Abstract
Organic aerosols are harmful to the environment because of their impact on air quality and visibility. They have serious effects not only on living beings and ecosystems because of their biological toxicity, but they also have an indirect effect on regional climate change as cloud condensation nuclei and radiation force. Many measures have been applied to decrease air pollution. Although the air quality has greatly improved, the standard of the World Health Organization (WHO) is far from being met at present. In this study, fine particulates were collected in Nanjing throughout 2019, and high-performance liquid chromatography–electrospray ion–mass spectrometry/mass spectrometry (HPLC-ESI-MS/MS) was carried out to determine 14 organic acids, 10 nitrated phenols, 1 aldehyde, and 1 ketone in aerosol samples. In this study, we further determined the changes in the pollutants in Nanjing in recent years compared to previous studies and characterized more kinds of species in the air. We found that different kinds of nitrated phenols showed similar trends of being abundant in winter and substituted in spring, autumn, and summer. 4-Nitrophenol was the most abundant species (2.83 ng m−3) among the nitrated phenols. p-Coumaric acid presented the highest level in summer with an average concentration of 1.55 ng m−3, indicating that grass burning was significant in summer, possibly due to wheat stalk and perennial ryegrass burning. The positive matrix fraction (PMF) model was applied to identify the sources of aerosols in Nanjing, including coal burning, grass burning, softwood burning, hardwood burning, anthropogenic secondary organic aerosols (SOAs), and biogenic SOAs. Coal burning and softwood burning contributed much more to the total determined species with values of 20.3% and 18.2%, respectively. Anthropogenic SOAs contributed 17.1%, and hardwood burning contributed 16.7%. The contribution of biogenic SOAs was 15%, and the grass-burning source contribution was the lowest, with 12.6%. With consideration of the large contribution from anthropogenic combustion activities, more strict measures are required to reduce emission pollutants in the future.
1. Introduction
Organic aerosols are harmful to the environment because of their impact on air quality and visibility. They have serious effects not only on living humans and ecosystems because of their biological toxicity, but they also have an indirect effect on regional climate change as cloud condensation nuclei and radiation force [1,2,3,4]. As is well known, with rapid economic growth, human and industrial activities will result in a large amount of aerosol particles, causing poor air quality and visibility. Many sources of organic aerosols can lead to air pollution, such as vehicle exhaust, industrial emissions, biogenic emissions, biomass burning, plant derails, soil dust, solvent production, and coal burning. Phthalic acid can be used as a tracer of SOAs [5] and a tracer of human-made secondary organic compounds, which may come from the oxidation reaction of naphthalene and some polycyclic aromatic hydrocarbons [6,7]. Terephthalic acid may come from primary emissions, such as vehicle exhaust and biomass combustion, and it was much higher than that in Athens (1.40 ng m−3) [8]. Previous studies have reported that isophthalic acid comes from material combustion (such as roadside waste incineration) and other primary sources [9]. Different sources mainly release various organic compounds, including nitrated phenols, organic acids, aldehydes, ketones, and so on. Sources’ contributions to species vary in different seasons. However, the understanding of aerosols at the molecular level is still very insufficient, and their composition characteristics, sources, and contributions are necessary to be studied.
Nitrated phenols, which are compounds comprising hydroxyl and nitro-functionalized benzene rings, have been investigated in the past decades because they are harmful to human and plant health, leading to genotoxicity and forest decline, respectively [10,11,12]. Nitrated phenols, such as methyl-nitrophenols, nitrophenol, methyl-nitrocatechols, and nitro-catechol, can cause air pollution all over the world [13,14,15]. Large amounts of nitrated phenols originate from the primary emissions of anthropogenic activities in the air. Previous studies have proven that nitrated phenols are prevalent in biomass-burning plumes [16,17,18]. Nitrated phenols can enter the environment via a number of sources. For example, they are used as raw materials in industries for producing phenol-formaldehyde resins, dyes, explosives, and disinfectants [19,20]. Nitrated phenols can also be formed in the combustion process of motor vehicles [20,21]. Coal and wood burning also releases these compounds into the air [20,21]. Another way nitrated phenols may enter the air is by the use of pesticides and herbicides in farmland, but this contributes small amounts of 4-nitrophenol to the atmosphere [20,21]. Other combustion activities, such as coal, gasoline, and diesel burning are possible sources of nitrated phenols [21,22,23]. Concentrations of nitrated phenols in the ambient atmosphere are not only the result of primary emissions of anthropogenic activities, but they are also secondary formation products from phenolic precursors [24]. Therefore, to gain a comprehensive understanding of nitrated phenols in the atmosphere, it is essential to determine the concentrations, chemical compositions, and origins.
Most studies on the components of organic acids are focused on distribution and seasonal variation [25]. Some organic acids can be not only directly discharged into the atmosphere via biomass burning and fossil fuel combustion but also generated in the atmosphere through the secondary photochemical oxidation of organic matter from human-made or natural sources. Benzoic acids, such as phthalic acid, terephthalic acid, isophthalic acid, and trimellitic acid, are mainly released from secondary sources. These are quite abundant in the atmosphere. Some isophthalic acid also comes from biomass combustion and other primary sources [9]. Terephthalic acid originates from primary emissions, such as vehicle exhaust and biomass combustion. Phthalic acid is used as a tracer of human-made secondary organic compounds formed by the oxidation reaction of naphthalene and some polycyclic aromatic hydrocarbons [6,7]. A previous study also showed that phthalic acid can be used as a tracer of SOAs [5]. Organic acids from biomass burning, especially for lignin combustion, were also investigated in this study. Lignin constitutes the main structure of vascular plants, making up about 18–25% of hardwood and 25–35% of softwood [26,27]. p-Coumaryl, coniferyl, and sinapyl alcohols polymerize to form lignin [28,29,30]. When lignin combusts under high temperatures, it will release a lot of organic acids, such as homovanillic acid, p-hydroxybenzoic acid, vanillic acid, and syingic acid [31,32]. Lignin combustion also discharges ketones and aldehydes, such as acetylsyringone, syringaldhyde, and vanillin. These species are specific tracers of biomass burning in the atmosphere. There have not been enough reports about these substances in China, especially regarding the application of source analysis.
In this study, we present the compositions, concentrations, and temporal variations of 10 nitrated phenols, 15 organic acids, 1 ketone, and 1 aldehyde in PM2.5 samples. Source apportionment was applied to better understand the sources of and contributions to the particulate matter.
2. Experiments
2.1. Sampling Site and Sample Collection
Ambient PM2.5 samples were collected on the roof of the library at the Nanjing University of Information Science and Technology (32°12′9″ N, 118°42′29″ E), 30 m above the ground. The sampling site is influenced by vehicle emissions, light chemistry industries, and residential emissions because of 10 industrial factories within 10 km and a road within 1.2 km. Aerosol samples were collected by a PM2.5 high-volume aerosol sampler (TISCH Environmental, Cleves, OH, USA) with a flow rate of 1.13 m3·min−1. The sample quartz filters had a total area of 414 cm2, and they were collected for 24 h (10:10–10:00). If it strongly rained or snowed, sampling was paused. The aerosol samples were collected from March to December 2019, while samples in May were lacking due to frequent precipitation and failure of the sampler. There are 112 aerosol samples collected. Before sampling, the filters were baked in a muffle furnace at 400 ℃ for about 4 h, then placed in a thermostatic chamber for 24 h, and weighed using an electronic balance (QUINTIX35-1CN, Sartorius, Göttingen, Germany). After sampling, they were weighed again and then stored at −20 °C in a refrigerator.
