Characterization of the Cultivable Microbiota Components of Marine Bioaerosols in the North Tropical Atlantic
Abstract
:1. Introduction
2. Materials and Methods
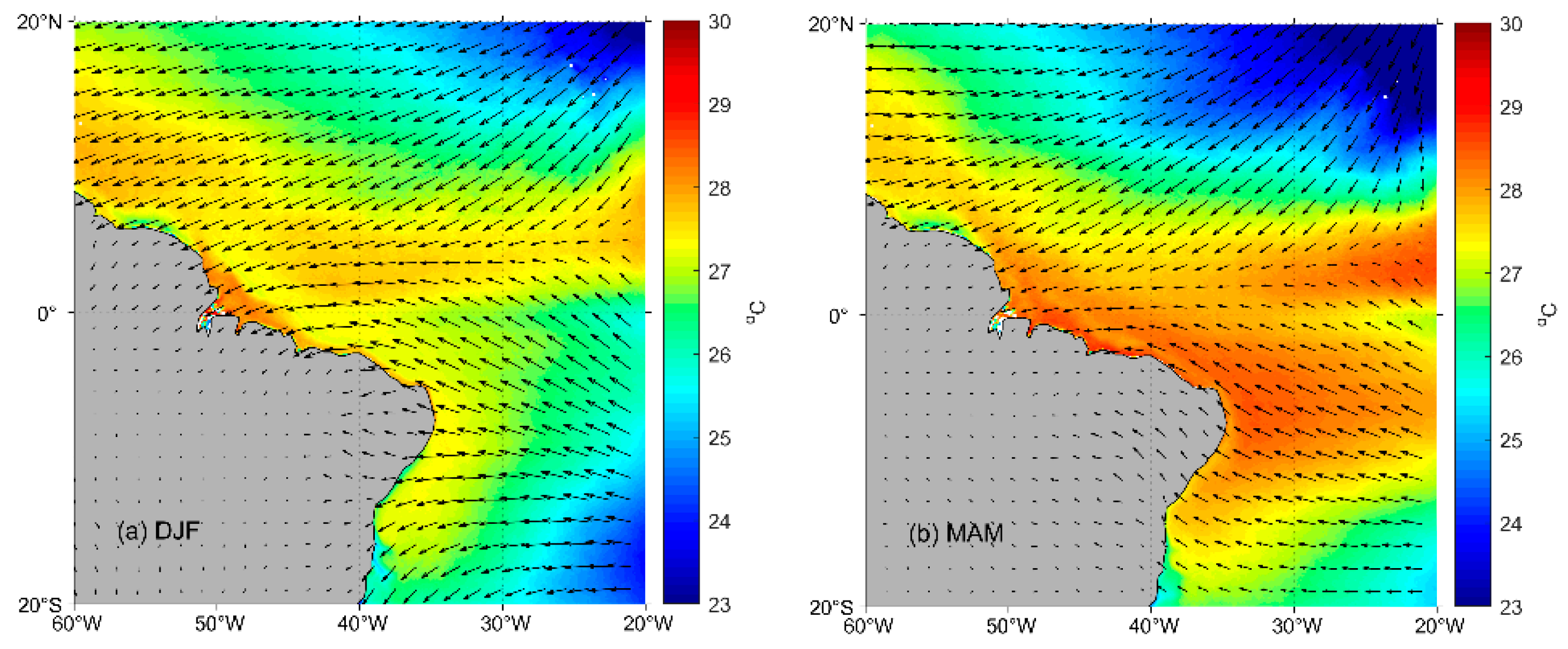
2.1. Study Area
2.2. Samplings
2.3. Sample Processing: Isolation and Identification of the Microbiota
2.4. Oceanographic and Meteorological Variables
2.5. Statistical Analysis
3. Results
3.1. Microbiota Cultivable in Marine Bioaerosols
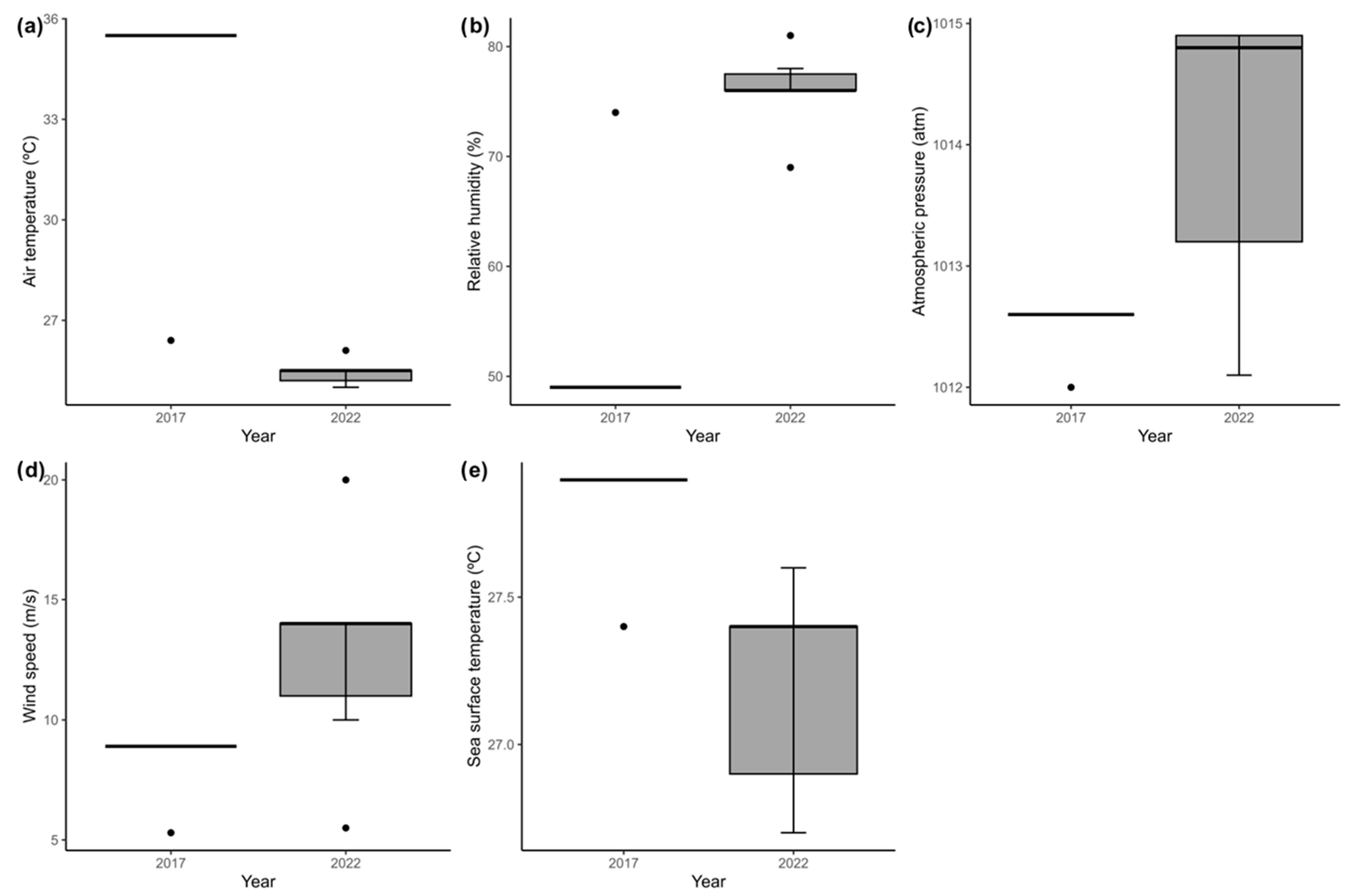
3.2. Oceanographic and Meteorological Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pady, S.M.; Kapica, L. Fungi in Air Over the Atlantic Ocean. Mycologia 1955, 47, 34–50. [Google Scholar] [CrossRef]
- Pady, S.M.; Kelly, C.D. Aerobiological studies of fungi and bacteria over the Atlantic Ocean. Can. J. Bot. 1954, 32, 202–212. [Google Scholar] [CrossRef]
- Seifried, J.S.; Wichels, A.; Gerdts, G. Spatial distribution of marine airborne bacterial communities. Microbiol. Open 2015, 4, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.A. Química entre a microcamada superficial oceânica e os aerossóis marinhos. Química Nova 2014, 37, 1382–1400. [Google Scholar] [CrossRef]
- Cho, B.C.; Hwang, C.Y. Prokaryotic abundance and 16S rRNA gene sequences detected in marine aerosols on the East Sea (Korea). FEMS Microbiol. Ecol. 2011, 76, 327–341. [Google Scholar] [CrossRef]
- Fröhlich-Nowoisky, J.; Burrows, S.M.; Xie, Z.; Engling, G.; Solomon, P.A.; Fraser, M.P.; Mayol-Bracero, O.L.; Artaxo, P.; Begerow, D.; Conrad, R.; et al. Biogeography in the air: Fungal diversity over land and oceans. Biogeosciences 2012, 9, 1125–1136. [Google Scholar] [CrossRef]
- Yao, M. Bioaerosol: A bridge and opportunity for many scientific research fields. J. Aerosol Sci. 2018, 115, 108–112. [Google Scholar] [CrossRef]
- Aller, J.Y.; Kuznetsova, M.R.; Jahns, C.J.; Kemp, P.F. The sea surface microlayer as a source of viral and bacterial enrichment in marine aerosols. J. Aerosol Sci. 2005, 36, 801–812. [Google Scholar] [CrossRef]
- Liss, P.S.; Duce, R.A. The Sea Surface and Global Change; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Ceter, T. Effects of global-warming and climate-changes on atmospheric fungi spores distribution. Commun. Fac. Sci. Univ. Ank. Ser. C Biol. Geol. Eng. Geophys. Eng. 2018, 27, 263–272. [Google Scholar] [CrossRef]
