Volatile Organic Compounds in the North China Plain: Characteristics, Sources, and Effects on Ozone Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Analysis
2.2. Data Processing Methods
2.2.1. Empirical Kinetic Modelling Approach (EKMA)
2.2.2. Positive Matrix Factorization (PMF)
3. Results and Discussion
3.1. General Characteristics of VOCs
VOCs’ Concentration and Chemical Composition
3.2. Sources of VOCs
3.3. Photochemical Reactivates
3.3.1. Reactivity Studies on VOCs
3.3.2. EKMA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, B.; Liu, C.Q.; Zhang, J.; Du, J.; Shi, K. The multifractal evaluation of PM2.5-O3 coordinated control capability in China. Ecol. Indic. 2021, 129, 107877. [Google Scholar] [CrossRef]
- Zhan, J.L.; Feng, Z.M.; Liu, P.F.; He, X.W.; He, Z.M.; Chen, T.Z.; Wang, Y.F.; He, H.; Mu, Y.J.; Liu, Y.C. Ozone and SOA formation potential based on photochemical loss of VOCs during the Beijing summer. Environ. Pollut. 2021, 285, 117444. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Sahu, L.K.; Beig, G.; Tripathi, N.; Maji, S.; Jaaffrey, S.N.A. The role of local meteorology on ambient particulate and gaseous species at an urban site of western India. Urban Climate. 2019, 28, 100449. [Google Scholar] [CrossRef]
- Hui, L.R.; Ma, T.; Gao, Z.J.; Gao, J.; Wang, Z.; Xue, L.K.; Liu, H.Q.; Liu, J.Y. Characteristics and sources of volatile organic compounds during high ozone episodes: A case study at a site in the eastern Guanzhong Plain, China. Chemosphere 2021, 265, 129072. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.H.; Huang, W.; Yao, D.; Zhao, S.M.; Wang, Y.H.; Ji, D.S.; Zhang, R.J.; Wang, Y.S. Parameterized atmospheric oxidation capacity and speciated OH reactivity over a suburban site in the North China Plain: A comparative study between summer and winter. Sci. Total Environ. 2021, 773, 145264. [Google Scholar] [CrossRef]
- Berezina, E.; Moiseenko, K.; Skorokhod, A.; Pankratova, N.V.; Belikov, I.; Belousov, V.; Elansky, N.F. Impact of VOCs and NOx on Ozone Formation in Moscow. Atmosphere 2020, 11, 11111262. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Xue, B.R.; Lv, Z.F.; Meng, Z.H.; Yang, X.F.; Xue, T.; Yu, Q.; He, K.B. Ground-level ozone pollution and its health impacts in China. Atmos. Environ. 2018, 173, 223–230. [Google Scholar] [CrossRef]
- Louie, P.K.K.; Ho, J.W.K.; Tsang, R.C.W.; Blake, D.R.; Lau, A.K.H.; Yu, J.Z.; Yuan, Z.B.; Wang, X.M.; Shao, M.; Zhong, L.J. VOCs and OVOCs distribution and control policy implications in Pearl River Delta region, China. Atmos. Environ. 2013, 76, 125–135. [Google Scholar] [CrossRef]
