Evasion of Gaseous Elemental Mercury from Forest and Urban Soils Contaminated by Historical and Modern Ore Roasting Processes (Idrija, Slovenia)
Abstract
1. Introduction
2. Materials and Methods
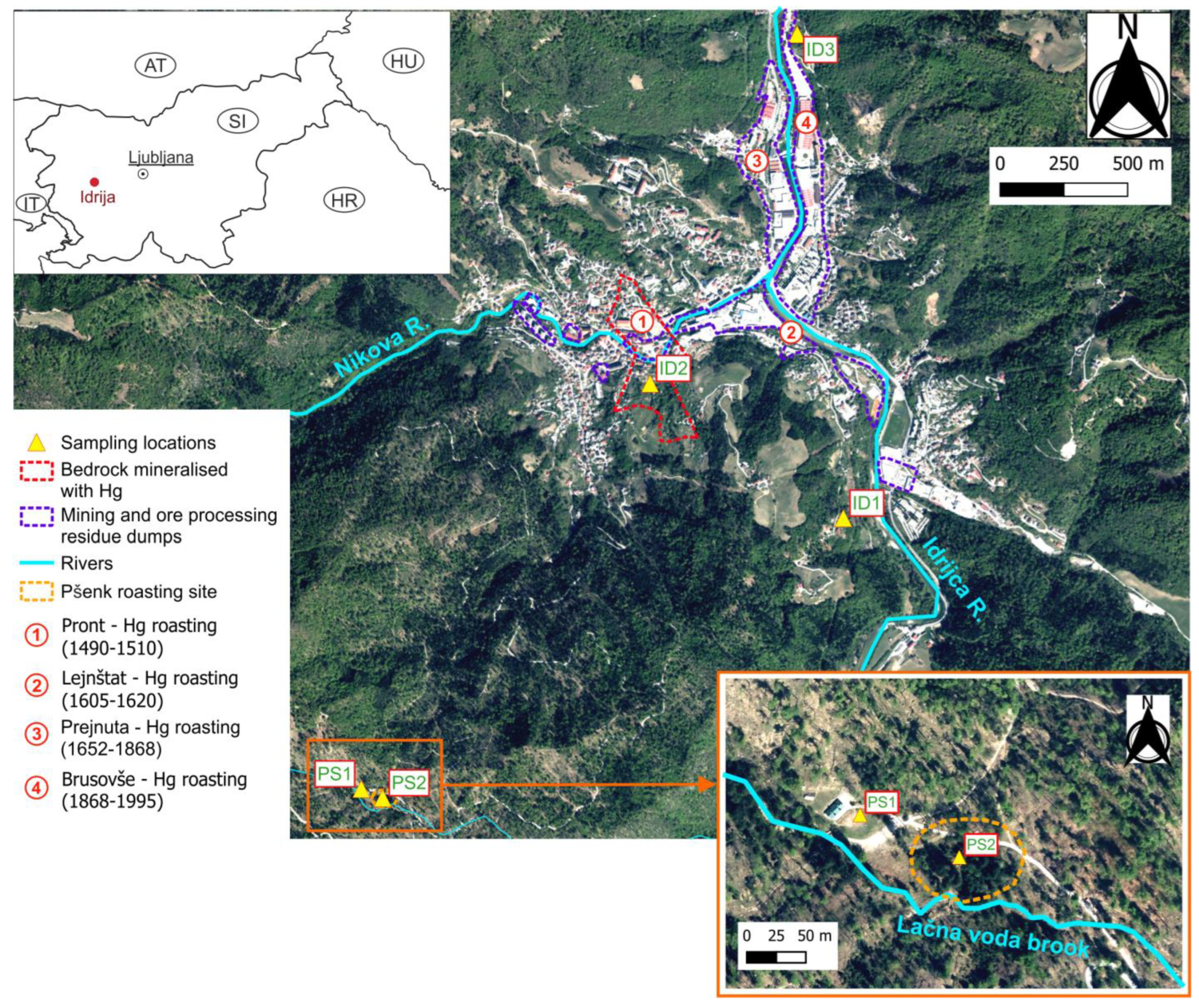
2.1. Environmental Settings and Experimental Sites
2.2. Field Activity
2.3. Analytical Determinations
3. Results
3.1. Soil Characteristics
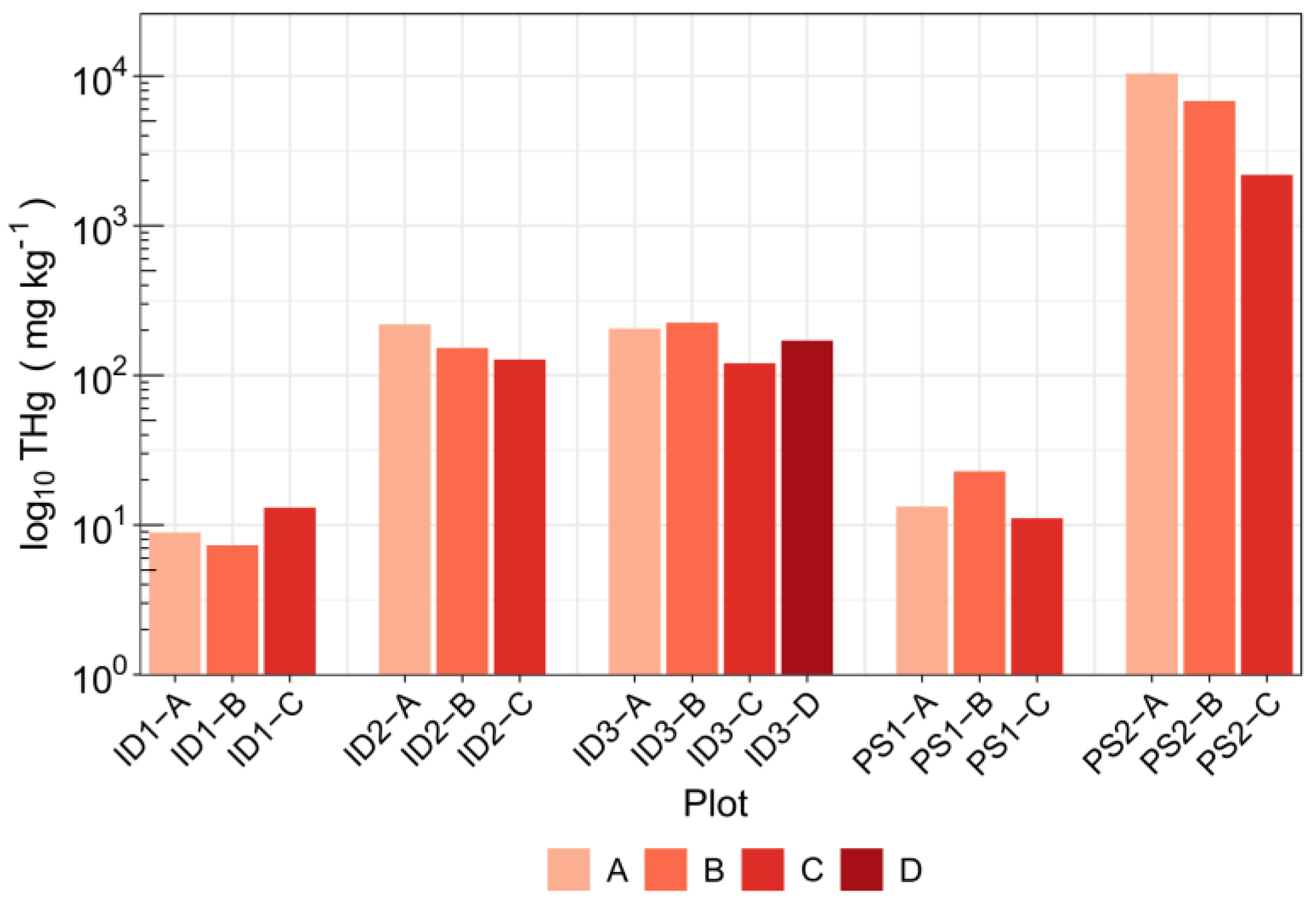
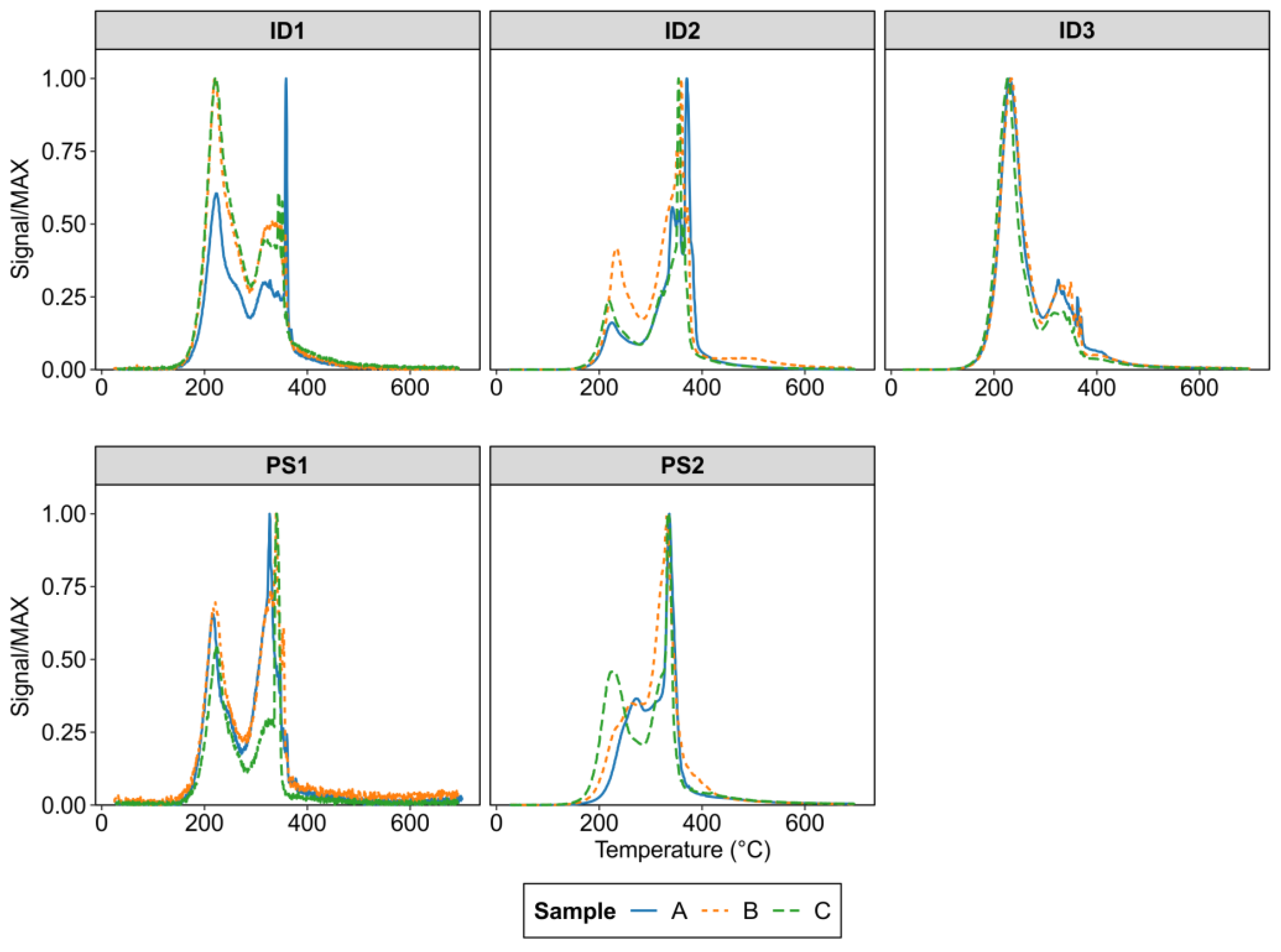
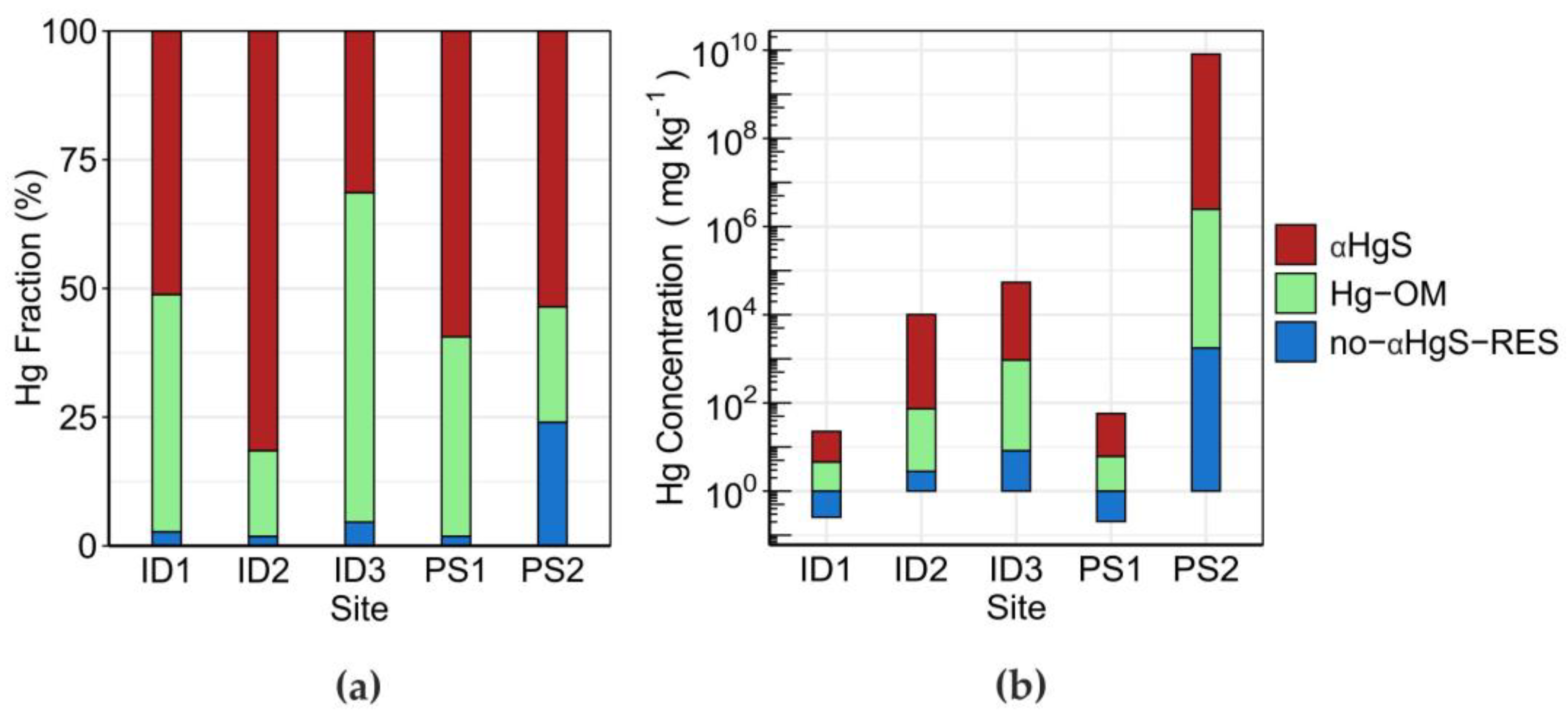
