Cardiovascular and Respiratory Health Effects of Fine Particulate Matters (PM2.5): A Review on Time Series Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Stage 1: Identifying the Research Question
2.2. Stage 2: Identifying Relevant Studies
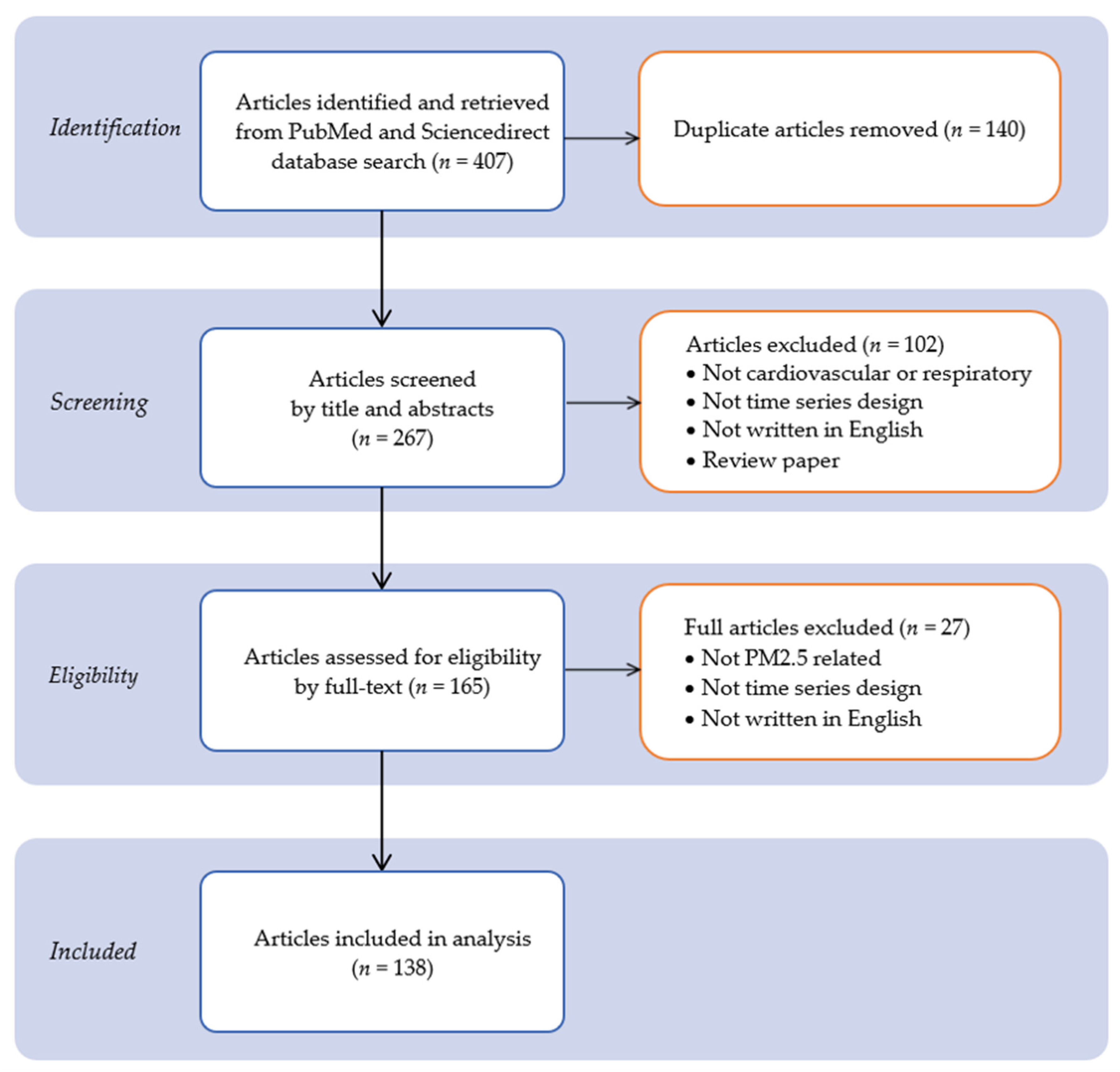
2.3. Stage 3: Study Selection
- Search strategy for identifying only articles published in English. Keywords used Boolean operators (OR, AND) during the search process;
- cardiovascular OR CVD, respiratory, cardiorespiratory, AND;
- PM2.5 OR particulate matter 2.5, AND;
- time series.
- 2.
- All articles were restricted to those that have been published from January 2016 to January 2021 to limit the search to recent publications within the previous 5 years;
- 3.
- To avoid duplications and biases, the researchers reviewed together each of the articles to screen titles and abstracts from the databases for eligibility. In the next step, two reviewers independently reviewed and evaluated the full-text articles. Only primary research articles were evaluated to confirm inclusion based on the methodology used on the exposure and association assessment criteria.
2.4. Stage 4: Charting the Data
2.5. Stage 5: Collating, Reporting, and Summarizing the Results
- Authors names;
- Year of publication;
- Duration and year of study;
- Study location by country and continent;
- Cardiovascular and respiratory diseases;
- International Statistical Classification of Diseases and Related Health Problems (ICD);
- Health endpoints (either mortality, hospital admissions, hospital visits, or emergency visits);
- Types of susceptible groups (either children, adults, the elderly, or not specified);
- The most frequent design used in time series analysis are the Generalized Additive Model (GAM), Generalized Linear Model (GLM), and Negative Binomial (NB);
- The descriptive of RRs associated with each disease.
2.6. Stage 6: Consultation with Experts
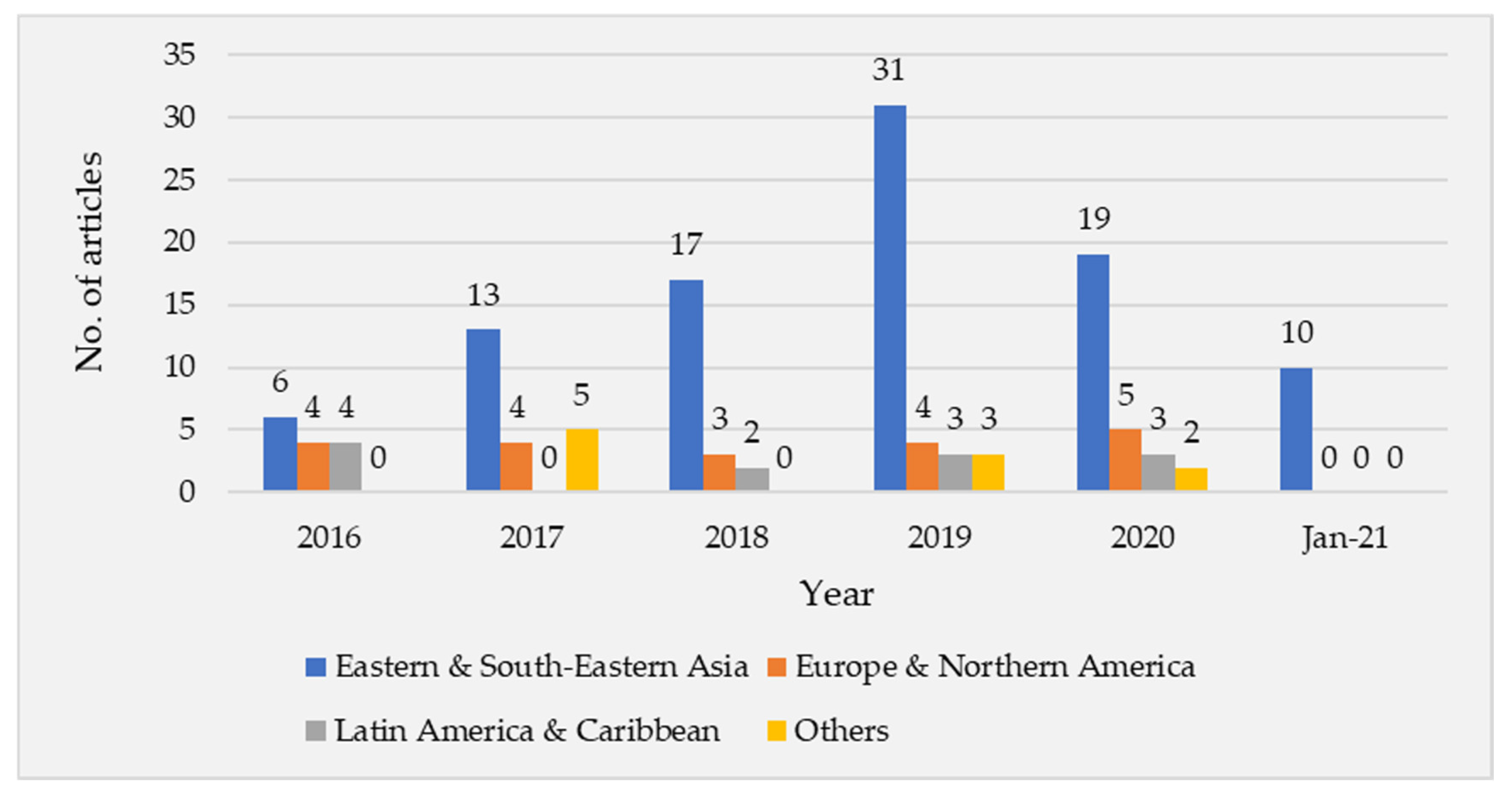
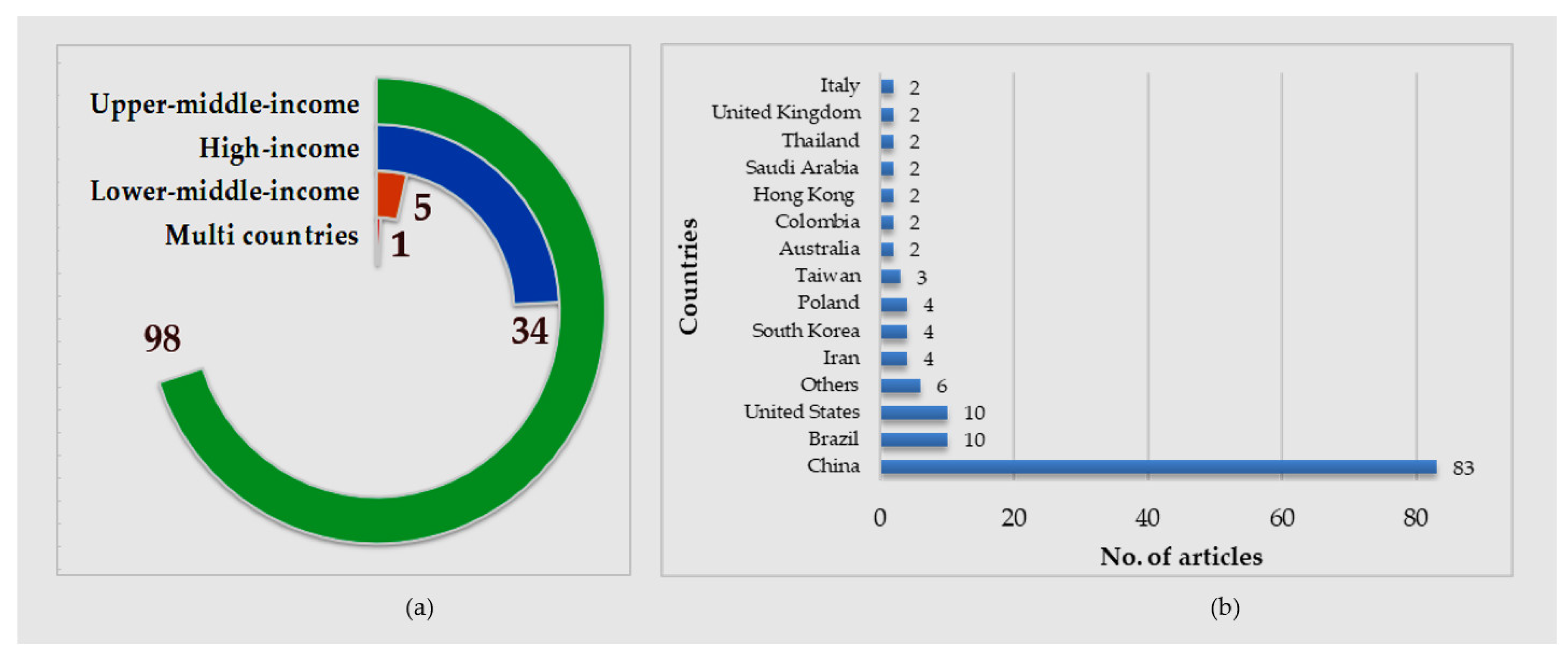
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Sun, J.; Wang, Y.; Li, W.; Zhang, Q.; Wang, W.; Quan, J.; Cao, G.; Wang, J.; Yang, Y.; et al. Factors Contributing to Haze and Fog in China. Chin. Sci. Bull. 2013, 58, 1178–1187. [Google Scholar] [CrossRef]
- Marcantonio, R.; Javeline, D.; Field, S.; Fuentes, A. Global Distribution and Coincidence of Pollution, Climate Impacts, and Health Risk in the Anthropocene. PLoS ONE 2021, 16, e0254060. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.H.; Shimadera, H.; Uranishi, K.; Matsuo, T.; Kondo, A. Numerical Assessment of PM2.5 and O3 Air Quality in Continental Southeast Asia: Impacts of Future Projected Anthropogenic Emission Change and Its Impacts in Combination with Potential Future Climate Change Impacts. Atmos. Environ. 2020, 226, 117398. [Google Scholar] [CrossRef]
- Snow, S.J.; De Vizcaya-Ruiz, A.; Osornio-Vargas, A.; Thomas, R.F.; Schladweiler, M.C.; McGee, J.; Kodavanti, U.P. The Effect of Composition, Size, and Solubility on Acute Pulmonary Injury in Rats Following Exposure to Mexico City Ambient Particulate Matter Samples. J. Toxicol. Environ. Health A 2014, 77, 1164–1182. [Google Scholar] [CrossRef]
- Araujo, J.A.; Nel, A.E. Particulate Matter and Atherosclerosis: Role of Particle Size, Composition and Oxidative Stress. Part. Fibre Toxicol. 2009, 6, 24. [Google Scholar] [CrossRef]
- Veremchuk, L.V.; Vitkina, T.I.; Barskova, L.S.; Gvozdenko, T.A.; Mineeva, E.E. Estimation of the Size Distribution of Suspended Particulate Matters in the Urban Atmospheric Surface Layer and Its Influence on Bronchopulmonary Pathology. Atmosphere 2021, 12, 1010. [Google Scholar] [CrossRef]
- Cachon, F.B.; Cazier, F.; Verdin, A.; Dewaele, D.; Genevray, P.; Delbende, A.; Ayi-Fanou, L.; Aïssi, F.; Sanni, A.; Courcot, D. Physicochemical Characterization of Air Pollution Particulate Matter (PM2.5 and PM > 2.5) in an Urban Area of Cotonou, Benin. Atmosphere 2023, 14, 201. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Mazzoli-Rocha, F.; Fernandes, S.; Einicker-Lamas, M.; Zin, W.A. Roles of Oxidative Stress in Signaling and Inflammation Induced by Particulate Matter. Cell. Biol. Toxicol. 2010, 26, 481–498. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Xing, Y.-F.; Xu, Y.-H.; Shi, M.-H.; Lian, Y.-X. The Impact of PM2.5 on the Human Respiratory System. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar]
