Removal and Resource Utilization of High Concentration Flue Gas Sulfur Dioxide Using Manganese Carbonate Ore
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Setup
2.3. Analytical Methods
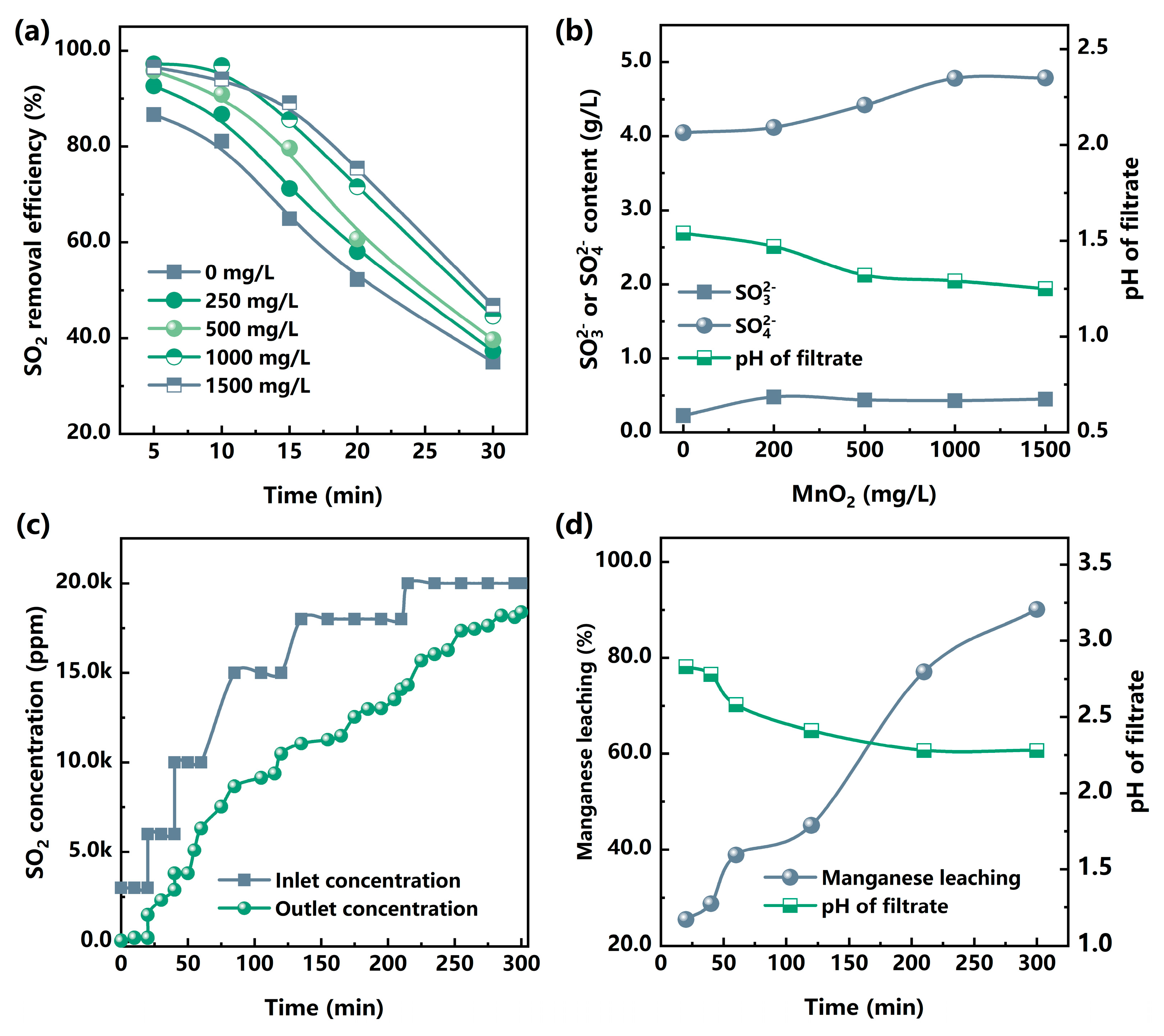
3. Results
3.1. Mechanism Study of MCO-FGD
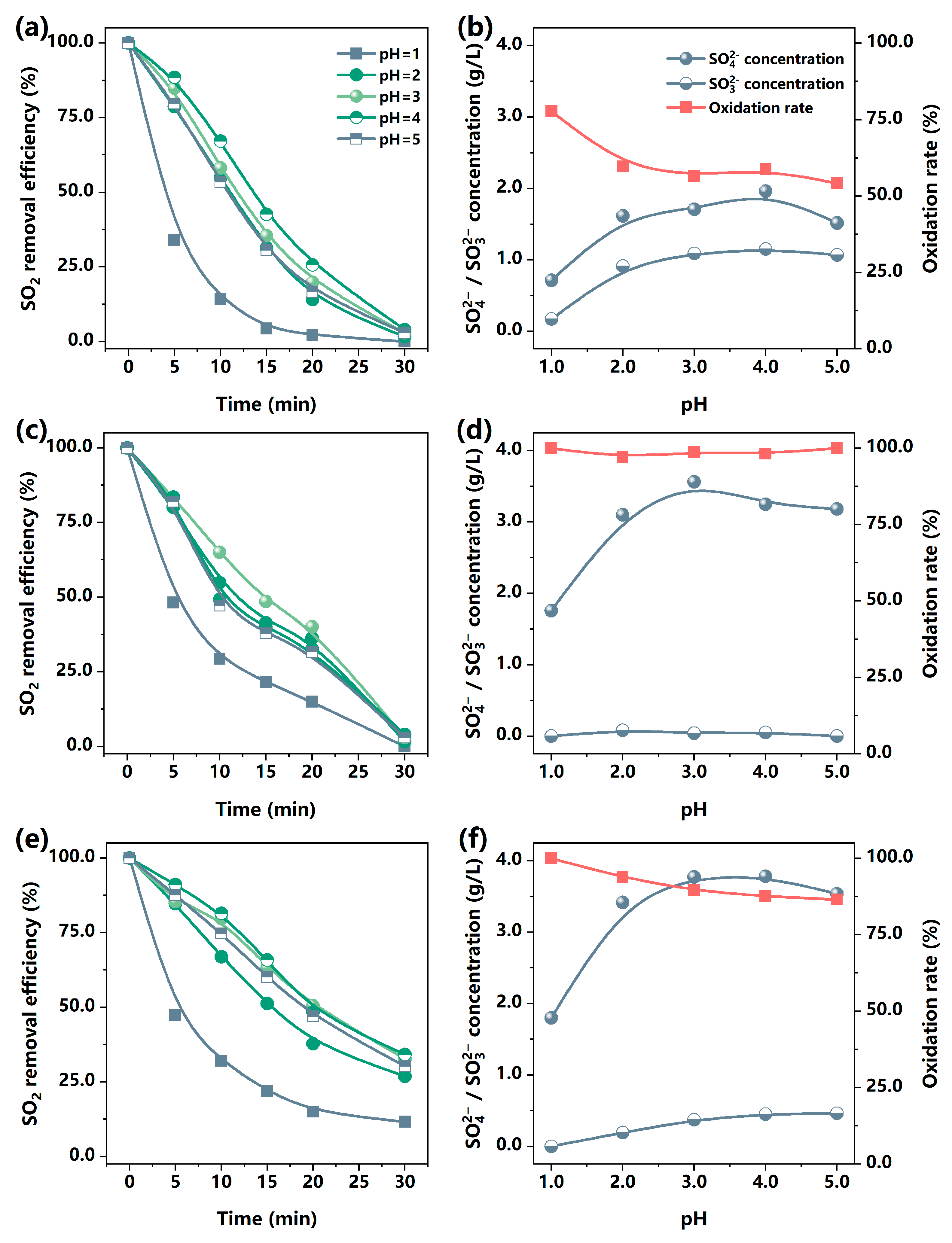
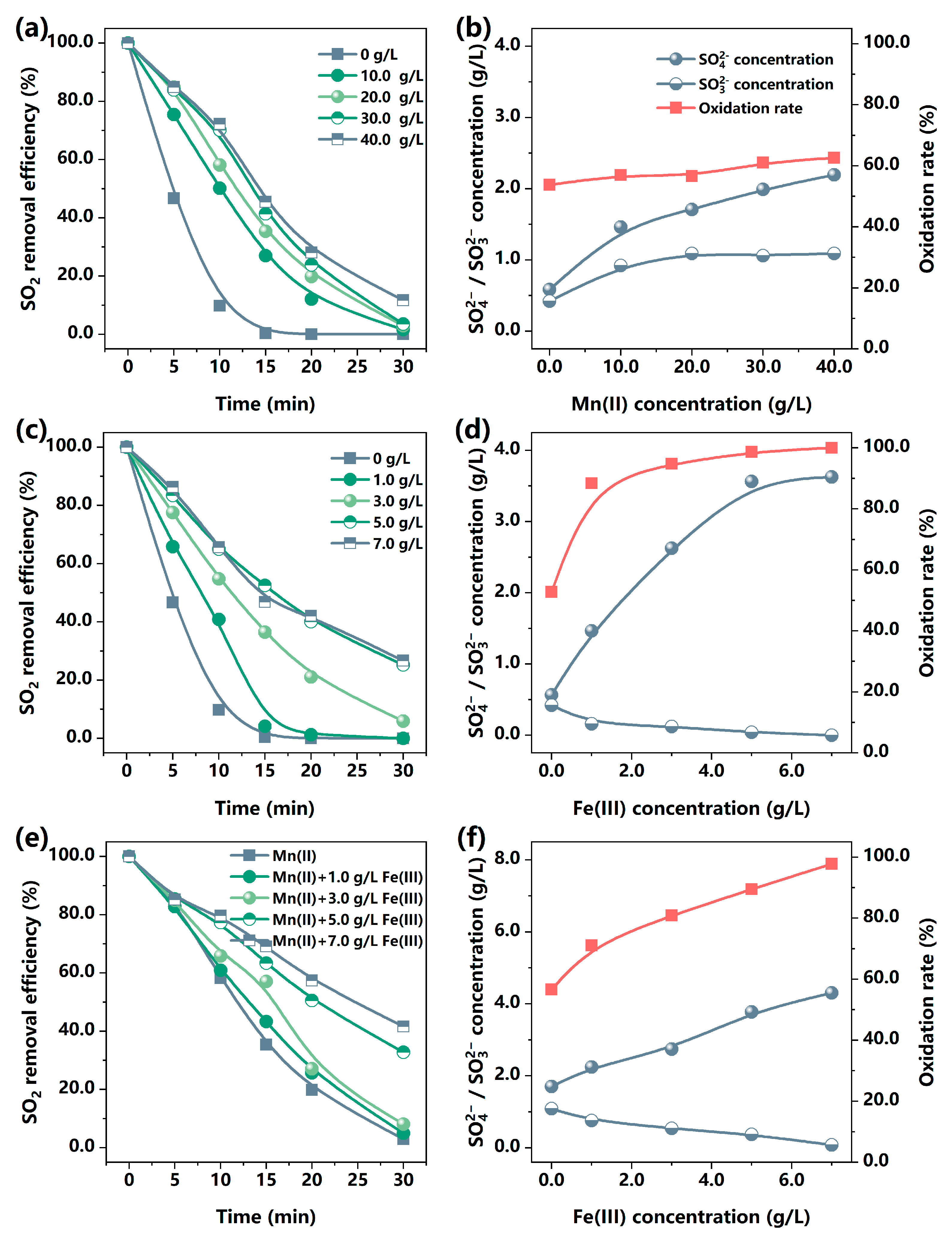
3.1.1. The Role of Mn(II) and Fe(III) in SO2 Removal