3.2. Topsoil Total Hg Concentration and Speciation
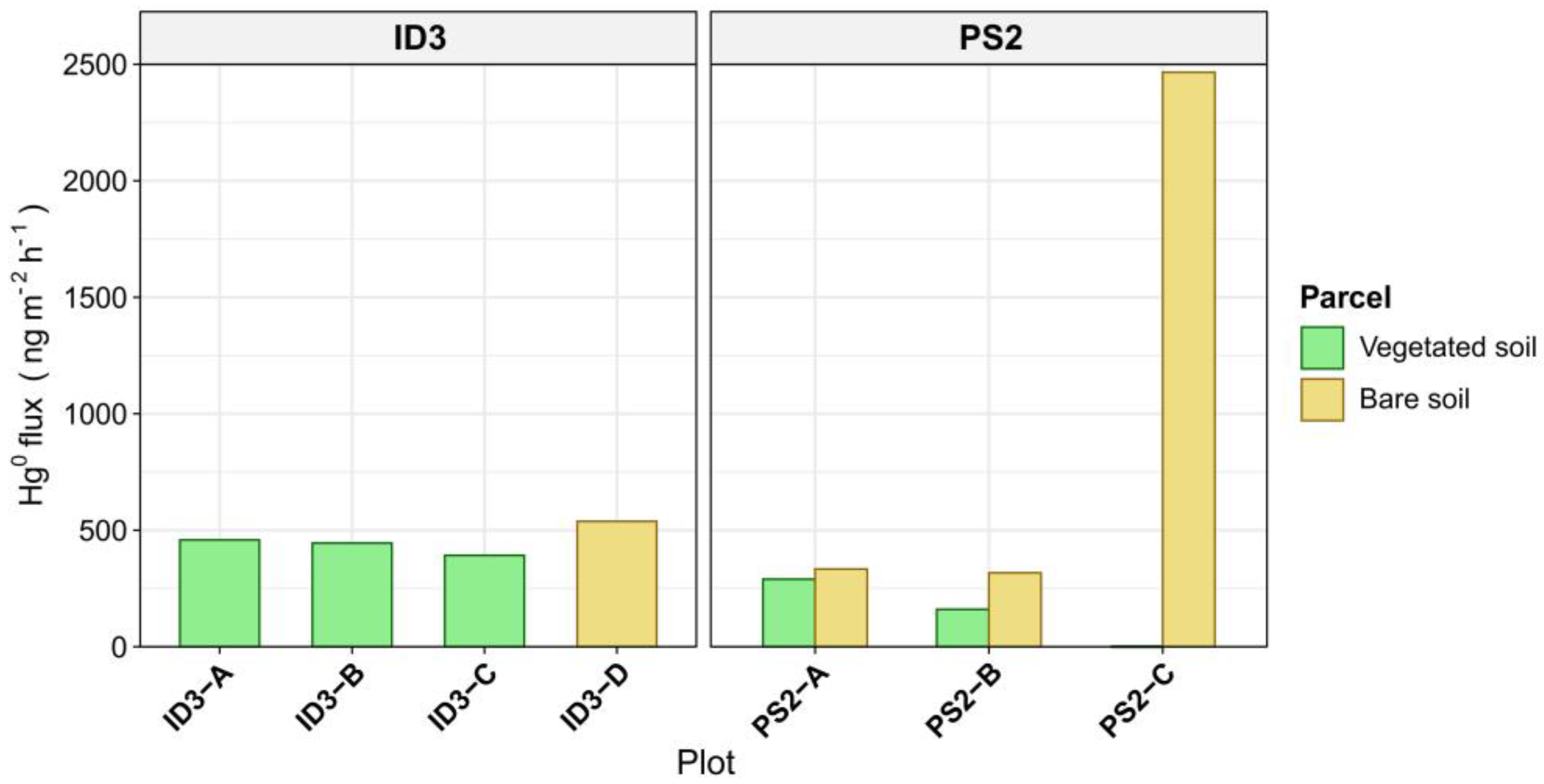
3.3. Elemental Mercury (Hg0) Fluxes at the Soil–Air Interface
4. Discussion
4.1. Elemental Mercury (Hg0) Fluxes and Soil Total Hg Concentrations
4.2. Elemental Mercury (Hg0) Fluxes and Hg Speciation in Soil
4.3. Elemental Mercury (Hg0) Fluxes and Meteorological Parameters
4.4. Elemental Mercury (Hg0) Fluxes and Vegetation Cover
5. Conclusions
- Measured Hg0 fluxes showed great spatial variability.