- Hu, R.Y.; Liu, G.J.; Zhang, H.; Xue, H.Q.; Wang, X. Levels, characteristics and health risk assessment of VOCs in different functional zones of Hefei. Ecotox. Environ. Safe. 2018, 160, 301–307. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.J.; Chai, X.L.; Xu, L.Z.; Zhang, L.F.; Ning, P.; Huang, J.H.; Tian, S.L. Interaction of inhalable volatile organic compounds and pulmonary surfactant: Potential hazards of VOCs exposure to lung. J. Hazard. Mater. 2019, 369, 512–520. [Google Scholar] [CrossRef]
- Liu, N.W.; Li, X.L.; Ren, W.H.; Li, L.G.; Su, C.C.; Wang, C. Concentration Characteristics and Photochemical Reactivities of VOCs in Shenyang, China. Atmosphere 2021, 12, 12101240. [Google Scholar] [CrossRef]
- Zhu, H.L.; Wang, H.L.; Jing, S.G.; Wang, Y.F.; Cheng, T.T.; Tao, S.K.; Lou, S.R.; Qiao, L.P.; Li, L.; Chen, J.M. Characteristics and sources of atmospheric volatile organic compounds (VOCs) along the mid-lower Yangtze River in China. Atmos. Environ. 2018, 190, 232–240. [Google Scholar] [CrossRef]
- Mo, Z.W.; Huang, S.; Yuan, B.; Pei, C.L.; Song, Q.C.; Qi, J.P.; Wang, M.; Wang, B.L.; Wang, C.; Shao, M. Tower-based measurements of NMHCs and OVOCs in the Pearl River Delta: Vertical distribution, source analysis and chemical reactivity. Environ. Pollut. 2022, 292, 11845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Tan, Q.W.; Deng, Y.; Lu, C.W.; Song, D.L.; Zhou, X.L.; Zhang, X.; Jiang, X. Source profiles and reactivity of volatile organic compounds from anthropogenic sources of a megacity in southwest China. Sci. Total Environ. 2021, 790, 148149. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.G.; Zhang, Y.Y.; Tan, Q.W.; Feng, M.; Qu, Y.; An, J.L.; Deng, Y.J.; Zhai, R.X.; Wang, Z.; et al. Characteristics, source apportionment and chemical conversions of VOCs based on a comprehensive summer observation experiment in Beijing. Atmos. Pollut. Res. 2021, 12, 183–184. [Google Scholar] [CrossRef]
- Tan, Y.; Han, S.W.; Chen, Y.; Zhang, Z.Z.; Li, H.W.; Li, W.Q.; Yuan, Q.; Li, X.W.; Wang, T.; Lee, S.C. Characteristics and source apportionment of volatile organic compounds (VOCs) at a coastal site in Hong Kong. Sci. Total Environ. 2021, 777, 146241. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Qu, K.; Ding, J.W.; Shang, Y.Q.; Liu, H.F.; Wei, M. Characteristics of Ozone Pollution, Regional Distribution and Causes during 2014–2018 in Shandong Province, East China. Atmosphere 2019, 10, 29. [Google Scholar] [CrossRef]
- Zhou, M.M.; Jiang, W.; Gao, W.D.; Zhou, B.H.; Liao, X.C. A high spatiotemporal resolution anthropogenic VOC emission inventory for Qingdao City in 2016 and its ozone formation potential analysis. Process. Safe. Environ. 2020, 139, 147–160. [Google Scholar] [CrossRef]
