Measurement of Atmospheric Volatile and Intermediate Volatility Organic Compounds: Development of a New Time-of-Flight Mass Spectrometer
Abstract
:1. Introduction
2. Instrument Description
2.1. Ionization Source
2.2. Radio Frequency Transfer Line
2.3. Time-of-Flight Mass Analyzer
3. Instrument Performance
3.1. Ionization of Species
3.2. Fragmentation of Detected Molecules
3.3. Quantification and Limit of Detection
3.4. Background Signals and Stress Tests
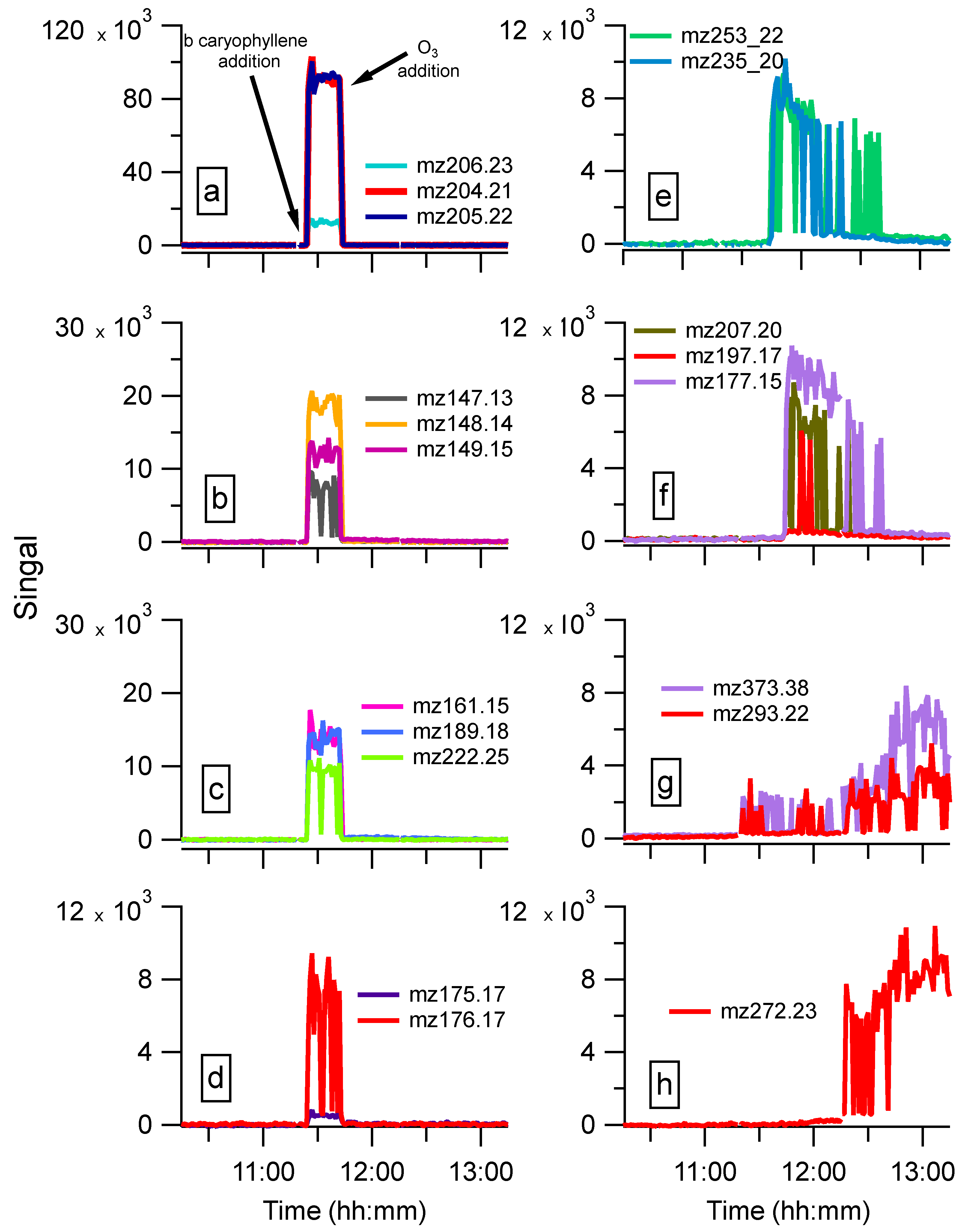
4. Application Examples
5. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goldstein, A.H.; Galbally, I.E. Known and unexplored organic constituents in the Earth’s Atmosphere. Environ. Sci. Technol. 2007, 41, 1515–1521. [Google Scholar] [CrossRef]
- Donahue, N.M.; Kroll, J.H.; Pandis, S.N.; Robinson, A.L. A two-dimensional volatility basis set—Part 2: Diagnostics of organic-aerosol evolution. Atmos. Chem. Phys. 2012, 12, 615–634. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kansal, A. Sources and reactivity of NMHCs and VOCs in the atmosphere: A review. J. Haz. Mat. 2009, 166, 17–26. [Google Scholar] [CrossRef]
- Calfapietra, C.; Fares, S.; Manes, F.; Morani, A.; Sgrigna, G.; Loreto, F. Role of biogenic volatile organic compounds (BVOC) emitted by urban trees on ozone concentration in cities: A review. Environ. Pollut. 2013, 183, 71–80. [Google Scholar] [CrossRef]
- Slowik, J.G.; Vlasenko, A.; McGuire, M.; Evans, G.J.; Abbatt, J.P.D. Simultaneous factor analysis of organic particle and gas mass spectra: AMS and PTR-MS measurements at an urban site. Atmos. Chem. Phys. 2010, 10, 1969–1988. [Google Scholar] [CrossRef]
- Crippa, M.; Canonaco, F.; Slowik, J.G.; El Haddad, I.; De-Carlo, P.F.; Mohr, C.; Heringa, M.F.; Chirico, R.; Marchand, N.; Temime-Roussel, B.; et al. Primary and secondary organic aerosol origin by combined gas-particle phase source apportionment. Atmos. Chem. Phys. 2013, 13, 8411–8426. [Google Scholar] [CrossRef]
- Kaltsonoudis, C.; Kostenidou, E.; Florou, K.; Psichoudaki, M.; Pandis, S.N. Temporal variability and sources of VOCs in urban areas of the eastern Mediterranean. Atmos. Chem. Phys. 2016, 16, 14825–14842. [Google Scholar] [CrossRef]
- Kaltsonoudis, C.; Kostenidou, E.; Florou, K.; Psichoudaki, M.; Pandis, S.N. Sources and chemical characterization of organic aerosol during the summer in the eastern Mediterranean. Atmos. Chem. Phys. 2015, 15, 11355–11371. [Google Scholar] [CrossRef]