- The release of Hg0 into the atmosphere depends on complex site-specific interactions between possible influencing factors.
- Considering measurements conducted on grasslands under direct solar irradiation, the total topsoil Hg content can be considered the main driver of Hg0 evasion.
- Gaseous Hg0 fluxes increase under high UV radiation and soil temperature, which in turn can increase the rate of Hg reduction reactions.
- Well-developed herbaceous vegetation can strongly limit Hg0 evasion from contaminated soil due to surface shading. This is confirmed by the increase in Hg0 fluxes observed from bare soil plots compared with vegetated plots and by relatively low Hg0 fluxes from shaded locations found at the extremely contaminated historic roasting site.
- Forests can significantly reduce the impact of heavy soil contamination in the local atmosphere. However, care should be taken in forest management practices, as clear-cut forests may expose contaminated soils to direct solar radiation, potentially resulting in high Hg0 emissions.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gosar, M.; Teršič, T. Environmental Geochemistry Studies in the Area of Idrija Mercury Mine, Slovenia. Environ. Geochem. Health 2012, 34, 27–41. [Google Scholar] [CrossRef]
- Martínez-Coronado, A.; Oyarzun, R.; Esbrí, J.M.; Llanos, W.; Higueras, P. Sampling High to Extremely High Hg Concentrations at the Cerco de Almadenejos, Almadén Mining District (Spain): The Old Metallurgical Precinct (1794 to 1861AD) and Surrounding Areas. J. Geochem. Explor. 2011, 109, 70–77. [Google Scholar] [CrossRef]
- Rimondi, V.; Gray, J.E.; Costagliola, P.; Vaselli, O.; Lattanzi, P. Concentration, Distribution, and Translocation of Mercury and Methylmercury in Mine-Waste, Sediment, Soil, Water, and Fish Collected near the Abbadia San Salvatore Mercury Mine, Monte Amiata District, Italy. Sci. Total Environ. 2012, 414, 318–327. [Google Scholar] [CrossRef]
- Clarkson, T.W.; Magos, L. The Toxicology of Mercury and Its Chemical Compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M.; Wu, M.; Gillette, C.; Blough, E.R. Environmental Mercury and Its Toxic Effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, Toxicity, and Remediation of Mercury: An Essence Review. Environ. Monit. Assess. 2019, 191, 566. [Google Scholar] [CrossRef]
- Beckers, F.; Rinklebe, J. Cycling of Mercury in the Environment: Sources, Fate, and Human Health Implications: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 693–794. [Google Scholar] [CrossRef]
- Yu, B.; Fu, X.; Yin, R.; Zhang, H.; Wang, X.; Lin, C.J.; Wu, C.; Zhang, Y.; He, N.; Fu, P.; et al. Isotopic Composition of Atmospheric Mercury in China: New Evidence for Sources and Transformation Processes in Air and in Vegetation. Environ. Sci. Technol. 2016, 50, 9362–9369. [Google Scholar] [CrossRef]
- Cheng, I.; Zhang, L.; Mao, H.; Blanchard, P.; Tordon, R.; Dalziel, J. Seasonal and Diurnal Patterns of Speciated Atmospheric Mercury at a Coastal-Rural and a Coastal-Urban Site. Atmos. Environ. 2014, 82, 193–205. [Google Scholar] [CrossRef]
- Schroeder, W.H.; Munthe, J. Atmospheric Mercury-An Overview. Atmos. Environ. 1998, 32, 809–822. [Google Scholar] [CrossRef]
- Lindberg, S.; Bullock, R.; Ebinghaus, R.; Engstrom, D.; Feng, X.; Fitzgerald, W.; Pirrone, N.; Prestbo, E.; Seigneur, C. A Synthesis of Progress and Uncertainties in Attributing the Sources of Mercury in Deposition. Ambio 2007, 36, 19–32. [Google Scholar] [CrossRef]
- Fitzgerald, W.F.; Engstrom, D.R.; Mason, R.P.; Nater, E.A. The Case for Atmospheric Mercury Contamination in Remote Areas. Environ. Sci. Technol. 1998, 32, 1–7. [Google Scholar] [CrossRef]
- Kalinchuk, V.V.; Mishukov, V.F.; Astakhov, A.S. Arctic Source for Elevated Atmospheric Mercury (Hg0) in the Western Bering Sea in the Summer of 2013. J. Environ. Sci. 2018, 68, 114–121. [Google Scholar] [CrossRef]
- Rolison, J.M.; Landing, W.M.; Luke, W.; Cohen, M.; Salters, V.J.M. Isotopic Composition of Species-Specific Atmospheric Hg in a Coastal Environment. Chem. Geol. 2013, 336, 37–49. [Google Scholar] [CrossRef]
- Agnan, Y.; Le Dantec, T.; Moore, C.W.; Edwards, G.C.; Obrist, D. New Constraints on Terrestrial Surface-Atmosphere Fluxes of Gaseous Elemental Mercury Using a Global Database. Environ. Sci. Technol. 2016, 50, 507–524. [Google Scholar] [CrossRef]
- Selin, H.; Keane, S.E.; Wang, S.; Selin, N.E.; Davis, K.; Bally, D. Linking Science and Policy to Support the Implementation of the Minamata Convention on Mercury. Ambio 2018, 47, 198–215. [Google Scholar] [CrossRef]
- Gustin, M.S.; Coolbaugh, M.F.; Engle, M.A.; Fitzgerald, B.C.; Keislar, R.E.; Lindberg, S.E.; Nacht, D.M.; Quashnick, J.; Rytuba, J.J.; Sladek, C.; et al. Atmospheric Mercury Emissions from Mine Wastes and Surrounding Geologically Enriched Terrains. Environ. Geol. 2003, 43, 339–351. [Google Scholar] [CrossRef]
- Fantozzi, L.; Ferrara, R.; Dini, F.; Tamburello, L.; Pirrone, N.; Sprovieri, F. Study on the Reduction of Atmospheric Mercury Emissions from Mine Waste Enriched Soils through Native Grass Cover in the Mt. Amiata Region of Italy. Environ. Res. 2013, 125, 69–74. [Google Scholar] [CrossRef]