- Kowalski, M.; Pastuszka, J.S. Effect of ambient air temperature and solar radiation on changes in bacterial and fungal aerosols concentration in the urban environment. Ann. Agric. Environ. Med. 2018, 25, 259–261. [Google Scholar] [CrossRef]
- Melo, A.D.; Cavalcanti, I.D.A.; Souza, P.P.D. Zona de convergência intertropical do Atlântico. In Tempo e Clima no Brasil; Cavalcanti, I.F.A., Ferreira, N.J., Silva, M.G.A.J., Dias, M.A.F.S., Eds.; Oficina de Textos: São Paulo, Brazil, 2009; pp. 25–41. [Google Scholar]
- Zhen, Q.; Deng, Y.; Wang, Y.; Wang, X.; Zhang, H.; Sun, X.; Ouyang, Z. Meteorological factors had more impact on airborne bacterial communities than air pollutants. Sci. Total Environ. 2017, 601–602, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Shao, Q.; Xu, W.; Gao, D.; Jin, C. Seasonal distribution of bioaerosols in the coastal region of Qingdao. J. Ocean Univ. China 2014, 13, 57–65. [Google Scholar] [CrossRef]
- Pasquarella, C.; Pitzurra, O.; Savino, A. The index of microbial air contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar] [CrossRef]
- Pasquarella, C.; Sansebastiano, G.E.; Ferretti, S.; Saccani, E.; Fanti, M.; Moscato, U.; Giannetti, G.; Fornia, S.; Cortellini, P.; Vitali, P.; et al. A mobile laminar airflow unit to reduce air bacterial contamination at surgical area in a conventionally ventilated operating theatre. J. Hosp. Infect. 2007, 66, 313–319. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A.; da Fonseca, F.G. Microbiologia de Brock, 12th ed.; Artmed: Porto Alegre, Brazil, 2010. [Google Scholar]
- Tortora, G.J.; Funke, B.R.; Case, C.L. Microbiologia, 10th ed.; Artmed: Porto Alegre, Brazil, 2012. [Google Scholar]
- Brenner, D.J.; Krieg, N.R.; Staley, J.T.; Garrity, G.M.; Boone, D.R.; Vos, P.; Goodfellow, M.; Rainey, F.A.; Schleifer, K.-H. (Eds.) Bergey’s Manual of Systematic Bacteriology: The Pseudomonadota, 2nd ed.; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Winn, W.; Allen, S.; Janda, W.; Koneman, E.; Procop, G.; Schreckenberger, P.; Woods, G. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology, 6th ed.; Lippincott Williams & Wilkins: Washington, DC, USA, 2006. [Google Scholar]
- Aamir, S.; Sutar, S.; Singh, S.K.; Baghela, A. A rapid and efficient method of fungal genomic DNA extraction, suitable for PCR based molecular methods. Plant Pathol. Quar. 2015, 5, 74–81. [Google Scholar] [CrossRef]
