google Scholar] [CrossRef]
- Bao, Y.; Song, Z.; Qiao, F. FIO-ESM version 2.0: Model description and evaluation. J. Geophys. Res. Ocean. 2020, 125, e2019JC016036. [Google Scholar] [CrossRef]
- Dunne, J.P.; Horowitz, L.W.; Adcroft, A.J.; Ginoux, P.; Held, I.M.; John, J.G.; Krasting, J.P.; Malyshev, S.; Naik, V.; Paulot, F.; et al. The GFDL Earth System Model version 4.1 (GFDL-ESM 4.1): Overall coupled model description and simulation characteristics. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002015. [Google Scholar] [CrossRef]
- Volodin, E.M.; Mortikov, E.V.; Kostrykin, S.V.; Galin, V.Y.; Lykossov, V.N.; Gritsun, A.S.; Diansky, N.A.; Gusev, A.V.; Iakovlev, N.G.; Shestakova, A.A.; et al. Simulation of the modern climate using the INM-CM48 climate model. Russ. J. Numer. Anal. Math. Model. 2018, 33, 367–374. [Google Scholar] [CrossRef]
- Boucher, O.; Servonnat, J.; Albright, A.L.; Aumont, O.; Balkanski, Y.; Bastrikov, V.; Bekki, S.; Bonnet, R.; Bony, S.; Bopp, L.; et al. Presentation and evaluation of the IPSL-CM6A-LR climate model. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002010. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F.; et al. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- Hajima, T.; Watanabe, M.; Yamamoto, A.; Tatebe, H.; Noguchi, M.A.; Abe, M.; Ohgaito, R.; Ito, A.; Yamazaki, D.; Okajima, H.; et al. Development of the MIROC-ES2L Earth system model and the evaluation of biogeochemical processes and feedbacks. Geosci. Model Dev. 2020, 13, 2197–2244. [Google Scholar] [CrossRef]
- Gutjahr, O.; Putrasahan, D.; Lohmann, K.; Jungclaus, J.H.; von Storch, J.-S.; Brüggemann, N.; Haak, H.; Stössel, A. Max planck institute earth system model (MPI-ESM1. 2) for the high-resolution model intercomparison project (HighResMIP). Geosci. Model Dev. 2019, 12, 3241–3281. [Google Scholar] [CrossRef]
- Mauritsen, T.; Bader, J.; Becker, T.; Behrens, J.; Bittner, M.; Brokopf, R.; Brovkin, V.; Claussen, M.; Crueger, T.; Esch, M.; et al. Developments in the MPI-M Earth System Model version 1.2 (MPI-ESM1. 2) and its response to increasing CO2. J. Adv. Model. Earth Syst. 2019, 11, 998–1038. [Google Scholar] [CrossRef]
- Yukimoto, S.; Kawai, H.; Koshiro, T.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M.; et al. The Meteorological Research Institute Earth System Model version 2.0, MRI-ESM2. 0: Description and basic evaluation of the physical component. J. Meteorol. Soc. Jpn. Ser. II 2019, 97, 931–965. [Google Scholar] [CrossRef]
- Cao, J.; Ma, L.; Liu, F.; Chai, J.; Zhao, H.; He, Q.; Wang, B.; Bao, Y.; Li, J.; Yang, Y.-M.; et al. NUIST ESM v3 data submission to CMIP6. Adv. Atmos. Sci. 2021, 38, 268–284. [Google Scholar] [CrossRef]