2.2. Sample Preparation and Chemical Analyses
A filter (20 cm2) was cut into small pieces, and they were put into 20 mL small glass beakers. Then, 350 μL of four internal standards, including 4-nitrophenol-2,3,5,6-d4, trans-cinnamic-d7, phthalic-3,4,5,6-d4 acid, and cis-cyclobutane-1,2-dicarboxylic acid, with concentrations of 1 μg·mL−1 were added to the filters. After 30 min, 10 mL of methanol was poured into glass beakers and the samples were extracted by sonication in an ice bath for 15 min. The above sonication procedure was repeated two times. The sample solution was filtered through a 0.22 μm PTFE filter and then dried under a gentle stream of high-purity nitrogen. The residue was re-dissolved in 500 μL of mixed solution (methanol/water = 1:1) and syringed into vials to analyze. The processing steps of the blank samples were the same. The details of the sample preparation procedures are presented in a previous study [33].
The nitrated phenols, organic acids, ketones, and aldehydes in the sample solutions were identified and quantified with high-performance liquid chromatography (HPLC, Thermo Ultimate 3000, Thermo Fisher, Waltham, MA, USA) coupled with a triple quadrupole mass spectrometer (QQQ, TSQ Quantum Access Max, Thermo Scientific, Waltham, MA, USA) equipped with an electrospray ionization (ESI) source. Chromatographic separation was achieved on an Atlantis T3 C18 column (2.1 mm × 150 mm, 2.1 μm particle size, Waters, Milford, MA, USA) at a flow rate of 0.3 mL min−1. The mobile phase contained (A) 0.1% formic acid in deionized water and (B) methanol. The applied gradient elution for this analysis started at 10% B and was maintained for 5 min, then increased to 90% B within 15 min and maintained for 7 min, and then returned to 10% B from 27 to 30 min and maintained until the next sample was analyzed.
2.3. Instrumental Conditions
Samples were separated and determined by HPLC-MS/MS under the following parameters: the spray voltage was −3800 V, the vapor temperature was 380 °C, the sheath gas was 35 psi, the aux gas was 10 psi, and the capillary temperature was 270 °C. All of the solvents used in the sample extraction processes and chemical analyses were HPLC-MS-grade solvents (Merck, Darmstadt, Germany). Water was freshly available with a Milli-Q 10 system (Millipore, MA, USA). The standard compounds were of high pure grade (>98%), and all of the elution solvents were of mass spectrum grade (>99.9%).
2.4. Source Analysis
The positive matrix factorization (PMF) model was deployed to identify the major sources and evaluate the contributions of the sources to the observed organic compounds. The PMF is an advanced source apportionment method that does not require prior information about the source profiles. The PMF algorithm decomposes the data matrix X of m rows and n columns (m and n are the numbers of samples and species, respectively) into a product of two matrices G (m × p) and F (p × n), plus a residual matric E [34]:
where Xij is the concentration of species j in sample I, p is the number of sources, gik is the contribution of source k to sample I, fkj is the concentration of species j in source k, and e is the residual.
In this work, the US EPA PMF 5.0 software package was used to conduct the analysis. All target compounds were input to the PMF model. The corresponding uncertainties were determined as: Mass concentrations × 0.05 + LOD. If the mass concentration was below LOD, ½ LOD was used as the uncertainty (such cases were, in fact, very rare). The PMF solutions were explored by varying the number of factors, 100 bootstrap replications, and an fpeak from −1 to 1, strictly following the user’s manual. Finally, five factors under an fpeak of −0.5 were chosen as the optimal solution.
3. Results and Discussion
3.1. Validation of HPLC-MS/MS
An internal method was used for quantification by comparing the target compound peak areas with the internal standards. To determine the standard curve, 2μg·L−1, 5 μg·L−1, 10 μg·L−1, 25 μg·L−1, 50 μg·L−1, 100 μg·L−1, 250 μg·L−1, 500 μg·L−1, 800 μg·L−1, 1000 μg·L−1, and 5000 μg·L−1 compounds were prepared with the addition of 700 μg·L−1 internal standards. The linear equations, coefficients of determination (R2), and linear ranges were obtained from the analytical curves of the target compounds, as summarized in Table S1. The R2 values ranged from 0.9950 to 0.9999. The limit of detection (LOD) and limit of quantification (LOQ) were calculated as the lowest concentration to produce a ratio of signal to noise between 3 and 10. The LODs and LOQs of the target compounds are presented in Table S1. The LODs of these carbohydrates ranged from 3 ng L−1 to 4.7 μg L−1.
The precision expressed by the relative standard deviation (RSD) was calculated by quantifying five replicates of the aerosol samples. Mixed standard carbohydrates with different concentration levels (10, 100, and 500 μg L−1) were added to aerosol blank matrix samples to calculate the recovery. Blank filters, which replaced the blank matrix samples because of a lack of aerosol blank matrix samples, were put on the higher-volume sampler beforehand. The recovery rate was determined by comparing the concentration of the extracted sample (the amount added before extraction) with the amount added after extraction. The recoveries (Table S2) were between 87% and 116%, with relative standard deviations (RSD) smaller than 20% for all target compounds. In addition, there were very small amounts of 2-methyl-5-nitrophenol, 4-nitrophenol, 3-hydroxybenzioc acid, 3-methyl-salalyacid, isophthalic acid, phthalic acid, and 4-nitrocatecol in the blank samples, with concentrations of 0.47 μg L−1, 0.11 μg L−1, 0.44 μg L−1, 0.78 μg L−1, 0.26 μg L−1, 0.76 μg L−1, and 0.23 μg L−1, respectively. The blank signals could be ignored because they were very low when compared to the samples. Overall, the above results indicate that the HPLCMS/MS method established in this study was effective for the quantitative analysis of the aerosol samples.
3.2. Concentrations and Compositions
3.2.1. Nitrated Phenols
Table S3 and Figure S1 present the daily and seasonal average concentrations of the total nitrated phenols in the PM2.5 samples. The average total concentrations of nitrated phenols in winter were about 5.2 times those in summer. Most nitrated phenols were present in the highest concentrations in winter and the lowest in summer. The distinct increases in the concentrations of nitrated phenols in winter and spring could be attributable to the intensified biomass burning and coal burning for heating during this season [24], which is a similar variation to that found in Shandong [35]. The relative proportions of nitrated phenols to the total nitrated phenols also changed greatly in different sample types and seasons. Figure 1 shows that 3,4-nitrophenol and 2-nitrophenol were the predominant species among the 10 detectable nitrated phenols in PM2.5 during the four seasons, accounting for 26–31% and 19–29%, respectively. Methyl-nitrophenols (i.e., 4M2NP, 2M4NP, 3M4NP, and 4M3NP) comprised 6–26% and were also the most prevalent of the 10 nitrated phenols, followed by 4-nitrocatechol (3–6%), 2,6-dimethyl-4-nitrophenol (1–2%), and 4-nitroguiacal (0–1%). Previous studies have reported that 4-nitrophenol was the richest species among the nitrated phenols in urban Beijing and Jinan [35,36], which is similar to our study.