- Sendrasoa, F.A.; Ratovonjanahary, V.T.; Rasamoelina, T.; Ramarozatovo, L.S.; Rapelanoro Rabenja, F. Treatment responses in patients with chromoblastomycosis to itraconazole in Madagascar. Med. Mycol. 2022, 60, myac086. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, A.; Sharma, A.; Shrimali, T. Pulmonary infection due to Acrophialophora fusispora in a patient with underlying mixed connective tissue disease and chronic pulmonary aspergillosis: A case report and review of literature. J. Mycol. Med. 2020, 30, 100932. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Zia, M.; Goli, M. Identification of saprophytic and allergenic fungi in indoor and outdoor environments. Environ. Monit. Assess. 2018, 190, 574. [Google Scholar] [CrossRef]
- Stanley, H.O.; Amesi, M.E. Air Quality Assessment of Port Harcourt Urban Slums and Health Implications. Int. J. Pathog. Res. 2021, 6, 50–57. [Google Scholar] [CrossRef]
- de Leeuw, G.; Guieu, C.; Arneth, A.; Bellouin, N.; Bopp, L.; Boyd, P.W.; van der Gon, H.A.D.; Desboeufs, K.V.; Dulac, F.; Facchini, M.C.; et al. Ocean–Atmosphere Interactions of Particles. In Ocean-Atmosphere Interactions of Gases and Particles, 1st ed.; Liss, P., Johnson, M.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 172–247. [Google Scholar] [CrossRef]
- Zrnčić, S. Microbial Diseases of Marine Organisms. J. Mar. Sci. Eng. 2022, 10, 1682. [Google Scholar] [CrossRef]
- Aprill, A. Marine Animal Microbiomes: Toward Understanding Host–Microbiome Interactions in a Changing Ocean. Front. Mar. Sci. 2017, 4, 222. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Li, Y.; Wang, B.; Shi, T. The Genus Chrysosporium: A Potential Producer of Natural Products. Fermentation 2023, 9, 76. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Y.; Wang, J.; Wang, L.; Li, X.; Zhang, D. Unique functional responses of fungal communities to various environments in the mangroves of the Maowei Sea in Guangxi, China. Mar. Pollut. Bull. 2021, 173, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Olsen, Y. Airborne Cladosporium and Alternaria spore concentrations through 26 years in Copenhagen, Denmark. Aerobiologia 2019, 36, 141–157. [Google Scholar] [CrossRef]
- Grinn-Gofroń, A.; Nowosad, J.; Bosiacka, B.; Camacho, I.; Pashley, C.; Belmonte, J.; De Linares, C.; Ianovici, N.; Manzano, J.M.M.; Sadyś, M.; et al. Airborne Alternaria and Cladosporium fungal spores in Europe: Forecasting possibilities and relationships with meteorological parameters. Sci. Total Environ. 2019, 653, 938–946. [Google Scholar] [CrossRef]