- Kostenidou, E.; Florou, K.; Kaltsonoudis, C.; Tsiflikiotou, M.; Vratolis, S.; Eleftheriadis, K.; Pandis, S.N. The contribution of wood burning and other pollution sources to wintertime organic aerosol levels in two Greek cities. Atmos. Chem. Phys. 2017, 17, 3145–3163. [Google Scholar] [CrossRef]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef] [PubMed]
- LeBouf, R.F.; Stefaniak, A.B.; Abbas-Virji, M. Validation of evacuated canisters for sampling volatile organic compounds in healthcare settings. J. Environ. Monit. 2012, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.; Cuadras, A.; Rovira, E.; Borrull, F.; Marcé-Recasens, R.M. Comparative study of solvent extraction and thermal desorption methods for determining a wide range of volatile organic compounds in ambient air. Talanta 2010, 82, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Król, S.; Zabiegała, B.; Namieśnik, J. Monitoring VOCs in atmospheric air II. Sample collection and preparation. Trends Anal. Chem. 2010, 29, 1101–1112. [Google Scholar] [CrossRef]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air Part 1: Sorbent-based air monitoring options. J. Chromatogr. A 2010, 1217, 2674–2684. [Google Scholar] [CrossRef]
- Woolfenden, E. Sorbent-based sampling methods for volatile and semi-volatile organic compounds in air. Part 2. Sorbent selection and other aspects of optimizing air monitoring methods. J. Chromatogr. A 2010, 1217, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Arnts, R.R. Evaluation of adsorbent sampling tube materials and Tenax-TA for analysis of volatile biogenic organic compounds. Atmos. Environ. 2010, 44, 1579–1584. [Google Scholar] [CrossRef]
- Tsiodra, I.; Grivas, G.; Tavernaraki, K.; Bougiatioti, A.; Apostolaki, M.; Paraskevopoulou, D.; Gogou, A.; Parinos, C.; Oikonomou, K.; Tsagkaraki, T.; et al. Annual exposure to polycyclic aromatic hydrocarbons in urban environments linked to wintertime wood-burning episodes. Atmos. Chem. Phys. 2021, 21, 17865–17883. [Google Scholar] [CrossRef]
- Vasilakopoulou, C.; Stavroulas, I.; Mihalopoulos, N.; Pandis, S.N. The effect of the averaging period for PMF analysis of aerosol mass spectrometer measurements during offline applications. Atmos. Meas. Tech. 2022, 15, 6419–6431. [Google Scholar] [CrossRef]