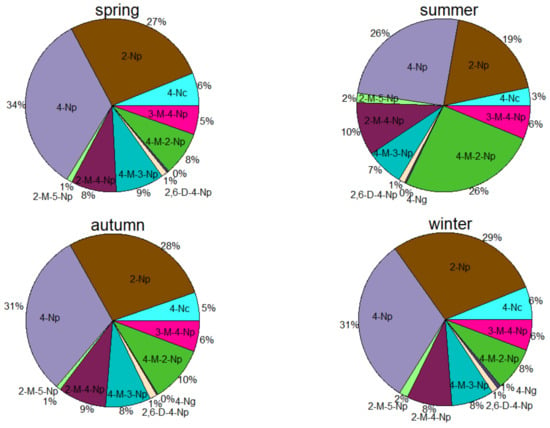
Figure 1.
Contributions of nitrophenols in four seasons. Notes: 2-Np: 2-nitrophenol, 4-Np: 4-nitrophenol, 4-Nc: 4-nitrocatechol, 3-M-4-Np: 3-methyl-4-nitrophenol, 4-M-2-Np: 4-methyl-2-nitrophenol, 4-M-3-Np: 4-methyl-3-nitrophenol, 2-M-4-Np: 2-methyl-4-nitrophenol, 2-M-5-Np: 2-methyl-5-nitrophenol.
It was noted that the ratios of 2-nitrophenol to 4-nitrophenol in the atmosphere were lower than 1 and ranged from 0.35 to 2.47 throughout the whole year of 2019, which is consistent with the study by Harrison et al., 2005. The ratios higher than 1 occurred on only five days: 25 and 31 March, 22 August, 23 September, and 10 December, and there was light pollution on 10 December. The much lower ratios of these two isomers were 0.48, 0.35, 0.67, 0.41, 0.58, 0.67, 0.56, and 0.41 on rainy days, which can be explained by a previous study that proved that 4-nitrophenol was prevalent in rainfall [20]. The concentration of 4-nitrophenol was correlated well with 2-nitrophenol (r = 0.97) (Figure S2), proving that the two isomers originated from the same sources. The dominance of 4-nitrophenol and 2-nitrophenol might arise from secondary formations of precursors and direct primary emissions, including from motor vehicles [35]. Good correlations between 4-nitrophenol (or 2-nitrophenol) and the temperature and NO2 were obtained in this study (with r > 0.62, r > 0.85), as shown in Figure S2. Previous studies have shown that nitrophenols and their derivatives can originate from the oxidation reactions of phenol and cresols in the presence of NO2 [37,38]. 4-Nitrophenol correlated well with tracers of biomass burning and vanillin, showing that 4-nitrophenol might come from biomass burning. A previous study proved that nitrophenols and their derivatives can be formed by the photo-oxidation of cresols that are emitted from biomass burning [18]. In addition, the total nitrated phenols correlated well with the temperature (r = 0.85) and NO2 (r = 0.65) (Figure S2), demonstrating that the formation of nitrated phenols was strongly related to the ambient temperature and the concentration of NO2. In addition, 4-nitrocatechol correlated well with 4-nitroguaiacol (r = 0.81), and 4-nitrocatechol presented a good correlation with phthalic acid (r = 0.65) (Figure S2), which could indicate that they originated from the same source, which is the same as the result of the PMF part in this article.
In the PM2.5 samples collected in Nanjing, 4-nitrophenol (2.83 ng m−3) and 2-nitrophenol (2.4 ng m−3) demonstrated comparable levels during the year 2019. 4-Nitrophenol showed the highest concentration among the nitrate phenols, which is consistent with the results reported in Shandong [21]. 2-Nitrophenol presented different seasonal variations of 4-nitrophenol, which showed the highest levels in spring (4.07 ng m−3), a relatively abundant concentration in autumn (3.25 ng m−3), and scarce levels in winter (1.06 ng m−3) and summer (0.66 ng m−3). The average concentrations of 4-nitrocatechol in the four seasons are presented in the tables. Its seasonal variation characteristics showed similar temporal changes to those in Flanders, Belgium in 2011 [39]. Compared to other cities, the concentrations of nitrated phenols in PM2.5 in Nanjing in 2019 were much lower than the observed concentrations in Jinan in summer and winter in 2014 (9.8 ng m−3, 48.4 ng m−3), respectively [34], but substantially higher than the observed concentrations in Hong Kong during 2009 to 2012 (1.99 ng m−3 and 11.63 ng m−3) [40]. Moreover, the summertime concentrations of nitrated phenols in fine particulate in Nanjing in this study were significantly lower than those observed in the rural sites of Yucheng (5.7 ng m−3) and in the urban site of Beijing (6.62 ng m−3), and higher than that in Xianghe (1.45 ng m−3) on the North China Plain in the past several years [34,36,41]. The total concentrations of fine particulate nitrate phenolic compounds in Nanjing are comparable to those in Strasbourg, France (16.4 ng m−3) [42].
3.2.2. Organic Acids
The concentrations of the organic acids were much higher than those of the nitrated phenols. The concentrations of the organic acids were 74 ng m−3, which was approximately 5 times that of the nitrated phenols during the studied year. The total average concentration of benzoic acids in PM2.5 showed obvious distinctions in the four seasons, and they were the highest in spring (68.59 ng m−3), followed by summer (38.51 ng m−3), autumn (33.26 ng m−3), and winter (29.30 ng m−3). The average concentration of the total benzoic acids was 42.1 ng m−3, which was 50% of the concentration of the organic acids. Compared to other locations, the concentrations of four benzoic acids in Nanjing (42.16 ng m−3) in 2019 were substantially lower than the observed concentrations in Guangzhou in 2006–2007 (187 ng m−3) and the concentrations in Hong Kong from 2006 to 2007 (143.5 ng m−3) [43]. Ten organic acids mainly from biomass burning were detected in the Nanjing PM2.5 samples. The average concentration of these total organic acids was 6.18 ng m−3 during the studied period, and they were the most abundant in winter, with 6.69 ng m−3, demonstrating that biomass burning contributed more in winter time, a conclusion that is significantly similar to that in our previous study in 2016 [33]. Figure 2 shows the relative fractions of the individual acids to the total organic acids amounts. Four benzoic acids were the most abundant organic compounds in PM2.5, accounting for 26–34% of the measured organic compounds during the year. PM2.5 was also abundant in terephthalic acid, with a proportion of 14–22%. Each of the three phthalic acids comprised ~10% of the detected benzoic acids, with the total contribution being up to ~30%. As shown in Figure 2, vanillic acid, cis-pinonic acid, and p-coumaric acid were the predominant species among the 10 detectable acids from biomass burning in PM2.5 during the year, accounting for 3%, 17%, and 23%, respectively. This can be explained by a recent study reporting that vanillic acid and p-coumaric acid were the most abundant biomass-burning tracers in the atmosphere [30]. It was noted that the proportions of p-coumaric acid in PM2.5 in summer in Nanjing increased from 3% to 34%, while the proportions of vanillin and vanillic acid decreased in summer (vanillin vs. vanillic acid, 2% vs. 1%), which indicates that there might be intense grass burning during the hottest season.