- Ferrara, R.; Maserti, B.E.; Andersson, M.; Edner, H.; Ragnarson, P.; Svanberg, S.; Hernandez, A. Atmospheric Mercury Concentrations and Fluxes in the Almaden District (Spain). Atmos. Environ. 1998, 32, 3897–3904. [Google Scholar] [CrossRef]
- Huremović, J.; Horvat, M.; Kotnik, J.; Kocman, D.; Žižek, S.; Ribeiro Guevara, S.; Muhić-Šarac, T.; Memić, M. Characterization of Mercury Contamination Surrounding a Chloralkali Production Facility in Tuzla, Bosnia and Herzegovina. Anal. Lett. 2017, 50, 1049–1064. [Google Scholar] [CrossRef]
- Wang, S.; Feng, X.; Qiu, G.; Wei, Z.; Xiao, T. Mercury Emission to Atmosphere from Lanmuchang Hg-Tl Mining Area, Southwestern Guizhou, China. Atmos. Environ. 2005, 39, 7459–7473. [Google Scholar] [CrossRef]
- Kocman, D.; Horvat, M.; Pirrone, N.; Cinnirella, S. Contribution of Contaminated Sites to the Global Mercury Budget. Environ. Res. 2013, 125, 160–170. [Google Scholar] [CrossRef]
- Osterwalder, S.; Huang, J.H.; Shetaya, W.H.; Agnan, Y.; Frossard, A.; Frey, B.; Alewell, C.; Kretzschmar, R.; Biester, H.; Obrist, D. Mercury Emission from Industrially Contaminated Soils in Relation to Chemical, Microbial, and Meteorological Factors. Environ. Pollut. 2019, 250, 944–952. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Contreras, F.; Adams, M.; Santos, F. Atmospheric Mercury Emissions from Polluted Gold Mining Areas (Venezuela). Environ. Geochem. Health 2006, 28, 529–540. [Google Scholar] [CrossRef]
- Cabassi, J.; Lazzaroni, M.; Giannini, L.; Mariottini, D.; Nisi, B.; Rappuoli, D.; Vaselli, O. Continuous and near Real-Time Measurements of Gaseous Elemental Mercury (GEM) from an Unmanned Aerial Vehicle: A New Approach to Investigate the 3D Distribution of GEM in the Lower Atmosphere. Chemosphere 2022, 288, 132547. [Google Scholar] [CrossRef]
- Floreani, F.; Acquavita, A.; Barago, N.; Klun, K.; Faganeli, J.; Covelli, S. Gaseous Mercury Exchange from Water—Air Interface in Differently Impacted Freshwater Environments. Int. J. Environ. Res. Public Health 2022, 19, 8149. [Google Scholar] [CrossRef]
- Kocman, D.; Horvat, M. A Laboratory Based Experimental Study of Mercury Emission from Contaminated Soils in the River Idrijca Catchment. Atmos. Chem. Phys. 2010, 10, 1417–1426. [Google Scholar] [CrossRef]
- Shi, T.; Gong, Y.; Ma, J.; Wu, H.; Yang, S.; Ju, T.; Qu, Y.; Liu, L. Soil-Air Exchange of Mercury from Agricultural Fields in Zhejiang, East China: Seasonal Variations, Influence Factors, and Models of Fluxes. Chemosphere 2020, 249, 126063. [Google Scholar] [CrossRef]
- Wallschläger, D.; Turner, R.R.; London, J.; Ebinghaus, R.; Kock, H.H.; Sommar, J.; Xiao, Z. Factors Affecting the Measurement of Mercury Emissions from Soils with Flux Chambers. J. Geophys. Res. Atmos. 1999, 104, 21859–21871. [Google Scholar] [CrossRef]
- Song, X.; Van Heyst, B. Volatilization of Mercury from Soils in Response to Simulated Precipitation. Atmos. Environ. 2005, 39, 7494–7505. [Google Scholar] [CrossRef]
- Gosar, M.; Šajn, R.; Miler, M.; Burger, A.; Bavec, S. Overview of Existing Information on Important Closed (or in Closing Phase) and Abandoned Mining Waste Sites and Related Mines in Slovenia. Geologija 2020, 63, 221–250. [Google Scholar] [CrossRef]
- Gosar, M.; Šajn, R. Mercury in Soil and Attic Dust as a Reflection of Idrija Mining and Mineralization (Slovenia) (Živo Srebro v Tleh in Podstrešnem Prahu v Idriji in Okolici Kot Posledica Orudenja in Rudarjenja). Geologija 2001, 44, 137–159. [Google Scholar]
- Gosar, M.; Šajn, R.; Biester, H. Binding of Mercury in Soils and Attic Dust in the Idrija Mercury Mine Area (Slovenia). Sci. Total Environ. 2006, 369, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Bavec, Š.; Gosar, M.; Biester, H.; Grčman, H. Geochemical Investigation of Mercury and Other Elements in Urban Soil of Idrija (Slovenia). J. Geochem. Explor. 2015, 154, 213–223. [Google Scholar] [CrossRef]
- Bavec, Š.; Gosar, M.; Miler, M.; Biester, H. Geochemical Investigation of Potentially Harmful Elements in Household Dust from a Mercury-Contaminated Site, the Town of Idrija (Slovenia). Environ. Geochem. Health 2017, 39, 443–465. [Google Scholar] [CrossRef]
- Čar, J. Mineralized Rocks and Ore Residues in the Idrija Region. In Proceedings of the Meeting of Researchers Entitled: Idrija as a Natural and Anthropogenic Laboratory, Mercury as a Global Pollutant, Idrija, Slovenia, 24 May 1996; pp. 10–15. [Google Scholar]
- Čar, J. Geological Structure of the Idrija—Cerkljansko Hills: Explanatory Book to the Geological Map of the Idrija—Cerkljansko Hills between Stopnik and Rovte 1:25.000; Geološki Zavod Slovenije: Ljubljana, Slovenia, 2010. [Google Scholar]
- Mlakar, I.; Čar, J. Geological Map of the Idrija—Cerkljansko Hills Between Stopnik and Rovte 1:25.000; Geološki Zavod Slovenije: Ljubljana, Slovenia, 2009. [Google Scholar]
- Gosar, M.; Čar, J. Vpliv Žgalnic Živosrebrove Rude Iz 16. in 17. Stoletja Na Razširjenost Živega Srebra v Okolici Idrije [Influence of Mercury Ore Roasting Sites from 16th and 17th Century on the Mercury Dispersion in Surroundings of Idrija]. Geologija 2006, 49, 91–101. [Google Scholar] [CrossRef]