- Król, S.; Zabiegała, B.; Namiesnik, J. Monitoring VOCs in atmospheric air: I. On-line gas analyzers. TrAC Trends Anal. Chem. 2010, 29, 1092–1100. [Google Scholar] [CrossRef]
- de Blas, M.; Navazo, M.; Alonso, L.; Durana, N.; Iza, J. Automatic on-line monitoring of atmospheric volatile organic compounds: Gas chromatography–mass spectrometry and gas chromatography–flame ionization detection as complementary systems. Sci. Total Environ. 2011, 409, 5459–5469. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of Proton-Transfer-Reaction Mass Spectrometry (PTR-MS) Medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- de Gouw, J.; Warneke, C. Measurements of volatile organic compounds in the earth’s atmosphere using proton-transfer-reaction mass spectrometry. Mass Spectrom. Rev. 2007, 26, 223–257. [Google Scholar] [CrossRef] [PubMed]
- Karl, T.G.; Christian, T.J.; Yokelson, R.J.; Artaxo, P.; Hao, W.M.; Guenther, A. The Tropical Forest and Fire Emissions Experiment: Method evaluation of volatile organic compound emissions measured by PTR-MS, FTIR, and GC from tropical biomass burning. Atmos. Chem. Phys. 2007, 7, 5883–5897. [Google Scholar] [CrossRef]
- Christian, T.J.; Kleiss, B.; Yokelson, R.; Holzinger, R.; Crutzen, P.J.; Hao, W.M.; Shirai, T.; Blake, D.R. Comprehensive laboratory measurements of biomass-burning emissions: 2. First intercomparison of open-path FTIR, PTR-MS, and GC-MS/FID/ECD. J. Geophys. Res. Atmos. 2004, 109, D02311. [Google Scholar] [CrossRef]
- Gkatzelis, G.I.; Coggon, M.M.; McDonald, B.C.; Peischl, J.; Aikin, K.C.; Gilman, J.B.; Trainer, M.; Warneke, C. Identifying volatile chemical product tracer compounds in U.S. cities. Environ. Sci. Technol. 2021, 55, 188–199. [Google Scholar] [CrossRef]
- Juran, S.; Pallozzi, E.; Guidolotti, G.; Fares, S.; Sigut, L.; Calfapietra, C.; Alivernini, A.; Savi, F.; Vecerova, K.; Krumal, K.; et al. Fluxes of biogenic volatile organic compounds above temperate Norway spruce forest of the Czech Republic. Agric. For. Meteorol. 2017, 232, 500–513. [Google Scholar] [CrossRef]
- Leglise, J.; Müller, M.; Piel, F.; Otto, T.; Wisthaler, A. Bulk organic aerosol analysis by proton-transfer-reaction mass spectrometry: An improved methodology for the determination of total organic mass, O:C and H:C elemental ratios, and the average molecular formula. Anal. Chem. 2019, 91, 12619–12624. [Google Scholar] [CrossRef]