- Yang, X.; An, X.Y.; Liu, Y.Q.; Jiang, C.M.; Zhang, P.C.; Li, L.J.; Zhao, S.Y.; Zhang, S.Y. Pollution Characteristic and Control Factor Analysis of Atmospheric Ozone during Summer Typical Periods in Linyi, Shandong. Environ. Sci. 2022, 43, 696–706. [Google Scholar]
- Shi, J.W.; Bao, Y.Z.; Xiang, F.; Wang, Z.J.; Ren, L.; Pang, X.C.; Wang, J.; Han, X.Y.; Ning, P. Pollution Characteristics and Health Risk Assessment of VOCs in Jinghong. Atmosphere 2022, 13, 15. [Google Scholar] [CrossRef]
- Simpson, I.J.; Blake, N.J.; Barletta, B.; Diskin, G.S.; Fuelberg, H.E.; Gorham, K.; Huey, L.G.; Meinardi, S.; Rowland, F.S.; Vay, S.A.; et al. Characterization of trace gases measured over Alberta oil sands mining operations: 76 speciated C2–C10 volatile organic compounds (VOCs), CO2, CH4, CO, NO, NO2, NOy, O3 and SO2. Atmos. Chem. Phys. 2010, 10, 11931–11954. [Google Scholar] [CrossRef]
- Chen, T.S.; Xue, L.K.; Zheng, P.G.; Zhang, Y.N.; Liu, Y.H.; Sun, J.J.; Han, G.X.; Li, H.Y.; Zhang, X.; Li, Y.F.; et al. Volatile organic compounds and ozone air pollution in an oil production region in northern China. Atmos. Chem. Phys. 2020, 20, 7069–7086. [Google Scholar] [CrossRef]
- USEPA (US Environemntal Protection Agency). Compendium Method TO-11A: Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography; US Environemntal Protection Agency, Center for Environmental Research Information Office of Research and Development: Cincinnati, OH, USA, 1999.
- Wang, Y.M.; Wang, Y.H.; Tang, G.Q.; Yang, Y.; Li, X.R.; Yao, D.; Wu, S.; Kang, Y.Y.; Wang, M.; Wang, Y.S. High gaseous carbonyl concentrations in the upper boundary layer in Shijiazhuang, China. Sci. Total Environ. 2021, 799, 138–149. [Google Scholar] [CrossRef]
- Yang, X.; Xue, L.K.; Yao, L.; Li, Q.Y.; Wen, L.; Zhu, Y.H.; Chen, T.S.; Wang, X.F.; Yang, L.X.; Wang, T.; et al. Carbonyl compounds at Mount Tai in the North China Plain: Characteristics, sources, and effects on ozone formation. Atmos. Res. 2017, 196, 53–61. [Google Scholar] [CrossRef]
- Yang, X.; Xue, L.K.; Wang, T.; Wang, X.F.; Gao, J.; Lee, S.C.; Blake, D.R.; Chai, F.H.; Wang, W.X. Observations and Explicit Modeling of Summertime Carbonyl Formation in Beijing: Identification of Key Precursor Species and Their Impact on Atmospheric Oxidation Chemistry. J. Geophys. Res.-Atmos. 2018, 123, 1426–1440. [Google Scholar] [CrossRef]
- Shen, H.Q.; Liu, Y.H.; Zhao, M.; Li, J.; Zhang, Y.N.; Yang, J.; Jiang, Y.; Chen, T.S.; Chen, M.; Huang, X.B.; et al. Significance of carbonyl compounds to photochemical ozone formation in a coastal city (Shantou) in eastern China. Sci. Total Environ. 2021, 764, 144031. [Google Scholar] [CrossRef] [PubMed]
- Cardelino, C.A.; Chameides, W.L. An Observation-Based Model for Analyzing Ozone Precursor Relationships in the Urban Atmosphere. J. Air Waste Manag. Assoc. 1995, 45, 161–180. [Google Scholar] [CrossRef]