- Gosar, M.; Teršič, T. Contaminated Sediment Loads from Ancient Mercury Ore Roasting Sites, Idrija Area, Slovenia. J. Geochem. Explor. 2015, 149, 97–105. [Google Scholar] [CrossRef]
- Teršič, T.; Gosar, M. Preliminary Results of Detailed Geochemical Study of Mercury at the Ancient Ore Roasting Site Pšenk (Idrija Area, Slovenia). Geologija 2009, 52, 79–86. [Google Scholar] [CrossRef]
- Teršič, T.; Gosar, M.; Biester, H. Distribution and Speciation of Mercury in Soil in the Area of an Ancient Mercury Ore Roasting Site, Frbejžene Trate (Idrija Area, Slovenia). J. Geochem. Explor. 2011, 110, 136–145. [Google Scholar] [CrossRef]
- Teršič, T.; Biester, H.; Gosar, M. Leaching of Mercury from Soils at Extremely Contaminated Historical Roasting Sites (Idrija Area, Slovenia). Geoderma 2014, 226–227, 213–222. [Google Scholar] [CrossRef]
- Biester, H.; Gosar, M.; Covelli, S. Mercury Speciation in Sediments Affected by Dumped Mining Residues in the Drainage Area of the Idrija Mercury Mine, Slovenia. Environ. Sci. Technol. 2000, 34, 3330–3336. [Google Scholar] [CrossRef]
- Gosar, M. Mercury in River Sediments, Floodplains and Plants Growing Thereon in Drainage Area of Idrija Mine, Slovenia. Pol. J. Environ. Stud. 2008, 17, 227–236. [Google Scholar] [CrossRef]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury Contamination of Coastal Sediments as the Result of Long-Term Cinnabar Mining Activity (Gulf of Trieste, Northern Adriatic Sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Horvat, M.; Covelli, S.; Faganeli, J.; Logar, M.; Mandić, V.; Rajar, R.; Širca, A.; Žagar, D. Mercury in Contaminated Coastal Environments; a Case Study: The Gulf of Trieste. Sci. Total Environ. 1999, 237–238, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, R.; Edner, H.; Svanberg, S.; Kotnik, J.; Horvat, M. Mercury Emissions from the Idrija Mercury Mine Measured by Differential Absorption Lidar Techniques and a Point Monitoring Absorption Spectrometer. Atmos. Environ. 2005, 39, 4067–4074. [Google Scholar] [CrossRef]
- Kocman, D.; Horvat, M. Non-Point Source Mercury Emission from the Idrija Hg-Mine Region: GIS Mercury Emission Model. J. Environ. Manag. 2011, 92, 2038–2046. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, J.; Horvat, M.; Dizdarevič, T. Current and Past Mercury Distribution in Air over the Idrija Hg Mine Region, Slovenia. Atmos. Environ. 2005, 39, 7570–7579. [Google Scholar] [CrossRef]
- Kocman, D.; Vreča, P.; Fajon, V.; Horvat, M. Atmospheric Distribution and Deposition of Mercury in the Idrija Hg Mine Region, Slovenia. Environ. Res. 2011, 111, 1–9. [Google Scholar] [CrossRef]
- Teršič, T.; Gosar, M.; Biester, H. Environmental Impact of Ancient Small-Scale Mercury Ore Processing at Pšenk on Soil (Idrija Area, Slovenia). Appl. Geochem. 2011, 26, 1867–1876. [Google Scholar] [CrossRef]
- Bavec, Š.; Biester, H.; Gosar, M. Urban Sediment Contamination in a Former Hg Mining District, Idrija, Slovenia. Environ. Geochem. Health 2014, 36, 427–439. [Google Scholar] [CrossRef]
- Baptista-Salazar, C.; Richard, J.H.; Horf, M.; Rejc, M.; Gosar, M.; Biester, H. Grain-Size Dependence of Mercury Speciation in River Suspended Matter, Sediments and Soils in a Mercury Mining Area at Varying Hydrological Conditions. Appl. Geochem. 2017, 81, 132–142. [Google Scholar] [CrossRef]
- Zupančič, M.; Šušteršič, M.; Bavec, Š.; Gosar, M. Oral and Inhalation Bioaccessibility of Potentially Toxic Elements in Household Dust from Former Hg Mining District, Idrija, Slovenia. Environ. Geochem. Health 2021, 43, 3505–3531. [Google Scholar] [CrossRef] [PubMed]
- Floreani, F.; Zappella, V.; Faganeli, J.; Covelli, S. Gaseous Mercury Evasion from Bare and Grass-Covered Soils Contaminated by Mining and Ore Roasting (Isonzo River Alluvial Plain, Northeastern Italy). Environ. Pollut. 2023, 318, 120921. [Google Scholar] [CrossRef]
- Gillis, A.; Miller, D.R. Some Potential Errors in the Measurement of Mercury Gas Exchange at the Soil Surface Using a Dynamic Flux Chamber. Sci. Total Environ. 2000, 260, 181–189. [Google Scholar] [CrossRef]
- Sholupov, S.; Pogarev, S.; Ryzhov, V. Zeeman Atomic Absorption Spectrometer RA-915 + for Direct Determination of Mercury in Air and Complex Matrix Samples. Fuel Process. Technol. 2004, 85, 473–485. [Google Scholar] [CrossRef]
- Mashyanov, N.; Obolkin, V.; Pogarev, S.; Ryzhov, V.; Sholupov, S.; Potemkin, V.; Molozhnikova, E.; Khodzher, T. Air Mercury Monitoring at the Baikal Area. Atmosphere 2021, 12, 807. [Google Scholar] [CrossRef]
- Kandel, T.P.; Lærke, P.E.; Elsgaard, L. Effect of Chamber Enclosure Time on Soil Respiration Flux: A Comparison of Linear and Non-Linear Flux Calculation Methods. Atmos. Environ. 2016, 141, 245–254. [Google Scholar] [CrossRef]
- Maier, M.; Weber, T.K.D.; Fiedler, J.; Fuß, R.; Glatzel, S.; Huth, V.; Jordan, S.; Jurasinski, G.; Kutzbach, L.; Schäfer, K.; et al. Introduction of a Guideline for Measurements of Greenhouse Gas Fluxes from Soils Using Non-Steady-State Chambers. J. Plant Nutr. Soil Sci. 2022, 185, 447–461. [Google Scholar] [CrossRef]