- Müller, M.; Eichler, P.; D’Anna, B.; Tan, W.; Wisthaler, A. Direct sampling and analysis of atmospheric particulate organic matter by proton-transfer-reaction mass spectrometry. Anal. Chem. 2017, 89, 10889–10897. [Google Scholar] [CrossRef]
- Eichler, P.; Müller, M.; D’Anna, B.; Wisthaler, A. A novel inlet system for online chemical analysis of semi-volatile submicron particulate matter. Atmos. Meas. Tech. 2022, 15, 6419–6431. [Google Scholar] [CrossRef]
- Kostenidou, E.; Martinez-Valiente, A.; R’Mili, B.; Marques, B.; Temime-Roussel, B.; Durand, A.; André, M.; Liu, Y.; Louis, C.; Vansevenant, B.; et al. Technical note: Emission factors, chemical composition, and morphology of particles emitted from Euro 5 diesel and gasoline light-duty vehicles during transient cycles. Atmos. Chem. Phys. 2021, 21, 4779–4796. [Google Scholar] [CrossRef]
- Marques, B.; Kostenidou, E.; Martinez-Valiente, A.; Vansevenant, B.; Sarica, T.; Fine, L.; Temime-Roussel, B.; Tassel, P.; Perret, P.; Liu, Y.; et al. Detailed speciation of non-methane volatile organic compounds in exhaust emissions from diesel and gasoline Euro 5 vehicles using online and offline measurements. Toxics 2022, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.A.; Mohr, C.; Schobesberger, S.; D’Ambro, E.L.; Lee, B.H.; Lopez-Hilfiker, F.D. Evaluating organic aerosol sources and evolution with a combined molecular composition and volatility framework using the Filter Inlet for Gases and Aerosols (FIGAERO). Acc. Chem. Res. 2020, 53, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Hilfiker, F.D.; Mohr, C.; Ehn, M.; Rubach, F.; Kleist, E.; Wildt, J.; Mentel, T.F.; Lutz, A.; Hallquist, M.; Worsnop, D.; et al. A novel method for online analysis of gas and particle composition: Description and evaluation of a Filter Inlet for Gases and AEROsols (FIGAERO). Atmos. Meas. Tech. 2014, 7, 983–1001. [Google Scholar] [CrossRef]
- Siegel, K.; Karlsson, L.; Zieger, P.; Baccarini, A.; Schmale, J.; Lawler, M.; Salter, M.; Leck, C.; Ekman, A.M.L.; Riipinen, I.; et al. Insights into the molecular composition of semi-volatile aerosols in the summertime central Arctic Ocean using FIGAERO-CIMS. Environ. Sci. Atmos. 2021, 1, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Smith, D. and Španěl, P: SIFT-MS and FA-MS methods for ambient gas phase analysis: Developments and applications in the UK. Analyst 2015, 140, 2573–2591. [Google Scholar] [CrossRef]