- Dodge, M. Combined Use of Modeling Techniques and Smog Chamber Data to Derive Ozone-Precursor Relationships. In International Conference on Photochemical Oxidant Pollution and Its Control: Proceedings; Environmental Sciences Research; US Environmental Protection Agency: Cincinnati, OH, USA, 1977; pp. 881–889. [Google Scholar]
- Ou, J.M.; Yuan, Z.B.; Zheng, J.Y.; Huang, Z.J.; Shao, M.; Li, Z.K.; Huang, X.B.; Guo, H.; Louie, P.K.K. Ambient Ozone Control in a Photochemically Active Region: Short Term Despiking or Long-Term Attainment? Environ. Sci. Technol. 2016, 50, 5720–5728. [Google Scholar] [CrossRef]
- Norris, G.; Duvall, R. EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide; U.S. Environmental Protection Agency Office of Research and Development: Washington, DC, USA, 2014. [Google Scholar]
- Ji, X.T.; Xu, K.; Liao, D.; Chen, G.J.; Liu, T.T.; Hong, Y.W.; Dong, S.J.; Choi, S.D.; Chen, J.S. Spatial-temporal Characteristics and Source Apportionment of Ambient VOCs in Southeast Mountain Area of China. Aerosol Air Qual. Res. 2022, 22, 15. [Google Scholar] [CrossRef]
- Vestenius, M.; Hopke, P.K.; Lehtipalo, K.; Petaja, T.; Hakola, H.; Hellen, H. Assessing volatile organic compound sources in a boreal forest using positive matrix factorization (PMF). Atmos. Environ. 2021, 259, 118503. [Google Scholar] [CrossRef]
- Hui, L.R.; Liu, X.G.; Tan, Q.W.; Feng, M.; An, J.L.; Qu, Y.; Zhang, Y.H.; Jiang, M.Q. Characteristics, source apportionment and contribution of VOCs to ozone formation in Wuhan, Central China. Atmos. Environ. 2018, 192, 55–71. [Google Scholar] [CrossRef]
- Li, J.; Zhai, C.Z.; Yu, J.Y.; Liu, R.L.; Li, Y.Q.; Zeng, L.M.; Xie, S.D. Spatiotemporal variations of ambient volatile organic compounds and their sources in Chongqing, a mountainous megacity in China. Sci. Total Environ. 2018, 627, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chuang, Y.C.; Hsieh, C.C.; Lee, C.S. VOC characteristics and source apportionment at a PAMS site near an industrial complex in central Taiwan. Atmos. Pollut. Res. 2019, 10, 1060–1074. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, H.L.; Jing, S.G.; Gao, Y.Q.; Peng, Y.R.; Lou, S.R.; Cheng, T.T.; Tao, S.K.; Li, L.; Li, Y.J.; et al. Characteristics and sources of volatile organic compounds (VOCs) in Shanghai during summer: Implications of regional transport. Atmos. Environ. 2019, 215, 116902. [Google Scholar] [CrossRef]
- Zou, Y.; Deng, X.J.; Zhu, D.; Gong, D.C.; Wang, H. Characteristics of 1 year of observational data of VOCs, NOx and O3 at a suburban site in Guangzhou, China. Atmos. Chem. Phys. 2015, 15, 6625–6636. [Google Scholar] [CrossRef]
