Abstract
In this trial, the effects of different nutritional treatments on the N balance parameters of fattening pigs in the 55–65 kg live weight category were evaluated. The following diets were used: control diet (C) and low-protein (LP) diet with 2% crude protein reduction, with and without 10% sugar beet pulp (S) or 0.5% benzoic acid supplementation. Six pigs per treatment with similar live weight were used, and in the context of the balance trial, the daily N intake, fecal and urinary N excretion and the pH of urine were measured. From the data N digestibility, the TAN % and N retention were calculated. Feeding LP diets reduced the fecal, urinary and total N excretion and also the pH of urine significantly compared with the control diet. Sugar beet pulp significantly increased the fecal N excretion and urinary pH, and it also reduced significantly N digestibility and the TAN ratio. Benzoic acid failed to reduce urinary pH. The measured N excretion, retention and TAN excretion values were more favorable than those that can be found in the different guidelines or those used in the Hungarian ammonia emission inventory.
1. Introduction
Ammonia emission is a major air quality concern at regional, national and global levels, and animal production is the main source of ammonia emission. It is estimated that 80–90% of ammonia emissions in the European Union are related to animal husbandry. In the case of Hungary, this value is around 70%, and the share of the pig sector is about 21% [,]. To improve air quality in the EU, the Directive 2016/2284 has been developed to reduce the national emissions of certain atmospheric pollutants. According to this, the member states must reach certain reductions. In Hungary, after 2030, a 32% reduction in ammonia emissions is expected compared to those of 2005 [,].
Several studies have reported that there are a number of feeding options available to reduce nitrogen (N) excretion of animals [,]. The amount of excreted ammonia can be reduced if the animals consume less and more balanced protein, if more accurate protein evaluation systems are used, if the diets are formulated based on digestible amino acids, if the so-called ideal protein concept is followed and if more detailed information is available on the protein, amino acid, and energy requirements of the animals of different genotypes and age categories. Among the dietary tools, multi-phase feeding can also increase the N retention of growing pigs [,,]. Feeding low-protein (LP), amino-acid-supplemented diets is already a common practice, since LP diets not only decrease the N excretion of the animals but in many cases are also cheaper [,,,,,,,,,]. In the case of growing pigs, about 2–3% reduction in crude protein content can be achieved, depending on the breed and age of the animals [,,,,,,,]. According to a recent meta-analysis [], if lysine, methionine, threonine, tryptophan and valine are supplemented, the protein content of the nursery, grower and finisher diets can be reduced to 18.3, 16.2 and 11.5%, respectively, without compromising the performance parameters. According to the literature data, 1% protein reduction can decrease ammonia emissions by about 10% [,,,]. From an ammonia emission point of view, the urinary nitrogen, mostly urea, is converted to ammonia in the barn, during the storage of manure or during the spreading of manure on the field. Therefore, modifying the excreted urinary and fecal nitrogen ratio can also affect ammonia emission. Nitrogen balance studies have shown that crude protein reduction has greater effect on the urinary than on the fecal N excretion [,,,]. This is favorable because of the lower amount of volatility properties [].
Pig diets containing higher fermentable non-starch polysaccharides (NSP) result in more intensive bacterial activity in the large intestine and increased synthesis of bacterial protein from the ammonia of the gut lumen. This way, the fermentable fiber decreases the ammonia absorption from the hind gut and the urea synthesis of liver. It results in lower urinary N excretion. Fermentable fiber also decreases the fecal pH through the increased volatile fatty acid production of hindgut bacteria [,,,,,,].
The pH of feces and urine can significantly reduce the NH3 emissions from manure during storage and application because both bacterial activity and the activity of urease enzyme decrease under acidic conditions []. The pH of urine can also be decreased by different nutritional techniques. The acidity of the urine can be decreased, for example, by adding acidifying Ca salts (CaSO4, CaCl2) to the feed instead of the basic calcium carbonate (CaCO3) [,,]. Benzoic acid or its salts are also commonly used feed additives, developed to decrease the pH of the urine. Benzoic acid is converted to hippuric acid during its metabolism and excreted via urine [].
Several studies have shown that feed and the above-discussed feeding techniques can reduce the ammonia emissions of pig farms. However, in many cases, the dietary modifications or the inclusion rates of the additives were above the practical levels [,,].
The objective of this study was to examine the impact of feeding a low-protein diet, using benzoic acid and sugar beet pulp supplementations, on the most important N balance parameters of fattening pigs. The dietary treatments were also used in combination, which has not been tested before. Our main goal was to attain further understanding on the efficiency of the investigated nutritional factors and use the results to improve the accuracy of the national ammonia emission inventory. We also want to implement these data into our ammonia emission advisory tool, developed recently [], and to use the results in our advisory work with farmers.
2. Materials and Methods
The experiment was carried out on the pig research farm of the Institute of Physiology and Nutrition, Hungarian University of Agriculture and Life Sciences, Georgikon Campus, Keszthely, Hungary. The trial was approved by the Food Chain Safety and Land Office Department of the Zala County Government Office (case number: ZAI/040/01010-7/2018).