- Pearce, D.; Bridge, P.; Hughes, K.; Sattler, B.; Psenner, R.; Russell, N. Microorganisms in the atmosphere over Antarctica. FEMS Microbiol. Ecol. 2009, 69, 143–157. [Google Scholar] [CrossRef]
- Damialis, A.; Kaimakamis, E.; Konoglou, M.; Akritidis, I.; Traidl-Hoffmann, C.; Gioulekas, D. Estimating the abundance of airborne pollen and fungal spores at variable elevations using an aircraft: How high can they fly? Sci. Rep. 2017, 7, 44535. [Google Scholar] [CrossRef]
- Klein, J. Role of Pde2 in the CO2 Sensing during Fruiting in Schizophyllum commune. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2022. [Google Scholar]
- Sanz-Sáez, I.; Salazar, G.; Sánchez, P.; Lara, E.; Royo-Llonch, M.; Sà, E.L.; Lucena, T.; Pujalte, M.J.; Vaqué, D.; Duarte, C.M.; et al. Diversity and distribution of marine heterotrophic bacteria from a large culture collection. BMC Microbiol. 2020, 20, 207. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhadury, P. Exploring biogeographic patterns of bacterioplankton communities across global estuaries. Microbiol. Open 2019, 8, e00741. [Google Scholar] [CrossRef]
- Urbano, R.; Palenik, B.; Gaston, C.J.; Prather, K.A. Detection and phylogenetic analysis of coastal bioaerosols using culture dependent and independent techniques. Biogeosciences 2011, 8, 301–309. [Google Scholar] [CrossRef]
- Begrem, S.; Jérôme, M.; Leroi, F.; Delbarre-Ladrat, C.; Grovel, O.; Passerini, D. Genomic diversity of Serratia proteamaculans and Serratia liquefaciens predominant in seafood products and spoilage potential analyses. Int. J. Food Microbiol. 2021, 354, 109326. [Google Scholar] [CrossRef] [PubMed]
- Oktariani, A.F.; Ramona, Y.; Sudaryatma, P.E.; Dewi, I.A.M.M.; Shetty, K. Role of Marine Bacterial Contaminants in Histamine Formation in Seafood Products: A Review. Microorganisms 2022, 10, 1197. [Google Scholar] [CrossRef]
- Hanson, C.; Fuhrman, J.; Horner-Devine, C.; Martiny, J. Beyond biogeographic patterns: Processes shaping the microbial landscape. Nat. Rev. Microbiol. 2012, 10, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Šantl-Temkiv, T.; Sikoparija, B.; Maki, T.; Carotenuto, F.; Amato, P.; Yao, M.; Morris, C.E.; Schnell, R.; Jaenicke, R.; Pöhlker, C.; et al. Bioaerosol field measurements: Challenges and perspectives in outdoor studies. Aerosol Sci. Technol. 2020, 54, 520–546. [Google Scholar] [CrossRef]