- Wang, Z.S.; Wang, H.Y.; Zhang, L.; Guo, J.; Li, Z.G.; Wu, K.; Zhu, G.Y.; Hou, D.L.; Su, H.Y.; Sun, Z.B.; et al. Characteristics of volatile organic compounds (VOCs) based on multisite observations in Hebei province in the warm season in 2019. Atmos. Environ. 2021, 256, 118435. [Google Scholar] [CrossRef]
- Chang, C.C.; Wang, J.L.; Liu, S.C.; Candice Lung, S.C. Assessment of vehicular and non-vehicular contributions to hydrocarbons using exclusive vehicular indicators. Atmos. Environ. 2006, 40, 6349–6361. [Google Scholar] [CrossRef]
- Wu, R.R.; Li, J.; Hao, Y.F.; Li, Y.Q.; Zeng, L.M.; Xie, S.D. Evolution process and sources of ambient volatile organic compounds during a severe haze event in Beijing, China. Sci. Total Environ. 2016, 560, 62–72. [Google Scholar] [CrossRef]
- Xuan, L.C.; Ma, Y.N.; Xing, Y.F.; Meng, Q.Q.; Song, J.; Chen, T.H.; Wang, H.; Wang, P.J.; Zhang, Y.F.; Gao, P. Source, temporal variation and health risk of volatile organic compounds (VOCs) from urban traffic in harbin, China. Environ. Pollut. 2021, 270, 116074. [Google Scholar] [CrossRef]
- Yang, Y.C.; Liu, X.G.; Zheng, J.; Tan, Q.W.; Feng, M.; Qu, Y.; An, J.L.; Cheng, N.L. Characteristics of one-year observation of VOCs, NOx, and O-3 at an urban site in Wuhan, China. J. Environ. Sci. 2019, 79, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Han, L.X.; Chen, L.H.; Li, K.W.; Bao, Z.E.; Zhao, Y.Y.; Zhang, X.; Azzi, M.; Cen, K.F. Source Apportionment of Volatile Organic Compounds (VOCs) during Ozone Polluted Days in Hangzhou, China. Atmosphere 2019, 10, 18. [Google Scholar] [CrossRef]
- Carrillo, E.R.; Hernandez, I.Y.; Mendoza, A. Use of Combined Observational-and Model-Derived Photochemical Indicators to Assess the O3-NOx-VOC System Sensitivity in Urban Areas. Atmosphere 2017, 8, 18. [Google Scholar]
- Yuan, B.; Shao, M.; de Gouw, J.; Parrish, D.D.; Lu, S.H.; Wang, M.; Zeng, L.M.; Zhang, Q.; Song, Y.; Zhang, J.B.; et al. Volatile organic compounds (VOCs) in urban air: How chemistry affects the interpretation of positive matrix factorization (PMF) analysis. J. Geophys. Res. Atmos. 2012, 117, 24302. [Google Scholar] [CrossRef]
- Yuan, B.; Shao, M.; Lu, S.H.; Wang, B. Source profiles of volatile organic compounds associated with solvent use in Beijing, China. Atmos. Environ. 2010, 44, 1919. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yang, L.X.; Chen, J.M.; Wang, X.F.; Xue, L.K.; Sui, X.; Wen, L.; Xu, C.H.; Yao, L.; Zhang, J.M.; et al. Characteristics of ambient volatile organic compounds and the influence of biomass burning at a rural site in Northern China during summer 2013. Atmos. Environ. 2016, 124, 156–165. [Google Scholar] [CrossRef]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total Environ. 