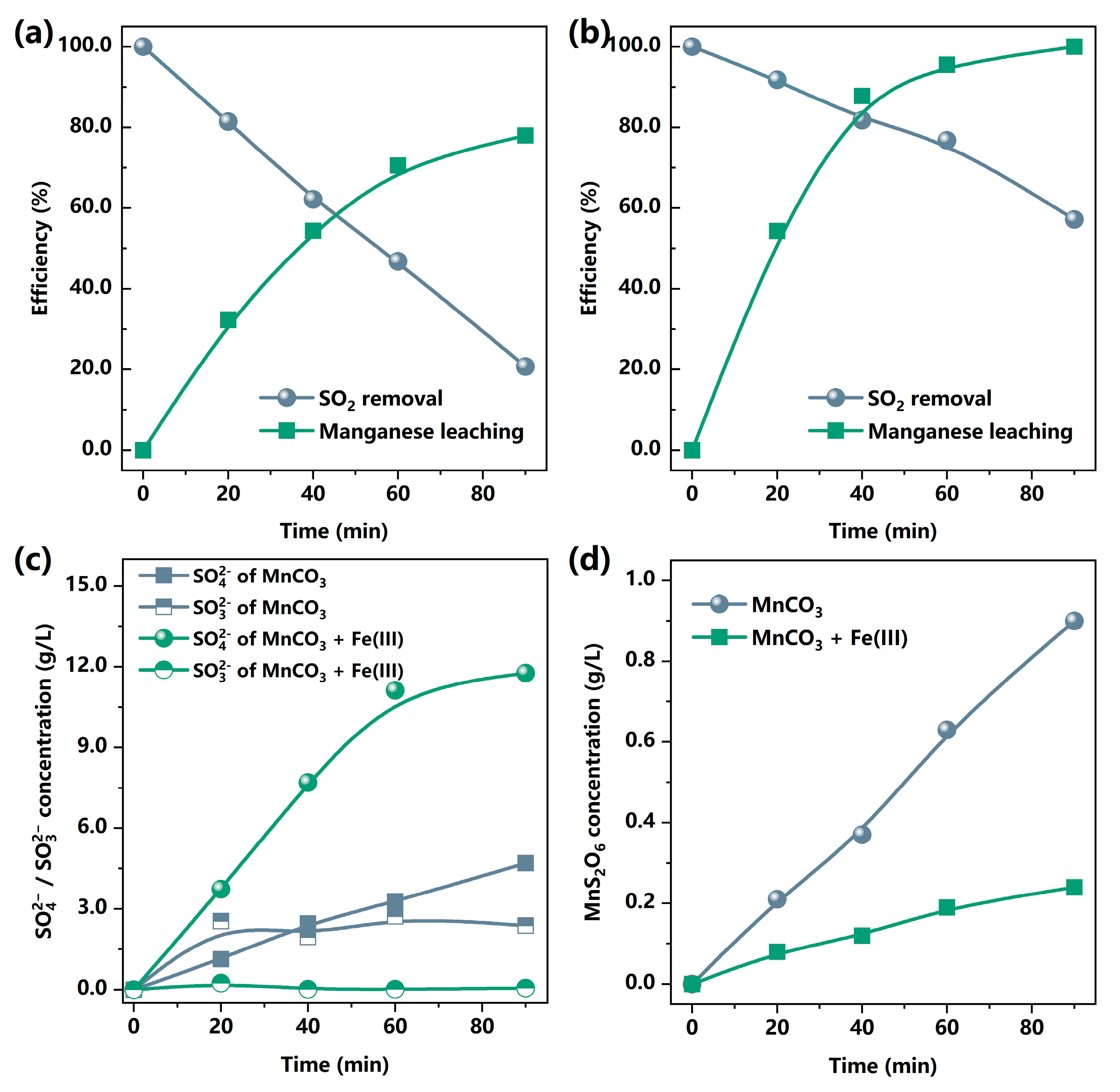
3.1.2. The Influences of Fe(III) on SO2 Removal by MnCO3
3.1.3. Mechanism Analysis
3.2. SO2 Removal of the Fe(III)-Enhanced MCO-FGD