- Kutzbach, L.; Schneider, J.; Sachs, T.; Giebels, M.; Nykänen, H.; Shurpali, N.J.; Martikainen, P.J.; Alm, J.; Wilmking, M. CO2 Flux Determination by Closed-Chamber Methods Can Be Seriously Biased by Inappropriate Application of Linear Regression. Biogeosciences 2007, 4, 1005–1025. [Google Scholar] [CrossRef]
- Chiodini, G.; Cioni, R.; Guidi, M.; Raco, B.; Marini, L. Soil CO2 Flux Measurements in Volcanic and Geothermal Areas. Appl. Geochem. 1998, 13, 543–552. [Google Scholar] [CrossRef]
- Bagnato, E.; Barra, M.; Cardellini, C.; Chiodini, G.; Parello, F.; Sprovieri, M. First Combined Flux Chamber Survey of Mercury and CO2 Emissions from Soil Diffuse Degassing at Solfatara of Pozzuoli Crater, Campi Flegrei (Italy): Mapping and Quantification of Gas Release. J. Volcanol. Geotherm. Res. 2014, 289, 26–40. [Google Scholar] [CrossRef]
- Heinemeyer, A.; McNamara, N.P. Comparing the Closed Static versus the Closed Dynamic Chamber Flux Methodology: Implications for Soil Respiration Studies. Plant Soil 2011, 346, 145–151. [Google Scholar] [CrossRef]
- Kyllönen, K.; Hakola, H.; Hellén, H.; Korhonen, M.; Verta, M. Atmospheric Mercury Fluxes in a Southern Boreal Forest and Wetland. Water. Air Soil Pollut. 2012, 223, 1171–1182. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Ding, L.; Wang, Z.; Teng, G.; Shi, Z.; Li, B. Influence of Deployment Time and Surface Wind Speed on the Accuracy of Measurements of Greenhouse Gas Fluxes Using a Closed Chamber Method under Low Surface Wind Speed. J. Air Waste Manag. Assoc. 2019, 69, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sigler, J.M.; Lee, X. Gaseous Mercury in Background Forest Soil in the Northeastern United States. J. Geophys. Res. Biogeosci. 2006, 111, G02007. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Z.; Zhang, X.; Driscoll, C.T. Measurement of the Vertical Distribution of Gaseous Elemental Mercury Concentration in Soil Pore Air of Subtropical and Temperate Forests. Environ. Sci. Technol. 2021, 55, 2132–2142. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on Ignition as a Method for Estimating Organic and Carbonate Content in Sediments: Reproducibility and Comparability of Results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Edmeades, D.C.; Wheeler, D.M. Measurement of Ph in New Zealand Soils: An Examination of the Effect of Electrolyte, Electrolyte Strength, and Soil:Solution Ratio. N. Zeal. J. Agric. Res. 1990, 33, 105–109. [Google Scholar] [CrossRef]
- Mashyanov, N.R.; Pogarev, S.E.; Panova, E.G.; Panichev, N.; Ryzhov, V. Determination of Mercury Thermospecies in Coal. Fuel 2017, 203, 973–980. [Google Scholar] [CrossRef]
- Petranich, E.; Predonzani, S.; Acquavita, A.; Mashyanov, N.; Covelli, S. Rapid Thermoscanning Technique for Direct Analysis of Mercury Species in Contaminated Sediments: From Pure Compounds to Real Sample Application. Appl. Geochem. 2022, 143, 105393. [Google Scholar] [CrossRef]
- Biester, H.; Scholz, C. Determination of Mercury Binding Forms in Contaminated Soils: Mercury Pyrolysis versus Sequential Extractions. Environ. Sci. Technol. 1997, 31, 233–239. [Google Scholar] [CrossRef]
- USDA Textural Soil Classification Study Guide. Soil Mechanics Level I Module 3; USDA Soil Conservation Service: Washington, DC, USA, 1987; pp. 1–53. [Google Scholar]
- Ballabio, C.; Jiskra, M.; Osterwalder, S.; Borrelli, P.; Montanarella, L.; Panagos, P. A Spatial Assessment of Mercury Content in the European Union Topsoil. Sci. Total Environ. 2021, 769, 144755. [Google Scholar] [CrossRef] [PubMed]
- Biester, H.U.; Muller, G.; Scholer, H.F. Binding and Mobility of Mercury in Soils Contaminated by Emissions from Chlor-Alkali Plants. Sci. Total Environ. 2002, 284, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Coufalík, P.; Komárek, J. The Use of Thermal Desorption in the Speciation Analysis of Mercury in Soil, Sediments and Tailings. J. Anal. Chem. 2014, 69, 1123–1129. [Google Scholar] [CrossRef]
- Reis, A.T.; Coelho, J.P.; Rucandio, I.; Davidson, C.M.; Duarte, A.C.; Pereira, E. Thermo-Desorption: A Valid Tool for Mercury Speciation in Soils and Sediments? Geoderma 2015, 237, 98–104. [Google Scholar] [CrossRef]
- Rumayor, M.; Lopez-Anton, M.A.; Díaz-Somoano, M.; Maroto-Valer, M.M.; Richard, J.H.; Biester, H.; Martínez-Tarazona, M.R. A Comparison of Devices Using Thermal Desorption for Mercury Speciation in Solids. Talanta 2016, 150, 272–277. [Google Scholar] [CrossRef]
- Yin, R.; Feng, X.; Wang, J.; Li, P.; Liu, J.; Zhang, Y.; Chen, J.; Zheng, L.; Hu, T. Mercury Speciation and Mercury Isotope Fractionation during Ore Roasting Process and Their Implication to Source Identification of Downstream Sediment in the Wanshan Mercury Mining Area, SW China. Chem. Geol. 2013, 336, 72–79. [Google Scholar] [CrossRef]
- Enescu, M.; Nagy, K.L.; Manceau, A. Nucleation of Mercury Sulfide by Dealkylation. Sci. Rep. 2016, 6, 39359. [Google Scholar] [CrossRef]
- Biester, H.; Gosar, M.; Müller, G. Mercury Speciation in Tailings of the Idrija Mercury Mine. J. Geochem. Explor. 1999, 65, 195–204. [Google Scholar] [CrossRef]