- Dadar, M.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Shahali, Y.; Dhama, K. Candida albicans—Biology, molecular characterization, pathogenicity, and advances in diagnosis and control—An update. Microb. Pathog. 2018, 117, 128–138. [Google Scholar] [CrossRef]
- Kaewkrajay, C.; Putchakarn, S.; Limtong, S. Cultivable yeasts associated with marine sponges in the Gulf of Thailand, South China Sea. Antonie van Leeuwenhoek 2021, 114, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Kurpas, M.; Zorena, K.; Wąż, P.; Marks, R. Mold and Yeast-Like Fungi in the Seaside Air of the Gulf of Gdańsk (Southern Baltic) after an Emergency Disposal of Raw Sewage. J. Fungi 2021, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Manamgoda, D.S.; Rossman, A.Y.; Castlebury, L.; Chukeatirote, E.; Hyde, K. A taxonomic and phylogenetic re-appraisal of the genus Curvularia (Pleosporaceae): Human and plant pathogens. Phytotaxa 2015, 212, 175–198. [Google Scholar] [CrossRef]
- Verma, P.; Singh, S.; Singh, R. Seven species of Curvularia isolated from three lakes of Bhopal. Adv. Life Sci. Technol. 2013, 8, 13–15. [Google Scholar]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Crous, P.W. Multi-locus phylogeny of the genus Curvularia and description of ten new species. Mycol. Progress 2020, 19, 559–588. [Google Scholar] [CrossRef]
- Parracha, J.L.; Borsoi, G.; Veiga, R.; Flores-Colen, I.; Nunes, L.; Viegas, C.A.; Moreira, L.M.; Dionísio, A.; Glória Gomes, M.; Faria, P. Durability assessment of external thermal insulation composite systems in urban and maritime environments. Sci. Total Environ. 2022, 849, 157828. [Google Scholar] [CrossRef] [PubMed]
- Babič, M.; Zupancic, J.; Gunde-cimerman, N.; Hoog, S. Ecology of the Human Opportunistic Black Yeast Exophiala dermatitidis Indicates Preference for Human-Made Habitats. Mycopathologia 2018, 183, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Yazdanparast, S.; Mohseni, S.; Hoog, G.; Aslani, N.; Sadeh, A.; Badali, H. Consistent high prevalence of Exophiala dermatitidis, a neurotropic opportunist, on railway sleepers. J. Mycol. Med. 2017, 27, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Thitla, T.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. First Report of Environmental Isolation of Exophiala spp. in Malaysia. Curr. Microbiol. 2020, 77, 2915–2924. [Google Scholar] [CrossRef]