- Smith, D.; Španěl, P. Ambient analysis of trace compounds in gaseous media by SIFT-MS. Analyst 2011, 136, 2009. [Google Scholar] [CrossRef]
- Paatero, P.; Tapper, U. Positive matrix factorization: A non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 1994, 5, 111–126. [Google Scholar] [CrossRef]
- Holzinger, R.; Acton, W.J.F.; Bloss, W.J.; Breitenlechner, M.; Crilley, L.R.; Dusanter, S.; Gonin, M.; Gros, V.; Keutsch, F.N.; Kiendler-Scharr, A.; et al. Validity and limitations of simple reaction kinetics to calculate concentrations of organic compounds from ion counts in PTR-MS. Atmos. Meas. Tech. 2019, 12, 6193–6208. [Google Scholar] [CrossRef]
- Gao, L.; Song, J.; Mohr, C.; Huang, W.; Vallon, M.; Jiang, F.; Leisner, T.; Saathoff, H. Kinetics, SOA yields, and chemical composition of secondary organic aerosol from β-caryophyllene ozonolysis with and without nitrogen oxides between 213 and 313 K. Atmos. Chem. Phys. 2022, 22, 6001–6020. [Google Scholar] [CrossRef]
- Wu, T.; Földes, T.; Lee, L.T.; Wagner, D.N.; Jiang, J.; Tasoglou, A.; Boor, B.E.; Blatchley, E.R. Real-time measurements of gas-phase trichloramine (NCl3) in an indoor aquatic center. Environ. Sci. Technol. 2021, 55, 8097–8107. [Google Scholar] [CrossRef]
- Pereira, M.F.; Apostolakis, A. NATO Science for Peace and Security Series—B: Physics and Biophysics Terahertz (THz), Mid Infrared (MIR) and Near Infrared (NIR) Technologies for Protection of Critical Infrastructures against Explosives and CBRN; Springer: Dordrecht, The Netherlands, 2021. [Google Scholar]
- Liangou, A.; Tasoglou, A.; Huber, H.J.; Wistrom, C.; Brody, K.; Menon, P.G.; Bebekoski, T.; Menschel, K.; Davidson-Fiedler, M.; DeMarco, K.; et al. Method for the identification of COVID-19 biomarkers in human breath using Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Eclinicalmedicine 2021, 42, 101207. [Google Scholar] [CrossRef]
- Spencer, S.E.; Tyler, C.A.; Tolocka, M.P.; Glish, G.L. Low-temperature plasma ionization-mass spectrometry for the analysis of compounds in organic aerosol particles. Anal. Chem. 2015, 87, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Neimira, B.A. Cold plasma decontamination of foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef]
- VonKeudell, A.; Awakowicz, P.; Benedikt, J.; Raballand, V.; Yanguas-Gil, A.; Opretzka, J.; Flötgen, C.; Reuter, R.; Byelykh, L.; Halfmann, H.; et al. Inactivation of bacteria and biomolecules by low-pressure plasma discharges. Plasma Med. 2010, 7, 327–352. [Google Scholar] [CrossRef]