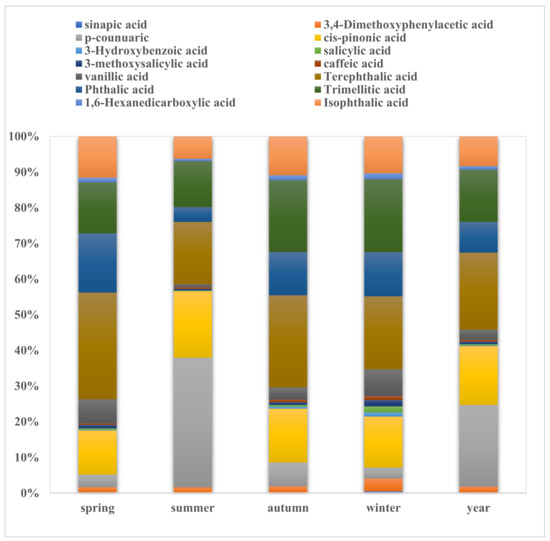
Figure 2.
Contributions of individual compounds in four seasons.
In this study, four benzoic acids showed above-average seasonal variation, but phthalic acid did not. Phthalic acid demonstrated the most abundant levels in spring (15.46 ng m−3) and scarce levels in summer (3.84 ng m−3), presenting comparable concentrations in autumn (5.74 ng m−3) and winter (5.57 ng m−3). The phthalic acid was much higher than that in Station Nord (2.22 ng m−3) and Zeppelin Mountain (2.22 ng m−3) in Arctic aerosols [44]. It was also much lower than that in PRD (81 ng m−3) and that observed in Tokyo (29 ng m−3) in summer [43,45]. The annual concentration of isophthalic acid was 6.5 ng m−3, which was much higher than that in Greece (1.81 ng m−3) [8], with an elevated concentration in spring (10.75 ng m−3), followed by summer (5.8 ng m−3), autumn (5.15 ng m−3), and winter (4.64 ng m−3). Finally, the annual concentration of trimellitic acid was 11.37 ng m−3, which was much higher than that in Greece (2.32 ng m−3) [8]. Its concentrations were comparable in spring and summer (13.29 ng m−3 vs. 11.98 ng m−3) and in autumn and winter (9.51 ng m−3 vs. 9.17 ng m−3). The correlations among phthalic acid, isophthalic acid, and terephthalic acid were good (r > 0.81) (Figure S2), showing the same sources. The concentration of trimellitic acid showed a poor correlation with them, indicating that the source of trimellitic acid was different from the other benzoic acids.
We discovered that the levels of most organic acids and their seasonal variation were similar, except for vanillic acid (Figure S1). Vanillic acid is derived from the precursor of coniferyl alcohol, which was the most abundant species among the organic acids from biomass burning, with a value of 0.98 ng m−3, contributing 3% of the total organic acids during the study period. The concentration of vanillic acid was the most abundant in winter (1.48 ng m−3), indicating that softwood burning mainly occurred in winter, which is consistent with the results of the concentration of vanillic acid in winter (10.5 ng m−3) in Lumbini due to harvest activities. The concentration of p-coumaric acid in summer was much higher than that in 2016 in Nanjing. This is because perennial ryegrass was introduced in Nanjing in recent years to be mixed with other kinds of cultivated grasses to keep them alive for a long time, but perennial ryegrass fades in summer. Therefore, perennial ryegrass burning was the main source of p-coumaric acid, causing the highest levels of this compound in the atmosphere in summer. p-Coumaric acid is a derivative of p-coumaryl alcohol, which is a unique unit of grass lignin and not prevalent in the lignins of hardwood or softwood [30]. The annual concentration of p-coumaric acid in this study was 1.14 ng m−3, which is much higher than that in the Arctic (2.0 pg m−3) [30]. The concentration of p-coumaric acid was highest in summer (1.55 ng m−3), followed by autumn (0.32 ng m−3) and spring (0.20 ng m−3), and the lowest in winter (0.07 ng m−3), demonstrating that grass burning activities mainly occurred in summer because of early wheat burning. Sinapic acid is mainly derived from the precursor sinapyl alcohol, which destroys hardwood lignin. The average concentration of sinapic acid was the highest in winter, with a value of 0.24 ng m−3, which is much lower than that in Dertenhausen, southern Germany in winter (48.83 ng m−3, 5.87 ng m−3), which is covered with a large amount of forest, and the surrounding residents cut down wood and burn it for cooking [46]. Sinapic acid was relatively high in autumn (0.25 ng m−3) and spring (0.15 ng m−3), but it was low in summer (0.02 ng m−3). These results can be used to tentatively deduce that hardwood burning contributed much higher levels in spring and autumn.
1,6-Hexanedicarboxylic acid and cis-pinonic acid were also determined in this study. The concentrations of these two species in different seasons are presented in Tables S3 and S5 (bold-sized parts). The average concentration of 1,6-hexanedicarboxylic acid was 0.77 ng m−3 during the year, which is higher than that in Station Nord (0.21 ng m−3) and Zeppelin Mountain (0.37 ng m−3) [44]. It was the most abundant in spring (1.25 ng m−3), followed by winter (0.76 ng m−3), autumn (0.66 ng m−3), and summer (0.63 ng m−3). The annual concentration of 1,6-hexanedicarboxylic acid correlated well with terephthalic acid (r = 0.77), phthalic acid (r = 0.86), and isophthalic acid (r = 0.83) (Figure S2), indicating that 1,6-hexanedicarboxylic acid entered the atmosphere via the same sources as three kinds of benzoic acids, including from motor vehicle emissions, oxidation reactions of naphthalene and some polycyclic aromatic hydrocarbons, and biomass burning in Nanjing, which is very consistent with a previous study. Kawamura proved that motor vehicle emissions were the main primary source of di-carboxylic acid, photo-oxidation reactions of aromatic hydrocarbon (toluene) were the main secondary source of di-carboxylic acid, and biomass burning was also one of the sources of di-carboxylic acid in the atmosphere [47,48].