- Ezekiel, C.; Kraak, B.; Sandoval-Denis, M.; Sulyok, M.; Oyedele, O.A.; Ayeni, K.; Makinde, O.; Akinyemi, O.; Krska, R.; Crous, P.; et al. Diversity and toxigenicity of fungi and description of Fusarium madaense sp. nov. from cereals, legumes and soils in north-central Nigeria. MycoKeys 2020, 67, 95–124. [Google Scholar] [CrossRef]
- Segretain, G.; Destombes, P. Description of a new agent for maduromycosis, Neotestudina rosatii, n. gen., n. sp., isolated in Africa. CR Hebd. Seances Acad. Sci. 1961, 253, 2577–2579. [Google Scholar]
- Baylet, R.; Camain, R.; Chabal, J.; Izarn, R. Recent contribution to the study of mycetoma in Senegal. Neotestudina rosatii, Pyrenochaeta romeroi, Aspergillus nidulans. Bull. Soc. Medicale D’Afr. Noire Lang. Francaise 1968, 13, 311–313. [Google Scholar]
- Destombes, P.; Mariat, F.; Rosati, L.; Segretain, G. Mycetoma in Somalia—Results of a survey done from 1959 to 1964. Acta Trop. 1977, 34, 355–373. [Google Scholar]
- Gonçalves, M.F.M.; Santos, L.; Silva, B.M.V.; Abreu, A.C.; Vicente, T.F.L.; Esteves, A.C.; Alves, A. Biodiversity of Penicillium species from marine environments in Portugal and description of Penicillium lusitanum sp. nov., a novel species isolated from sea water. Int. J. Syst. Evol. Microbiol. 2019, 69, 3014–3021. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, X.; Li, G.; Feng, Z.; Xu, J. Pestalotiopisorin B, a new isocoumarin derivative from the mangrove endophytic fungus Pestalotiopsis sp. HHL101. Nat. Prod. Res. 2019, 34, 1002–1007. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, D.Y.; Xu, J. Two new polyketides from endophytic fungus Pestalotiopsis sp. HQD-6 isolated from the Chinese mangrove plant Rhizophora mucronate. J. Asian Nat. Prod. Res. 2021, 24, 52–58. [Google Scholar] [CrossRef]
- Wu, C.; Wang, Y.; Yang, Y. Pestalotiopsis Diversity: Species, Dispositions, Secondary Metabolites, and Bioactivities. Molecules 2022, 27, 8088. [Google Scholar] [CrossRef] [PubMed]
- Al-Hosni, K.; Shahzad, R.; Latif Khan, A.; Muhammad Imran, Q.; Al Harrasi, A.; Al Rawahi, A.; Asaf, S.; Kang, S.M.; Yun, B.W.; Lee, I.J. Preussia sp. BSL-10 producing nitric oxide, gibberellins, and indole acetic acid and improving rice plant growth. J. Plant Interact. 2018, 13, 112–118. [Google Scholar] [CrossRef]