- Burlica, R.; Shih, K.-Y.; Locke, B.R. Formation of H2 and H2O2 in a water-spray gliding arc nonthermal plasma reactor. Ind. Eng. Chem. Res. 2010, 49, 6342–6349. [Google Scholar] [CrossRef]
- Cappellin, L.; Makhoul, S.; Schuhfried, E.; Romano, A.; Pulgar, J.S.; Aprea, E.; Farneti, B.; Costa, F.; Gasperi, F.; Biasioli, F. Ethylene: Absolute real-time high-sensitivity detection with PTR/SRI-MS. The example of fruits, leaves and bacteria. Int. J. Mass Spectrom. 2014, 365–366, 33–41. [Google Scholar] [CrossRef]
- Papanastasiou, D.; Kounadis, D.; Orfanopoulos, I.; Lekkas, A.; Zacharos, A.; Raptakis, E.; Gini, M.; Eleftheriadis, K.; Nikolos, I. Experimental and numerical investigations of under-expanded gas flows for optimal operation of a novel multipole differential ion mobility filter in the first vacuum-stage of a mass spectrometer. Int. J. Mass Spectrom. 2021, 465, 1387–3806. [Google Scholar] [CrossRef]
- Papanastasiou, D.; McMahon, A. Correlated phase space distributions of ions in an orthogonal time-of-flight mass spectrometer. Int. J. Mass Spectrom. 2006, 254, 20–27. [Google Scholar] [CrossRef]
- Loos, M.; Gerber, C.; Corona, F.; Hollender, J.; Singer, H. Accelerated isotope fine structure calculation using pruned transition trees. Anal. Chem. 2015, 87, 5738–5744. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jorga, S.D.; Pierce, J.R.; Donahue, N.M.; Pandis, S.N. Particle wall-loss correction methods in smog chamber experiments. Atmos. Meas. Tech. 2018, 11, 6577–6588. [Google Scholar] [CrossRef]
- Kanawati, B.; Herrmann, F.; Joniec, S.; Winterhalter, R.; Moortgat, G.K. Mass spectrometric characterization of b-caryophyllene ozonolysis products in the aerosol studied using an electrospray triple quadrupole and time-of-flight analyzer hybrid system and density functional theory. Rapid Commun. Mass Spectrom. 2008, 22, 165–186. [Google Scholar] [CrossRef]
- Winterhalter, R.; Herrmann, F.; Kanawati, B.; Nguyen, T.L.; Peeters, J.; Vereecken, L.; Moortgat, G.K. The gas-phase ozonolysis of b-caryophyllene (C15H24). Part I: An experimental study. Phys. Chem. Chem. Phys. 2009, 11, 4152–4172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chen, Q.; Guzman, M.I.; Chan, C.K.; Martin, S.T. Second-generation products contribute substantially to the particle-phase organic material produced by β-caryophyllene ozonolysis. Atmos. Chem. Phys. 2011, 11, 121–132. [Google Scholar] [CrossRef]