2016, 541, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Li, H.; Wu, Z.H.; Zhang, W.Q.; Liu, K.K.; Cheng, X.; Zhang, Y.J.; Li, B.; Chen, Y.Z. Characteristics of atmospheric volatile organic compounds in urban area of Beijing: Variations, photochemical reactivity and source apportionment. J. Environ. Sci. 2020, 95, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Deng, S.X.; Li, G.H.; Lu, Z.Z.; Song, H.; Gao, J.; Sun, Z.G.; Xu, K. VOCs characteristics and their ozone and SOA formation potentials in autumn and winter at Weinan, China. Environ. Res. 2022, 203, 111821. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zang, T.T.; Yan, B.; Wei, C.H. Distribution Characteristics of Volatile Organic Compounds and Contribution to Ozone Formation in a Coking Wastewater Treatment Plant. Int. J. Environ. Res. Public Health 2020, 17, 13. [Google Scholar] [CrossRef]
- Wang, X.D.; Yin, S.S.; Zhang, R.Q.; Yuan, M.H.; Ying, Q. Assessment of summertime O3 formation and the O3-NOX-VOC sensitivity in Zhengzhou, China using an observation-based model. Sci. Total Environ. 2022, 813, 152449. [Google Scholar] [CrossRef]
- Fan, M.Y.; Zhang, Y.L.; Lin, Y.C.; Li, L.; Xie, F.; Hu, J.L.; Mozaffar, A.; Cao, F. Source apportionments of atmospheric volatile organic compounds in Nanjing, China during high ozone pollution season. Chemosphere 2021, 263, 128025. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, W.T.; Zhang, L.; Qin, W.; Zhang, Y.; Zhang, X.Z.; Xie, X. Ozone pollution characteristics and sensitivity analysis using an observation-based model in Nanjing, Yangtze River Delta Region of China. J. Environ. Sci. 2020, 93, 13–22. [Google Scholar] [CrossRef] [PubMed]
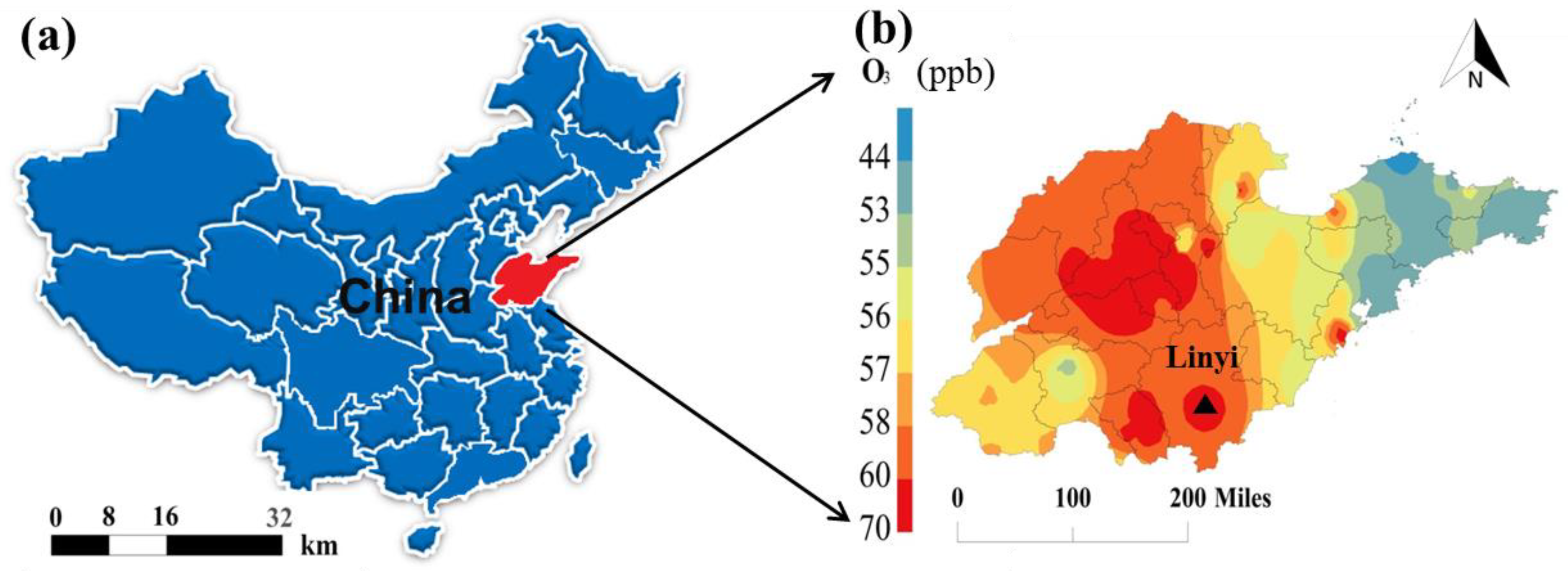


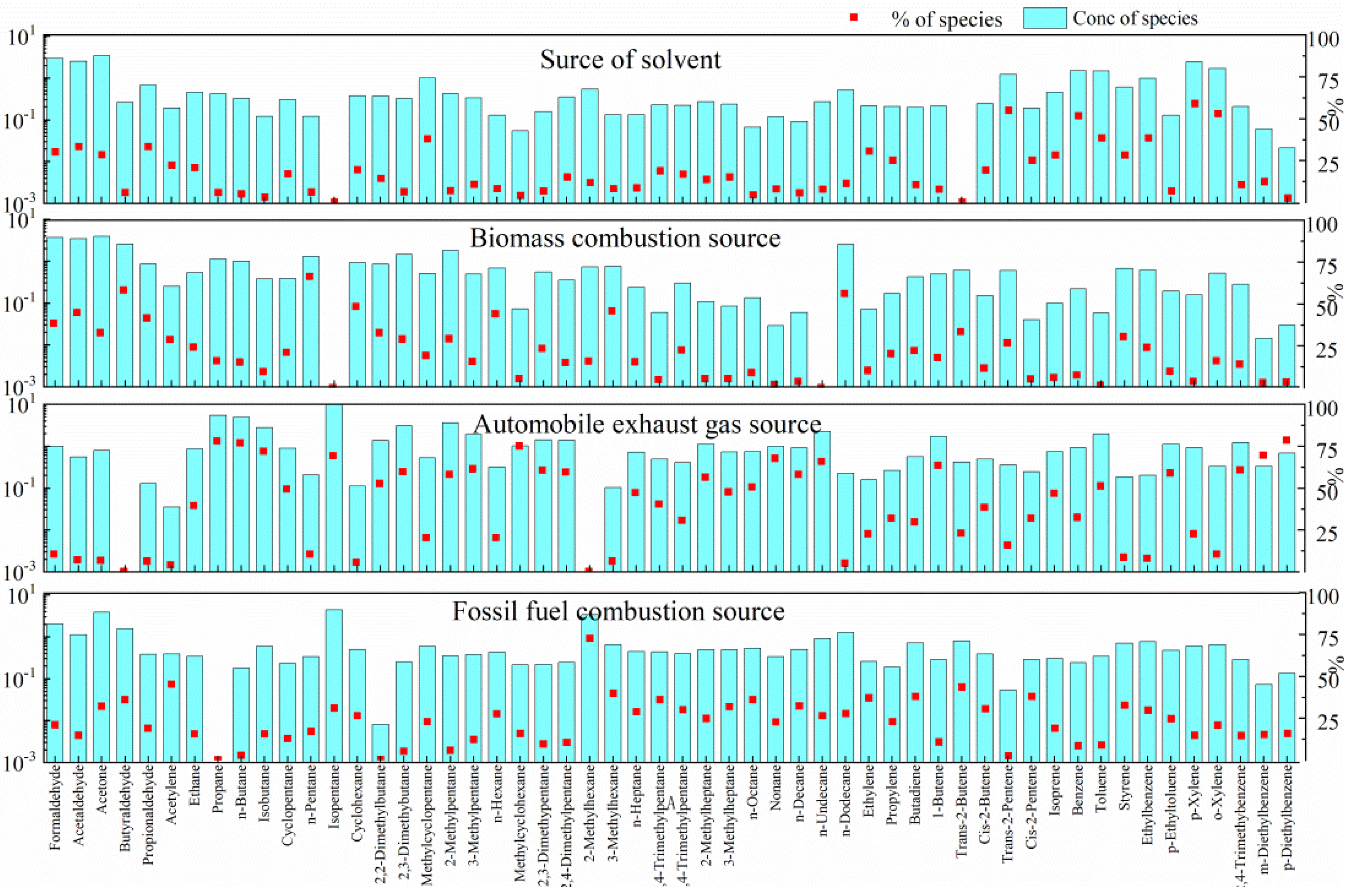
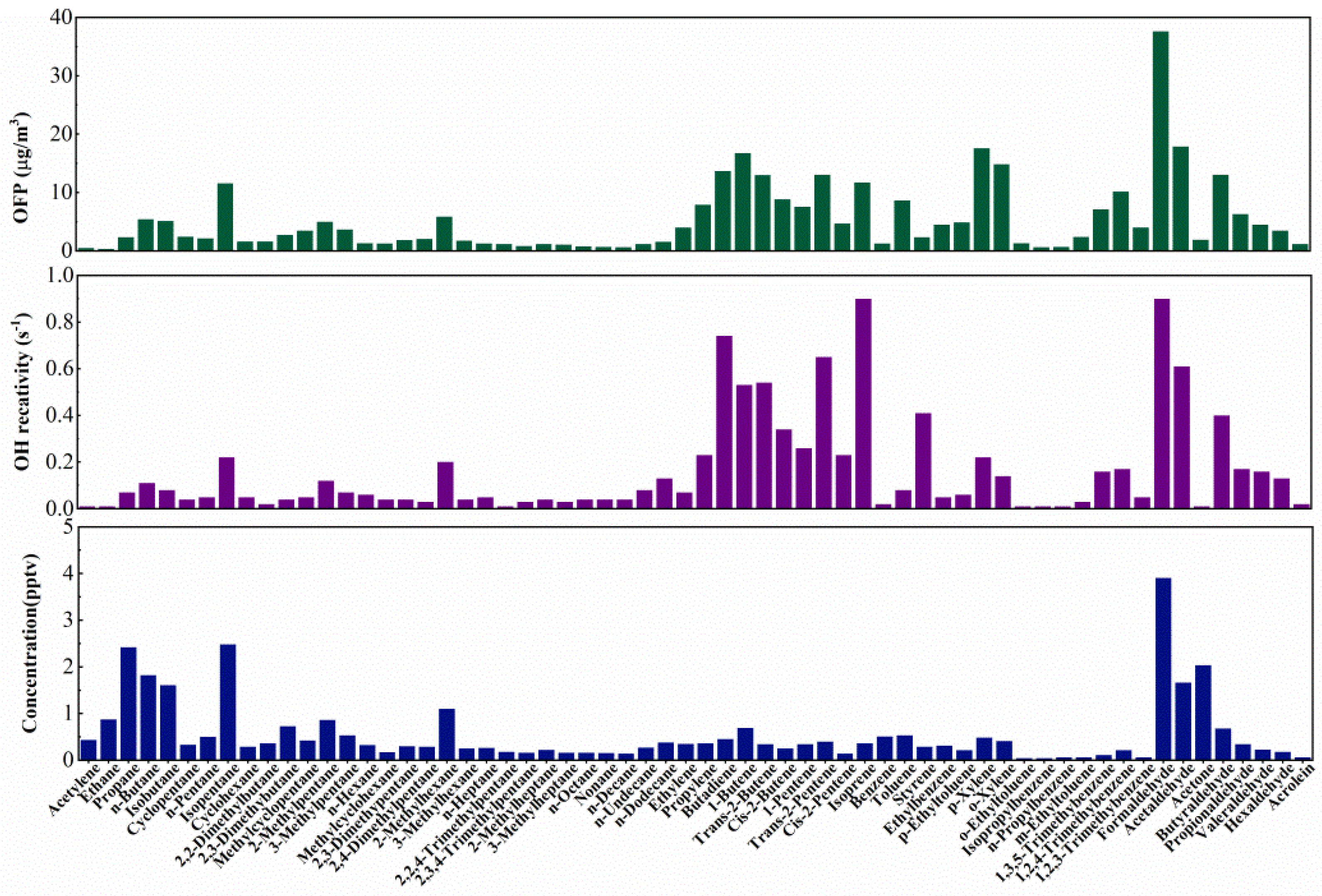
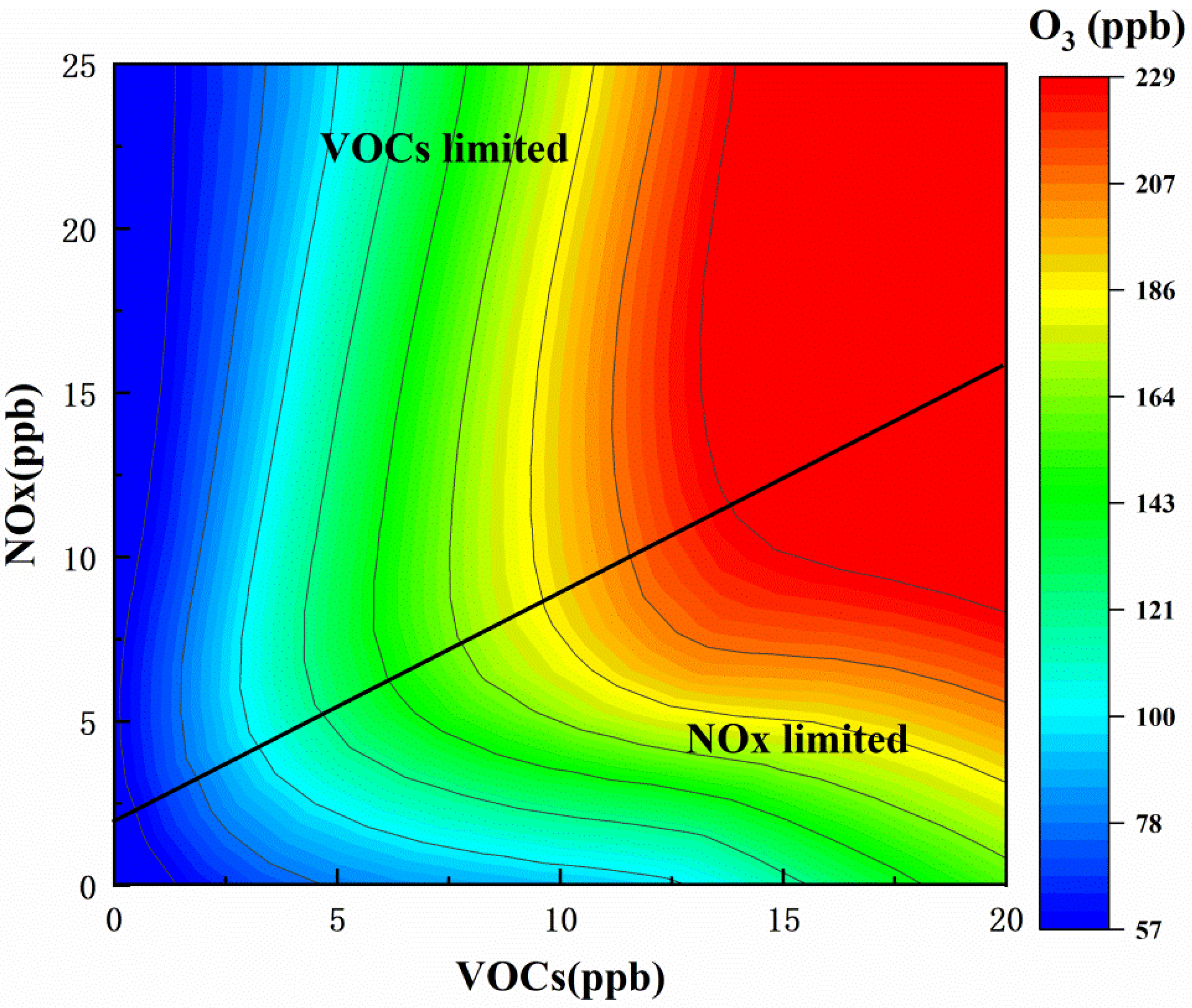
| Species a | ppb-Average (Mean ± SD) | Range |
|---|---|---|
| O3 | 48 ± 29 | 1–145 |
| CO | 0.61 ± 0.32 | 0.08–2.32 |
| NO | 2.22 ± 3.46 | 0.75–60.48 |
| NO2 | 14.05 ± 8.57 | 4.38–60.38 |
| SO2 | 2.21 ± 1.54 | 0.35–10.15 |
| TEMP (°C) | 24.5 ± 4.4 | 16.7–37.6 |
| RH (%) | 67 ± 18 | 15–89 |
| Alkanes | 15.41 ± 9.09 | 4.27–35.27 |
| Alkenes | 3.70 ± 0.90 | 2.22–7.09 |
| Alkyne | 0.43 ± 0.18 | 0.14–0.85 |
| Aromatics | 3.32 ± 0.88 | 2.00–7.90 |
| OVOCs | 9.62 ± 5.49 | 2.94–53.48 |
| Reference | Sampling Period | Sampling Site | Type of Site | Average of VOCs Species | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TVOCs | Ethylene | Isopentane | Propane | Ethyne | Benzene | Isoprene | Acetone | Acetaldehyde | ||||