Cis-pinonic acid, a secondary organic compound formed from α-pinene released from plants, is always regarded as an indicator of biogenic SOA. The average concentration of cis-pinonic acid was 8.07 ng m−3 during the year in Nanjing, which is much higher than that in Arctic areas, such as Station Nord (0.06 ng m−3) and Zeppelin Mountain (0.26 ng m−3) [44]. The elevated concentration of cis-pinonic acid in summer was due to abundant sunlight and increased amounts of precursors (α-pinene) released from different plants, initiating a series of photochemical reactions.
3.2.3. Ketone and Aldehyde
The average concentrations of acetosyringone and vanillin were 0.16 ng m−3 and 1.52 ng m−3 during the year. Acetosyringone and vanillin are tracers of hardwood burning and softwood burning. The average concentration of vanillin was the most abundant in winter, with a value of 1.58 ng m−3, which further demonstrates that softwood burning contributed more than in any other season. The average concentration of acetosyringone was also most abundant in winter, with a value of 0.24 ng m−3. The fractions of the two species in different seasons are displayed in Figure 3. Vanillin showed comparable contributions in the four seasons (~25%), and acetosyringone showed similar contributions in spring and summer (~24% and ~26%), a high proportion in winter (36%), and the lowest contribution in autumn (14%). The total organic acids contributed similarly in spring and summer (34%, 33%) and similarly in autumn and winter (18%, 15%). The total nitrated phenols contributed lower in summer (7%), higher in winter (36%), and similarly in spring and autumn (29%, 28%).
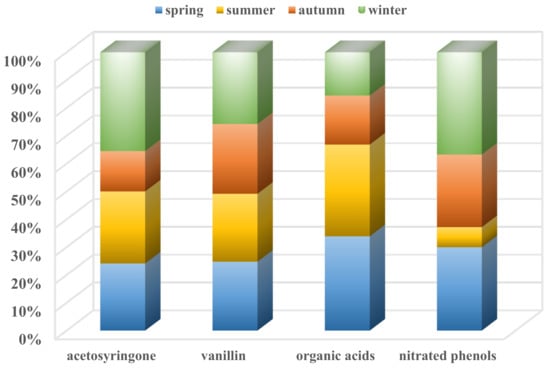
Figure 3.
Fractions of four kinds of species in four seasons.
3.3. Source Apportionment
3.3.1. Source Identification
To elucidate the primary origins of nitrated phenols, organic acids, aldehydes, and ketones in Nanjing, the PMF receptor model was applied to the input concentrations of these compositions in PM2.5. Six source contributors were identified based on the PMF outputs (Figure 4).
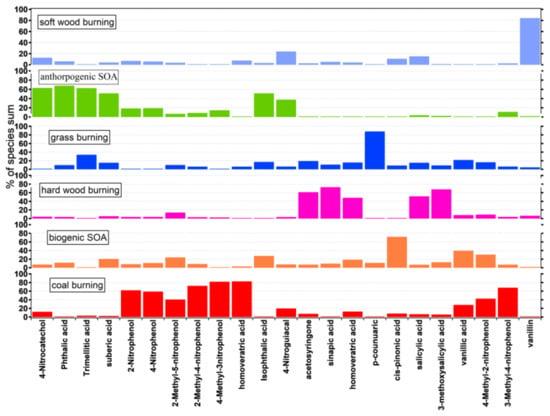
Figure 4.
Source profiles of six factors applied to PMF model.
Factor 1 was defined as “coal burning” and contained significant amounts of 2,6-dimethyl-4-nitrophenol (82.8%), 4-methyl-3-nitrophenol (81.9%), and 2-methyl-4-nitrophenol (72.5%), with relatively high 2-nitrophenol, 4-nitrophenol, and 3-methyl-4-nitrophenol. The significant correlations among the 2, 6-dimethyl-4-nitrophenol, 4-methyl-3-nitrophenol, and 2-methyl-4-nitrophenol suggest the same source. Six species (nitrophenols and methyl-nitrophenols) were identified as tracers of coal burning from nearby thermal power plants, industries, and residential cooking and heating use [49]. In particular, the combustion of anthracite has been shown to emit a large amount of particulate 4-nitrophenol. Coal burning was always used in Nanjing in winter during the study period (43%) (Figure 5).
Factor 2 was characterized by the overwhelming dominance of p-coumaric acid (88.1% of the total) and minor contributions from vanillin and sinapic acid. p-Coumaric acid is not prevalent in softwood and hardwood but is a unique tracer of grass lignin [30], and grass burning can release a lesser vanillin and sinapic acid [28]. This factor was thus defined as “grass burning”. Figure 5 shows that it contributed the most in summer with a percentage of 78% because perennial ryegrass burning was the dominant biomass burning in summer in Nanjing during the study period. This is similar to the results in Willamette, Oregon, in the northwestern United States, where nearly one million tons of straw are produced each year from wheatgrass and grain. The main source of straw is single-year plants of perennial ryegrass. Most of this straw is burned in the fields at the end of summer and scattered in the air, causing air pollution. The grass-burning contribution results are comparable with our previous study [33].
Factor 3 featured high contributions of cis-pinonic acid (71.7%), which is a significant marker of biogenic SOAs. Cis-pinonic acid is an oxidation product of precursor compounds released by plant reactions via a series of photochemical reactions in the atmosphere. Mass loadings of this factor were also high during summer, matching the abundant precursor released by abundant plants and enough sunlight, easily causing photochemical reactions during the daytime. Indeed, this factor’s contribution dominated in summer (31.6%), and in winter, its contribution was minor (15.3%) (Figure 5).
Factor 4 was identified as originating from mixed SOAs, as the predominant species were phthalic acid (68%), trimellitic acid (62.1%), and 4-nitrocatechol (63.3%), with relatively high concentrations of 1,6-hexanedicarboxylic acid and 4-nitroguiacol. Phthalic acid and trimellitic acid can be indicators of anthropogenic sources of secondary organic aerosol [50]. For example, vehicle exhaust can also emit polycyclic aromatic hydrocarbons, which can produce phthalic acid through a series of oxidation reactions in the atmosphere. 4-Nitrocatechol is produced by the primary emissions from biomass burning reacting with a series of chemicals in the atmosphere; here, we regarded it as a tracer of biomass-burning SOAs [18]. In addition, good correlations among 4-nitrophenol, phthalic acid, and isophthalic acid further prove that biomass burning can contribute to them. Therefore, mixed SOAs included anthropogenic SOAs and biomass-burning SOAs. Figure 5 demonstrates that mixed SOAs contributed much more in spring, with 49.9%, which could be attributed to a large amount of vehicle exhaust emissions and biomass burning emissions.
Factor 5 was ascribed to “softwood burning” because of the high concentration of vanillin (82%), and the fact that vanillin can be used as a tracer for softwood lignin burning [28,33]. Note that the appearance of some 4-nitroguaicol (23.4%) and salicylic acid (14.9%) might also be attributed to softwood lignin. For example, pinewood burning emits mainly vanillin and lesser amounts of other products. Softwood burning contributed much in spring (46.8%) and winter (34%).