- Bé, A.G.; Chase, H.M.; Liu, Y.; Upshur, M.A.; Zhang, Y.; Tuladhar, A.; Chase, Z.A.; Bellcross, A.D.; Wang, H.-F.; Wang, Z.; et al. Atmospheric β-caryophyllene-derived ozonolysis products at interfaces. ACS Earth Space Chem. 2019, 3, 158–169. [Google Scholar] [CrossRef]

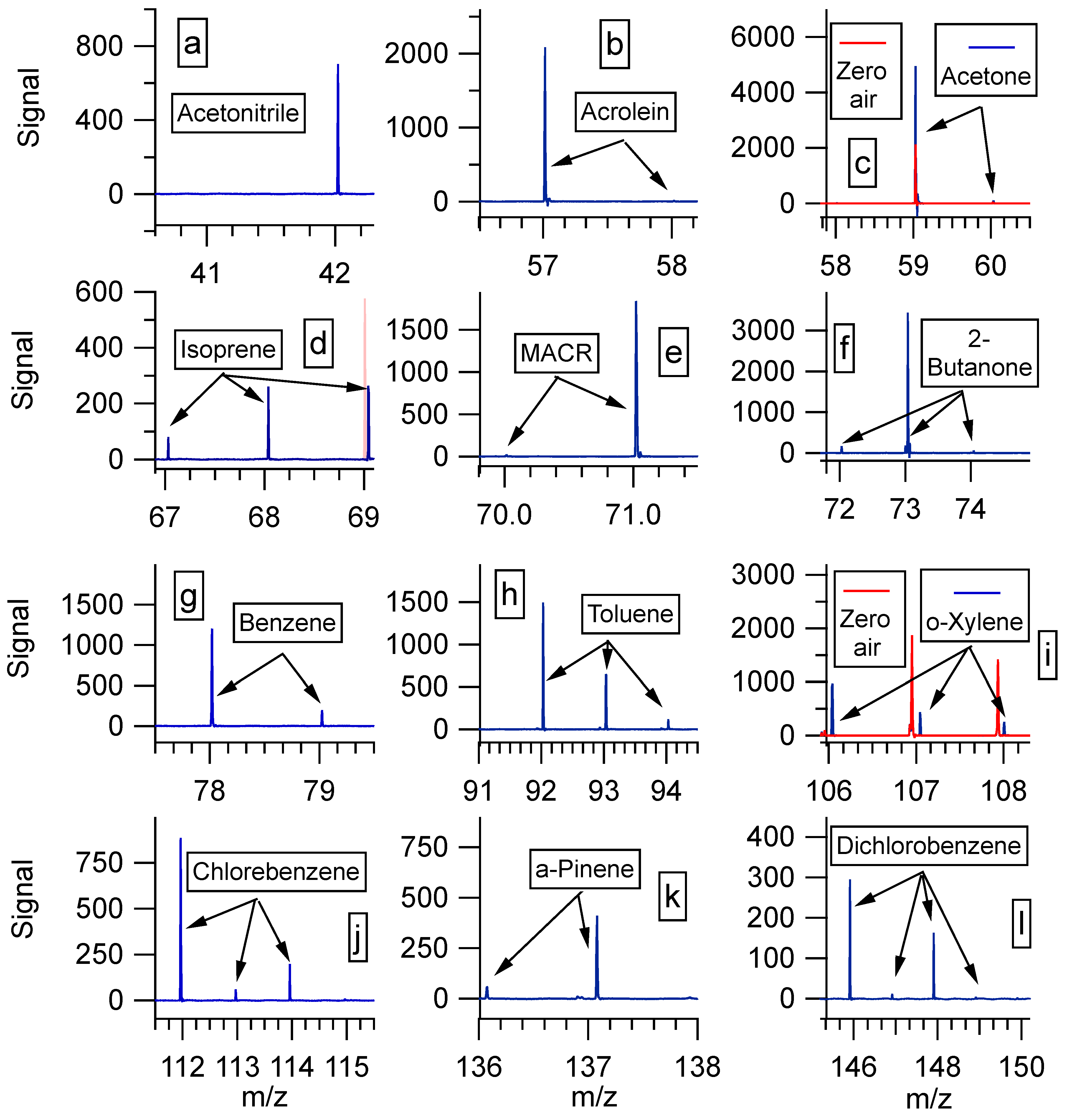


| Compound | Chemical Formula | Theoretical Masses for Monoisotopic Peak and the Second Isotope (Percentage Abundance) | Formulas for Ionic Compounds Observed Experimentally (Theoretical Masses for the Monoisotopic Peak and the Second Isotope) | Experimental m/z of Detected Peaks (Percentage Abundance) | Δppm |
|---|---|---|---|---|---|
| Acetonitrile | C2H3N | 41.0265 (100) ǂ | [C2H3N+H]+ (42.0338) | 42.0342 (100) | 9.5 |
| 42.0299 (2.2) | [C2H3N+H]+ (43.0371) * | N/D | N/A | ||
| Acrolein | C3H4O | 56.0262 (100) ǂ | [C3H4O+H]+ (57.0335) | 57.0333 (100) Ɫ | −3.5 |
| 57.0295 (3.2) | [C3H4O+H]+ (58.0368) * | 58.0366 (0.6) | −3.4 | ||
| Acetone | C3H6O | 58.0418 (100) ǂ | [C3H6O+H]+ (59.0491) | 59.0493 (100) Ɫ | 3.4 |
| 59.0452 (3.2) | [C3H6O+H]+ (60.0525) * | 60.0529 (2) | 6.7 | ||
| Isoprene | C5H8 | 68.0626 (100) ǂ | [C5H8-H]+ (67.0542) C5H8+ (68.0621) [C5H8+H]+ (69.0699) | 67.0546 (30) 68.0617 (99) 69.0697 (100) Ɫ | 6 −5.9 −2.9 |
| 69.0659 (5.4) | [C5H8-H]+ (68.05760) * C5H8+ (69.0654) * [C5H8+H]+ (70.0732) * | N/D N/D N/D | N/A N/A N/A | ||
| Methacrolein | C4H6O | 70.0418 (100) ǂ | C4H6O+ (70.0413) [C4H6O+H]+ (71.0491) | 70.0409 (1) 71.0488 (100) Ɫ | −5.7 −4.2 |
| 71.0452 (4.3) | [C4H6O+H]+ (72.0525) * | N/D | N/A | ||
| 2-butanone | C4H8O | 72.0575 (100) ǂ | C4H8O+ (72.0570) [C4H8O+H]+ (73.0648) | 72.056 (5) 73.0644 (100) Ɫ | −13.9 −5.5 |
| 73.0608 (4.3) | [C4H8O+H]+ (74.0681) * | 74.0677 (1.6) | −5.4 | ||
| Benzene | C6H6 | 78.0469 (100) ǂ | C6H6+ ( 78.0464) [C6H6+H]+ (79.0542) | 78.0467 (100) Ɫ 79.0543 (16.5) | 3.8 1.3 |
| 79.0503 (6.5) | C6H6+ (79.0498) * | N/D | N/A | ||
| Toluene | C7H8 | 92.062 (100) ǂ | C7H8+ (92.0621) [C7H8+H]+ (93.0699) | 92.0621 (100) Ɫ 93.0698 (43) | 0.0 −1.1 |
| 93.0659 (7.6) | C7H8+ (93.0654) * [C7H8+H]+ (94.0732) * | N/D N/D | N/A N/A | ||
| O-xylene | C8H10 | 106.0782 (100) ǂ | C8H10+ (106.0777) [C8H10+H]+ (107.0855) | 106.0784 (100) Ɫ 107.0849 (45) | 6.6 5.6 |