| This study | June 2020 | Linyi | Urban | 36.55 ± 10.82 | 0.35 ± 0.18 | 2.47 ± 2.10 | 2.42 ± 2.79 | 0.43 ± 0.19 | 0.51 ± 0.21 | 0.36 ± 0.20 | 2.03 ± 0.84 | 1.66 ± 0.99 |
| [16] | August–October 2018 | Hongkong | Urban | - | - | - | - | - | 0.29 ± 0.20 | 0.47 ± 0.47 | - | - |
| [34] | August 2016–2017 | Wuhan | Urban | 11.2 ± 5.7 | 2.62 | 1.08 | 5.40 | 2.35 | 0.73 | - | 2.27 | - |
| [35] | August–September 2015 | Chongqing | Rural | 13.0 ± 9.6 | 3.0 ± 1.8 | - | 3.3 ± 4.0 | - | 1.0 ± 0.5 | 0.4 ± 0.4 | 0.8 ± 0.3 | 2.2 ± 2.4 |
| [36] | 2016 | Taixi (Taiwan) | Urban | 11.20 | 0.93 | 0.51 | 1.42 | 0.83 | 0.25 | - | - | - |
| [37] | May 2017 | Shanghai | Urban | 42.7 ± 23.0 | - | - | - | 1.2 ± 0.9 | - | - | - | - |
| [38] | June–August 2011 | Guangzhou | Rural | 40.55 | 2.99 | - | 4.34 | - | 0.62 | 1.14 | - | - |
| [39] | May–September 2019 | Handan | Urban | - | 0.41 | - | 1.60 | 1.02 | 1.10 | - | - | - |
| VOCs Species | VOCs Concentration (ppbv) | KOH × 10−12 | LOH | MIR | OFP (μg/m3) |
|---|---|---|---|---|---|
| Acetylene | 0.43 | 0.9 | 0.01 | 0.95 | 0.48 |
| Alkanes | |||||
| Ethane | 0.87 | 0.26 | 0.01 | 0.28 | 0.33 |
| Propane | 2.42 | 1.15 | 0.07 | 0.49 | 2.33 |
| n-Butane | 1.82 | 2.54 | 0.11 | 1.15 | 5.42 |
| Isobutane | 1.61 | 2.12 | 0.08 | 1.23 | 5.12 |
| Cyclopentane | 0.44 | 5.16 | 0.04 | 2.39 | 2.46 |
| n-Pentane | 0.50 | 3.94 | 0.05 | 1.31 | 2.12 |
| Isopentane | 2.48 | 3.6 | 0.22 | 1.45 | 11.55 |
| Cyclohexane | 0.47 | 7.49 | 0.05 | 1.46 | 1.61 |
| 2, 2-Dimethylbutane | 0.36 | 2.32 | 0.02 | 1.17 | 1.60 |
| 2, 3-Dimethylbutane | 0.73 | 2.32 | 0.04 | 0.97 | 2.71 |
| Methylcyclopentane | 0.53 | 5.16 | 0.05 | 2.19 | 3.46 |
| 2-Methylpentane | 0.86 | 5.6 | 0.12 | 1.5 | 4.95 |
| 3-Methylpentane | 0.53 | 5.7 | 0.07 | 1.8 | 3.64 |
| n-Hexane | 0.32 | 7.49 | 0.06 | 1.07 | 1.31 |
| Methylcyclohexane | 0.41 | 10.4 | 0.04 | 1.7 | 1.25 |
| 2, 3-Dimethylpentane | 0.43 | 5.7 | 0.04 | 1.34 | 1.81 |
| 2, 4-Dimethylpentane | 0.40 | 4.77 | 0.03 | 1.55 | 2.02 |
| 2-Methylhexane | 1.10 | 7.49 | 0.20 | 1.19 | 5.83 |
| 3-Methylhexane | 0.25 | 7.15 | 0.04 | 1.61 | 1.76 |
| n-Heptane | 0.26 | 7.15 | 0.05 | 1.07 | 1.25 |
| 2,2,4-trimethylpentane | 0.25 | 3.34 | 0.01 | 1.26 | 1.15 |
| 2,3,4-trimethylpentane | 0.31 | 6.6 | 0.03 | 1.03 | 0.82 |
| 2-Methylheptane | 0.38 | 7.15 | 0.04 | 1.07 | 1.19 |
| 3-Methylheptane | 0.33 | 7.15 | 0.03 | 1.24 | 1.04 |
| Octane | 0.16 | 8.68 | 0.04 | 0.9 | 0.75 |
| Nonane | 0.15 | 9.7 | 0.04 | 0.78 | 0.68 |
| Decane | 0.14 | 11.6 | 0.04 | 0.68 | 0.61 |
| n-undecane | 0.27 | 12.3 | 0.08 | 0.61 | 1.14 |
| n-Dodecane | 0.38 | 13.9 | 0.13 | 0.55 | 1.57 |
| Alkenes | |||||
| Ethylene | 0.35 | 8.52 | 0.07 | 9 | 3.96 |
| Propylene | 0.36 | 26.3 | 0.23 | 11.66 | 7.89 |
| 1, 3-Butadiene | 0.45 | 66.6 | 0.74 | 12.61 | 13.68 |