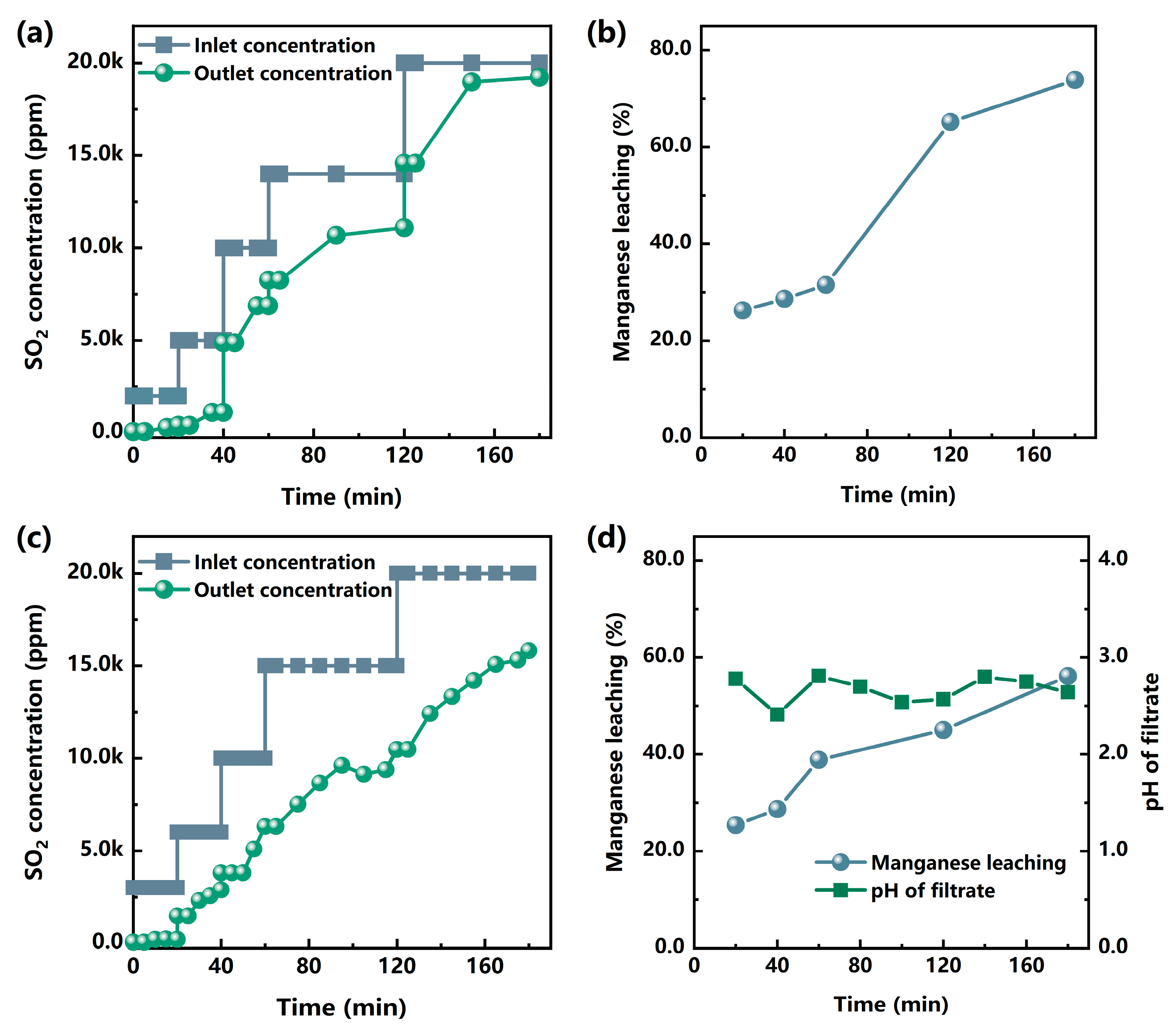
3.3. Technical Study of MCO-FGD
3.3.1. Fe(III) Promoted Multi-Stage MCO-FGD
3.3.2. Mn(II)-Fe(III) Promoted Multi-Stage MCO-FGD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics. China Statistics Yearbook 2013~2019; China Statistics Press: Beijing, China, 2013–2019. Available online: http://www.stats.gov.cn/sj/ndsj/ (accessed on 9 April 2023).