- Youn, U.; Seo, S.; Yim, J.; Kim, C.; Han, S. Chemical constituents from the culture filtrate of a Himalayan soil fungus, Preussia sp. and their anti-inflammatory activity. Korean J. Microbiol. 2018, 54, 18–23. [Google Scholar] [CrossRef]
- Heitger, M.; Baltar, F. Respiration, Production, and Growth Efficiency of Marine Pelagic Fungal Isolates. J. Fungi 2023, 9, 417. [Google Scholar] [CrossRef]
- Sarkar, A.; Philip, A.M.; Thakker, D.P.; Wagh, M.S.; Rao, K.B. In vitro Antioxidant activity of extracellular L-glutaminase enzyme isolated from marine yeast Rhodotorula sp. DAMB1. Res. J. Pharm. Technol. 2020, 13, 209–215. [Google Scholar] [CrossRef]
- Miranda, A.F.; Nham Tran, T.L.; Abramov, T.; Jehalee, F.; Miglani, M.; Liu, Z.; Rochfort, S.; Gupta, A.; Cheirsilp, B.; Adhikari, B.; et al. Marine Protists and Rhodotorula Yeast as Bio-Convertors of Marine Waste into Nutrient-Rich Deposits for Mangrove Ecosystems. Protist 2020, 171, 125738. [Google Scholar] [CrossRef]
- Martinez-Bracero, M.; Markey, E.; Clancy, J.H.; McGillicuddy, E.J.; Sewell, G.; O’Connor, D.J. Airborne Fungal Spore Review, New Advances and Automatisation. Atmosphere 2022, 13, 308. [Google Scholar] [CrossRef]



| P1 | P2 | P3 | P4 | P5 | ||
|---|---|---|---|---|---|---|
| Bacteria | Pseudomonadota | X | * | * | X | |
| Enterobacteriaceae | X | X | X | |||
| Bacillota | X | X | X | |||
| S. liquefaciens | X | X | ||||
| Bacillus | X | X | ||||
| Fungi | Aspergillus sp. | X | X | X | ||
| Fonsecaea sp. | X | X | ||||
| Rhodotorula sp. | X | |||||
| Stemphylium sp. | X | |||||
| Acrophialophora sp. | X | |||||
| Chrysosporium sp. | X | |||||
| Schizophyllum sp. | X | |||||
| Cladosporium sp. | X | X | ||||
| Preussia sp. | X | |||||
| Neotestudina sp. | X | X | ||||
| Preussia sp. | X | |||||
| Curvularia sp. | X | X | ||||
| Penicullium sp. | X | X | ||||
| Exophiala dermatitidis | X | |||||
| Candida sp. | X | X | ||||
| Pestalotiopsis sp. | X | |||||
| Cystobasidium sp. | X | |||||
| Mucor sp. | X |
| Collection Point | Code Sample | Identification | Similarity | Access (GenBank) | Nucleotide Coverage (%) | E-Value |
|---|---|---|---|---|---|---|
| P1 | 1 | Aspergillus sp. | 95% | MN788648.1 | 100 | 0 |
| 12 | Neotestudina sp. | 97% | OW983104.1 | 100 | 0 | |
| P2 | 06.1 | Rhodotorula sphaerocarpa | 86% | MT355634.1 | 100 | 0 |
| 7 | Aspergillus sp. | 89% | MK725871.1 | 100 | 0 | |
| 5 | Preussia sp. | 95% | KC013967.1 | 100 | 0 | |