| 1-butene | 0.69 | 31.4 | 0.53 | 9.73 | 16.75 |
| trans-2-butene | 0.34 | 64 | 0.54 | 15.16 | 12.99 |
| cis-2-butene | 0.25 | 56.4 | 0.34 | 14.24 | 8.83 |
| 1-pentene | 0.34 | 31.4 | 0.26 | 7.21 | 7.56 |
| trans-2-pentene | 0.40 | 67 | 0.65 | 10.56 | 13.04 |
| cis-2-pentene | 0.14 | 65 | 0.23 | 10.38 | 4.67 |
| Isoprene | 0.36 | 101 | 0.90 | 10.61 | 11.70 |
| Aromatics | |||||
| Benzene | 0.51 | 1.23 | 0.02 | 0.72 | 1.28 |
| Toluene | 0.53 | 5.96 | 0.08 | 4 | 8.63 |
| Styrene | 0.29 | 58 | 0.41 | 1.73 | 2.31 |
| Ethylbenzene | 0.31 | 7.1 | 0.05 | 3.04 | 4.50 |
| p-Ethyltoluene | 0.21 | 12.1 | 0.06 | 4.44 | 4.88 |
| p/m-xylene | 0.48 | 19 | 0.22 | 7.8 | 17.54 |
| o-xylene | 0.41 | 13.7 | 0.14 | 7.64 | 14.82 |
| o-Ethyltoluene | 0.04 | 12.3 | 0.01 | 5.59 | 1.29 |
| Isopropylbenzene | 0.04 | 6.5 | 0.01 | 2.52 | 0.60 |
| n-propylbenzene | 0.06 | 6 | 0.01 | 2..03 | 0.69 |
| m-Ethyltoluene | 0.06 | 19.2 | 0.03 | 7.39 | 2.38 |
| 1,3,5-Trimethylbenzene | 0.11 | 57.5 | 0.16 | 11.76 | 7.08 |
| 1,2,4-Trimethylbenzene | 0.21 | 32.5 | 0.17 | 8.87 | 10.17 |
| 1,2,3-Trimethylbenzene | 0.06 | 32.7 | 0.05 | 11.97 | 3.96 |
| OVOCs | |||||
| Formaldehyde | 3.89 | 9.37 | 0. 90 | 7.2 | 37.60 |
| Acetaldehyde | 1.66 | 15 | 0.61 | 5.5 | 17.88 |
| Acetone | 2.03 | 0.17 | 0.0003 | 0.36 | 1.89 |
| n-Butyraldehyde | 0.68 | 24 | 1.20 | 5.97 | 13.03 |
| Propionaldehyde | 0.34 | 20 | 0.17 | 7.08 | 6.27 |
| Valeraldehyde | 0.23 | 28 | 0.16 | 5.08 | 4.48 |
| n-Hexanal | 0.18 | 30 | 0.03 | 4.35 | 3.45 |
| Acrolein | 0.06 | 13.6 | 0.02 | 7.45 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Gao, L.; Zhao, S.; Pan, G.; Fan, G.; Xia, Z.; Sun, X.; Xu, H.; Chen, Y.; Jin, X. Volatile Organic Compounds in the North China Plain: Characteristics, Sources, and Effects on Ozone Formation. Atmosphere 2023, 14, 318. https://doi.org/10.3390/atmos14020318
Yang X, Gao L, Zhao S, Pan G, Fan G, Xia Z, Sun X, Xu H, Chen Y, Jin X. Volatile Organic Compounds in the North China Plain: Characteristics, Sources, and Effects on Ozone Formation. Atmosphere. 2023; 14(2):318. https://doi.org/10.3390/atmos14020318
Chicago/Turabian StyleYang, Xue, Luhong Gao, Shiyang Zhao, Guang Pan, Guolan Fan, Zhiyong Xia, Xiaoyan Sun, Hongyu Xu, Yanjun Chen, and Xiaolong Jin. 2023. "Volatile Organic Compounds in the North China Plain: Characteristics, Sources, and Effects on Ozone Formation" Atmosphere 14, no. 2: 318. https://doi.org/10.3390/atmos14020318
APA StyleYang, X., Gao, L., Zhao, S., Pan, G., Fan, G., Xia, Z., Sun, X., Xu, H., Chen, Y., & Jin, X. (2023). Volatile Organic Compounds in the North China Plain: Characteristics, Sources, and Effects on Ozone Formation. Atmosphere, 14(2), 318. https://doi.org/10.3390/atmos14020318