- Dastoorpoor, M.; Khanjani, N.; Moradgholi, A.; Sarizadeh, R.; Cheraghi, M.; Estebsari, F. Prenatal Exposure to Ambient Air Pollution and Adverse Pregnancy Outcomes in Ahvaz, Iran: A Generalized Additive Model. Int. Arch. Occup. Environ. Health 2021, 94, 309–324. [Google Scholar] [CrossRef]
- Phosri, A.; Ueda, K.; Phung, V.L.H.; Tawatsupa, B.; Honda, A.; Takano, H. Effects of Ambient Air Pollution on Daily Hospital Admissions for Respiratory and Cardiovascular Diseases in Bangkok, Thailand. Sci. Total. Environ. 2019, 651, 1144–1153. [Google Scholar] [CrossRef]
- Slama, A.; Śliwczyński, A.; Woźnica, J.; Zdrolik, M.; Wiśnicki, B.; Kubajek, J.; Turżańska-Wieczorek, O.; Gozdowski, D.; Wierzba, W.; Franek, E. Impact of Air Pollution on Hospital Admissions with a Focus on Respiratory Diseases: A Time-Series Multi-City Analysis. Environ. Sci. Pollut. Res. 2019, 26, 16998–17009. [Google Scholar] [CrossRef]
- Brook, R.D.; Franklin, B.; Cascio, W.; Hong, Y.; Howard, G.; Lipsett, M.; Luepker, R.; Mittleman, M.; Samet, J.; Smith, S.C.; et al. Air Pollution and Cardiovascular Disease: A Statement for Healthcare Professionals From the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation 2004, 109, 2655–2671. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Mutlu, G.M. Particulate Matter Air Pollution: Effects on the Cardiovascular System. Front. Endocrinol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Mei, X.-D.; Feng, D. Air Pollution and Chronic Airway Diseases: What Should People Know and Do? J. Thorac. Dis. 2016, 8, E31–E40. [Google Scholar]
- Lee, B.-J.; Kim, B.; Lee, K. Air Pollution Exposure and Cardiovascular Disease. Toxicol. Res. 2014, 30, 71–75. [Google Scholar] [CrossRef]
- Kloog, I.; Ridgway, B.; Koutrakis, P.; Coull, B.A.; Schwartz, J.D. Long- and Short-Term Exposure to PM2.5 and Mortality. Epidemiology 2013, 24, 555–561. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Zhang, L.; Yu, C.; Wang, X.; Shi, Z.; Hu, J.; Zhang, Y. Assessing Short-Term Impacts of PM2.5 Constituents on Cardiorespiratory Hospitalizations: Multi-City Evidence from China. Int. J. Hyg. Environ. Health 2022, 240, 113912. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Krewski, D.; Chen, Y.; Burnett, R.; Cakmak, S. Comparison of Time Series and Case-Crossover Analyses of Air Pollution and Hospital Admission Data. Int. J. Epidemiol. 2003, 32, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Slama, A.; Śliwczyński, A.; Woźnica-Pyzikiewicz, J.; Zdrolik, M.; Wiśnicki, B.; Kubajek, J.; Turżańska-Wieczorek, O.; Studnicki, M.; Wierzba, W.; Franek, E. The Short-Term Effects of Air Pollution on Respiratory Disease Hospitalizations in 5 Cities in Poland: Comparison of Time-Series and Case-Crossover Analyses. Environ. Sci. Pollut. Res. Int. 2020, 27, 24582–24590. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Agathokleous, E.; Anenberg, S.C.; De Marco, A.; Paoletti, E.; Calatayud, V. Trends in Urban Air Pollution over the Last Two Decades: A Global Perspective. Sci. Total Environ. 2023, 858, 160064. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, D. Exposure to Outdoor Air Pollution and Its Human Health Outcomes: A Scoping Review. PLoS ONE 2019, 14, e0216550. [Google Scholar] [CrossRef]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological Time Series Studies of PM2.5 and Daily Mortality and Hospital Admissions: A Systematic Review and Meta-Analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.S.Z.S.; Saldiva, P.H.N.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- World Bank How Does the World Bank Classify Countries?—World Bank Data Help Desk. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/378834-how-does-the-world-bank-classify-countries (accessed on 16 February 2022).