- Seland, Ø.; Bentsen, M.; Olivié, D.; Toniazzo, T.; Gjermundsen, A.; Graff, L.S.; Debernard, J.B.; Gupta, A.K.; He, Y.-C.; Kirkevåg, A.; et al. Overview of the Norwegian Earth System Model (NorESM2) and key climate response of CMIP6 DECK, historical, and scenario simulations. Geosci. Model Dev. 2020, 13, 6165–6200. [Google Scholar] [CrossRef]
- Sellar, A.A.; Jones, C.G.; Mulcahy, J.P.; Tang, Y.; Yool, A.; Wiltshire, A.; O’Connor, F.M.; Stringer, M.; Hill, R.; Palmieri, J.; et al. UKESM1: Description and evaluation of the UK Earth System Model. J. Adv. Model. Earth Syst. 2019, 11, 4513–4558. [Google Scholar] [CrossRef]
- Chatterton, N.J.; Bennett, J.H.; Thornley, W.R. Fructan, starch, and sucrose concentrations in crested wheatgrass and redtop as aff ected by temperature. Plant Physiol. Biochem. 1987, 25, 617–623. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT User’s Guide; SAS Institute: Cary, NC, USA, 2006. [Google Scholar]
- Murkute, A.A.; Sharma, S.; Singh, S.K. Studies on salt stress tolerance of citrus rootstock genotypes with arbuscular mycorrhizal fungi. Hortic. Sci. 2006, 33, 70–76. [Google Scholar] [CrossRef]
- Zittis, G.; Hadjinicolaou, P.; Lelieveld, J. Future precipitation projections for the Eastern Mediterranean: Assessment of different RCMs and emission scenarios. Theor. Appl. Climatol. 2018, 131, 1171–1185. [Google Scholar]
- Lelieveld, J.; Hadjinicolaou, P.; Kostopoulou, E.; Chenoweth, J.; El Maayar, M.; Giannakopoulos, C.; Hannides, C.; Lange, M.A.; Tanarhte, M.; Tyrlis, E.; et al. Climate change and impacts in the Eastern Mediterranean and the Middle East. Clim. Change 2016, 136, 5–29. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Akin, M.; Toplu, C.; Erdogan-Orhan, I.; Kocak, M.S. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2017, 11, 63–66. [Google Scholar]
- Jarvis, D.I.; Hodgkin, T.; Sthapit, B.R.; Fadda, C.; Lopez-Noriega, I. An heuristic framework for identifying multiple ways of supporting the conservation and use of traditional crop varieties within the agricultural production system. Crit. Rev. Plant Sci. 2011, 30, 125–176. [Google Scholar] [CrossRef]
- Naor, V.; Kigel, J. Temperature affects plant development, flowering and tuber dormancy in calla lily (Zantedeschia). J. Hortic. Sci. Biotechnol. 2002, 77, 170–176. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Yadollahi, A.; Arzani, K.; Ebadi, A.; Wirthensohn, M.; Karimi, S. The response of different almond genotypes to moderate and severe water stress in order to screen for drought tolerance. Sci. Hortic. 2011, 129, 403–413. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Lenis, J.I.; Calle, F.; Jaramillo, G.; Perez, J.C.; Ceballos, H.; Cock, J.H. Leaf retention and cassava productivity. Field Crop. Res. 2006, 95, 126–134. [Google Scholar] [CrossRef]