2.1. Animals, Experimental Design, Diets and Housing
In the experiment, 60 crossbred (Topigs 20 × Danbred Duroc) weaned male piglets were selected to have similar live weight and placed into 6 floor pens of 10 pigs per pen. The size of the pens was 3.5 × 3.4 m. Wheat straw was used as bedding material, and the pens were equipped with self-feeders and -waterers. The animals were fed ad libitum, and the water was also available without constraints. The manure was removed daily. In the grower phase, the animals were adapted to the diets, and the N balance trials were carried out in the subsequent fattening phase.
The grower and fattening diets were fed in the 30–40 kg and 40–80 kg live weight categories, respectively. A two-factorial design was used, with dietary protein content and feed additives as main factors. The following treatments were applied:
- −
- A commercial, maize, soybean meal-based control diet (C);
- −
- Low-protein diets with 2% protein reduction and amino acid supplementation (LP);
- −
- Sugar-beet-pulp-supplemented diets (S);
- −
- Benzoic-acid-supplemented diets (B).
The composition and the measured nutrient content of the grower and fattening diets are shown in Table 1 and Table S1, respectively. The proximate analysis of the compound feeds was carried out with the official methods. The amino acid analysis was carried out with an automatic amino acid analyzer (INGOS AAA 400). The DE content of diets were calculated according to the equation of Noblet and Perez []. From Table 1, it can be seen that LP diets contained 5–9% less soybean meal and more crystalline amino acids. In addition to lysine and methionine, the LP diets also contained threonine, valine and tryptophan. The sugar beet pulp incorporation rate was 10%. Its lower energy content was compensated with more sunflower oil. Benzoic acid was used in the form of its Ca-benzoate salt at 0.5%.

Table 1.
Composition and measured nutrient content of the fattening diets.
In the fattening phase, 6 animals from each treatment groups were selected with similar live weight and transferred to a different room, containing specific balance cages. Since only 24 balance cages were available, the trial was carried out over 2 subsequent weeks. In the first week, the diets “C + B”, “C + S” and “LP + S” were fed, while in the second week, the remaining 3 diets were fed. The average body weight of the animals at the start and end of the balance trial can be seen in Table 2.

Table 2.
Live weight of pigs at the beginning and at the end of the balance trial.
The cages were equipped to measure exactly the feed intake and to collect separately the total amounts of urine and feces. The amount of daily feed was calculated as 95% of the ad libitum feed intake according to the recommendations of the latest NRC [] for the 55–60 kg live weight category. The daily feed ratio of 1.9 kg was distributed into two equal portions and given to the animals at 7.00 a.m. and 3.00 p.m. Water was provided ad libitum via nipple self-waterers. To reduce the nitrogen loss from the urine, 20 mL of 5% sulfuric acid was poured into the urine containers. In the balance cage room, heat blowers were used for heating and the room temperature was set to 16 ± 2 °C. The balance experiment took 7 days. After 2 days adaptation, in the subsequent 5 days, the total amounts of feces and urine were collected daily in the morning and stored at −10 °C.
2.2. Measurement of Nitrogen Forms and Calculations
Before the analytical procedure, the daily feces and urine samples from every pig were mixed and homogenized, and a representative sample of about 500 mL urine and 500 g feces was used for nitrogen analysis. From the diet, feces and urine samples, their N contents were determined according to the Kjeldahl method with a Foss–Kjeltec 8400 Analyzer (Nils Foss Allé 1, DK-3400 Hilleroed, Denmark). From the data, the daily N intake, the daily fecal N excretion, the daily urinary N excretion, also called total ammoniacal N (TAN), the N digestibility, the daily total N excretion, the daily amount of retained N and the ratio of the N retention were calculated as follows:
N digestibility (%) = ((N intake (g/day) − fecal N excretion (g/day))/(N intake (g/day))
TAN (%) = urinary N excretion (g/day)/(fecal N excretion (g/day) + urinary N excretion (g/day))
N retention (g/day) = N intake (g/day) − fecal N excretion (g/day) − urinary N excretion (g/day)
N retention (%) = N retention (g/day)/N intake (g/day)
Annual N excretion (kg/animal/year) = daily N excretion (g/animal/day) ∗ 365/1000
annual TAN excretion (kg/animal/year) = annual N excretion (kg/animal/year) ∗ TAN of the total N excretion (%)
2.3. pH Measurements
Urine pH was measured from sulfuric acid-free fresh samples every morning after feeding. The measurement was carried out by Adwa Waterproof AD12 instrument (Adwa Hungary Kft. 6726 Szeged, Alsó-Kikötő sor 11.C., Hungary)
2.4. Statistical Analysis
All the measured and calculated parameters were evaluated with two-way analysis of variance (ANOVA), using the dietary protein content and feed additives as main factors. The differences were considered as significant at a level of p ≤ 0.05. Data were expressed as means ± SEM.