- Vuckovic, M.; Schmidt, J. Visual Analytics for Climate Change Detection in Meteorological Time-Series. Forecasting 2021, 3, 276–289. [Google Scholar] [CrossRef]
- Pan, A.; Sarnat, S.E.; Chang, H.H. Time-Series Analysis of Air Pollution and Health Accounting for Covariate-Dependent Overdispersion. Am. J. Epidemiol. 2018, 187, 2698–2704. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman and Hall/CRC: London, UK, 1989; ISBN 978-0-412-31760-6. [Google Scholar]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-0-412-34390-2. [Google Scholar]
- Chai, G.; He, H.; Sha, Y.; Zhai, G.; Zong, S. Effect of PM2.5 on Daily Outpatient Visits for Respiratory Diseases in Lanzhou, China. Sci. Total Environ. 2019, 649, 1563–1572. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Q.; Xu, B.; Xu, Y.; Ding, Z.; Sun, H. Air Pollution and Cardiovascular Mortality in Nanjing, China: Evidence Highlighting the Roles of Cumulative Exposure and Mortality Displacement. Chemosphere 2021, 265, 129035. [Google Scholar] [CrossRef]
- Xu, J.; Geng, W.; Geng, X.; Cui, L.; Ding, T.; Xiao, C.; Zhang, J.; Tang, J.; Zhai, J. Study on the Association between Ambient Air Pollution and Daily Cardiovascular Death in Hefei, China. Environ. Sci. Pollut. Res. 2020, 27, 547–561. [Google Scholar] [CrossRef]
- Lin, H.; Ma, W.; Qiu, H.; Vaughn, M.G.; Nelson, E.J.; Qian, Z.; Tian, L. Is Standard Deviation of Daily PM2.5 Concentration Associated with Respiratory Mortality? Environ. Pollut. 2016, 216, 208–214. [Google Scholar] [CrossRef]
- Lin, H.; Ma, W.; Qiu, H.; Wang, X.; Trevathan, E.; Yao, Z.; Dong, G.-H.; Vaughn, M.G.; Qian, Z.; Tian, L. Using Daily Excessive Concentration Hours to Explore the Short-Term Mortality Effects of Ambient PM2.5 in Hong Kong. Environ. Pollut. 2017, 229, 896–901. [Google Scholar] [CrossRef]
- Yap, J.; Ng, Y.; Yeo, K.K.; Sahlén, A.; Lam, C.S.P.; Lee, V.; Ma, S. Particulate Air Pollution on Cardiovascular Mortality in the Tropics: Impact on the Elderly. Environ. Health 2019, 18, 34. [Google Scholar] [CrossRef]
- Kwon, O.K.; Kim, S.-H.; Kang, S.-H.; Cho, Y.; Oh, I.-Y.; Yoon, C.-H.; Kim, S.-Y.; Kim, O.-J.; Choi, E.-K.; Youn, T.-J.; et al. Association of Short- and Long-Term Exposure to Air Pollution with Atrial Fibrillation. Eur. J. Prev. Cardiol. 2019, 26, 1208–1216. [Google Scholar] [CrossRef]
- Oh, J.; Han, C.; Lee, D.-W.; Jang, Y.; Choi, Y.-J.; Bae, H.J.; Kim, S.; Ha, E.; Hong, Y.-C.; Lim, Y.-H. Short-Term Exposure to Fine Particulate Matter and Hospitalizations for Acute Lower Respiratory Infection in Korean Children: A Time-Series Study in Seven Metropolitan Cities. Int. J. Environ. Res. Public. Health 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Bai, C.-H.; Chuang, K.-J.; Fan, Y.-C.; Chang, T.-P.; Yim, S.H.-L.; Ho, K.-F. Association of Ambient Non-Methane Hydrocarbons Exposure with Respiratory Hospitalizations: A Time Series Study in Taipei, Taiwan. Sci. Total Environ. 2020, 729, 139010. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, C.X.; Dennekamp, M.; Dimitriadis, C.; Straney, L.; Ikin, J.; Abramson, M.J. The Association of Coal Mine Fire Smoke with Hospital Emergency Presentations and Admissions: Time Series Analysis of Hazelwood Health Study. Chemosphere 2020, 253, 126667. [Google Scholar] [CrossRef] [PubMed]
- Salimi, F.; Henderson, S.B.; Morgan, G.G.; Jalaludin, B.; Johnston, F.H. Ambient Particulate Matter, Landscape Fire Smoke, and Emergency Ambulance Dispatches in Sydney, Australia. Environ. Int. 2017, 99, 208–212. [Google Scholar] [CrossRef]
- Strosnider, H.M.; Chang, H.H.; Darrow, L.A.; Liu, Y.; Vaidyanathan, A.; Strickland, M.J. Age-Specific Associations of Ozone and Fine Particulate Matter with Respiratory Emergency Department Visits in the United States. Am. J. Respir. Crit. Care Med. 2019, 199, 882–890. [Google Scholar] [CrossRef]
- Krall, J.R.; Mulholland, J.A.; Russell, A.G.; Balachandran, S.; Winquist, A.; Tolbert, P.E.; Waller, L.A.; Sarnat, S.E. Associations between Source-Specific Fine Particulate Matter and Emergency Department Visits for Respiratory Disease in Four U.S. Cities. Environ. Health Perspect. 2017, 125, 97–103. [Google Scholar] [CrossRef]
- Ye, D.; Klein, M.; Mulholland, J.A.; Russell, A.G.; Weber, R.; Edgerton, E.S.; Chang, H.H.; Sarnat, J.A.; Tolbert, P.E.; Ebelt Sarnat, S. Estimating Acute Cardiovascular Effects of Ambient PM2.5 Metals. Environ. Health Perspect. 2018, 126, 027007. [Google Scholar] [CrossRef]
- Ebisu, K.; Malig, B.; Hasheminassab, S.; Sioutas, C. Age-Specific Seasonal Associations between Acute Exposure to PM2.5 Sources and Cardiorespiratory Hospital Admissions in California. Atmos. Environ. 2019, 218, 117029. [Google Scholar] [CrossRef]
- Blomberg, A.J.; Coull, B.A.; Jhun, I.; Vieira, C.L.Z.; Zanobetti, A.; Garshick, E.; Schwartz, J.; Koutrakis, P. Effect Modification of Ambient Particle Mortality by Radon: A Time Series Analysis in 108 U.S. Cities. J. Air Waste Manag. Assoc. 2019, 69, 266–276. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Hwang, S.-A.; Kinney, P.L.; Lin, S. Seasonal and Temperature Modifications of the Association between Fine Particulate Air Pollution and Cardiovascular Hospitalization in New York State. Sci. Total Environ. 2017, 578, 626–632. [Google Scholar] [CrossRef]
- Bi, J.; D’Souza, R.R.; Rich, D.Q.; Hopke, P.K.; Russell, A.G.; Liu, Y.; Chang, H.H.; Ebelt, S. Temporal Changes in Short-Term Associations between Cardiorespiratory Emergency Department Visits and PM2.5 in Los Angeles, 2005 to 2016. Environ. Res. 2020, 190, 109967. [Google Scholar] [CrossRef]
- Qiu, X.; Wei, Y.; Wang, Y.; Di, Q.; Sofer, T.; Awad, Y.A.; Schwartz, J. Inverse Probability Weighted Distributed Lag Effects of Short-Term Exposure to PM2.5 and Ozone on CVD Hospitalizations in New England Medicare Participants—Exploring the Causal Effects. Environ. Res. 2020, 182, 109095. [Google Scholar] [CrossRef]
- Yitshak-Sade, M.; Bobb, J.F.; Schwartz, J.D.; Kloog, I.; Zanobetti, A. The Association between Short and Long-Term Exposure to PM2.5 and Temperature and Hospital Admissions in New England and the Synergistic Effect of the Short-Term Exposures. Sci. Total Environ. 2018, 639, 868–875. [Google Scholar] [CrossRef]
- Pearce, J.L.; Neelon, B.; Bozigar, M.; Hunt, K.J.; Commodore, A.; Vena, J. Associations between Multipollutant Day Types and Select Cardiorespiratory Outcomes in Columbia, South Carolina, 2002 to 2013. Environ. Epidemiol. 2018, 2, e030. [Google Scholar] [CrossRef]
- Solimini, A.; Renzi, M. Association between Air Pollution and Emergency Room Visits for Atrial Fibrillation. Int. J. Environ. Res. Public Health 2017, 14, 661. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Struniawski, K.; Pogorzelski, S.; Bachórzewska-Gajewska, H.; Dobrzycki, S. Gender Differences in Association between Air Pollution and Daily Mortality in the Capital of the Green Lungs of Poland–Population-Based Study with 2,953,000 Person-Years of Follow-Up. J. Clin. Med. 2020, 9, 2351. [Google Scholar] [CrossRef]
- Kollanus, V.; Tiittanen, P.; Niemi, J.V.; Lanki, T. Effects of Long-Range Transported Air Pollution from Vegetation Fires on Daily Mortality and Hospital Admissions in the Helsinki Metropolitan Area, Finland. Environ. Res. 2016, 151, 351–358. [Google Scholar] [CrossRef]
- Borsi, S.H.; Khanjani, N.; Nejad, H.Y.; Riahi, A.; Sekhavatpour, Z.; Raji, H.; Dastoorpoor, M. Air Pollution and Hospital Admissions Due to Deep Vein Thrombosis (DVT) in Ahvaz, Iran. Heliyon 2020, 6, e04814. [Google Scholar] [CrossRef]
- Tapia, V.; Steenland, K.; Sarnat, S.E.; Vu, B.; Liu, Y.; Sánchez-Ccoyllo, O.; Vasquez, V.; Gonzales, G.F. Time-Series Analysis of Ambient PM2.5 and Cardiorespiratory Emergency Room Visits in Lima, Peru during 2010–2016. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 680–688. [Google Scholar] [CrossRef]
- Zhang, J.; Mauzerall, D.L.; Zhu, T.; Liang, S.; Ezzati, M.; Remais, J. Environmental Health in China: Challenges to Achieving Clean Air and Safe Water. Lancet 2010, 375, 1110–1119. [Google Scholar] [CrossRef]
- Mou, Y.; Song, Y.; Xu, Q.; He, Q.; Hu, A. Influence of Urban-Growth Pattern on Air Quality in China: A Study of 338 Cities. Int. J. Environ. Res. Public. Health 2018, 15, 1805. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, R.; Zhou, Y.; Lin, B.; Fu, L.; He, K.; Hao, J. On-Road Vehicle Emission Control in Beijing: Past, Present, and Future. Environ. Sci. Technol. 2011, 45, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Health Effects Institute Burden of Disease Attributable to Coal-Burning and Other Air Pollution Sources in China. Available online: https://www.healtheffects.org/publication/burden-disease-attributable-coal-burning-and-other-air-pollution-sources-china (accessed on 14 February 2022).