- Gibon, Y.; Sulpice, R.; Larher, F. Proline accumulation in canola leaf discs subjected to osmotic stress is related to the loss of chlorophylls and to the decrease of mitochondrial activity. Physiol. Plant. 2000, 110, 469–476. [Google Scholar] [CrossRef]
- Din, J.; Khan, S.U.; Ali, I.; Gurmani, A.R. Physiological and agronomic response of canola varieties to drought stress. J. Anim. Plant Sci. 2011, 21, 78–82. [Google Scholar]
- Liu, D.; Pei, Z.F.; Naeem, M.S.; Ming, D.F.; Liu, H.B.; Khan, F.; Zhou, W.J. 5-Aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J. Agron. Crop Sci. 2011, 197, 284–295. [Google Scholar] [CrossRef]
- Boyer, J.S. Leaf enlargement and metabolic rates in corn, soybean, and sunflower at various leaf water potentials. Plant Physiol. 1970, 46, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Lecoeur, J.; Wery, J.; Turc, O.; Tardieu, F. Expansion of pea leaves subjected to short water deficit: Cell number and cell size are sensitive to stress at different periods of leaf development. J. Exp. Bot. 1995, 46, 1093–1101. [Google Scholar] [CrossRef]
- Hessini, K.; Martínez, J.P.; Gandour, M.; Albouchi, A.; Soltani, A.; Abdelly, C. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ. Exp. Bot. 2009, 67, 312–319. [Google Scholar] [CrossRef]
- Grant, O.M.; Johnson, A.W.; Davies, M.J.; James, C.M.; Simpson, D.W. Physiological and morphological diversity cultivated strawberry (Fragaria × ananasa) in response to water deficit. Environ. Exp. Bot. 2010, 68, 264–272. [Google Scholar] [CrossRef]
- Klamkowski, K.; Treder, W. Response to drought stress of three strawberry cultivars grown under greenhouse conditions. J. Fruit Ornam. Plant Res. 2008, 16, 79–188. [Google Scholar]
- Boutraa, T.; Akhkha, A.; Al-Shoaibi, A.A.; Alhejeli, A.M. Effect of water stress on growth and water use efficiency (WUE) of some wheat cultivars (Triticum durum) grown in Saudi Arabia. J. Taibah Univ. Sci. 2010, 3, 39–48. [Google Scholar] [CrossRef]
- Madramootoo, C.A.; Rigby, M. Effects of trickle irrigation on the growth and sunscald of bell peppers (Capsicum annuum L.) in southern Quebec. Agric. Water Manag. 1991, 19, 181–189. [Google Scholar] [CrossRef]