3. Results
The animals consumed entirely their daily ratios, so the N intake was influenced only by the crude protein content of the feed. The N intake and N excretion results are summarized in Table 3. As expected, the N intake of pigs was about 7 g lower when LP diets were fed, and as a result, the fecal, urinary and total N excretion were significantly lower with this treatment. The dietary supplements modified only the fecal N excretion of animals. Feeding sugar beet pulp increased this parameter significantly. Significant interaction was found between the two main factors in the case of fecal N excretion. The reason for this was that treatment S increased the fecal excretion in the control diet, but this was not the case when sugar beet pulp was mixed into the LP diet.

Table 3.
The effect of the treatments on the nitrogen intake and nitrogen excretion.
The protein content of diets modified only urinary pH (Table 4). On the other hand, sugar-beet-pulp-containing diets decreased N digestibility and the TAN% and increased urinary pH significantly compared with the two other treatments. The reason for the significant interactions between the main factors in this case was that sugar beet pulp decreased the digestibility and TAN% in the control diet but not in the LP feed. On the other hand, sugar beet pulp had no impact on the pH of urine in the control group but increased that of the LP-diet-fed animals.

Table 4.
The effect of the treatments on the N digestibility, TAN ratio, N retention and urinary pH.
In the ammonia emission inventories, the EU member countries can use different methods to calculate their emissions. There are default values for the N excretions of the different farm animal species and also for the TAN content of the excreted N [,]. In addition to the default values, country-specific-emission factors can be developed and used to improve the accuracy of the inventories. The nitrogen excretion calculation is based on the number of animals and the amount of the annual excretion. In order to compare our results with those of the national ammonia emission inventory, the daily N excretion values were recalculated to annual values by multiplying the daily excretions by 365 days. The annual results of excretion are shown in Figure 1. In Hungary, the N excretion of fattening pigs above 50 kg live weight is 12.5 kg/animal/year. The figure shows that all treatments resulted in lower excretion than the default value and feeding LP diets can further reduce this parameter in this live weight category. The two additives did not affect this parameter.
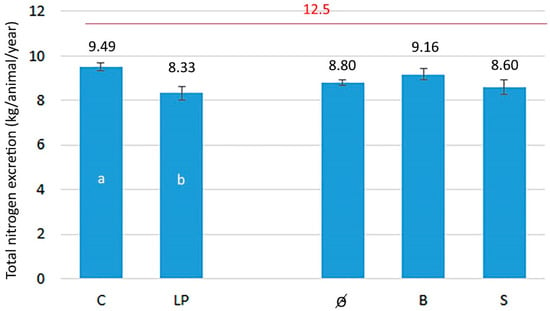
Figure 1.
The effect of the treatments on the annual N excretion. C—control diet, LP—2% reduced crude protein diets, C + S—the control diet with sugar beet pulp supplementation, C + B—the control diet with Ca-benzoate supplementation, LP + S—the LP diet with sugar beet pulp supplementation, LP + B—the LP diet with Ca-benzoate supplementation. The data were evaluated with two-way analysis of variance, using the dietary N content and feed additives as main factors. The differences were considered significant at a level of p ≤ 0.05. Data are expressed as means ± SEM. Red line indicates the excretion level used recently in the Hungarian ammonia emission inventory.
The TAN excretion was also calculated from the data of this trial and compared with the actual calculation used in the national inventory (Figure 2). In Hungary, 70% TAN is still used in the calculation, which means 8.75 kg annual excretion for this pig category. The data of Figure 2 show that since most of the measured TAN percentages were below 70% in this trial, the TAN excretion results of animals were even more favorable than those for N excretion. TAN excretion of pigs consuming the LP diets was significantly lower than that of the control group. Of course, animals with higher body weight excrete more N and TAN, but according to our previous findings with pigs of different live weight categories [], the excretion data for the whole fattening are also lower than the default value.
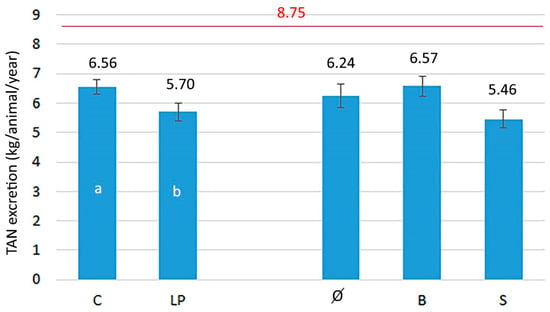
Figure 2.
The effect of the treatments on the TAN excretion. C—control diet, LP—2% reduced crude protein diets, C + S—the control diet with sugar beet pulp supplementation, C + B—the control diet with Ca-benzoate supplementation, LP + S—the LP diet with sugar beet pulp supplementation, LP + B—the LP diet with Ca-benzoate supplementation. The data were evaluated with two-way analysis of variance, using the dietary N content and feed additives as main factors. The differences were considered significant at a level of p ≤ 0.05. Data are expressed as means ± SEM. Red line indicates the excretion level used recently in the Hungarian ammonia emission inventory.