| 107.0816 (8.7) | C8H10+ (107.0811) * [C8H10+H]+ (108.0889) * | N/D 108.087 (26) | N/A −17.6 | ||
| Chlorobenzene | C6H5Cl | 112.0079 (100) | C6H5Cl+ (112.0074) | 112.0079 (100) Ɫ | 4.5 |
| 113.0113 (6.5) | 2nd*C6H5Cl+ (113.0108) * | 113.0118 (6.7) | 8.8 | ||
| 114.0050 (32.4) | 3rd*C6H5Cl+ (114.0045) ** | 114.0051 (13.5) | 5.3 | ||
| α-Pinene | C10H16 | 136.1252 (100) ǂ | C10H16+ (136.1247) [C10H16+H]+ (137.1325) | 136.1258 (14) 137.1326 (100) Ɫ | 8.1 0.7 |
| 137.1285 (10.8) | [C10H16+H]+ (138.1358) * | BQL | N/A | ||
| Dichlorobenzene | C6H4Cl2 | 145.9690 (100) ǂ | C6H4Cl2+ (145.9685) | 145.9695 (100) Ɫ | 6.9 |
| 146.9724 (6.5) | C6H4Cl2+ (146.9718) * | 146.9732 (3.9) | 9.5 | ||
| 147.9661 (64.8) | C6H4Cl2+ (147.9655) ** | 147.964 (55) | −10.7 | ||
| 148.9694 (4.2) | C6H4Cl2+ (148.9689) *** | 148.9697 (1.5) | 5.4 | ||
| 149.9631 (10.5) | C6H4Cl2+ (149.9626) **** | N/D | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsonoudis, C.; Zografou, O.; Matrali, A.; Panagiotopoulos, E.; Lekkas, A.; Kosmopoulou, M.; Papanastasiou, D.; Eleftheriadis, K.; Pandis, S.N. Measurement of Atmospheric Volatile and Intermediate Volatility Organic Compounds: Development of a New Time-of-Flight Mass Spectrometer. Atmosphere 2023, 14, 336. https://doi.org/10.3390/atmos14020336
Kaltsonoudis C, Zografou O, Matrali A, Panagiotopoulos E, Lekkas A, Kosmopoulou M, Papanastasiou D, Eleftheriadis K, Pandis SN. Measurement of Atmospheric Volatile and Intermediate Volatility Organic Compounds: Development of a New Time-of-Flight Mass Spectrometer. Atmosphere. 2023; 14(2):336. https://doi.org/10.3390/atmos14020336
Chicago/Turabian StyleKaltsonoudis, Christos, Olga Zografou, Angeliki Matrali, Elias Panagiotopoulos, Alexandros Lekkas, Mariangela Kosmopoulou, Dimitris Papanastasiou, Konstantinos Eleftheriadis, and Spyros N. Pandis. 2023. "Measurement of Atmospheric Volatile and Intermediate Volatility Organic Compounds: Development of a New Time-of-Flight Mass Spectrometer" Atmosphere 14, no. 2: 336. https://doi.org/10.3390/atmos14020336
APA StyleKaltsonoudis, C., Zografou, O., Matrali, A., Panagiotopoulos, E., Lekkas, A., Kosmopoulou, M., Papanastasiou, D., Eleftheriadis, K., & Pandis, S. N. (2023). Measurement of Atmospheric Volatile and Intermediate Volatility Organic Compounds: Development of a New Time-of-Flight Mass Spectrometer. Atmosphere, 14(2), 336. https://doi.org/10.3390/atmos14020336