- Kirnak, H.; Tas, I.; Kaya, C.; Higgs, D. Effects of deficit irrigation on growth, yield, and fruit quality of eggplant under semi-arid conditions. Aust. J. Agric. Res. 2002, 53, 1367–1373. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Tardieu, F.; Granier, C.; Muller, B. Modelling leaf expansion in a fluctuating environment: Are changes in specific leaf area a consequence of changes in expansion rate? New Phytol. 1999, 143, 33–43. [Google Scholar] [CrossRef]
- Passioura, J.B. Environmental biology and crop improvement. Funct. Plant Biol. 2002, 29, 537–546. [Google Scholar] [CrossRef]
- Schuppler, U.; He, P.H.; John, P.C.L.; Munns, R. Effect of water stress on cell division and cell-division-cycle 2-like cell cycle kinase activity in wheat leaves. Plant Physiol. 1998, 117, 667–678. [Google Scholar] [CrossRef]
- Razmjoo, K.; Heydarizadeh, P.; Sabzalian, M.R. Effect of salinity and drought stresses on growth parameters and essential oil content of Matricaria chamomile. Int. J. Agric. Biol. 2008, 10, 451–454. [Google Scholar]
- Shihab, U.; Shahnaj, P.; Awal, M.A. Morpo–Aspets of Mungbean (Vigna radiata L.) in Response to Water Stress. IJASR 2013, 3, 137–148. [Google Scholar]
- Baher, Z.F.; Mirza, M.; Ghorbanli, M.; Rezaii, M.B. The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortansis L. Flavour Frag. J. 2002, 17, 275–277. [Google Scholar] [CrossRef]
- Colom, M.R.; Vazzana, C. Water stress effects on three cultivars of Eragrostis curvula. Italy J. Agron. 2002, 6, 127–132. [Google Scholar]
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260. [Google Scholar] [CrossRef]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in non-halophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Marcum, K.B. Salinity tolerance mechanisms of grasses in the subfamily Chloridoideae. Crop Sci. 1999, 39, 1153–1160. [Google Scholar] [CrossRef]
- Shahba, M.A. Interaction effects of salinity and mowing on performance and physiology of bermudagrass cultivars. Crop Sci. 2010, 50, 2620–2631. [Google Scholar] [CrossRef]
- Shahba, M.A.; Alshammary, S.F.; Abbas, M.S. Effects of salinity on seashore paspalum cultivars at different mowing heights. Crop Sci. 2012, 52, 1358–1370. [Google Scholar] [CrossRef]
- Shahba, M.A.; Abbas, M.S.; Alshammary, S.F. Drought resistance strategies of seashore paspalum cultivars at different mowing heights. HortScience 2014, 49, 221–229. [Google Scholar] [CrossRef]
- Rozema, J.; Visser, M. The applicability of the rooting technique measuring salt resistance in populations of Festuca rubra and Juncus species. Plant Soil 1981, 62, 479–485. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2003, 25, 239–250. [Google Scholar] [CrossRef]