Factor 6 was considered to be “hardwood burning” due to high concentrations of acetosyringone (61.4%), sinapic acid (73%), salicylic acid (59.1%), 3-methoxysalicylic acid (67.9%), and 3,4-dimethoxyphenylacetic acid (56.8%) and given that hardwood lignin burning can release amounts of acetosyringone, sinapic acid, salicylic acid, and 3-methoxysalicylic acid, with a lot of 3,4-dimethoxyphenylacetic acid [28,51]. Hardwood burning contributed obviously in spring (34.6%) and winter (38%), which was similar to softwood burning.
3.3.2. Source Contributions in Different Seasons
Figure 5 shows the average annual and seasonal contributions to the total measured species. On an annual basis, the contributions from these six sources were different (mixed SOA: 31.42%, grass burning: 23.84%, softwood burning: 22.5%, coal burning: 13.65%, hardwood burning: 5.11%, biogenic SOA: 3.48%), while their contributions differed significantly in the four seasons. Coal burning seemed to be the most abundant source among the six sources in autumn (31.4%) and winter (31.0%), but it was the lowest contributor in spring (15.8%) and summer (3.8%), indicating that coal was always used by residents heating and cooking in the cold seasons. Grass burning contributed mostly in summer (42.9%), followed by winter (4.3%), autumn (2.2%), and spring (0.9%). Hardwood burning and softwood burning were obviously different in spring (17.5% vs. 27.9%), summer (17.8% vs. 4%), and autumn (9.6% vs. 17.5%), but not in winter (22.1% vs. 23.4%), indicating that there were obvious distinctions between softwood burning and hardwood burning in the four seasons except for in winter, when softwood burning contributed comparably more. Mixed SOAs were the most abundant source in spring (26.6%), but the lowest contributor in summer (6.3%).
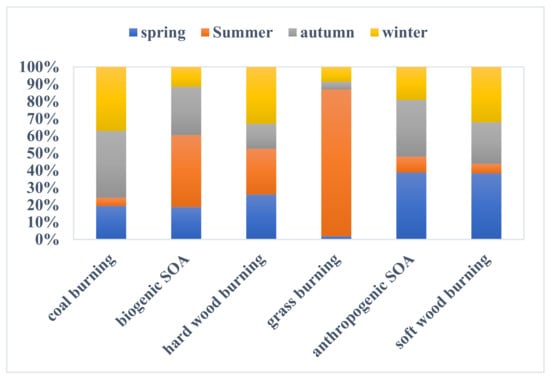

Figure 5.
Source contributions in four seasons.
3.3.3. Source Contributions to Different Organic Components
In Figure 5 and Figure 6, we can see the contributions of each pollutant source to different species and the contributions of pollutant sources in different seasons. It can be seen in Figure 6 that coal burning, anthropogenic SOAs, and biogenic SOAs contributed very much to nitrophenol compounds, with 60%, 16%, and 11%, respectively. Grass burning, anthropogenic SOAs, and biogenic SOAs contributed more to organic acids (39%, 27%, and 21%, respectively). Hardwood burning and grass burning contributed very much to ketones, with 61% and 19%, respectively. The contributions of biogenic SOAs, coal burning, and grass burning to aldehydes were very large, with 40%, 28%, and 22% respectively. The major contributions of coal burning in autumn and winter were 39% and 37%, respectively. Biological sources were the main contributors in summer and autumn, accounting for 42% and 27% respectively. The contributions of anthropogenic emissions in spring and autumn were 39% and 32%, respectively. The burning contribution of grass was highest in summer, accounting for 85%, while the contributions of hardwood and softwood were the highest in winter. The government can control the activities of coal burning and anthropogenic and biological combustion through macro-control policies to reduce their impact on human beings. Three suggestions are provided as follows. Firstly, softwood, hardwood, and grass should be processed via new methods, such as selecting them as original sources to produce paper without burning. Attention should especially be paid to the treatment of straw and withered grass in summer. Secondly, the emissions of coal burning by residents should be controlled, especially in autumn and winter. People are encouraged to choose environmental or reclaimed coal for heating and cooking or change their methods indoors, such as by using electricity. If possible, renewable energy resources can replace a part of fossil combustion to produce electricity. Finally, artificial sources should be paid more attention, especially in summer, because of the abundant mixed SOAs that occur. For example, restrict vehicular access and encourage people to ride electro mobiles or bicycles, take the common bus or metro, or walk.
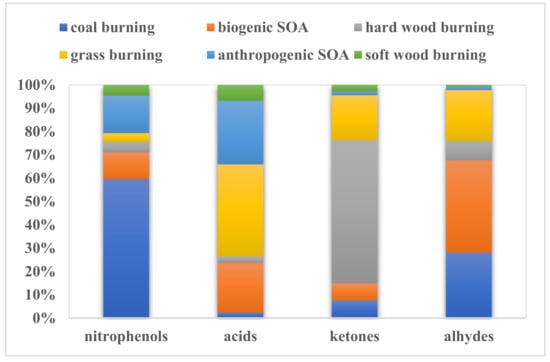
Figure 6.
Source contributions to different organic components.
4. Conclusions
This work presents the measurement results of 4 aromatic acids, 10 nitrated phenols, and 12 biomass-burning tracers in PM2.5 samples collected in Nanjing in 2019. The annual mean concentration of the total aromatic acids, and nitrated phenols, biomass burning tracers were 42.16 ng m−3, 12.06 ng m−3, and 9.44 ng m−3, respectively. The PMF results indicate that coal burning was the dominant contributor to 2-nitrophenol, 4-nirophenol, 2-methyl-4-nirophenol, 4-methyl-3-nitrophenol, 2,6-dimethyl-4-nirophenol, 4-methyl-4-nitrophenol, and 3-methyl-4-nitrophenol, whereas mixed SOAs contributed the most 4-nitrocatechol, phthalic acid, trimellitic acid, 1,6-hexanedicarboxylic acid, isophalic acid, and 4-nitroguiacal. Cis-pinonic acid was mainly derived from biogenic SOAs, p-coumaric acid was derived from grass lignin burning, and vanillic acid was dominantly from softwood burning. Hardwood burning was the main source of 3,5-dimethoxy-4-hydroxyl-acetophenone, sinapic acid, 3,4-dimethyodyphenylacid. Furthermore, coal burning contributed significant amounts of the total determined compounds in winter and autumn, indicating that residential heating and increasing industry operation were the main sources of nitrated phenols. Softwood and hardwood burning were key sources in spring and winter, which was different from coal burning. Mixed SOAs were identified as the dominant contributors in spring and autumn, suggesting that the primary emissions occurred from photochemical reactions under special meteorological conditions, strong light, and abundant precursors, producing phthalic acid and dicarboxylic acid. Grass burning contributed more in summer, and biogenic SOAs contributed relatively higher in winter than in other seasons. Overall, our findings here provide useful information to make proper suggestions to control air pollution in this region. We highlight that biomass can be selected as an original source or fuel to reuse, and traffic flow should be controlled to decrease the primary pollutant emissions and suppress the formation of secondary organic pollutants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/atmos14060971/s1, Table S1: Standard curve, range, r2, LOD and LOQ (limit of detection, limit of quantification) of this method, Table S2: Concentrations of blank samples, recovery and precision of this method, Table S3: Concentration of nitrophenols in four seasons (Unit: ng m−3), Table S4: Concentration of benzoic acids in four seasons (Unit: ng m−3), Table S5: Concentration of organic acids, ketones and aldhyde in four seasons (Unit: ng m−3), Figure S1: Temporal variation of individual species, Figure S2: Correlations of different compounds.