- Huang, R.-J.; Zhang, Y.; Bozzetti, C.; Ho, K.-F.; Cao, J.-J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High Secondary Aerosol Contribution to Particulate Pollution during Haze Events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zheng, L.; Lu, M.; Gui, L.; Xu, D.; Wu, W.; Liu, Y. Acute Effects of Ambient Particulate Matter Pollution on Hospital Admissions for Mental and Behavioral Disorders: A Time-Series Study in Shijiazhuang, China. Sci. Total. Environ. 2018, 636, 205–211. [Google Scholar] [CrossRef]
- Requia, W.J.; Jhun, I.; Coull, B.A.; Koutrakis, P. Climate Impact on Ambient PM2.5 Elemental Concentration in the United States: A Trend Analysis over the Last 30 Years. Environ. Int. 2019, 131, 104888. [Google Scholar] [CrossRef]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-Term Air Pollution Exposure and Cardio- Respiratory Mortality: A Review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef]
- Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Xun, W.W.; Katsouyanni, K.; Dimakopoulou, K.; Brunekreef, B.; Weinmayr, G.; Hoffmann, B.; et al. Long-Term Exposure to Air Pollution and Cardiovascular Mortality: An Analysis of 22 European Cohorts. Epidemiology 2014, 25, 368–378. [Google Scholar] [CrossRef]
- Bell, M.L.; Samet, J.M.; Dominici, F. Time-Series Studies of Particulate Matter. Annu. Rev. Public. Health 2004, 25, 247–280. [Google Scholar] [CrossRef]
- WHO. Ambient (Outdoor) Air Quality Database: Summary Results, Update 2018; World Health Organization (WHO): Geneva, Switzerland, 2018. [Google Scholar]
- Department of Environment; Ministry of Natural Resources, Environment and Climate Change; Department of Environmen Air Quality Standards. Available online: https://www.doe.gov.my/en/2021/12/15/air-quality-standards/ (accessed on 3 March 2023).
- Rahman, E.A.; Hamzah, F.M.; Latif, M.T.; Dominick, D. Assessment of PM2.5 Patterns in Malaysia Using the Clustering Method. Aerosol Air Qual. Res. 2022, 22, 210161. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Pinheiro, S.; Junger, W.; Ignotti, E.; Hacon, S. Variabilidade Climática Aumenta a Morbimortalidade Associada Ao Material Particulado. Rev. Saúde Pública 2017, 51, 91. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A Global Response Is Needed. Bull. World Health Organ. 2016, 94, 634–634A. [Google Scholar] [CrossRef]
- Topaz, M.; Shafran-Topaz, L.; Bowles, K.H. ICD-9 to ICD-10: Evolution, Revolution, and Current Debates in the United States. Perspect. Health Inf. Manag. 2013, 10, 1d. [Google Scholar]
- Otero Varela, L.; Doktorchik, C.; Wiebe, N.; Quan, H.; Eastwood, C. Exploring the Differences in ICD and Hospital Morbidity Data Collection Features across Countries: An International Survey. BMC Health Serv. Res. 2021, 21, 308. [Google Scholar] [CrossRef]
- Ito, K.; Mathes, R.; Ross, Z.; Nádas, A.; Thurston, G.; Matte, T. Fine Particulate Matter Constituents Associated with Cardiovascular Hospitalizations and Mortality in New York City. Environ. Health Perspect. 2011, 119, 467–473. [Google Scholar] [CrossRef]
- Chu, H.; Xin, J.; Yuan, Q.; Zhang, X.; Pan, W.; Zeng, X.; Chen, Y.; Ma, G.; Ge, Y.; Du, M.; et al. Evaluation of Vulnerable PM2.5-Exposure Individuals: A Repeated-Measure Study in an Elderly Population. Environ. Sci. Pollut. Res. Int. 2018, 25, 11833–11840. [Google Scholar] [CrossRef]
- Li, S.; Cao, S.; Duan, X.; Zhang, Y.; Gong, J.; Xu, X.; Guo, Q.; Meng, X.; Bertrand, M.; Zhang, J.J. Long-Term Exposure to PM2.5 and Children’s Lung Function: A Dose-Based Association Analysis. J. Thorac. Dis. 2020, 12, 6379–6395. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Kwong, J.C.; Burnett, R.T.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Bai, L.; McLaughlin, J.; Chen, H. Long-Term Exposure to Air Pollution and Mortality in a Prospective Cohort: The Ontario Health Study. Environ. Int. 2021, 154, 106570. [Google Scholar] [CrossRef]
- Bravo, M.A.; Ebisu, K.; Dominici, F.; Wang, Y.; Peng, R.D.; Bell, M.L. Airborne Fine Particles and Risk of Hospital Admissions for Understudied Populations: Effects by Urbanicity and Short-Term Cumulative Exposures in 708 U.S. Counties. Environ. Health Perspect. 2017, 125, 594–601. [Google Scholar] [CrossRef]
- Çapraz, Ö.; Deniz, A.; Doğan, N. Effects of Air Pollution on Respiratory Hospital Admissions in İstanbul, Turkey, 2013 to 2015. Chemosphere 2017, 181, 544–550. [Google Scholar] [CrossRef]
- Chen, R.; Gao, Q.; Sun, J.; Yang, H.; Li, Y.; Kang, F.; Wu, W. Short-Term Effects of Particulate Matter Exposure on Emergency Room Visits for Cardiovascular Disease in Lanzhou, China: A Time Series Analysis. Environ. Sci. Pollut. Res. 2020, 27, 9327–9335. [Google Scholar] [CrossRef]
- Kowalska, M.; Skrzypek, M.; Kowalski, M.; Cyrys, J. Effect of NOx and NO2 Concentration Increase in Ambient Air to Daily Bronchitis and Asthma Exacerbation, Silesian Voivodeship in Poland. Int. J. Environ. Res. Public Health 2020, 17, 754. [Google Scholar] [CrossRef] [PubMed]
- Leepe, K.A.; Li, M.; Fang, X.; Hiyoshi, A.; Cao, Y. Acute Effect of Daily Fine Particulate Matter Pollution on Cerebrovascular Mortality in Shanghai, China: A Population-Based Time Series Study. Environ. Sci. Pollut. Res. Int. 2019, 26, 25491–25499. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yang, H.; Wang, J.; An, Z.; Li, J.; Wu, Z.; Zhao, Q.; Li, H.; Zhai, D.; Liu, Y.; et al. Acute Effects of Ambient Air Pollution on Outpatients with Chronic Rhinitis in Xinxiang, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 9889–9897. [Google Scholar] [CrossRef] [PubMed]
- De Moura Menezes, R.A.; Pavanitto, D.R.; Nascimento, L.F.C. Different Response to Exposure to Air Pollutants in Girls and Boys. Rev. Paul Pediatr. 2019, 37, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Nayebare, S.R.; Aburizaiza, O.S.; Siddique, A.; Carpenter, D.O.; Zeb, J.; Aburizaiza, A.J.; Pantea, C.; Hussain, M.M.; Khwaja, H.A. Association of Fine Particulate Air Pollution with Cardiopulmonary Morbidity in Western Coast of Saudi Arabia. Saudi Med. J. 2017, 38, 905–912. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, L.; Zhou, L.; Pan, J. Coarse Particles (PM2.5-10) and Cause-Specific Hospitalizations in Southwestern China: Association, Attributable Risk and Economic Costs. Environ. Res. 2020, 190, 110004. [Google Scholar] [CrossRef]
- Shan, W.; Lu, Y.; Guo, Y.; Li, Y.; Xu, L.; Cao, L. Short-Term Association between Particular Matter Air Pollution and Pediatric Clinical Visits for Wheezing in a Subarea of Shanghai. Environ. Sci. Pollut. Res. Int. 2016, 23, 19201–19211. [Google Scholar] [CrossRef]
- Vahedian, M.; Khanjani, N.; Mirzaee, M.; Koolivand, A. Associations of Short-Term Exposure to Air Pollution with Respiratory Hospital Admissions in Arak, Iran. J. Environ. Health Sci. Eng. 2017, 15, 17. [Google Scholar] [CrossRef]
- Wang, M.; Chen, J.; Zhang, Z.; Yu, P.; Gan, W.; Tan, Z.; Bao, J. Associations between Air Pollution and Outpatient Visits for Arrhythmia in Hangzhou, China. BMC Public Health 2020, 20, 1524. [Google Scholar] [CrossRef]
- Wu, T.; Ma, Y.; Wu, X.; Bai, M.; Peng, Y.; Cai, W.; Wang, Y.; Zhao, J.; Zhang, Z. Association between Particulate Matter Air Pollution and Cardiovascular Disease Mortality in Lanzhou, China. Environ. Sci. Pollut. Res. Int. 2019, 26, 15262–15272. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, A.; Liang, S.; Qi, Q.; Jiang, L.; Ye, Y. The Association between Air Pollution and Population Health Risk for Respiratory Infection: A Case Study of Shenzhen, China. Int. J. Environ. Res. Public Health 2017, 14, 950. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Chen, Q.; Jiang, Y.; Chu, C.; Ding, Y.; Yu, Y.; Fan, Y.; Shi, J.; Luo, Y.; et al. The Relationship between Air Quality and Respiratory Pathogens among Children in Suzhou City. Ital. J. Pediatr. 2019, 45, 123. [Google Scholar] [CrossRef]
- Zhang, Z.; Chai, P.; Wang, J.; Ye, Z.; Shen, P.; Lu, H.; Jin, M.; Gu, M.; Li, D.; Lin, H.; et al. Association of Particulate Matter Air Pollution and Hospital Visits for Respiratory Diseases: A Time-Series Study from China. Environ. Sci. Pollut. Res. Int. 2019, 26, 12280–12287. [Google Scholar] [CrossRef]
- Zhu, L.; Ge, X.; Chen, Y.; Zeng, X.; Pan, W.; Zhang, X.; Ben, S.; Yuan, Q.; Xin, J.; Shao, W.; et al. Short-Term Effects of Ambient Air Pollution and Childhood Lower Respiratory Diseases. Sci. Rep. 2017, 7, 4414. [Google Scholar] [CrossRef]
- Bono, R.; Romanazzi, V.; Bellisario, V.; Tassinari, R.; Trucco, G.; Urbino, A.; Cassardo, C.; Siniscalco, C.; Marchetti, P.; Marcon, A. Air Pollution, Aeroallergens and Admissions to Pediatric Emergency Room for Respiratory Reasons in Turin, Northwestern Italy. BMC Public Health 2016, 16, 722. [Google Scholar] [CrossRef]