4. Discussion
In this trial, the effects of feeding LP diets, using sugar beet pulp as a fermentable fiber source and benzoic acid, a feed additive that can reduce urinary pH, were compared. There are plenty of research data on the effects of these techniques on the production traits and N metabolism of pigs [,,,,,]. The novelties of our trial were that we used only such treatments as can be applied under practical conditions and we evaluated also the combinations of dietary protein content, and the two most frequently used feed additives.
The N intake of pigs was only influenced by the protein content of the diets. As expected, feeding LP diets had great influence on all N excretion parameters. Nitrogen balance studies have shown that crude protein reduction has greater effect on the urinary than the fecal excretion [,,,]. This is favorable for ammonia emissions due to the fewer volatility properties. In this trial, the reductions in fecal and urinary N excretions were almost identical, 9% and 8.7%, respectively. Our results on sugar beet pulp are in agreement with the previous findings that fermentable fiber can really push N excretion towards the fecal excretion [,]. Therefore, this technique can also be used in the ammonia emission mitigation programs, but a balance is needed between the emission mitigation and the potential negative effects of the impaired digestion. Benzoic acid failed to modify the N excretion of pigs. The total N excretion was also influenced mostly by the N intake, and in this trial, the lowest excretion values were obtained if LP diets were fed. The dietary CP level was decreased from 16.5% to 14.6% in the LP diet. This 2% reduction in crude protein resulted in a 12.2% decrease in the total N excretion compared with the control. It is generally assumed that reducing the initial protein level of feeds by 1% reduces the nitrogen excretion by up to 10% []. According to these results and our previous findings [], such a strict correlation between protein reduction and N and TAN excretion cannot be declared. It depends on several factors, for example, the rate of protein reduction, the age and breed of the animals and other dietary factors such as fiber content or exogenous enzymes [,,,,].
Feeding sugar beet pulp at 10% reduced the N digestibility significantly, by about 6%. This is not new information, although in the previous trials, higher inclusion rates were usually used [,]. The reason for this is that fibers above a certain amount can decrease the digestion and also the absorption of the nutrients []. This is true also for the protein and amino acids. Our results on the sugar-beet-pulp-supplemented diets are also in agreement with those given in the literature. For example, O’Shea et al. [] performed experiments with 63 ± 1.3 kg, Large White × (Large White × Landrace) pigs. They fed 200 g/kg beet pulps and obtained similar excretion and digestibility values (9.7 g/day fecal N excretion, 16.8 g/day urinary N excretion, 26.5 g/day total N excretion and 82.2% N digestibility).
In the ammonia inventories, TAN mainly indicates the urinary N that can be converted quickly to ammonia from manure. The default TAN ratio of pig manure is 70% [,,]. According to the results of this trial, only sugar beet pulp has significant TAN-decreasing effect. This finding is in accordance with some previous studies. The range of TAN decrease changes according to the protein decrease and the incorporation rate and the sources of the fermentable fiber [,,,]. The results are of course also affected by the age of pigs, since older animals with higher body weight have more developed bacterial communities in the large intestine.
In balance trials, N retention depends on the N intake and total N excretion of pigs. This parameter was not affected by the treatments. However, the measured N retention values are better than the default value (34%) of the inventory [].
The pH of urine and faeces is important for the volatilization of N, whereas the growth of urea-degrading bacteria and urease enzyme activity is reduced by low pH. The pH optimum for urease is between 6 and 9.
The pH of urine is determined by the ratio of the excreted acidic and basic substances [,]. Low urine pH also lowers the pH of the manure, even after a certain period of storage. This pH effect can also significantly reduce NH3 emissions from manure during storage and application []. The urine can be acidified, for example, by adding CaSO4 or CaCl2 to the feed instead of the basic calcium carbonate (CaCO3) [,,] or feeding benzoic acid, a feed additive developed for this purpose. Several studies reported that 1% benzoic acid reduced the pH of urine [,,,,]. However, one report concluded that the higher level of benzoic acid (2.5%) can have negative effects on the health of animals, through reducing the number of white blood cells and globulin and causing spleen injury []. In our experiment, to avoid the depressing effects, only 0.5% incorporation rate was used, according to the practical recommendations. Based on our results, feeding 0.5% benzoic acid is not enough to decrease the pH of urine. The reason for this could be that a higher incorporation rate is needed for more hippuric acid conversion and excretion. Therefore, higher inclusion is needed from this feed additive, but its potential negative effects should be taken into account.
On the other hand, feeding LP diets significantly reduced the urinary pH. The pH-lowering effect of LP diets can be found in the literature [,,,,]. According to these data, the decrease in pH is due to the change in the electrolyte balance of the animals. The kidney plays an important role in regulating the constant pH of the blood, and the cation–anion ratio of feed affects the pH of urine and slurry. Canh et al. [] and Portejoie et al. [] also found strong correlation between the electrolyte balance and the urinary and manure pH. Potassium, which is present in higher amounts in protein-rich feedstuffs, such as legume seeds, plays an important role in the electrolyte balance of animals. The lower potassium content of LP diets affects the ratio of Na+ and K+ excretion and thus the pH of the urine.