- Esbrí, J.M.; Bernaus, A.; Ávila, M.; Kocman, D.; García-Noguero, E.M.; Guerrero, B.; Gaona, X.; Álvarez, R.; Perez-Gonzalez, G.; Valiente, M.; et al. XANES Speciation of Mercury in Three Mining Districts—Almadén, Asturias (Spain), Idria (Slovenia). J. Synchrotron Radiat. 2010, 17, 179–186. [Google Scholar] [CrossRef]
- Wallschläger, D.; Kock, H.H.; Schroeder, W.H.; Lindberg, S.E.; Ebinghaus, R.; Wilken, R.D. Estimating Gaseous Mercury Emissions from Contaminated Floodplain Soils to the Atmosphere with Simple Field Measurement Techniques. Water. Air Soil Pollut. 2002, 135, 39–54. [Google Scholar] [CrossRef]
- Rinklebe, J.; During, A.; Overesch, M.; Wennrich, R.; Stärk, H.J.; Mothes, S.; Neue, H.U. Optimization of a Simple Field Method to Determine Mercury Volatilization from Soils-Examples of 13 Sites in Floodplain Ecosystems at the Elbe River (Germany). Ecol. Eng. 2009, 35, 319–328. [Google Scholar] [CrossRef]
- Eckley, C.S.; Tate, M.T.; Lin, C.J.; Gustin, M.; Dent, S.; Eagles-Smith, C.; Lutz, M.A.; Wickland, K.P.; Wang, B.; Gray, J.E.; et al. Surface-Air Mercury Fluxes across Western North America: A Synthesis of Spatial Trends and Controlling Variables. Sci. Total Environ. 2016, 568, 651–665. [Google Scholar] [CrossRef]
- Edwards, G.C.; Howard, D.A. Air-Surface Exchange Measurements of Gaseous Elemental Mercury over Naturally Enriched and Background Terrestrial Landscapes in Australia. Atmos. Chem. Phys. 2013, 13, 5325–5336. [Google Scholar] [CrossRef]
- Lin, C.J.; Gustin, M.S.; Singhasuk, P.; Eckley, C.; Miller, M. Empirical Models for Estimating Mercury Flux from Soils. Environ. Sci. Technol. 2010, 44, 8522–8528. [Google Scholar] [CrossRef]
- Fernández-Martínez, R.; Esbrí, J.M.; Higueras, P.; Rucandio, I. Comparison of Mercury Distribution and Mobility in Soils Affected by Anthropogenic Pollution around Chloralkali Plants and Ancient Mining Sites. Sci. Total Environ. 2019, 671, 1066–1076. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.G.; Rinklebe, J. Mercury Speciation, Transformation, and Transportation in Soils, Atmospheric Flux, and Implications for Risk Management: A Critical Review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, K. Review: Evaporation of Mercury from Soils. An Integration and Synthesis of Current Knowledge. Environ. Geol. 2000, 39, 249–271. [Google Scholar] [CrossRef]
- Jiskra, M.; Wiederhold, J.G.; Skyllberg, U.; Kronberg, R.M.; Hajdas, I.; Kretzschmar, R. Mercury Deposition and Re-Emission Pathways in Boreal Forest Soils Investigated with Hg Isotope Signatures. Environ. Sci. Technol. 2015, 49, 7188–7196. [Google Scholar] [CrossRef]
- Gabriel, M.C.; Williamson, D.G. Principal Biogeochemical Factors Affecting the Speciation and Transport of Mercury through the Terrestrial Environment. Environ. Geochem. Health 2004, 26, 421–434. [Google Scholar] [CrossRef]
- Eckley, C.S.; Blanchard, P.; McLennan, D.; Mintz, R.; Sekela, M. Soil-Air Mercury Flux near a Large Industrial Emission Source before and after Closure (Flin Flon, Manitoba, Canada). Environ. Sci. Technol. 2015, 49, 9750–9757. [Google Scholar] [CrossRef]
- Pannu, R.; Siciliano, S.D.; O’Driscoll, N.J. Quantifying the Effects of Soil Temperature, Moisture and Sterilization on Elemental Mercury Formation in Boreal Soils. Environ. Pollut. 2014, 193, 138–146. [Google Scholar] [CrossRef]
- Carpi, A.; Lindberg, S.E. Application of a Teflon® Dynamic Flux Chamber for Quantifying Soil Mercury Flux: Tests and Results over Background Soil. Atmos. Environ. 1998, 32, 873–882. [Google Scholar] [CrossRef]
- Gustin, M.S.; Biester, H.; Kim, C.S. Investigation of the Light-Enhanced Emission of Mercury from Naturally Enriched Substrates. Atmos. Environ. 2002, 36, 3241–3254. [Google Scholar] [CrossRef]
- Llanos, W.; Kocman, D.; Higueras, P.; Horvat, M. Mercury Emission and Dispersion Models from Soils Contaminated by Cinnabar Mining and Metallurgy. J. Environ. Monit. 2011, 13, 3460–3468. [Google Scholar] [CrossRef]
- Gómez-Armesto, A.; Martínez-Cortizas, A.; Ferro-Vázquez, C.; Méndez-López, M.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Modelling Hg Mobility in Podzols: Role of Soil Components and Environmental Implications. Environ. Pollut. 2020, 260, 114040. [Google Scholar] [CrossRef]
- Bavec, Š.; Gosar, M. Speciation, Mobility and Bioaccessibility of Hg in the Polluted Urban Soil of Idrija (Slovenia). Geoderma 2016, 273, 115–130. [Google Scholar] [CrossRef]
- Ma, M.; Sun, T.; Du, H.; Wang, D. A Two-Year Study on Mercury Fluxes from the Soil under Different Vegetation Cover in a Subtropical Region, South China. Atmosphere 2018, 9, 30. [Google Scholar] [CrossRef]
- Moore, C.; Carpi, A. Mechanisms of the Emission of Mercury from Soil: Role of UV Radiation. J. Geophys. Res. Atmos. 2005, 110, D24302. [Google Scholar] [CrossRef]
- Choi, H.D.; Holsen, T.M. Gaseous Mercury Fluxes from the Forest Floor of the Adirondacks. Environ. Pollut. 2009, 157, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, X.; Wang, S. Exchange Fluxes of Hg between Surfaces and Atmosphere in the Eastern Flank of Mount Gongga, Sichuan Province, Southwestern China. J. Geophys. Res. Atmos. 2008, 113, D20306. [Google Scholar] [CrossRef]