- Cai, J.; Yu, S.; Pei, Y.; Peng, C.; Liao, Y.; Liu, N.; Ji, J.; Cheng, J. Association between Airborne Fine Particulate Matter and Residents’ Cardiovascular Diseases, Ischemic Heart Disease and Cerebral Vascular Disease Mortality in Areas with Lighter Air Pollution in China. Int. J. Environ. Res. Public Health 2018, 15, 1918. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, H.; Zhao, Y. Ambient Air Pollution and Daily Hospital Admissions for Respiratory System–Related Diseases in a Heavy Polluted City in Northeast China. Environ. Sci. Pollut. Res. 2020, 27, 10055–10064. [Google Scholar] [CrossRef]
- Ferreira, T.; Forti, M.; de Freitas, C.; Nascimento, F.; Junger, W.; Gouveia, N. Effects of Particulate Matter and Its Chemical Constituents on Elderly Hospital Admissions Due to Circulatory and Respiratory Diseases. Int. J. Environ. Res. Public Health 2016, 13, 947. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Wu, C.; Lin, X.; Zhou, Q.; Ji, S.; Yang, S.; Zhang, X.; Liu, B. Temporal Cross-Correlations between Air Pollutants and Outpatient Visits for Respiratory and Circulatory System Diseases in Fuzhou, China. BMC Public Health 2020, 20, 1131. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Chen, R.; Zhou, Y.; Meng, X.; Hong, J.; Cao, L.; Lu, Y.; Dong, X.; Xia, M.; et al. Associations of Short-Term Exposure to Air Pollution and Emergency Department Visits for Pediatric Asthma in Shanghai, China. Chemosphere 2021, 263, 127856. [Google Scholar] [CrossRef]
- Luong, L.T.M.; Dang, T.N.; Thanh Huong, N.T.; Phung, D.; Tran, L.K.; Van Dung, D.; Thai, P.K. Particulate Air Pollution in Ho Chi Minh City and Risk of Hospital Admission for Acute Lower Respiratory Infection (ALRI) among Young Children. Environ. Pollut. 2020, 257, 113424. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yue, L.; Liu, J.; He, X.; Li, L.; Niu, J.; Luo, B. Association of Air Pollution with Outpatient Visits for Respiratory Diseases of Children in an Ex-Heavily Polluted Northwestern City, China. BMC Public Health 2020, 20, 816. [Google Scholar] [CrossRef] [PubMed]
- Nayebare, S.R.; Aburizaiza, O.S.; Siddique, A.; Carpenter, D.O.; Arden Pope, C.; Mirza, H.M.; Zeb, J.; Aburiziza, A.J.; Khwaja, H.A. Fine Particles Exposure and Cardiopulmonary Morbidity in Jeddah: A Time-Series Analysis. Sci. Total Environ. 2019, 647, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.C.; Nascimento, L.F.C.; Almeida, A.A.; dos Santos Targa, M.; Cesar, A.C.G. Fine Particulate Matter and Ischemic Heart Diseases Inrelation to Sex. An Ecological Time Series Study. Sao Paulo Med. J. 2019, 137, 60–65. [Google Scholar] [CrossRef]
- Song, J.; Lu, M.; Zheng, L.; Liu, Y.; Xu, P.; Li, Y.; Xu, D.; Wu, W. Acute Effects of Ambient Air Pollution on Outpatient Children with Respiratory Diseases in Shijiazhuang, China. BMC Pulm. Med. 2018, 18, 150. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Wu, Y.; Si, Y.; Li, M.; Wu, Y.; Wang, X.; Wang, M.; Chen, L.; Wei, C.; et al. Ambient Particulate Matter Pollution and Adult Hospital Admissions for Pneumonia in Urban China: A National Time Series Analysis for 2014 through 2017. PLoS Med. 2019, 16, e1003010. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Su, H.; Ho, H.C.; Song, Y.; Zheng, H.; Hossain, M.Z.; Khan, M.A.; Bogale, D.; Zhang, H.; et al. Ambient Particulate Matter (PM1, PM2.5, PM10) and Childhood Pneumonia: The Smaller Particle, the Greater Short-Term Impact? Sci. Total Environ. 2021, 772, 145509. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Zhang, Y.; Huang, X.; Duan, X.; Chen, D.; Ou, Y.; Tang, L.; Liu, S.; Hu, W.; et al. Association of Change in Air Quality with Hospital Admission for Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Guangdong, China: A Province-Wide Ecological Study. Ecotoxicol. Environ. Saf. 2021, 208, 111590. [Google Scholar] [CrossRef]
- Yang, H.; Yan, C.; Li, M.; Zhao, L.; Long, Z.; Fan, Y.; Zhang, Z.; Chen, R.; Huang, Y.; Lu, C.; et al. Short Term Effects of Air Pollutants on Hospital Admissions for Respiratory Diseases among Children: A Multi-City Time-Series Study in China. Int. J. Hyg. Environ. Health 2021, 231, 113638. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, S.; Dong, H.; Ji, M.; Chen, Z.; Li, G.; Yao, X.; Wang, S.-L.; Zhang, Z. Short-Term Effects of Ambient Air Pollutants and Myocardial Infarction in Changzhou, China. Environ. Sci. Pollut. Res. 2018, 25, 22285–22293. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Gou, K.; Wang, R.; Wang, J. The Impact of Air Pollution on Outpatient Visits of Children with Asthma in Xi’an, China. Wilderness Environ. Med. 2021, 32, 47–54. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Lang, L.; Huang, C.; Ma, W.; Lin, H. Ambient Fine and Coarse Particulate Matter Pollution and Respiratory Morbidity in Dongguan, China. Environ. Pollut. 2017, 222, 126–131. [Google Scholar] [CrossRef]
- Zhou, H.; Geng, H.; Dong, C.; Bai, T. The Short-Term Harvesting Effects of Ambient Particulate Matter on Mortality in Taiyuan Elderly Residents: A Time-Series Analysis with a Generalized Additive Distributed Lag Model. Ecotoxicol. Environ. Saf. 2021, 207, 111235. [Google Scholar] [CrossRef]
- Zuo, B.; Liu, C.; Chen, R.; Kan, H.; Sun, J.; Zhao, J.; Wang, C.; Sun, Q.; Bai, H. Associations between Short-Term Exposure to Fine Particulate Matter and Acute Exacerbation of Asthma in Yancheng, China. Chemosphere 2019, 237, 124497. [Google Scholar] [CrossRef]
- Bai, L.; Su, X.; Zhao, D.; Zhang, Y.; Cheng, Q.; Zhang, H.; Wang, S.; Xie, M.; Su, H. Exposure to Traffic-Related Air Pollution and Acute Bronchitis in Children: Season and Age as Modifiers. J. Epidemiol. Community Health 2018, 72, 426–433. [Google Scholar] [CrossRef]
- Chen, R.; Yin, P.; Meng, X.; Liu, C.; Wang, L.; Xu, X.; Ross, J.A.; Tse, L.A.; Zhao, Z.; Kan, H.; et al. Fine Particulate Air Pollution and Daily Mortality. A Nationwide Analysis in 272 Chinese Cities. Am. J. Respir. Crit. Care Med. 2017, 196, 73–81. [Google Scholar] [CrossRef]
- Gong, T.; Sun, Z.; Zhang, X.; Zhang, Y.; Wang, S.; Han, L.; Zhao, D.; Ding, D.; Zheng, C. Associations of Black Carbon and PM2.5 with Daily Cardiovascular Mortality in Beijing, China. Atmos. Environ. 2019, 214, 116876. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Y.; Feng, W.; Wu, J.; Fu, C.; Deng, H.; Huang, J.; Wang, L.; Zheng, M.; Liu, H. Ambient Air Pollution and Risk for Ischemic Stroke: A Short-Term Exposure Assessment in South China. Int. J. Environ. Res. Public Health 2017, 14, 1091. [Google Scholar] [CrossRef]
- Guo, P.; Feng, W.; Zheng, M.; Lv, J.; Wang, L.; Liu, J.; Zhang, Y.; Luo, G.; Zhang, Y.; Deng, C.; et al. Short-Term Associations of Ambient Air Pollution and Cause-Specific Emergency Department Visits in Guangzhou, China. Sci. Total Environ. 2018, 613–614, 306–313. [Google Scholar] [CrossRef]
- Li, D.; Wang, J.; Zhang, Z.; Shen, P.; Zheng, P.; Jin, M.; Lu, H.; Lin, H.; Chen, K. Effects of Air Pollution on Hospital Visits for Pneumonia in Children: A Two-Year Analysis from China. Environ. Sci. Pollut. Res. 2018, 25, 10049–10057. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Yin, P.; Wang, L.; Zhou, M. Ambient Fine Particulate Matter Pollution and Years of Life Lost from Cardiovascular Diseases in 48 Large Chinese Cities: Association, Effect Modification, and Additional Life Gain. Sci. Total Environ. 2020, 735, 139413. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, J.; Yang, H.; Zhao, L.; Liu, Y.; Xu, H.; Fan, Y.; Hong, J.; Long, Z.; Li, X.; et al. Short-Term Exposure to Ambient Particulate Matter and Outpatient Visits for Respiratory Diseases among Children: A Time-Series Study in Five Chinese Cities. Chemosphere 2021, 263, 128214. [Google Scholar] [CrossRef]