Comparing our results with the Hungarian ammonia inventory, we currently use 34% N retention in Hungary for fattening pigs over 50 kg live weight and TAN percentage of 70%. Based on the present results, the true N retention of the recent pig genotypes is more favorable, which is also confirmed by other literature data [,,]. In the papers of Figueroa et al. [], Gloaguen et al. [] and Mroz et al. [], N retention values were published of 58.01–62.41%, 63.9–72.2% and 47.4–53.2%, respectively. The higher N retention results lower TAN % mainly in the younger age categories [], and the TAN can also be further reduced by feeding LP diets. This means that, in pig fattening, it is worth developing country-specific N excretion and retention factors, taking into account the live weight and genotype of animals and the effects of the different dietary factors. This can improve the accuracy of the national inventories. In most cases, these new factors result in more favorable ammonia emission compared with the default values that can be found in the different recommendations [,].
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/atmos14050776/s1. Table S1: Composition and measured nutrient content of the grower diet.
Author Contributions
Conceptualization, K.D. and Z.B.; methodology, K.D. and Z.B.; software, I.A.G.-K.; validation, J.P. and I.A.G.-K.; formal analysis, I.A.G.-K. and N.S.; investigation, Á.B., I.A.G.-K., Z.B., L.P. and Á.K.; resources, K.D.; data curation, I.A.G.-K.; writing—original draft preparation, I.A.G.-K., N.S. and K.D.; writing—review and editing, I.A.G.-K. and K.D.; visualization, I.A.G.-K. and N.S.; supervision, L.P. and K.D.; project administration, K.D.; funding acquisition, Z.B. and K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hungarian Ministry of Agriculture (AKGF/31/2022).
Institutional Review Board Statement
The animal experiment was approved by the Institutional Ethics Committee (Animal Welfare Committee, Georgikon Campus, Hungarian University of Agriculture and Life Sciences) under the license number ZAI/040/01010-7/2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated or analyzed during this study are included in this published article.
Conflicts of Interest
All authors declare no conflict of interest.
References
- IIR. Hungary Informative Inventory Report 1990–2019. Available online: https://www.ceip.at/status-of-reporting-and-review-results/2021-submission (accessed on 18 July 2021).
- Geicsnek-Koltay, I.A.; Benedek, Z.; Baranyai, N.H.; Such, N.; Pál, L.; Wágner, L.; Bartos, Á.; Kovács, Á.; Poór, J.; Dublecz, K. Impacts of Age, Genotype and Feeding Low-Protein Diets on the N-Balance Parameters of Fattening Pigs. Agriculture 2022, 12, 94. [Google Scholar] [CrossRef]
- National Emission Ceilings (NEC) Directive—(EU) 2016/2284; European Union: Brussels, Belgium, 2016.
- Aarnink, A.J.A.; Verstegen, M.W.A. Nutrition, Key Factor to Reduce Environmental Load from Pig Production. Livest. Sci. 2007, 109, 194–203. [Google Scholar] [CrossRef]
- Bittman, S.; Dedina, M.; Howard, C.M.; Oenema, O.; Sutton, M.A. (Eds.) Options for Ammonia Mitigation Guidance from the UNECE Task Force on Reactive Nitrogen; Centre for Ecology and Hydrology: Edinburgh, UK, 2014. [Google Scholar]
- Boisen, S.; Fernandez, J.A.; Madsen, A. Studies on Ideal Protein Requirement of Pigs From20 to 95 Kg Live Weight. In Proceedings of the 6th International Symposium on Protein Metabolism and Nutrition, Herning, Denmark, 9–14 June 1991; p. 299. [Google Scholar]
- Koch, F. Amino Acid Formulation to Improve Carcass Quality and Limit Nitrogen Load in Waste. In Proceedings of the Carolina Swine Nutrition Conference; Carolina Feed Industry Association: Raleigh, NC, USA, 1990; pp. 76–95. [Google Scholar]
- Van der Peet-Schwering, C.; Voermans, M. Effects of Feeding and HousiI He Ammonia Emission of Growing and Finishing Pig Facilities. In Proceedings of the Rep. Exp. Pig Stn.; Rosmalen, The Netherlands, 1996; pp. 17–19.