Author Contributions
Methodology, S.G.; software, S.G.; validation, A.W. and Z.X.; writing—original draft preparation W.L.; writing—review and editing, X.W.; project administration, M.C.; funding acquisition, M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant numbers 22176100, 21976094) and the National Key Research and Development Project 9 (grant number 2018YFC0213802).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is unavailable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Feng, Y.; Ramanathan, V.; Kotamarthi, V.R. Brown carbon: A significant atmospheric absorber of solar radiation. Atmos. Chem. Phys. 2013, 13, 8607–8621. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.H.; Surratt, J.D.; Zotter, P.; Prévôt, A.S.; Weber, R.J. Light-absorbing soluble organic aerosol in Los Angeles and Atlanta: A contrast in secondary organic aerosol. Geophys. Res. Lett. 2011, 38, 21. [Google Scholar] [CrossRef]
- Laskin, A.; Laskin, J.; Nizkorodov, S.A. Chemistry of Atmospheric Brown Carbon. Chem. Rev. 2015, 115, 4335. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Lee, A.K.; Huang, L.; Li, X.; Yang, F.; Abbatt, J.P. Photochemical processing of aqueous atmospheric brown carbon. Atmos. Chem. Phys. 2015, 15, 6087–6100. [Google Scholar] [CrossRef]
- Fine, P.M.; Chakrabarti, B.; Krudysz, M.; Schauer, J.J.; Sioutas, C. Diurnal Variations of Individual Organic Compound Constituents of Ultrafine and Accumulation Mode Particulate Matter in the Los Angeles Basin. Environ. Sci. Technol. 2004, 38, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Schauer, J.J.; Fraser, M.P.; Cass, G.R.; Simoneit, B. Source Reconciliation of Atmospheric Gas-Phase and Particle-Phase Pollutants during a Severe Photochemical Smog Episode. Environ. Sci. Technol. 2002, 36, 3806–3814. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Cass, G.R.; Schauer, J.J.; Edgerton, E.S. Source apportionment of PM2.5 in the Southeastern United States using solvent-extractable organic compounds as tracers. Environ. Sci. Technol. 2002, 36, 2361. [Google Scholar] [CrossRef]
- Kanellopoulos, P.G.; Chrysochou, E.; Koukoulakis, K.; Bakeas, E. Secondary organic aerosol markers and related polar organic compounds in summer aerosols from a sub-urban site in Athens: Size distributions, diurnal trends and source apportionment. Atmos. Pollut. Res. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Simoneit, B.; Medeiros, P.M.; Didyk, B.M. Combustion products of plastics as indicators for refuse burning in the atmosphere. Environ. Sci. Technol. 2005, 39, 6961–6970. [Google Scholar] [CrossRef]
- Blank, L.W. A new type of forest decline in Germany. Nature 1985, 314, 311–314. [Google Scholar] [CrossRef]
- Grosjean, D. Atmospheric fate of toxic aromatic compounds. Sci. Total Environ. 1991, 100, 367–414. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, L.; Han, S. The genotoxicity of substtitued nitrobenzenes and the quanttative structure activity relationship studies. Chemosphere 1995, 30, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Trautner, F.; Hutzinger, O.; Richartz, H.; Reischl, A. Nitrated phenols in fog. Atmos. Environ. Part A Gen. Top. 1990, 24, 3067–3071. [Google Scholar]
- Morville, S.; Scheyer, A.; Mirabel, P.; Millet, M. Spatial and Geographical Variations of Urban, Suburban and Rural Atmospheric Concentrations of Phenols and Nitrophenols (7 pp). Environ. Sci. Pollut. Res. 2006, 13, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Belloli, R.; Barletta, B.; Bolzacchini, E.; Meinardi, S.; Orlandi, M.; Rindone, B. Determination of toxic nitrophenols in the atmosphere by high-performance liquid chromatography. J. Chromatogr. A 1999, 846, 277–281. [Google Scholar] [CrossRef]
- Mohr, C.; Lopez-Hilfiker, F.D.; Zotter, P.; Prevot, A.S.; Xu, L.; Ng, N.L.; Herndon, S.C.; Williams, L.R.; Franklin, J.P.; Zahniser, M.S.; et al. Contribution of nitrated phenols to wood burning brown carbon light absorption in Detling, United Kingdom during winter time. Environ. Sci. Technol. 2013, 47, 6316–6324. [Google Scholar] [CrossRef]
- Sophie, T. One-year study of polycyclic aromatic compounds at an urban site in Grenoble (France): Seasonal variations, gas/particle partitioning and cancer risk estimation. Sci. Total Environ. 2016, 565, 1071–1083. [Google Scholar]
- Iinuma, Y.; Boge, O.; Graefe, R.; Herrmann, H. Methyl-Nitrocatechols: Atmospheric Tracer Compounds for Biomass Burning Secondary Organic Aerosols. Environ. Sci. Technol. 2010, 44, 8453. [Google Scholar] [CrossRef]
- Nojima, K.; Fukaya, K.; Fukui, S.; Kanno, S. Studies on photochemistry of aromatic hydrocarbons II: The formation of nitrophenols and nitrobenzene by the photochemical reaction of benzene in the presence of nitrogen monoxide. Chemosphere 1975, 4, 77–82. [Google Scholar] [CrossRef]
- Harrison, M.; Barra, S.; Borghesi, D.; Vione, D.; Arsene, C.; Olariu, R.I. Nitrated phenols in the atmosphere: A review. Atmos. Environ. 2005, 39, 231–248. [Google Scholar] [CrossRef]
- Desyaterik, Y.; Sun, Y.; Shen, X.; Lee, T.; Wang, X.; Wang, T.; Collett, J.L. Speciation of “brown” carbon in cloud water impacted by agricultural biomass burning in eastern China. J. Geophys. Res. Atmos. 2013, 118, 7389–7399. [Google Scholar] [CrossRef]
- Yan, L.; Bai, Y.; Zhao, R.; Fan, L.; Xie, K. Correlation between coal structure and release of the two organic compounds during pyrolysis. Fuel 2015, 145, 12–17. [Google Scholar] [CrossRef]
- Tremp, J.; Mattrel, P.; Fingler, S.; Giger, W. Phenols and nitrophenols as tropospheric pollutants: Emissions from automobile exhausts and phase transfer in the atmosphere. Water Air Soil Pollut. 1993, 68, 113–123. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Gu, R.; Wang, H.; Yao, L.; Wen, L.; Zhu, F.; Wang, W.; Xue, L.; Yang, L.; et al. Observations of fine particulate nitrated phenols in four sites in northern China: Concentrations, source apportionment, and secondary formation. Atmos. Chem. Phys. 2018, 18, 4349–4359. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, J.; Qin, W.; Cheng, S.; Zhang, Y.; Ahmad, M.; Ouyang, W. Characteristics and secondary formation of water-soluble organic acids in PM1, PM2.5 and PM10 in Beijing during haze episodes. Sci. Total Environ. 2019, 669, 175–184. [Google Scholar] [CrossRef]