- Shahba, M.A.; Qian, Y.L.; Hughes, H.G.; Koski, A.J.; Christensen, D. Relationships of soluble carbohydrates and freeze tolerance in saltgrass. Crop Sci. 2003, 43, 2148–2153. [Google Scholar] [CrossRef]
- Popp, M.; Smirnoff, N. Polyol accumulation and metabolism during water defi cit. In Environment and Plant Metabolism Flexibility and Acclimation; Smirnoff, N., Ed.; Bios Scientific: Oxford, UK, 1995; pp. 199–215. [Google Scholar]
- Lee, G.J.; Carrow, R.N.; Duncan, R.R. Identification of new soluble sugars accumulated in a halophytic Seashore paspalum ecotype under salinity stress. Hortic. Environ. Biotechnol. 2008, 49, 13–19. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Levitt, J. Salt stresses. In Responses of Plants to Environmental Stresses; Academic Press: Cambridge, MA, USA, 1980; Volume II, pp. 365–454. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Role of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Maggio, A.; Miyazaki, S.; Veronese, P.; Fujita, T.; Ibeas, J.I.; Damsz, B.; Narasimhan, M.L.; Hasegawa, P.M.; Joly, R.J.; Bressan, R.A. Does proline accumulation play an active role in stress induced growth reduction? Plant J. 2002, 31, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Huang, B. Mechanisms and strategies for improving drought resistance in turfgrass. Acta Hortic. 2008, 783, 221. [Google Scholar] [CrossRef]
- Sharp, R.E.; Hsiao, C.T.; Silk, W.K. Growth of the maize primary root at low water potentials. II Role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiol. 1990, 93, 1337–1346. [Google Scholar] [CrossRef]
- DaCosta, M.; Huang, B. Osmotic adjustment associated with variation in bentgrass tolerance to drought stress. J. Am. Soc. Hortic. Sci. 2006, 131, 338–344. [Google Scholar] [CrossRef]
- Kim, K.S.; Beard, J.B. Comparative turfgrass evapotranspiration rates and associated plant morphological characteristics. Crop Sci. 1988, 28, 328–331. [Google Scholar] [CrossRef]
- Arunyanark, A.; Jogloy, S.; Akkasaeng, C.; Vorasoot, N.; Kesmala, T.; Nageswara Rao, R.C.; Wright, G.C.; Patanothai, A.; Arunyanark, A.; Jogloy, S.; et al. Chlorophyll stability is an indicator of drought tolerance in peanut. J. Agron. Crop Sci. 2008, 194, 113–125. [Google Scholar] [CrossRef]
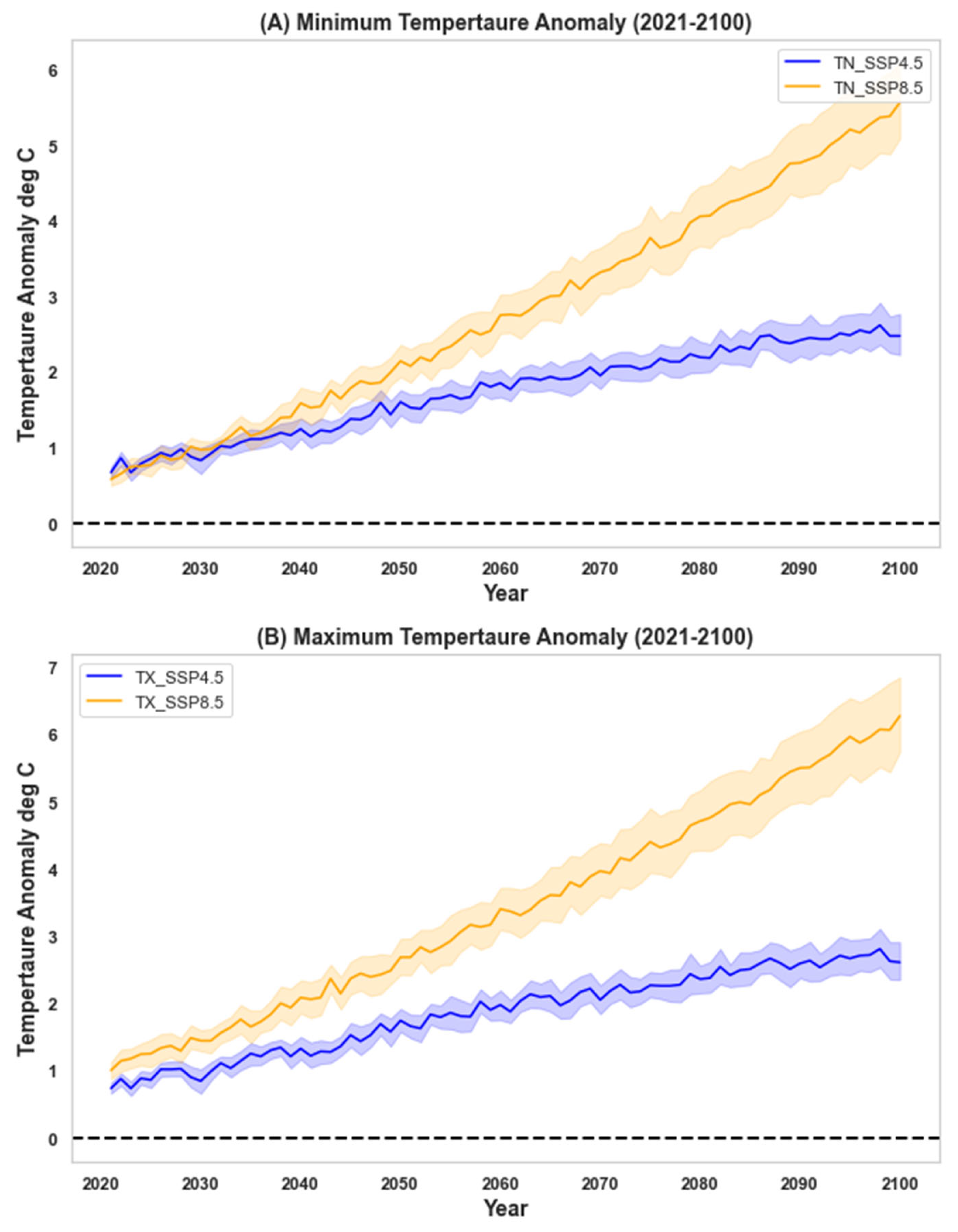

| Scenario | Period | Mean | StDev | Skewness | Kurtosis | Difference | Trend yr−1 | |
|---|---|---|---|---|---|---|---|---|
| TN | SSP2-4.5 | 2031–2060 | 12.49 | 0.29 | −0.03 | −1.28 | 2.95 | 0.03 |
| 2061–2100 | 13.39 | 0.24 | −0.23 | −1.31 | 3.85 | 0.02 | ||
| SSP5-8.5 | 2031–2060 | 12.90 | 0.49 | 0.09 | −1.17 | 4.36 | 0.06 | |