- Santonja, G.G.; Georgitzikis, K.; Scalet, B.M.; Montobbio, P.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for the Intensive Rearing of Poultry or Pigs; Join Research Center: Seville, Spain, 2017. [Google Scholar]
- Canh, T.T.; Aarnink, A.J.A.; Mroz, Z.; Jongbloed, W.; Schrama, J.W.; Verstegen, M.W.A. Influence of Electrolyte Balance and Acidifying Calcium Salts in the Diet of Growing Finishing Pigs on Urinary PH, Slurry PH, and Ammonia Volatilisation from Slurry. Livest. Prod. Sci. 1998, 56, 1–13. [Google Scholar] [CrossRef]
- Carter, S.D.; Kim, H.J. Technologies to Reduce Environmental Impact of Animal Wastes Associated with Feeding for Maximum Productivity. Anim. Front. 2013, 3, 42–47. [Google Scholar] [CrossRef]
- Gatel, F.; Grosjean, F. Effect of Protein Content of the Diet on Nitrogen Excretion by Pigs. Livest. Prod. Sci. 1992, 31, 109–120. [Google Scholar] [CrossRef]
- Kerr, B.J. Dietary Manipulation to Reduce Environmental Impact. In Proceedings of the 9th International Symposium on Digestive Physiology in Pigs, Banff, AB, Canada, 14 May 2003; pp. 139–158. [Google Scholar]
- Niyazov, N.; Ostrenko, K. Effect of Low-Protein Diets on the Nitrogen Balance and Productivity of Pigs. J. Livest. Sci. 2020, 11, 106–109. [Google Scholar] [CrossRef]
- Powers, W.J.; Zamzow, S.B.; Kerr, B.J. Reduced Crude Protein Effects on Aerial Emissions from Swine. Appl. Eng. Agric. 2007, 23, 539–546. [Google Scholar] [CrossRef]
- Schutte, J.B.; De Jong, J.; Van Kempen, G.J.M. Dietary Protein Is Relation to Requirement and Pollution in Pigs during the Body Weight Range of 20–40 kg. In Nitrogen Flow in Pig Production and Environmental Consequences; Verstegen, M.W.A., den Hartog, L.A., van Kempen, G.J.M., Metz, J.H.M., Eds.; Pudoc: Wageningen, The Netherlands, 1993; pp. 259–263. [Google Scholar]
- Li, Q.F.; Trottier, N.; Powers, W. Feeding Reduced Crude Protein Diets with Crystalline Amino Acids Supplementation Reduce Air Gas Emissions from Housing. J. Anim. Sci. 2015, 93, 721–730. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Wang, G.; Cai, S.; Zeng, X.; Qiao, S. Advances in Low-Protein Diets for Swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. [Google Scholar] [CrossRef]
- FVM. Vidékfejlesztési Képzési és Szaktanácsadási Intézet Az Állattartás Környezeti Hatásai, Helyzete És Viszonya a Hazai És EU Szabályozáshoz. Available online: https://docplayer.hu/1648861-Az-allattartas-kornyezeti-hatasai-helyzete-es-viszonya-a-hazai-es-eu-1-szabalyozashoz.html (accessed on 6 December 2021).
- Gay, S.W. Ammonia emissions and animal agriculture. In Virginia Cooperative Extension; VirginiaTech: Blacksburg, VA, USA, 2009; pp. 442–445. [Google Scholar]
- Canh, T.T.; Aarnink, A.J.A.; Schutte, J.B.; Sutton, A.; Langhout, D.J.; Verstegen, M.W.A. Dietary Protein Affects Nitrogen Excretion and Ammonia Emission from Slurry of Growing-Finishing Pigs. Livest. Prod. Sci. 1998, 56, 181–191. [Google Scholar] [CrossRef]
- Dourmad, J.Y.; Henry, Y.; Bourdon, D.; Quiniou, N.; Guillou, D. Effect of Growth Potential and Dietary Protein Input on Growth Performance, Carcass Characteristics and Nitrogen Output in Growing-Finishing Pigs. In Proceedings of the Proceedings Congress on Ni-trogen Flow in Pig Production and Environmental Consequences, Wageningen, The Netherlands, 8 June 1993; pp. 206–211. [Google Scholar]
- Lenis, N.P.; Schutte, J.B. Aminozuurvoorziening van Biggen En Vleesvarkens in Relatie Tot de Stikstofuitscheiding. In Mestproblematiek: Aanpak via de Voeding van Varkens en Pluimvee. Onderzoek Inzake de Mest en Ammoniakproblematiek in de Veehouderij; Jongbloed, A.W., Coppoolse, J., Eds.; Dienst Landbouwkundig Onderzoek: Wageningen, The Netherlands, 1990; Volume 4. [Google Scholar]
- Han, I.K.; Lee, J.H. The Role of Synthetic Amino Acids in Monogastric Animal Production-review. Asian-Australas. J. Anim. Sci. 2000, 13, 543–560. [Google Scholar] [CrossRef]
- Carpenter, D.A.; O’Mara, F.P.; O’Doherty, J.V. The Effect of Dietary Crude Protein Concentration on Growth Performance, Carcass Composition and Nitrogen Excretion in Entire Grower-Finisher Pigs. Irish J. Agric. Food Res. 2004, 43, 227–236. [Google Scholar]
- Zhao, Y.; Tian, G.; Chen, D.; Zheng, P.; Yu, J.; He, J.; Mao, X.; Yu, B. Effects of Varying Levels of Dietary Protein and Net Energy on Growth Performance, Nitrogen Balance and Faecal Characteristics of Growing-Finishing Pigs. Rev. Bras. Zootec. 2019, 48, 20180021. [Google Scholar] [CrossRef]
- Rocha, G.C.; Duarte, M.E.; Kim, S.W. Advances, Implications, and Limitations of Low-Crude-Protein Diets in Pig Production. Animals 2022, 12, 3478. [Google Scholar] [CrossRef]
- Babcsány, I.; Nyári, E.; Warning, S.; Lynott, D.; Csizmazia, L.; Szélesné Kutas, B.; Csáki, Z.S.; Mayer, A.; Demeter, J.; Ács, P.; et al. Útmutató az Elérhető Legjobb Technika Meghatározásához; Integrált Szennyezés-Megelő Zési És Környezet-Egészségügyi Főosztály: Budapest, Hungary, 2004. [Google Scholar]