- Hyder, M.; Genberg, J.; Sandahl, M.; Swietlicki, E.; Joensson, J.A. Yearly trend of dicarboxylic acids in organic aerosols from south of Sweden and source attribution. Atmos. Environ. 2012, 57, 197–204. [Google Scholar] [CrossRef]
- Impoinvil, D.; Tom, S.; William, S.W.; Ajit, R.; Padarath, B.R.; Geeta, S.; Cyril, C.; Matthew, B.; Cowling, B.J. The Spatial Heterogeneity between Japanese Encephalitis Incidence Distribution and Environmental Variables in Nepal. PLoS ONE 2011, 6, e022192. [Google Scholar] [CrossRef]
- Simoneit, B. Biomass burning-a review of organic tracers for smoke from incomplete combustion. Appl. Geochem. 2002, 17, 129–162. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef]
- Zangrando, R.; Barbaro, E.; Zennaro, P.; Rossi, S.; Kehrwald, N.M.; Gabrieli, J.; Barbante, C.; Gambaro, A. Molecular markers of biomass burning in arctic aerosols. Environ. Sci. Technol. 2013, 47, 8565–8574. [Google Scholar] [CrossRef]
- Oros, D.R.; Simoneit, B. Identification and emission factors of molecular tracers in organic aerosols from biomass burning Part 1 Temperate climate conifers. Appl. Geochem. 2001, 16, 1544. [Google Scholar] [CrossRef]
- Simoneit, B. A review of biomarker compounds as source indicators and tracers for air pollution. Environ. Sci. Pollut. Res. 1999, 6, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Qi, L.; Yu, W.; Nie, D.; Shi, S.; Gu, C.; Ge, X.; Chen, M. Molecular characterization of biomass burning tracer compounds in fine particles in Nanjing, China. Atmos. Environ. 2020, 240, 117837. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Yuan, B. Secondary formation of nitrated phenols: Insights from observations during the Uintah Basin Winter Ozone Study (UBWOS) 2014. Atmos. Chem. Physic 2016, 16, 2139–2153. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Wang, Y.; Zheng, J.; Yu, J.Z. The formation of nitro-aromatic compounds under high NO x and anthropogenic VOC conditions in urban Beijing, China. Atmos. Chem. Phys. 2019, 19, 7649–7665. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Lu, C.; Li, R.; Zhang, J.; Dong, S.; Yang, L.; Xue, L.; Chen, J.; Wang, W. Nitrated phenols and the phenolic precursors in the atmosphere in urban Jinan, China. Sci. Total Environ. 2020, 714, 136760. [Google Scholar] [CrossRef]
- Olariu, R.I.; Klotz, B.; Barnes, I.; Becker, K.H.; Mocanu, R. FT-IR study of the ring-retaining products from the reaction of OH radicals with phenol, o-, m-, and p-cresol. Atmos. Environ. 2002, 36, 3685–3697. [Google Scholar] [CrossRef]
- Kahnt, A.; Behrouzi, S.; Vermeylen, R.; Shalamzari, M.S.; Vercauteren, J.; Roekens, E.; Claeys, M.; Maenhaut, W. One-year study of nitro-organic compounds and their relation to wood burning in PM10 aerosol from a rural site in Belgium. Atmos. Environ. 2013, 81, 561–568. [Google Scholar] [CrossRef]
- Chow, K.S.; Huang, X.H.H.; Yu, J.Z. Quantification of nitroaromatic compounds in atmospheric fine particulate matter in Hong Kong over 3 years: Field measurement evidence for secondary formation derived from biomass burning emissions. Environ. Chem. 2016, 13, 665–673. [Google Scholar] [CrossRef]
- Teich, M.; van Pinxteren, D.; Wang, M.; Kecorius, S.; Wang, Z.; Müller, T.; Mocnik, G.; Herrmann, H. Contributions of nitrated aromatic compounds to the light absorption of water-soluble and particulate brown carbon in different atmospheric environments in Germany and China 2017. Atmos. Chem. Phys. 2017, 17, 1653–1672. [Google Scholar] [CrossRef]
- Delhomme, O.; Morville, S.; Millet, M. Seasonal and diurnal variations of atmospheric concentrations of phenols and nitrophenols measured in the Strasbourg area, France. Atmos. Pollut. Res. 2010, 1, 16–22. [Google Scholar] [CrossRef]
- Ho, K.F.; Ho, S.S.H.; Lee, S.C.; Kawamura, K.; Zou, S.C.; Cao, J.J.; Xu, H.M. Summer and winter variations of dicarboxylic acids, fatty acids and benzoic acid in PM2.5 in Pearl Delta River Region, China. Atmos. Chem. Phys. 2011, 11, 2197–2208. [Google Scholar] [CrossRef]
- Hansen, A.; Kristensen, K.; Nguyen, Q.T.; Zare, A.; Glasius, M. Organosulfates and organic acids in Arctic aerosols: Speciation, annual variation and concentration levels. Atmos. Chem. Phys. 2014, 14, 7807–7823. [Google Scholar] [CrossRef]
- Kawamura, K.; Yasui, O. Diurnal changes in the distribution of dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban Tokyo atmosphere. Atmos. Environ. 2005, 39, 1945–1960. [Google Scholar] [CrossRef]
- Bari, M.A.; Baumbach, G.; Kuch, B.; Scheffknecht, G. Temporal variation and impact of wood smoke pollution on a residential area in southern Germany. Atmos. Environ. 2010, 44, 3823–3832. [Google Scholar] [CrossRef]
- Kawamura, K.; Kasukabe, H.; Barrie, L.A. Source and reaction pathways of dicarboxylic acids, ketoacids and dicarbonyls in arctic aerosols: One year of observations. Atmos. Environ. 1996, 30, 1722. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikushima, K. Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere. Environ. Sci. Technol 1993, 27, 2227–2235. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Ren, Y.; Wang, J.; Wu, C.; Han, Y.; Zhang, L.; Cheng, C.; Meng, J. Identification of chemical compositions and sources of atmospheric aerosols in Xi’an, inland China during two types of haze events. Sci. Total Environ. 2016, 566–567, 230–237. [Google Scholar] [CrossRef]
- Soja, A.J. Intercomparison of near-real-time biomass burning emissions estimates constrained by satellite fire data. J. Appl. Remote Sens. 2008, 2, 142–154. [Google Scholar]
- Oros, D.R.; Simoneit, B. Identification and emission factors of molecular tracers in organic aerosols from biomass burning Part 2. Deciduous trees. Appl. Geochem. 2001, 16, 1545–1565. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).