- Liu, Y.; Zhou, M.; Lu, K. Compilation of reaction kinetics parameters determined in the key development project for air pollution formation mechanism and control technologies in china. J. Environ. Sci. 2023, 123, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Li, L.; Ren, Q.; Yang, X.; Jiang, Z.; Zhang, Z. Utilization of low-quality desulfurized ash from semi-dry flue gas desulfurization by mixing with hemihydrate gypsum. Fuel 2019, 255, 115783. [Google Scholar] [CrossRef]
- Fang, D.; Liao, X.; Zhang, X.; Teng, A.; Xue, X. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: As a reductant to remove chromium and vanadium from vanadium industrial wastewater. J. Hazard. Mater. 2018, 342, 436–445. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amorós, D.; Morallón, E. Catalytic oxidation ofsulfur dioxide by activated carbon: A physical chemistry experiment. J. Chem. Educ. 1999, 76, 958. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; He, G.; Wang, H.; Zhang, X.; Lin, J.; Qi, Y.; Liang, X. Challenges and opportunities for carbon neutrality in China. Nat. Rev. Earth Environ. 2022, 3, 141–155. [Google Scholar] [CrossRef]
- Mahboob, I.; Shafiq, I.; Shafique, S.; Akhter, P.; Amjad, U.-e.-S.; Hussain, M.; Park, Y.-K. Effect of active species scavengers in photocatalytic desulfurization of hydrocracker diesel using mesoporous Ag3VO4. Chem. Eng. J. 2022, 441, 136063. [Google Scholar] [CrossRef]
- Yao, L.; Xin, G.; Pu, P.; Yang, L.; Jiang, X.; Jiang, Z.; Jiang, W. Promotion of manganese extraction and flue gas desulfurization with manganese ore by iron in the anodic solution of electrolytic manganese. Hydrometallurgy 2021, 199, 105542. [Google Scholar] [CrossRef]
- Xie, C.; Li, T.; Deng, C.; Song, Y.; Zhang, H.; Li, X. A highly reversible neutral zinc/manganese battery for stationary energy storage. Energy Environ. Sci. 2020, 13, 135–143. [Google Scholar] [CrossRef]
- Lu, J.; Dreisinger, D.; Glück, T. Manganese electrodeposition—A literature review. Hydrometallurgy 2014, 141, 105–116. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Lu, M.; Su, Z.; Li, G.; Jiang, T. Extraction and separation of manganese and iron from ferruginous manganese ores: A review. Miner. Eng. 2019, 131, 286–303. [Google Scholar] [CrossRef]
- Singh, V.; Chakraborty, T.; Tripathy, S.K. A Review of Low Grade Manganese Ore Upgradation Processes. Miner. Process. Extr. Metall. Rev. 2020, 41, 417–438. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Wu, X.; Du, J.; Tao, C. Electric field enhancement in leaching of manganese from low-grade manganese dioxide ore: Kinetics and mechanism study. J. Electroanal. Chem. 2017, 788, 165–174. [Google Scholar] [CrossRef]
- Santos, O.d.S.H.; Carvalho, C.d.F.; Silva, G.A.d.; Santos, C.G.d. Manganese ore tailing: Optimization of acid leaching conditions and recovery of soluble manganese. J. Environ. Manag. 2015, 147, 314–320. [Google Scholar] [CrossRef]