- Figueroa, J.; Lewis, A.; Miller, P.S. Nitrogen Balance and Growth Trials with Pigs Fed Low-Crude Protein, Amino Acid-Supplemented Diets. Neb. Swine Rep. 2000, 110, 26–28. [Google Scholar]
- O’Shea, C.J.; Lynch, B.; Lynch, M.B.; Callan, J.J.; O’Doherty, J.V. Ammonia Emissions and Dry Matter of Separated Pig Manure Fractions as Affected by Crude Protein Concentration and Sugar Beet Pulp Inclusion of Finishing Pig Diets. Agric. Ecosyst. Environ. 2009, 131, 154–160. [Google Scholar] [CrossRef]
- Portejoie, S.; Dourmad, J.Y.; Martinez, J.; Lebreton, Y. Effect of Lowering Dietary Crude Protein on Nitrogen Excretion, Manure Composition and Ammonia Emission from Fattening Pigs. Livest. Prod. Sci. 2004, 1–2, 45–55. [Google Scholar] [CrossRef]
- O’Connell, J.M.; Callan, J.J.; O’Doherty, J.V. The Effect of Dietary Crude Protein Level, Cereal Type and Exogenous Enzyme Supplementation on Nutrient Digestibility, Nitrogen Excretion, Faecal Volatile Fatty Acid Concentration and Ammonia Emissions from Pigs. Anim. Feed Sci. Technol. 2006, 127, 73–88. [Google Scholar] [CrossRef]
- Aarnink, A.J.A.; Cahn, T.T.; Mroz, Z. Reduction of Ammonia Volatilization by Housing and Feeding in Fattening Pig-Geries. In Ammonia and Odour Emission from Animal Production Facilities; Voermans, J.A.M., Monteney, G.J., Eds.; Animal Nutrition, WIAS: Vinkeloord, The Netherlands, 1997; pp. 283–291. [Google Scholar]
- Clark, O.G.; Moehn, S.; Edeogu, I.; Price, J.; Leonard, J. Manipulation of Dietary Protein and Nonstarch Polysaccharide to Control Swine Manure Emissions. J. Environ. Qual. 2005, 34, 1461–1466. [Google Scholar] [CrossRef]
- Jarret, G.; Cerisuelo, A.; Peu, P.; Martinez, J.; Dourmad, J.Y. Impact of Pig Diets with Different Fibre Contents on the Composition of Excreta and Their Gaseous Emissions and Anaerobic Digestion. Agric. Ecosyst. Environ. 2012, 160, 51–58. [Google Scholar] [CrossRef]
- Philippe, F.X.; Cabaraux, J.F.; Nicks, B. Ammonia Emissions from Pig Houses: Influencing Factors and Mitigation Techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Low, A.G. Role of Diet. In Recent Advances in Animal Nutrition; Haresign, W., Cole, D.J.A., Eds.; Butterworths: London, UK, 1985; p. 87. [Google Scholar]
- Philippe, F.-X.; Laitat, M.; Wavreille, J.; Nicks, B.; Cabaraux, J.-F.; Philippe, F.-X.; Laitat, M.; Wavreille, J.; Nicks, B.; Cabaraux, J.-F. Effects of a High-Fibre Diet on Ammonia and Greenhouse Gas Emissions from Gestating Sows and Fattening Pigs. Atmos. Environ. 2015, 109, 197–204. [Google Scholar] [CrossRef]
- Nørgaard, J.V.; Fernández, J.A.; Eriksen, J.; Olsen, O.H.; Carlson, D.; Poulsen, H.D. Urine Acidification and Mineral Metabolism in Growing Pigs Fed Diets Supplemented with Dietary Methionine and Benzoic Acid. Livest. Sci. 2010, 134, 113–115. [Google Scholar] [CrossRef]
- Bühler, K.; Wenk, C.; Broz, J.; Gebert, S. Influence of Benzoic Acid and Dietary Protein Level on Performance, Nitrogen Metabolism and Urinary PH in Growing-Finishing Pigs. Arch. Anim. Nutr. 2006, 60, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Le Dinh, P.; van der Peet-Schwering, C.M.C.; Ogink, N.W.M.; Aarnink, A.J.A. Effect of Diet Composition on Excreta Composition and Ammonia Emissions from Growing-Finishing Pigs. Animals 2022, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Le Dinh, P.; Aarnink, A. Nutritional Strategies to Reduce Emissions from Waste in Pig Production Phung Lê Đình, Hue University of Agriculture and Forestry, University and Research, The Netherlands. In Achieving Sustainable Production of Pig Meat; Burleigh Dodds Science Publishing: Wageningen, The Netherlands, 2018; Volume 1, pp. 243–264. ISBN 9781351114493. [Google Scholar]
- Magyar, M.; Pirkó, B.; Seenger, J.K.; Baranyai, N.H.; Dublecz, K.; Vojtela, T.; Rák, R.; Borka, G.; Szabó, A.; Benedek, Z. Advisory and Knowledge Transfer Tool for Ammonia Emission Mitigation on Pig Farms in Hungary. Appl. Sci. 2021, 11, 5970. [Google Scholar] [CrossRef]
- Noblet, J.; Perez, J.M. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J. Anim. Sci. 1993, 77, 3389–3398. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Swine: Eleventh Revised Edition; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-48903-4. [Google Scholar]
- Publications Office of the EU. EMEP/EEA Air Pollutant Emission Inventory Guidebook; Publications Office of the EU: Luxembourg, 2019. [Google Scholar]
- Intergovernmental Panel on Climate Change Emissions from Livestock and Manure Management. 2019 Refinement 2006 IPCC Greenhause Gas Guidelines. 2019. Available online: https://www.ipcc.ch/report/2019-refinement-to-the-2006-ipcc-guidelines-for-national-greenhouse-gas-inventories/ (accessed on 6 February 2023).