| 2061–2100 | 15.20 | 0.85 | 0.07 | −1.21 | 5.67 | 0.07 | ||
| TX | SSP2-4.5 | 2031–2060 | 18.72 | 0.29 | −0.02 | −1.33 | 0.69 | 0.03 |
| 2061–2100 | 19.61 | 0.23 | −0.23 | −1.32 | 1.57 | 0.02 | ||
| SSP5-8.5 | 2031–2060 | 19.21 | 0.48 | 0.09 | −1.18 | 1.09 | 0.05 | |
| 2061–2100 | 21.37 | 0.84 | 0.07 | −1.22 | 3.34 | 0.07 | ||
| Pr | SSP2-4.5 | 2031–2060 | 2.54 | 0.02 | 0.13 | −0.67 | −1.04 | −0.001 |
| 2061–2100 | 2.58 | 0.02 | −0.44 | 1.36 | −1.08 | −0.001 | ||
| SSP5-8.5 | 2031–2060 | 2.56 | 0.02 | 0.13 | −1.22 | −1.06 | −0.002 | |
| 2061–2100 | 2.61 | 0.02 | 0.34 | −0.63 | −1.11 | −0.002 |
| Parameters | Water Regimes |
|---|---|
| Leaf color (0–10 scale) | 65.1 * |
| Leaf area (cm2) | 4.11 * |
| Plant height (cm) | 2.66 * |
| TNC (mg g−1 dry wt.) | 711.0 ** |
| RSC (mg g−1 dry wt.) | 92.0 ** |
| Proline content (µg g−1 fresh wt.) | 1337 ** |
| Total ET (mm d−1) | 5.1 ** |
| Parameter | Water Regimes (% of Total ET) | Regression | R2 | |||
|---|---|---|---|---|---|---|
| C | 75 | 50 | 25 | |||
| Leaf color (0–10 scale) | 9.4 a | 9.0 a | 7.5 b | 3.8 c | Y = 7.4 − 0.6 X | 0.84 ** |
| Leaf area (cm2) | 21.6 a | 20.5 a | 15.6 b | 8.5 c | Y = 110.8 − 2.5 X | 0.88 ** |
| Plant height (cm) | 22.0 a | 20.5 a | 15.6 b | 10.5 c | Y = 102.2 − 2.1 X | 0.90 ** |
| ET rate (mmd−1) | 4.4 a | 4.0 a | 2.7 b | 2.2 c | Y = 9.7 − 0.6 X | 0.75 * |
| TNC (mg g−1 dry wt.) | 112.8 a | 105.6 a | 69.5 b | 55.9 c | Y = 102.7 − 1.6 X | 0.84 ** |
| RSC (mg g−1 dry wt.) | 15.5 d | 16.8 a | 22.8 b | 30.2 c | Y = 9.8 + 0.23 X | 0.86 ** |
| Proline content (µg g−1 fresh wt.) | 223.2 d | 240.0 a | 849.0 b | 1155.0 c | Y = 129.6 + 12.5 X | 0.91 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubaira, M.; Shahba, M.; Gamal, G. Future Implications of Climate Change on Arum palaestinum Boiss: Drought Tolerance, Growth and Production. Atmosphere 2023, 14, 1361. https://doi.org/10.3390/atmos14091361
Abubaira M, Shahba M, Gamal G. Future Implications of Climate Change on Arum palaestinum Boiss: Drought Tolerance, Growth and Production. Atmosphere. 2023; 14(9):1361. https://doi.org/10.3390/atmos14091361
Chicago/Turabian StyleAbubaira, Mabruka, Mohamed Shahba, and Gamil Gamal. 2023. "Future Implications of Climate Change on Arum palaestinum Boiss: Drought Tolerance, Growth and Production" Atmosphere 14, no. 9: 1361. https://doi.org/10.3390/atmos14091361
APA StyleAbubaira, M., Shahba, M., & Gamal, G. (2023). Future Implications of Climate Change on Arum palaestinum Boiss: Drought Tolerance, Growth and Production. Atmosphere, 14(9), 1361. https://doi.org/10.3390/atmos14091361