- Sun, D.; Li, M.L.; Li, C.H.; Cui, R.; Zheng, X.Y. A Green Enriching Process of Mn from Low Grade Ore of Manganese Carbonate. Appl. Mech. Mater. 2014, 644–650, 5427–5430. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, Y.; Liu, X.; Tang, J.; Zhou, C.; Guo, L.; Yang, J. Synergistic leaching mechanism of chloride ions for extracting manganese completely from manganese carbonate ores. J. Environ. Chem. Eng. 2021, 9, 104918. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.; Kim, J.K.; Lee, B.W. The smelting of manganese carbonate ore. In Proceedings of the Smelting of Manganese Carbonate Ore, Cape Town, South Africa, 1–4 February 2004; pp. 223–230. [Google Scholar]
- Zhang, W.; Cheng, C.Y. Manganese metallurgy review. Part I: Leaching of ores/secondary materials and recovery of electrolytic/chemical manganese dioxide. Hydrometallurgy 2007, 89, 137–159. [Google Scholar] [CrossRef]
- Hagelstein, K. Globally sustainable manganese metal production and use. J. Environ. Manag. 2009, 90, 3736–3740. [Google Scholar] [CrossRef]
- Sun, W.; Su, S.; Wang, Q.; Ding, S. Lab-scale circulation process of electrolytic manganese production with low-grade pyrolusite leaching by SO2. Hydrometallurgy 2013, 133, 118–125. [Google Scholar] [CrossRef]
- Sun, W.; Ding, S.; Zeng, S.; Su, S.; Jiang, W. Simultaneous absorption of NOx and SO2 from flue gas with pyrolusite slurry combined with gas-phase oxidation of NO using ozone. J. Hazard. Mater. 2011, 192, 124–130. [Google Scholar] [CrossRef]
- Sun, D.; Yang, L.; Liu, N.; Jiang, W.; Jiang, X.; Li, J.; Yang, Z.; Song, Z. Sulfur resource recovery based on electrolytic manganese residue calcination and manganese oxide ore desulfurization for the clean production of electrolytic manganese. Chin. J. Chem. Eng. 2020, 28, 864–870. [Google Scholar] [CrossRef]
- Yuan, J.; Jiang, X.; Liu, Y.; Yao, L.; Jiang, W. Mechanism and influence factors of flue gas desulfurization by symbiotic manganese oxides ore. Chin. J. Environ. Eng. 2018, 12, 1104–1111. (In Chinese) [Google Scholar] [CrossRef]
- Ibusuki, T.; Takeuchi, K. Sulfur dioxide oxidation by oxygen catalyzed by mixtures of manganese(II) and iron(III) in aqueous solutions at environmental reaction conditions. Atmos. Environ. 1987, 21, 1555–1560. [Google Scholar] [CrossRef]
- Brandt, C.; van Eldik, R. Transition metal-catalyzed oxidation of sulfur(IV) oxides. Atmospheric-relevant processes and mechanisms. Chem. Rev. 1995, 95, 119–190. [Google Scholar] [CrossRef]
- Li, H.; Shan, C.; Li, W.; Pan, B. Peroxymonosulfate activation by iron(III)-tetraamidomacrocyclic ligand for degradation of organic pollutants via high-valent iron-oxo complex. Water Res. 2018, 147, 233–241. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, Y.; Ma, S.; Fu, Y. Flue gas desulfurization based on Mn-Fe synergistis catalyzed oxifation. J. North China Electr. Power Univ. 2001, 28, 80–83. [Google Scholar]
- Qu, Y.; Guo, J.; Chu, Y.; Sun, M.; Yin, H. The influence of Mn species on the SO2 removal of Mn-based activated carbon catalysts. Appl. Surf. Sci. 2013, 282, 425–431. [Google Scholar] [CrossRef]
- GB/T 1056−2016; Manganese Ores—Determination of Manganese Content—Potentiometric Method and Ammonium Iron (II) Sulphate Titrimetric Method. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2016.