- Mroz, Z.; Jongbloed, A.W.; Partanen, K.H.; Vreman, K.; Kemme, P.A.; Jogut, J. The Effects of Calcium Benzoate in Diets with or without Organic Acids on Dietary Buffering Capacity, Apparent Digestibility, Retention of Nutrients and Manure Characteristics in Swine. J. Anim. Sci. 2000, 78, 2622–2632. [Google Scholar] [CrossRef] [PubMed]
- Canh, T.T.; Verstegen, M.W.A.; Aarnink, A.J.A.; Schrama, J.W. Influence of Dietary Factors on Nitrogen Partitioning and Composition of Urine and Feces of Fattening Pigs. J. Anim. Sci. 1997, 75, 700–706. [Google Scholar] [CrossRef]
- Canh, T.T.; Sutton, A.L.; Aarnink, A.J.A.; Verstegen, M.W.A.; Schrama, J.W.; Bakker, G.C.M. Dietary Carbohydrates Alter the Fecal Composition and PH and the Ammonia Emission from Slurry of Growing Pigs. J. Anim. Sci. 1998, 76, 1887–1895. [Google Scholar] [CrossRef]
- EU 2017/302 Best Available Techniques (BAT) Conclusions, under Directive 2010/75/EU of the European Parliament and of the Council, for the Intensive Rearing of Poultry or Pigs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2017.043.01.0231.01.ENG (accessed on 6 February 2023).
- Dublecz, K.; Husvéth, F.; Wágner, L.; Márton, A.; Koltay, I.; Such, N.; Rawash, M.A.; Mezőlaki, Á.; Pál, L.; Molnár, A. Feeding Low Protein Diets Poultry and Pig Diets—Physiological, Economic and Environmental Aspects. In Proceedings of the International Symposium on Animal Science, Herceg Novi, Montenegro, 3–8 June 2019; pp. 20–29. [Google Scholar]
- Shriver, J.A.; Carter, S.D.; Sutton, A.L.; Richert, B.T.; Senne, B.W.; Pettey, L.A. Effects of Adding Fiber Sources to Reduced-Crude Protein, Amino Acid-Supplemented Diets on Nitrogen Excretion, Growth Performance, and Carcass Traits of Finishing Pigs. J. Anim. Sci. 2003, 81, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Aarnink, A.J.A.; Hol, J.M.G.; Nijeboer, G.M. Het Effect van Toevoeging van Benzoëzuur (1% VevoVitall®) Aan Vleesvarkensvoer Op de Ammoniakemissiereductie Is Bepaald En Bedroeg Gemiddeld 15.8% Ten Opzichte van Voer Zonder VevoVitall®; Wageningen University and Research Centre: Wageningen, The Netherlands, 2008. [Google Scholar]
- Guingand, N.; Demerson, L. Jiri Broz Incidence de l’incorporation d’acide Benzoïque Dans l’alimentation Des Porcs Charcutiers Sur Les Performances Zootechniques et l’émission d’ammoniac. J. Rech. Porc. 2005, 37, 1–6. [Google Scholar]
- Daumer, M.L.; Guiziou, F.; Dourmad, J.-Y. Influence de La Teneur En Protéines de l’aliment et de l’addition d’acide Benzoïque et de Phytase Microbienne Sur Les Caractéristiques des Effluents Chez Le Porc à l’engraissement. J. Rech. Porc. 2007, 39, 13–22. [Google Scholar]
- Shu, Y.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Yuan, Z.; Chen, D.; Mao, X. Excess of Dietary Benzoic Acid Supplementation Leads to Growth Retardation, Hematological Abnormality and Organ Injury of Piglets. Livest. Sci. 2016, 190, 94–103. [Google Scholar] [CrossRef]
- Gloaguen, M.; Le Floc’h, N.; Corrent, E.; Primot, Y.; van Milgen, J. The Use of Free Amino Acids Allows Formulating Very Low Crude Protein Diets for Piglets. J. Anim. Sci. 2014, 92, 637–644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).