- Liang, H.; Qiu, H.; Tian, L. Short-Term Effects of Fine Particulate Matter on Acute Myocardial Infraction Mortality and Years of Life Lost: A Time Series Study in Hong Kong. Sci. Total Environ. 2018, 615, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Ratnapradipa, K.; Wang, X.; Zhang, Y.; Xu, Y.; Yao, Z.; Dong, G.; Liu, T.; Clark, J.; Dick, R.; et al. Hourly Peak Concentration Measuring the PM2.5-Mortality Association: Results from Six Cities in the Pearl River Delta Study. Atmos. Environ. 2017, 161, 27–33. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Zhou, Y.; Feng, A.; Wang, C.; Shi, T. Short-Term Effect of Relatively Low Level Air Pollution on Outpatient Visit in Shennongjia, China. Environ. Pollut. 2019, 245, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xue, X.; Zhou, B.; Zhang, Y.; Sun, B.; Chen, J.; Li, X. Population Susceptibility Differences and Effects of Air Pollution on Cardiovascular Mortality: Epidemiological Evidence from a Time-Series Study. Environ. Sci. Pollut. Res. 2019, 26, 15943–15952. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Y.; Jiang, J.; Luan, H.; Yu, C.; Nan, P.; Luo, B.; You, M. Short-Term Effects of Ambient Air Pollution on Hospitalization for Respiratory Disease in Taiyuan, China: A Time-Series Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2160. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Yu, H.; Wang, L.; Zhu, X.; Chen, M.; Zhou, L.; Deng, R.; Zhang, Y.; Pu, X.; Pan, J. The Burden of Overall and Cause-Specific Respiratory Morbidity Due to Ambient Air Pollution in Sichuan Basin, China: A Multi-City Time-Series Analysis. Environ. Res. 2018, 167, 428–436. [Google Scholar] [CrossRef]
- Qu, F.; Liu, F.; Zhang, H.; Chao, L.; Guan, J.; Li, R.; Yu, F.; Yan, X. Comparison of Air Pollutant-Related Hospitalization Burden from AECOPD in Shijiazhuang, China, between Heating and Non-Heating Season. Environ. Sci. Pollut. Res. 2019, 26, 31225–31233. [Google Scholar] [CrossRef]
- Rodríguez-Villamizar, L.A.; Rojas-Roa, N.Y.; Fernández-Niño, J.A. Short-Term Joint Effects of Ambient Air Pollutants on Emergency Department Visits for Respiratory and Circulatory Diseases in Colombia, 2011–2014. Environ. Pollut. 2019, 248, 380–387. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, J.; Zhang, Q.; Sun, S.; Lei, R.; Zhang, C.; Cheng, H.; Ding, L.; Ding, R.; Xiao, C.; et al. The Short-Term Effect of PM2.5/O3 on Daily Mortality from 2013 to 2018 in Hefei, China. Environ. Geochem. Health 2021, 43, 153–169. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Wu, Y.; Si, Y.; Song, J.; Cao, Y.; Li, M.; Wu, Y.; Wang, X.; Chen, L.; et al. Association between Ambient Fine Particulate Pollution and Hospital Admissions for Cause Specific Cardiovascular Disease: Time Series Study in 184 Major Chinese Cities. BMJ 2019, l6572. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, Y.; Huang, L.; Zhang, H.; Wang, C.; Hu, J. Associations between Daily Outpatient Visits for Respiratory Diseases and Ambient Fine Particulate Matter and Ozone Levels in Shanghai, China. Environ. Pollut. 2018, 240, 754–763. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Zhang, Y.; Huang, X.; Duan, X.; Ou, Y.; Liu, S.; Hu, W.; Liao, C.; Zheng, Y.; et al. Association of Hospital Admission for Bronchiectasis with Air Pollution: A Province-Wide Time-Series Study in Southern China. Int. J. Hyg. Environ. Health 2021, 231, 113654. [Google Scholar] [CrossRef]
- Yao, C.; Wang, Y.; Williams, C.; Xu, C.; Kartsonaki, C.; Lin, Y.; Zhang, P.; Yin, P.; Lam, K.B.H. The Association between High Particulate Matter Pollution and Daily Cause-Specific Hospital Admissions: A Time-Series Study in Yichang, China. Environ. Sci. Pollut. Res. 2020, 27, 5240–5250. [Google Scholar] [CrossRef]
- Yoo, S.-E.; Park, J.-S.; Lee, S.H.; Park, C.-H.; Lee, C.-W.; Lee, S.-B.; Yu, S.D.; Kim, S.-Y.; Kim, H. Comparison of Short-Term Associations between PM2.5 Components and Mortality across Six Major Cities in South Korea. Int. J. Environ. Res. Public Health 2019, 16, 2872. [Google Scholar] [CrossRef]
- Zheng, P.; Wang, J.; Zhang, Z.; Shen, P.; Chai, P.; Li, D.; Jin, M.; Tang, M.-L.; Lu, H.; Lin, H.; et al. Air Pollution and Hospital Visits for Acute Upper and Lower Respiratory Infections among Children in Ningbo, China: A Time-Series Analysis. Environ. Sci. Pollut. Res. 2017, 24, 18860–18869. [Google Scholar] [CrossRef]
- Amsalu, E.; Wang, T.; Li, H.; Liu, Y.; Wang, A.; Liu, X.; Tao, L.; Luo, Y.; Zhang, F.; Yang, X.; et al. Acute Effects of Fine Particulate Matter (PM2.5) on Hospital Admissions for Cardiovascular Disease in Beijing, China: A Time-Series Study. Environ. Health 2019, 18, 70. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Samoli, E.; Analitis, A.; Fuller, G.W.; Green, D.C.; Anderson, H.R.; Purdie, E.; Dunster, C.; Aitlhadj, L.; Kelly, F.J.; et al. Short-Term Associations between Particle Oxidative Potential and Daily Mortality and Hospital Admissions in London. Int. J. Hyg. Environ. Health 2016, 219, 566–572. [Google Scholar] [CrossRef]
- Cai, J.; Peng, C.; Yu, S.; Pei, Y.; Liu, N.; Wu, Y.; Fu, Y.; Cheng, J. Association between PM2.5 Exposure and All-Cause, Non-Accidental, Accidental, Different Respiratory Diseases, Sex and Age Mortality in Shenzhen, China. Int. J. Environ. Res. Public Health 2019, 16, 401. [Google Scholar] [CrossRef]
- César, A.C.G.; Nascimento, L.F. Coarse Particles and Hospital Admissions Due to Respiratory Diseases in Children. An Ecological Time Series Study. Sao Paulo Med. J. 2018, 136, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, P.; Lan, L.; Zhou, L.; Liu, R.; Sun, Q.; Ban, J.; Wang, W.; Xu, D.; Li, T. Short-Term Exposures to PM2.5 and Cause-Specific Mortality of Cardiovascular Health in China. Environ. Res. 2018, 161, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yin, P.; Meng, X.; Wang, L.; Liu, C.; Niu, Y.; Liu, Y.; Liu, J.; Qi, J.; You, J.; et al. Associations between Coarse Particulate Matter Air Pollution and Cause-Specific Mortality: A Nationwide Analysis in 272 Chinese Cities. Environ. Health Perspect. 2019, 127, 017008. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, F.; Lei, R.; Shen, C.; Liu, J.; Yang, M.; Ding, R.; Cao, J. Associations of Ambient PM2.5 and O3 with Cardiovascular Mortality: A Time-Series Study in Hefei, China. Int. J. Biometeorol. 2019, 63, 1437–1447. [Google Scholar] [CrossRef]
- Davila Cordova, J.E.; Tapia Aguirre, V.; Vasquez Apestegui, V.; Ordoñez Ibarguen, L.; Vu, B.N.; Steenland, K.; Gonzales, G.F. Association of PM2.5 Concentration with Health Center Outpatient Visits for Respiratory Diseases of Children under 5 Years Old in Lima, Peru. Environ. Health 2020, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Li, C.; Ji, J.; Yang, Y.; Wang, S.; Tian, X.; Xu, K.-F. Short-Term Effects of Ambient Air Pollution on Chronic Obstructive Pulmonary Disease Admissions in Beijing, China (2013–2017). Int. J. Chron Obs. Pulmon. Dis. 2019, 14, 297–309. [Google Scholar] [CrossRef]
- Gu, J.; Shi, Y.; Zhu, Y.; Chen, N.; Wang, H.; Zhang, Z.; Chen, T. Ambient Air Pollution and Cause-Specific Risk of Hospital Admission in China: A Nationwide Time-Series Study. PLoS Med. 2020, 17, e1003188. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Chiang, H.-C.; Chen, M.-J.; Chuang, C.-Y.; Tsen, C.-M.; Fang, G.-C.; Tsai, Y.-I.; Chen, N.-T.; Lin, T.-Y.; Lin, S.-L.; et al. Ambient PM2.5 in the Residential Area near Industrial Complexes: Spatiotemporal Variation, Source Apportionment, and Health Impact. Sci. Total Environ. 2017, 590–591, 204–214. [Google Scholar] [CrossRef]
- Lanzinger, S.; Schneider, A.; Breitner, S.; Stafoggia, M.; Erzen, I.; Dostal, M.; Pastorkova, A.; Bastian, S.; Cyrys, J.; Zscheppang, A.; et al. Associations between Ultrafine and Fine Particles and Mortality in Five Central European Cities—Results from the UFIREG Study. Environ. Int. 2016, 88, 44–52. [Google Scholar] [CrossRef]
- Lin, H.; Tao, J.; Du, Y.; Liu, T.; Qian, Z.; Tian, L.; Di, Q.; Rutherford, S.; Guo, L.; Zeng, W.; et al. Particle Size and Chemical Constituents of Ambient Particulate Pollution Associated with Cardiovascular Mortality in Guangzhou, China. Environ. Pollut. 2016, 208, 758–766. [Google Scholar] [CrossRef]
- Liu, G.; Sun, B.; Yu, L.; Chen, J.; Han, B.; Liu, B.; Chen, J. Short-Term Exposure to Ambient Air Pollution and Daily Atherosclerotic Heart Disease Mortality in a Cool Climate. Environ. Sci. Pollut. Res. 2019, 26, 23603–23614. [Google Scholar] [CrossRef]
- Mokoena, K.K.; Ethan, C.J.; Yu, Y.; Shale, K.; Liu, F. Ambient Air Pollution and Respiratory Mortality in Xi’an, China: A Time-Series Analysis. Respir. Res. 2019, 20, 139. [Google Scholar] [CrossRef]
- Patto, N.V.; Nascimento, L.F.C.; Mantovani, K.C.C.; Vieira, L.C.P.F.S.; Moreira, D.S. Exposure to Fine Particulate Matter and Hospital Admissions Due to Pneumonia: Effects on the Number of Hospital Admissions and Its Costs. Rev. Assoc. Med. Bras. (1992) 2016, 62, 342–346. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; Limsukon, A.; Tajarernmuang, P.; Phetsuk, N. Acute Effects of Air Pollutants on Daily Mortality and Hospitalizations Due to Cardiovascular and Respiratory Diseases. J. Thorac. Dis. 2019, 11, 3070–3083. [Google Scholar] [CrossRef]
- Pothirat, C.; Chaiwong, W.; Liwsrisakun, C.; Bumroongkit, C.; Deesomchok, A.; Theerakittikul, T.; Limsukon, A.; Tajarernmuang, P.; Phetsuk, N. The Short-Term Associations of Particular Matters on Non-Accidental Mortality and Causes of Death in Chiang Mai, Thailand: A Time Series Analysis Study between 2016–2018. Int. J. Environ. Health Res. 2021, 31, 538–547. [Google Scholar] [CrossRef]