- Soffer, N. The determination of dithionate, sulphite and sulphate in manganese leach liquors. Analyst 1961, 86, 843–849. [Google Scholar] [CrossRef]
- Brent Brent Smith, C.; Freeman, H.S. 9—Water and wastewater analysis. In Chemical Testing of Textiles; Fan, Q., Ed.; Woodhead Publishing: Sawston, UK, 2005; pp. 242–269. [Google Scholar] [CrossRef]
- Sun, W.; Su, S.; Sanglan, D.; Jiang, W.; Xu, Y. Decomposition characteristics of manganese dithionate in absorption solution of flue gas desulfurization and denitration with pyrolusite slurry. J. Sichuan Univ. Eng. Sci. Ed. 2011, 43, 166–170. [Google Scholar]
- Huss, A.; Lim, P.K.; Eckert, C.A. Oxidation of aqueous sulfur dioxide. 2. High-pressure studies and proposed reaction mechanisms. J. Phys. Chem. 1982, 86, 4229–4233. [Google Scholar] [CrossRef]
- Chen, Z.; Tong, Z. Removal of SO2 from flue gas by Mn2+ catalytic oxidation. Chin. J. Environ. Sci. 1995, 16, 32–34. (In Chinese) [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Yao, L.; Jiang, W.; Jiang, X.; Li, J. Removal of manganous dithionate (MnS2O6) with MnO2 from the desulfurization manganese slurry. RSC Adv. 2020, 10, 1430–1438. [Google Scholar] [CrossRef]
- Connick, R.E.; Zhang, Y. Kinetics and mechanism of the oxidation of HSO3- by O2. 2. The manganese(II)-catalyzed reaction. Inorg. Chem. 1996, 35, 4613–4621. [Google Scholar] [CrossRef]
- Chen, J.; Guo, X.J.; Li, H. Implementation and practice of an integrated process to recover copper from low grade ore at Zijinshan mine. Hydrometallurgy 2020, 195, 105394. [Google Scholar] [CrossRef]
- Novič, M.; Grgić, I.; Poje, M.; Hudnik, V. Iron-catalyzed oxidation of s(IV) species by oxygen in aqueous solution: Influence of pH on the redox cycling of iron. Atmos. Environ. 1996, 30, 4191–4196. [Google Scholar] [CrossRef]
- Pan, D.; Yang, L.; Wu, H.; Huang, R.; Zhang, Y. Formation and removal characteristics of sulfuric acid mist in a wet flue gas desulfurization system. J. Chem. Technol. Biot. 2017, 92, 598–604. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, W. Process and mechanism of catalyzed oxidation of SO2 with pyrolusite slurry. Environ. Sci. Technol. 2008, 31, 13–15+37. (In Chinese) [Google Scholar] [CrossRef]



| Components | Mn | O | C | Si | Al | Fe |
| Content (%) | 24.97 | 46.27 | 9.88 | 7.41 | 2.07 | 1.19 |
| Components | K | Na | Ca | Mg | S | Ti |
| Content (%) | 0.41 | 0.32 | 4.72 | 2.39 | 0.11 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Z.; Wang, Y.; Liu, J.; Xu, B.; Ding, L.; Huang, W.; Dai, Z.; Jiang, W.; Yao, L.; Yang, L. Removal and Resource Utilization of High Concentration Flue Gas Sulfur Dioxide Using Manganese Carbonate Ore. Atmosphere 2023, 14, 835. https://doi.org/10.3390/atmos14050835
Tang Z, Wang Y, Liu J, Xu B, Ding L, Huang W, Dai Z, Jiang W, Yao L, Yang L. Removal and Resource Utilization of High Concentration Flue Gas Sulfur Dioxide Using Manganese Carbonate Ore. Atmosphere. 2023; 14(5):835. https://doi.org/10.3390/atmos14050835
Chicago/Turabian StyleTang, Zhaotong, Yuchen Wang, Jie Liu, Bo Xu, Lin Ding, Wenfeng Huang, Zhongde Dai, Wenju Jiang, Lu Yao, and Lin Yang. 2023. "Removal and Resource Utilization of High Concentration Flue Gas Sulfur Dioxide Using Manganese Carbonate Ore" Atmosphere 14, no. 5: 835. https://doi.org/10.3390/atmos14050835
APA StyleTang, Z., Wang, Y., Liu, J., Xu, B., Ding, L., Huang, W., Dai, Z., Jiang, W., Yao, L., & Yang, L. (2023). Removal and Resource Utilization of High Concentration Flue Gas Sulfur Dioxide Using Manganese Carbonate Ore. Atmosphere, 14(5), 835. https://doi.org/10.3390/atmos14050835