- Kuiken, T.; Zhang, H.; Gustin, M.; Lindberg, S. Mercury Emission from Terrestrial Background Surfaces in the Eastern USA. Part I: Air/Surface Exchange of Mercury within a Southeastern Deciduous Forest (Tennessee) over One Year. Appl. Geochem. 2008, 23, 345–355. [Google Scholar] [CrossRef]
- Windmöller, C.C.; Durão, W.A.; de Oliveira, A.; do Valle, C.M. The Redox Processes in Hg-Contaminated Soils from Descoberto (Minas Gerais, Brazil): Implications for the Mercury Cycle. Ecotoxicol. Environ. Saf. 2015, 112, 201–211. [Google Scholar] [CrossRef]
- Higueras, P.; Llanos, W.; García, M.E.; Millán, R.; Serrano, C. Mercury Vapor Emissions from the Ingenios in Potosí (Bolivia). J. Geochem. Explor. 2012, 116–117, 1–7. [Google Scholar] [CrossRef]
- Jiang, T.; Skyllberg, U.; Wei, S.; Wang, D.; Lu, S.; Jiang, Z.; Flanagan, D.C. Modeling of the Structure-Specific Kinetics of Abiotic, Dark Reduction of Hg(II) Complexed by O/N and S Functional Groups in Humic Acids While Accounting for Time-Dependent Structural Rearrangement. Geochim. Cosmochim. Acta 2015, 154, 151–167. [Google Scholar] [CrossRef]
- Fu, X.; Feng, X.; Zhang, H.; Yu, B.; Chen, L. Mercury Emissions from Natural Surfaces Highly Impacted by Human Activities in Guangzhou Province, South China. Atmos. Environ. 2012, 54, 185–193. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, X.; Lin, C.J.; Sommar, J.; Lu, Z.; Feng, X. Process Factors Driving Dynamic Exchange of Elemental Mercury Vapor over Soil in Broadleaf Forest Ecosystems. Atmos. Environ. 2019, 219, 117047. [Google Scholar] [CrossRef]
- Gruba, P.; Socha, J.; Pietrzykowski, M.; Pasychnyk, D. Tree Species Affects the Concentration of Total Mercury (Hg) in Forest Soils: Evidence from a Forest Soil Inventory in Poland. Sci. Total Environ. 2019, 647, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.B.; Friedland, A.J. Mercury in Coniferous and Deciduous Upland Forests in Northern New England, USA: Implications of Climate Change. Biogeosciences 2015, 12, 6737–6749. [Google Scholar] [CrossRef]
- Schuster, E. The Behavior of Mercury in the Soil with Special Emphasis on Complexation and Adsorption Processes—A Review of the Literature. Water Air Soil Pollut. 1991, 56, 667–680. [Google Scholar] [CrossRef]
- Yang, Y.K.; Zhang, C.; Shi, X.; Lin, T.; Wang, D. yong Effect of Organic Matter and PH on Mercury Release from Soils. J. Environ. Sci. 2007, 19, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.O.; Harris, L.A.; Turner, R.R.; Stevenson, R.J.; Henson, T.J.; Melton, R.C.; Hoffman, D.P. Formation of Mercuric Sulfide in Soil. Environ. Sci. Technol. 1997, 31, 3037–3043. [Google Scholar] [CrossRef]
- Carpi, A.; Fostier, A.H.; Orta, O.R.; dos Santos, J.C.; Gittings, M. Gaseous Mercury Emissions from Soil Following Forest Loss and Land Use Changes: Field Experiments in the United States and Brazil. Atmos. Environ. 2014, 96, 423–429. [Google Scholar] [CrossRef]
- Mazur, M.; Mitchell, C.P.J.; Eckley, C.S.; Eggert, S.L.; Kolka, R.K.; Sebestyen, S.D.; Swain, E.B. Gaseous Mercury Fluxes from Forest Soils in Response to Forest Harvesting Intensity: A Field Manipulation Experiment. Sci. Total Environ. 2014, 496, 678–687. [Google Scholar] [CrossRef]
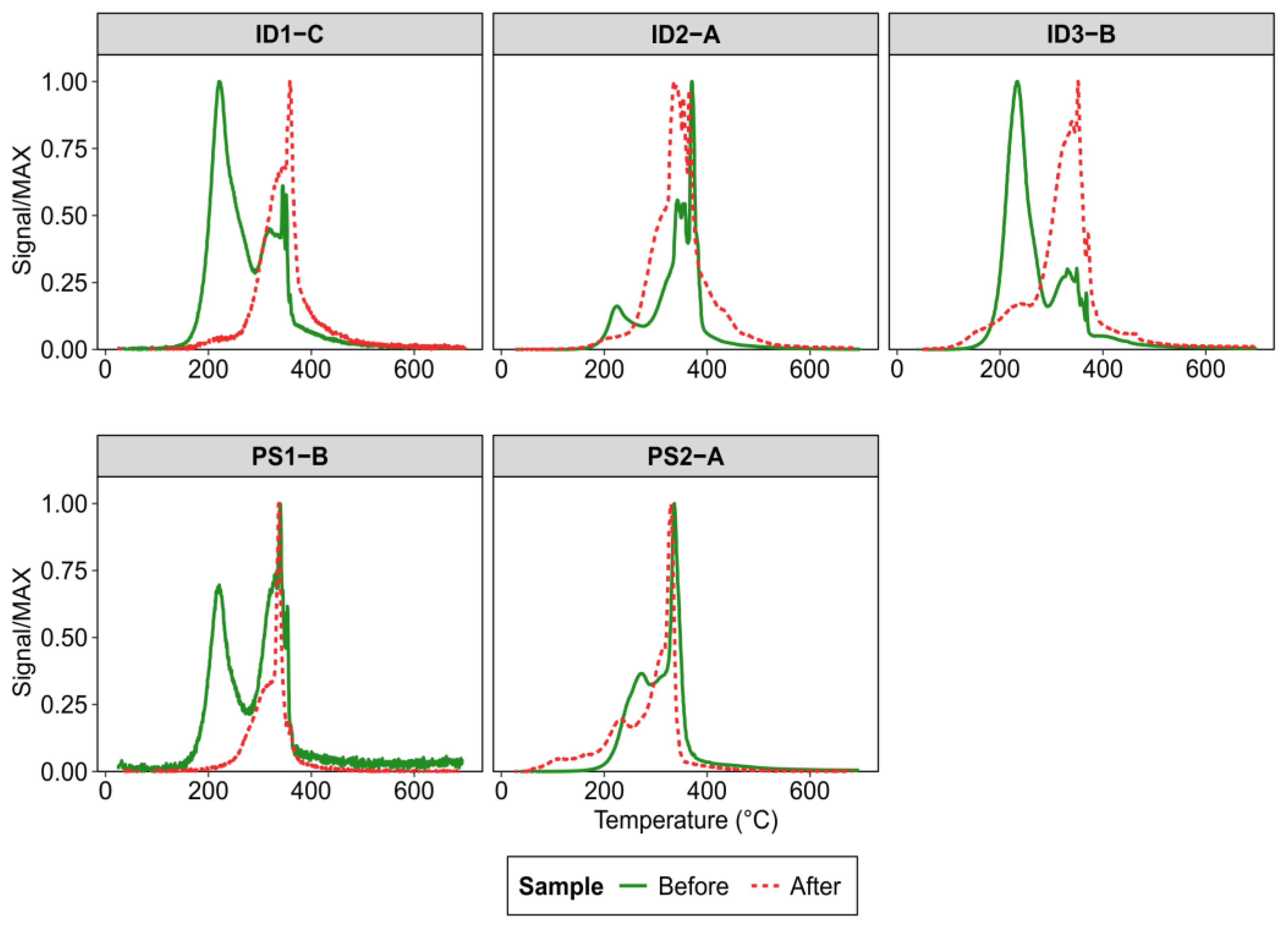
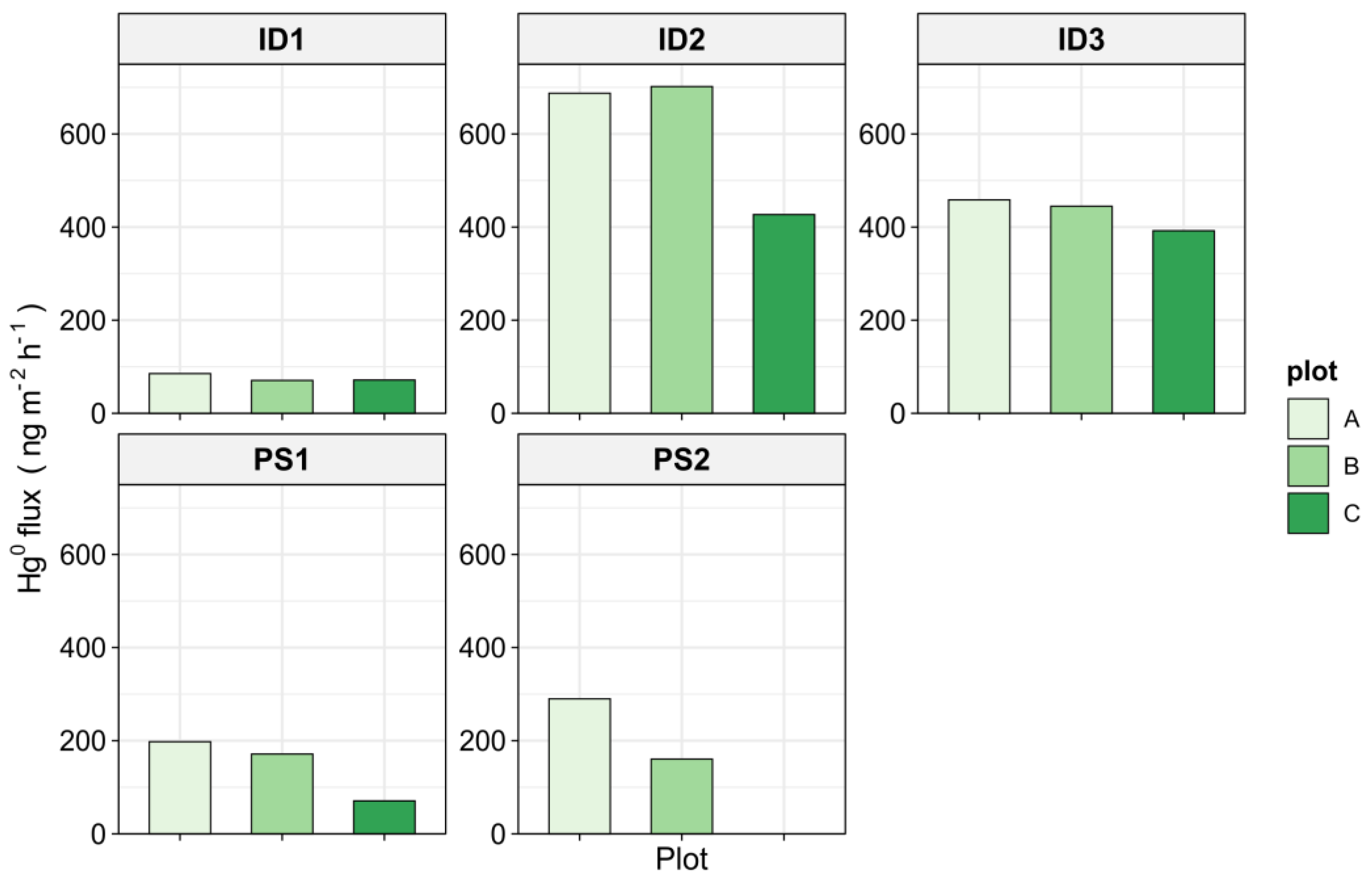
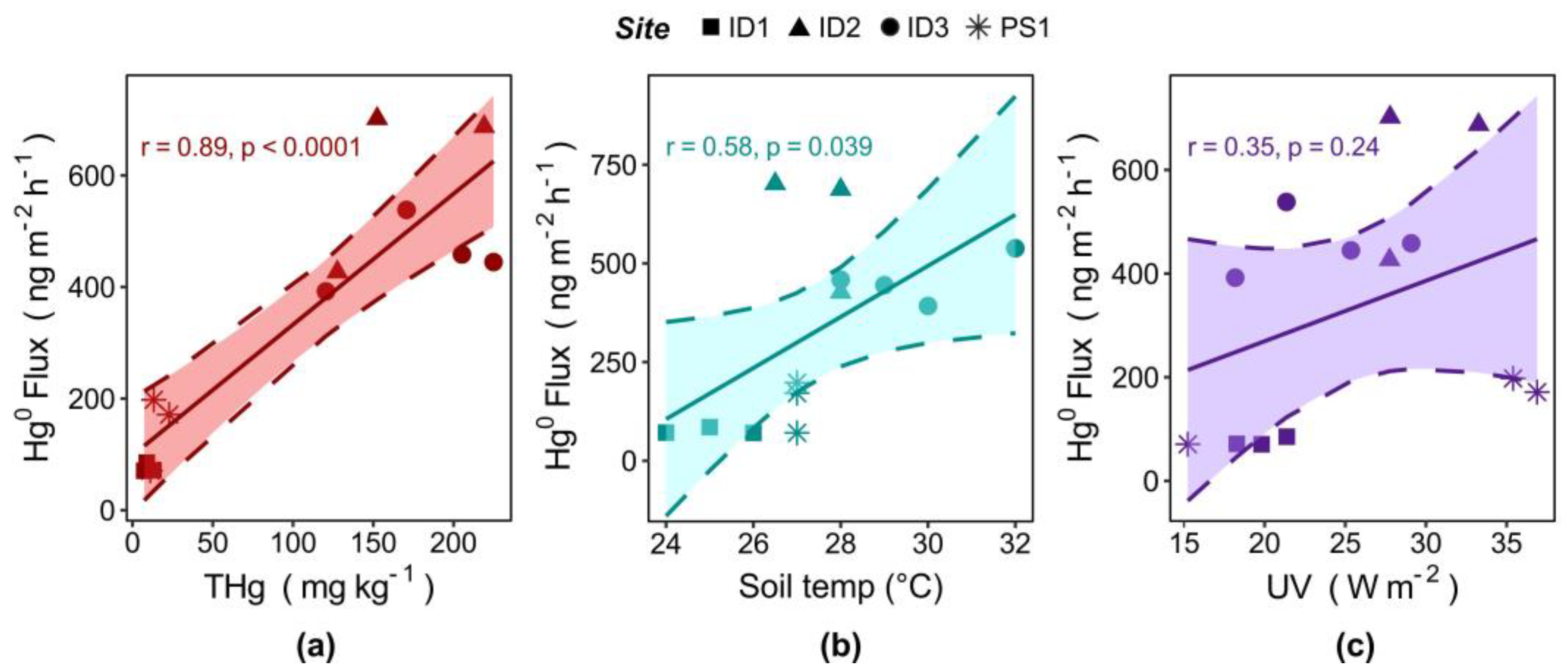
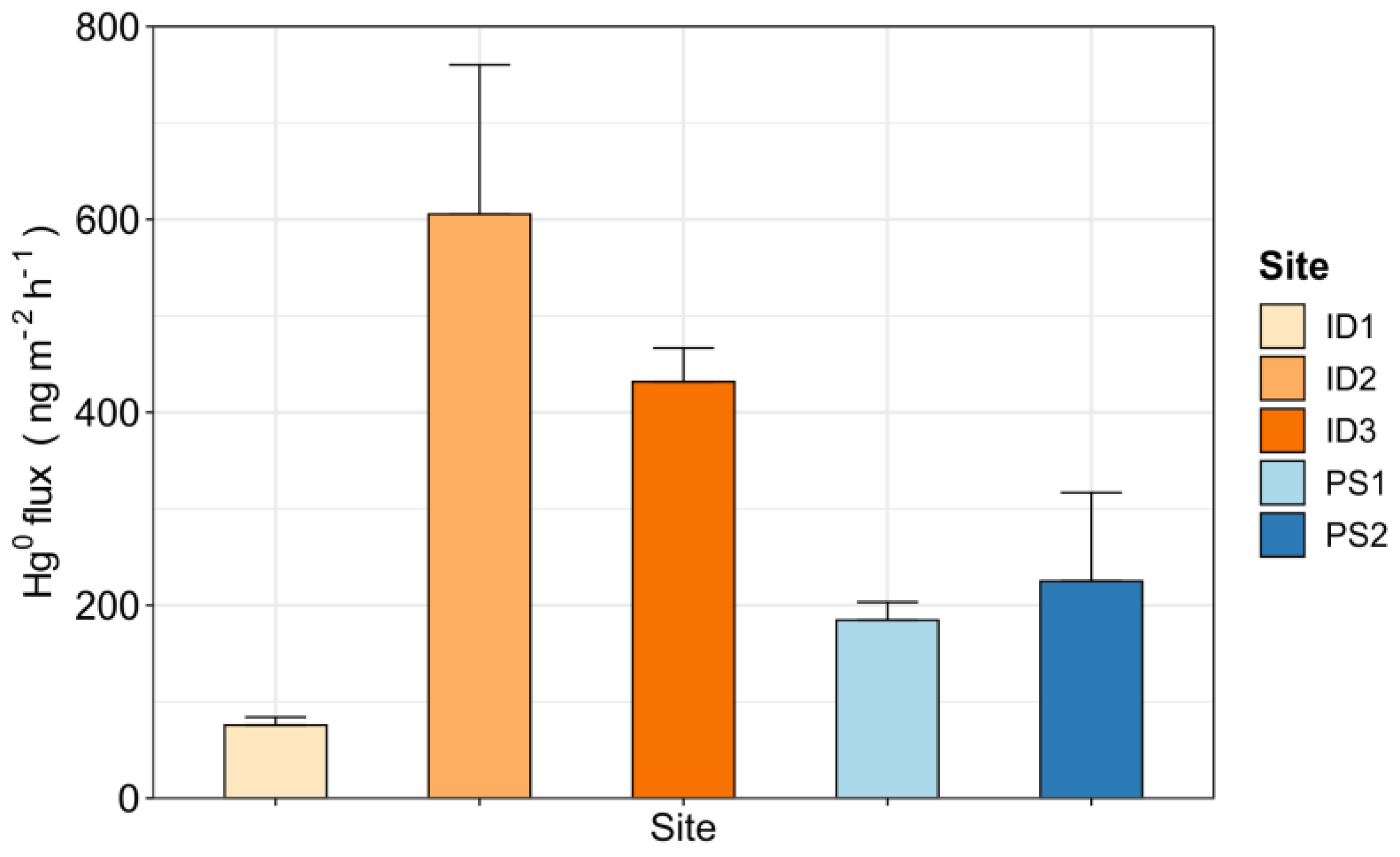
| Parameter | ID1 | ID2 | ID3 | PS1 | PS2 |
|---|---|---|---|---|---|
| pH | 7.72 ± 0.09 | 7.89 ± 0.08 | 7.19 ± 0.50 | 7.75 ± 0.05 | 4.72 ± 1.10 |
| LOI550 (%) | 11.5 ± 1.0 | 8.88 ± 1.73 | 13.4 ± 1.2 | 11.1 ± 1.0 | 39.0 ± 24.8 |
| Sand (%) | 40.7 ± 3.4 | 34.8 ± 4.2 | 41.9 ± 0.7 | 40.3 ± 5.7 | 61.1 ± 18.8 |
| Silt (%) | 55.0 ± 2.9 | 60.9 ± 3.6 | 53.9 ± 0.9 | 54.4 ± 5.8 | 35.9 ± 15.8 |
| Clay (%) | 4.3 ± 0.5 | 4.3 ± 0.6 | 4.2 ± 0.3 | 5.3 ± 0.6 | 2.9 ± 3.0 |
| THg (mg kg−1) | 9.76 ± 2.96 | 166 ± 47 | 180 ± 46 | 15.7 ± 6.3 | 6466 ± 4119 |
| α-HgS (%) | 51.2 ± 2.0 | 81.5 ± 5.1 | 31.4 ± 7.2 | 59.4 ± 6.1 | 53.6 ± 6.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floreani, F.; Pavoni, E.; Gosar, M.; Covelli, S. Evasion of Gaseous Elemental Mercury from Forest and Urban Soils Contaminated by Historical and Modern Ore Roasting Processes (Idrija, Slovenia). Atmosphere 2023, 14, 1036. https://doi.org/10.3390/atmos14061036
Floreani F, Pavoni E, Gosar M, Covelli S. Evasion of Gaseous Elemental Mercury from Forest and Urban Soils Contaminated by Historical and Modern Ore Roasting Processes (Idrija, Slovenia). Atmosphere. 2023; 14(6):1036. https://doi.org/10.3390/atmos14061036
Chicago/Turabian StyleFloreani, Federico, Elena Pavoni, Mateja Gosar, and Stefano Covelli. 2023. "Evasion of Gaseous Elemental Mercury from Forest and Urban Soils Contaminated by Historical and Modern Ore Roasting Processes (Idrija, Slovenia)" Atmosphere 14, no. 6: 1036. https://doi.org/10.3390/atmos14061036
APA StyleFloreani, F., Pavoni, E., Gosar, M., & Covelli, S. (2023). Evasion of Gaseous Elemental Mercury from Forest and Urban Soils Contaminated by Historical and Modern Ore Roasting Processes (Idrija, Slovenia). Atmosphere, 14(6), 1036. https://doi.org/10.3390/atmos14061036