- Pu, X.; Wang, L.; Chen, L.; Pan, J.; Tang, L.; Wen, J.; Qiu, H. Differential Effects of Size-Specific Particulate Matter on Lower Respiratory Infections in Children: A Multi-City Time-Series Analysis in Sichuan, China. Environ. Res. 2021, 193, 110581. [Google Scholar] [CrossRef]
- Qu, Y.; Pan, Y.; Niu, H.; He, Y.; Li, M.; Li, L.; Liu, J.; Li, B. Short-Term Effects of Fine Particulate Matter on Non-Accidental and Circulatory Diseases Mortality: A Time Series Study among the Elder in Changchun. PLoS ONE 2018, 13, e0209793. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, C.; Chen, R.; Wang, C.; Li, J.; Sun, J.; Kan, H.; Cao, J.; Bai, H. Association of Fine Particulate Matter on Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Yancheng, China. Sci. Total Environ. 2019, 650, 1665–1670. [Google Scholar] [CrossRef]
- Teng, B.; Zhang, X.; Yi, C.; Zhang, Y.; Ye, S.; Wang, Y.; Tong, D.; Lu, B. The Association between Ambient Air Pollution and Allergic Rhinitis: Further Epidemiological Evidence from Changchun, Northeastern China. Int. J. Environ. Res. Public Health 2017, 14, 226. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Chen, K. The Impact of Ambient Particulate Matter on Hospital Outpatient Visits for Respiratory and Circulatory System Disease in an Urban Chinese Population. Sci. Total Environ. 2019, 666, 672–679. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, S.; Dong, H.; Wang, L.; Wang, C.; Ji, X.; Ji, M.; Yao, X.; Zhang, Z. Association between Short-Term Exposure to Particulate Matter Air Pollution and Cause-Specific Mortality in Changzhou, China. Environ. Res. 2019, 170, 7–15. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Zhang, X.; Dong, M.; Wu, J.; Dong, Y.; Chen, R.; Ding, X.; Huang, C.; Zhang, Q.; et al. The Burden of Ambient Air Pollution on Years of Life Lost in Wuxi, China, 2012–2015: A Time-Series Study Using a Distributed Lag Non-Linear Model. Environ. Pollut. 2017, 224, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Dong, J.; Liu, X.; Tan, E.; Shu, J.; Li, S. Association between Ambient Particulate Matter and Hospital Outpatient Visits for Chronic Obstructive Pulmonary Disease in Lanzhou, China. Environ. Sci. Pollut. Res. 2020, 27, 22843–22854. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-H.; Hsu, S.-C.; Bai, K.-J.; Huang, S.-K.; Hsu, C.-W. Association of Time-Serial Changes in Ambient Particulate Matters (PMs) with Respiratory Emergency Cases in Taipei’s Wenshan District. PLoS ONE 2017, 12, e0181106. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xu, D.; He, M.Z.; Wang, Y.; Du, Z.; Du, Y.; Qian, Y.; Ji, D.; Li, T. Fine Particle Constituents and Mortality: A Time-Series Study in Beijing, China. Environ. Sci. Technol. 2018, 52, 11378–11386. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mayvaneh, F.; Baaghideh, M.; Entezari, A.; Ho, H.C.; Xiang, Q.; Jiao, A.; Zhang, F.; Hu, K.; Chen, G.; et al. Utilizing Daily Excessive Concentration Hours to Estimate Cardiovascular Mortality and Years of Life Lost Attributable to Fine Particulate Matter in Tehran, Iran. Sci. Total Environ. 2020, 703, 134909. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Fang, B.; Wang, C.; Xia, T.; Bottai, M.; Fang, F.; Cao, Y. Comparison of Frequentist and Bayesian Generalized Additive Models for Assessing the Association between Daily Exposure to Fine Particles and Respiratory Mortality: A Simulation Study. Int. J. Environ. Res. Public Health 2019, 16, 746. [Google Scholar] [CrossRef]
- GUO, J.; MA, M.; XIAO, C.; ZHANG, C.; CHEN, J.; LIN, H.; DU, Y.; LIU, M. Association of Air Pollution and Mortality of Acute Lower Respiratory Tract Infections in Shenyang, China: A Time Series Analysis Study. Iran J. Public Health 2018, 47, 1261–1271. [Google Scholar]
- Huang, F.; Chen, R.; Shen, Y.; Kan, H.; Kuang, X. The Impact of the 2013 Eastern China Smog on Outpatient Visits for Coronary Heart Disease in Shanghai, China. Int. J. Environ. Res. Public Health 2016, 13, 627. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Lee, J.Y.; Yi, S.-M.; Kim, H. Associations of Particulate Matter and Its Components with Emergency Room Visits for Cardiovascular and Respiratory Diseases. PLoS ONE 2017, 12, e0183224. [Google Scholar] [CrossRef]
- Jiang, J.; Niu, Y.; Liu, C.; Chen, R.; Cao, J.; Kan, H.; Cheng, Y. Short-Term Exposure to Coarse Particulate Matter and Outpatient Visits for Cardiopulmonary Disease in a Chinese City. Ecotoxicol. Environ. Saf. 2020, 199, 110686. [Google Scholar] [CrossRef]
- Li, Y.R.; Xiao, C.C.; Li, J.; Tang, J.; Geng, X.Y.; Cui, L.J.; Zhai, J.X. Association between Air Pollution and Upper Respiratory Tract Infection in Hospital Outpatients Aged 0–14 Years in Hefei, China: A Time Series Study. Public Health 2018, 156, 92–100. [Google Scholar] [CrossRef]
- Lin, H.; Tao, J.; Qian, Z.; Ruan, Z.; Xu, Y.; Hang, J.; Xu, X.; Liu, T.; Guo, Y.; Zeng, W.; et al. Shipping Pollution Emission Associated with Increased Cardiovascular Mortality: A Time Series Study in Guangzhou, China. Environ. Pollut. 2018, 241, 862–868. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Yu, Q.; Huo, X.; Ming, X.; Wang, J.; Zhou, Y.; Peng, Z.; Zhang, H.; Cui, X.; et al. Short-Term Effects of Ambient Air Pollution on Pediatric Outpatient Visits for Respiratory Diseases in Yichang City, China. Environ. Pollut. 2017, 227, 116–124. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, L.; Hou, L.; Yu, C.; Tao, N.; Liu, J.; Li, Y.; Zhou, C.; Yang, G.; Li, H. Ambient Air Pollution Exposures and Newly Diagnosed Pulmonary Tuberculosis in Jinan, China: A Time Series Study. Sci. Rep. 2018, 8, 17411. [Google Scholar] [CrossRef]
- Mokoena, K.K.; Ethan, C.J.; Yu, Y.; Shale, K.; Fan, Y.; Liu, F.; Rong, J. The Effect of Ambient Air Pollution on Circulatory Mortality: A Short-Term Exposure Assessment in Xi’an, China. Environ. Sci. Pollut. Res. 2019, 26, 22512–22521. [Google Scholar] [CrossRef]
- Nascimento, L.F.C.; Vieira, L.C.P.F.; Mantovani, K.C.C.; Moreira, D.S. Air Pollution and Respiratory Diseases: Ecological Time Series. Sao Paulo Med. J. 2016, 134, 315–321. [Google Scholar] [CrossRef]
- Rodríguez-Villamizar, L.; Rojas-Roa, N.; Blanco-Becerra, L.; Herrera-Galindo, V.; Fernández-Niño, J. Short-Term Effects of Air Pollution on Respiratory and Circulatory Morbidity in Colombia 2011–2014: A Multi-City, Time-Series Analysis. Int. J. Environ. Res. Public Health 2018, 15, 1610. [Google Scholar] [CrossRef]
- Sacramento, D.S.; Martins, L.C.; Arbex, M.A.; Pamplona, Y.d.A.P. Atmospheric Pollution and Hospitalization for Cardiovascular and Respiratory Diseases in the City of Manaus from 2008 to 2012. Sci. World J. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Tian, Q.; Li, M.; Montgomery, S.; Fang, B.; Wang, C.; Xia, T.; Cao, Y. Short-Term Associations of Fine Particulate Matter and Synoptic Weather Types with Cardiovascular Mortality: An Ecological Time-Series Study in Shanghai, China. Int. J. Environ. Res. Public Health 2020, 17, 1111. [Google Scholar] [CrossRef]
- Vahedian, M.; Khanjani, N.; Mirzaee, M.; Koolivand, A. Ambient Air Pollution and Daily Hospital Admissions for Cardiovascular Diseases in Arak, Iran. ARYA Atheroscler 2017, 13, 117–134. [Google Scholar] [PubMed]
- Wang, C.; Hao, L.; Liu, C.; Chen, R.; Wang, W.; Chen, Y.; Yang, Y.; Meng, X.; Fu, Q.; Ying, Z.; et al. Associations between Fine Particulate Matter Constituents and Daily Cardiovascular Mortality in Shanghai, China. Ecotoxicol. Environ. Saf. 2020, 191, 110154. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, F.; Guo, M.; Fan, W.; Ji, W.; Dong, Z. Associations between PM1 Exposure and Daily Emergency Department Visits in 19 Hospitals, Beijing. Sci. Total Environ. 2021, 755, 142507. [Google Scholar] [CrossRef]
- Zhang, D.; Tian, Y.; Zhang, Y.; Cao, Y.; Wang, Q.; Hu, Y. Fine Particulate Air Pollution and Hospital Utilization for Upper Respiratory Tract Infections in Beijing, China. Int. J. Environ. Res. Public Health 2019, 16, 533. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Wu, C.; Zhang, M.; Feng, H.; Li, D.; Zhu, W. Acute Effects of Ambient Air Pollution on Clinic Visits of College Students for Upper Respiratory Tract Infection in Wuhan, China. Environ. Sci. Pollut. Res. 2021, 28, 29820–29830. [Google Scholar] [CrossRef] [PubMed]

| Categories | N (%) |
|---|---|
| Study duration | |
| Less than 7 years | 111 (80.4) |
| 7 years and more | 27 (19.6) |
| Type of time series analysis | |
| GAM and Poisson | 103 (74.6) |
| GLM and Poisson | 24 (17.4) |
| Negative Binomial | 2 (1.4) |
| Others | 9 (6.5) |
| ICD codes | |
| ICD-10 | 98 (71.0) |
| ICD-9 | 11 (8.0) |
| Both ICD | 5 (3.6) |
| No classification | 24 (17.4) |
| Outcomes | Children (Only) (n = 17) | Elderly (Only) (n = 58) | Children & Elderly (n = 14) | All Ages (n = 49) |
|---|---|---|---|---|
| Health outcomes | ||||
| Mortality | 0 | 26 | 0 | 15 |
| Hospital admission | 6 | 16 | 6 | 13 |
| Clinic or ED visits | 11 | 11 | 6 | 14 |
| Combined | 0 | 5 | 2 | 7 |
| Disease | ||||
| Cardiovascular | 0 | 21 | 1 | 15 |
| Respiratory | 17 | 18 | 8 | 19 |
| Combined | 0 | 19 | 5 | 15 |
| Author (Reference) | Location | Study Period | Health Outcome | Diseases (ICD Classification) | Key Findings | Vulnerable Groups |
|---|---|---|---|---|---|---|
| Eastern & South-Eastern Asia | ||||||
| Chai et al., 2019 [37] | Lanzhou, China | 10 years (2007–2016) | Hospital visits | RD (ICD-10: J00–J99) |
| Each 10-μg/m3 increase in PM2.5 had a cumulative effect on RD visits
|
| Chen et al., 2021 [38] | Nanjing, China | 16 years (2004–2019 | Hospital admission | CVD (ICD-10: I00–I99), IHD (ICD-10: I20–I25), and CBVD (ICD-10: I60–I69). | Cumulative effect estimates for PM2.5 on IHD mortality were elevated and statistically significant within 27 (2.11%; 95% CI: 0.12–.27%) and 22 (2.63%; 95% CI: 0.39–4.91%) days |
|
| Xu et al., 2019 [39] | Heifei, China | 11 years (2007–2016) | Mortality | CVD (ICD-10: I00–I99), IHD (ICD-10: I20–I25), and CBVD (ICD-10: I60–I69) |
|
|
| Lin et al., 2016 [40] | Hong Kong | 14 years (1998–2011) | Mortality | RD (ICD-10: J00–J99) | A positive but non-significant synergistic interaction between daily mean and variation on RD and pneumonia mortality |
|
| Lin et al., 2017 [41] | Hong Kong | 11 years (2001–2011) | Mortality | CVD (ICD-9: 390–459 or ICD-10: I00–I99) RD (ICD-9: 460–519 or ICD-10: J00–J99) | PM2.5 was significantly associated with mortality; the highest increase in daily mean PM2.5 at lag03 corresponded to ER of 2.77% (95% CI: 1.50–4.05%) increase in CVD mortality and 2.07% (95% CI: 0.49–3.67%) increase in RD mortality. |
|
| Yap et al., 2019 [42] | Singapore | 13 years (2001–2013) | Mortality | CVD (ICD-9390–459) and (ICD-10 I00–I99) Non-accidental deaths (ICD-9000–799) and (ICD-10: A00–R99) | An increase of 10 μg/m3 in PM2.5 was associated with significant increases in non-accidental mortality ER: 0.660%; 95% CI: 0.204–1.118%) and CVD mortality (ER: 0.883%; 95% CI: 0.121–1.621%). |
|
| Kwon et al., 2019 [43] | Seoul, South Korea | 9 years (2007–2015) | Hospital admission | CVD: Atrial fibrillation (AF) and a primary diagnosis of CVD (ICD-10: I00–I99), | A 10-μg/m3 increase in ambient PM2.5 showed significantly increased admissions RR = 1.045; (95% CI: 1.002–1.089) at lag 3 |
|
| Oh et al., 2020 [44] | South Korea (seven metropolitan cities) | 8 years (2008–2016) | Hospital admission | ALRI | A 10 μg/m3 increase in the 7-day moving average of PM2.5 was associated with a 1.20% (95% CI: 0.71–1.71) increase in ALRI hospitalization |
|
| Qiu et al., 2020 [45] | Taipei, Taiwan | 8 years (2010–2017) | Hospital admission | RD (ICD-10: J00–J99), pneumonia (ICD-10: J12–J18), COPD (ICD-10: J40–J44), asthma (ICD-10: J45–J46) | A strong association of PM2.5 with all-RD and asthma admissions; percentage change for RD in association with an IQR increased at different lags (Lag02 and Lag03) |
|
| Oceania | ||||||
| Guo et al., 2020 [46] | Hazelwood, Australia | 7 years (2009–2015) | EDV and hospital admission | CVD (ICD-10: I00–I99); CBVD (ICD-10: I61–I69), IHD (I ICD-10: 20–I25) RD (ICD-10: J00–J99); COPD (J41–J44), asthma (J45–J46). |
|
|
| Salimi et al., 2017 [47] | Sydney, Australia | 11 years (2004–2015) | EAD | RD & CVD: breathing problems, chest pain, cardiac or respiratory arrest, and death, stroke or CBVD |
|
|
| Europe & Northern America | ||||||
| Strosnider et al., 2019 [48] | United States (17 states) | 15 years (2000–2014) | EDV | RD (ICD-9: 460–519) |
|
|
| Krall et al., 2017 [49] | United States (Atlanta, Birmingham, St. Louis, Dallas) | 11 years (1999–2009) | EDV | RD: pneumonia (ICD-9: 480–486), COPD (ICD-9: 491, 492, 496), URI (ICD-9: 460–465, 466.0, 477), and asthma and/or wheeze (ICD-9: 493, 786.07) |
|
|
| Ye et al., 2018 [50] | Atlanta, United States | 16 years (1998–2013) | EDV | CVD: IHD (ICD-9: 410–414), cardiac dysrhythmias (ICD-9: 427), CHF (ICD-9: 428), or CBVD (ICD-9: 433–437, 443–445, 451–453). |
|
|
| Ebisu et al., 2019 [51] | California, United States | 8 years (2002–2009) | Hospital admission | CVD: (ICD-9: 390–459) RD: (ICD-9: 460–519) | Exposure to an increase in PM2.5 vehicular emissions associated with increased risk for CVD admission and RD hospitalizations in specific groups | Each IQR increase in PM2.5 emissions is associated with:
|
| Blomberg et al., 2019 [52] | United States (108 cities) | 14 years (1999–2013) | Mortality | CVD (ICD-10: I01–I59) RD (ICD-10: J00–J99) |
|
|
| Hsu et al., 2017 [53] | New York, United States | 16 years (1991–2006) | Hospital admission | CVD: CRHD (ICD-9: 393–396), hypertension (401–405), IHD (410–414), cardiac dysrhythmias (427), heart failure (428), and other CVD (430–434, 436–438) |
|
|
| Bi et al., 2020 [54] | Los Angeles, United States | 12 years (2005–2016) | EDV and hospital admission | CVD (ICD-10: I20–I79) RD (ICD-10: J45–J46) |
|
|
| Qiu et al., 2020 [55] | New England, United States | 13 years (2000–2012) | Hospital admission | CVD specific on AMI, CHF and IS |
| Each 10 μg/m3 increase in PM2.5 associated with:
|
| Yitshak-Sade et al., 2018 [56] | New England, United States | 11 years (2001–2011) | Hospital admission | CVD (ICD 9: 390–429) or ischemic stroke (ICD 9: 432–435) RD (ICD 9: 460–519) |
|
|
| Pearce et al., 2018 [57] | South Carolina, United States | 12 years (2002–2013) | EDV and hospital admission | CVD: URI (ICD-9: 460–466, 477; CHF (ICD-9: 428); IHD (ICD-9: 410–414) RD: Asthma (ICD-9: 493, 786.07) |
|
|
| Solimini and Renzi, 2017 [58] | Rome, Italy | 14 years (2001–2014) | EDV | Atrial fibrillation (AF) with ICD-9: 427.31 |
|
|
| Kuźma et al., 2020 [59] | Bialystok, Poland | 10 years (2008–2017) | Mortality | CVD (ICD-10: I01–I59) |
|
|
| Kollanus et al., 2016 [60] | Helsinki, Finland | 10 years (2001–2010) | Hospital admission and mortality | CVD (ICD-10: I01–I59) RD (ICD-10: J00–J64, J65–J99) |
|
|
| Central & Southern Asia | ||||||
| Borsi et al., 2020 [61] | Ahvaz, Iran | 11 years (2008–2018) | Hospital admission | CVD: deep venous thrombosis (DVT) |
| Each 10 μg/m3 increase in PM2.5, increased risk of DVT admissions:
|
| Latin America & Caribbean | ||||||
| Tapia et al., 2020 [62] | Lima, Peru | 7 years (2010–2016) | Hospital visit | CVD (ICD-10: I20–I25, I63–I67) RD (ICD-10: J0–J45, J00–J06, J09–J22, J30–J45) |
|
|
| Multiple Countries | ||||||
| Liu et al., 2019 [31] | Multi countries (652 cities, 24 countries) | 30 years (1986–2015) | Mortality | CVD (I00–I99), and RD (J00–J99) |
|
|
| Diseases Outcomes | Short-Term (Less than 7 Years) | Long-Term (7 Years & above) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Q1 | Q2 (Median) | Q3 | Min | Max | Mean (SD) | Q1 | Q2 (Median) | Q3 | Min | Max | |
| Cardiovascular | 1.0378 (0.066) | 1.0073 | 1.0239 | 1.0485 | 0.8200 | 1.2880 | 1.0503 (0.0603) | 1.0149 | 1.0297 | 1.0582 | 1.0050 | 1.2700 |
| Respiratory | 1.0493 (0.0751) | 1.0082 | 1.0169 | 1.0723 | 0.9132 | 1.3800 | 1.0391 (0.0382) | 1.0139 | 1.0355 | 1.0407 | 1.0074 | 1.1580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Mahiyuddin, W.R.; Ismail, R.; Mohammad Sham, N.; Ahmad, N.I.; Nik Hassan, N.M.N. Cardiovascular and Respiratory Health Effects of Fine Particulate Matters (PM2.5): A Review on Time Series Studies. Atmosphere 2023, 14, 856. https://doi.org/10.3390/atmos14050856
Wan Mahiyuddin WR, Ismail R, Mohammad Sham N, Ahmad NI, Nik Hassan NMN. Cardiovascular and Respiratory Health Effects of Fine Particulate Matters (PM2.5): A Review on Time Series Studies. Atmosphere. 2023; 14(5):856. https://doi.org/10.3390/atmos14050856
Chicago/Turabian StyleWan Mahiyuddin, Wan Rozita, Rohaida Ismail, Noraishah Mohammad Sham, Nurul Izzah Ahmad, and Nik Muhammad Nizam Nik Hassan. 2023. "Cardiovascular and Respiratory Health Effects of Fine Particulate Matters (PM2.5): A Review on Time Series Studies" Atmosphere 14, no. 5: 856. https://doi.org/10.3390/atmos14050856
APA StyleWan Mahiyuddin, W. R., Ismail, R., Mohammad Sham, N., Ahmad, N. I., & Nik Hassan, N. M. N. (2023). Cardiovascular and Respiratory Health Effects of Fine Particulate Matters (PM2.5): A Review on Time Series Studies. Atmosphere, 14(5), 856. https://doi.org/10.3390/atmos14050856