| 7 | Cladosporium sp. | 84% | KX788174.1 | 100 | 0 | |
| 16 | Curvularia sp. | 86% | MN215656.1 | 100 | 0 | |
| 23 | Penicillium sp. | 82% | MN634532.1 | 100 | 0 | |
| 06.2 | Neotestudina sp. | 92% | OW983104.1 | 100 | 0 | |
| P3 | 8 | Penicillium citrinum | 87% | OP163524.1 | 100 | 0 |
| 9 | Aspergillus sp. | 93% | KY203991.1 | 100 | 0 | |
| 11 | Candida sp. | 95% | KY911171.1 | 100 | 0 | |
| 14 | Aspergillus sp. | 92% | MN700120.1 | 100 | 0 | |
| 15 | Aspergillus japonicus | 83% | GQ359413.1 | 100 | 0 | |
| 16 | Curvularia sp. | 73% | MN540246.1 | 100 | 0 | |
| 18 | Pestalotiopsis sp. | 81% | ON681710.1 | 100 | 0 | |
| P4 | 26 | Cystobasidium sp. | 87% | LC424143.1 | 100 | 0 |
| P5 | 29 | Candida sp. | 88% | KP131656.1 | 100 | 0 |
| 30 | Exophiala dermatitidis | 86% | MN410630.1 | 100 | 0 |
| Environmental Parameters | Collect Points 2017 | Collect Points 2022 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P1 | P2 | P3 | P4 | P5 | |
| Air Temperature (°C) | 28.4 | 26.4 | 35.5 | 25.7 | 26.5 | 25.2 | 25.5 | 25.1 | 26.1 | 25 |
| Relative humidity (%) | 63 | 74 | 49 | 82 | 78 | 69.0 | 76.0 | 85.0 | 78.0 | 81.0 |
| Atmospheric Pressure (hPa) | 1013 | 1012 | 1012.6 | 1008.6 | 1008.5 | 1014.7 | 1014.9 | 1012.9 | 1012.1 | 1012.7 |
| Wind Speed (knots) | 2.4 | 5.3 | 8.9 | 15.6 | 18 | 20 | 14 | 9 | 5.5 | 10 |
| Sea Surface Temperature (°C) | 26.9 | 27.4 | 27.9 | 27.6 | 26.7 | 25.4 | 25.95 | 27.35 | 28.16 | 27.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moura, G.C.C.; Ayres, Y.M.; Brito, A.L.d.C.; Júnior, E.F.d.S.; Rocha, R.d.S.; De Sousa, P.M.V.; Ferreira, A.G.; Sousa, O.V.d.; Veleda, D. Characterization of the Cultivable Microbiota Components of Marine Bioaerosols in the North Tropical Atlantic. Atmosphere 2023, 14, 1470. https://doi.org/10.3390/atmos14101470
Moura GCC, Ayres YM, Brito ALdC, Júnior EFdS, Rocha RdS, De Sousa PMV, Ferreira AG, Sousa OVd, Veleda D. Characterization of the Cultivable Microbiota Components of Marine Bioaerosols in the North Tropical Atlantic. Atmosphere. 2023; 14(10):1470. https://doi.org/10.3390/atmos14101470
Chicago/Turabian StyleMoura, Gabriela Cristina Chagas, Yasmin Marques Ayres, Anna Luisa de Carvalho Brito, Edmilson Ferreira de Souza Júnior, Rafael dos Santos Rocha, Paulo Miguel Vieira De Sousa, Antônio Geraldo Ferreira, Oscarina Viana de Sousa, and Doris Veleda. 2023. "Characterization of the Cultivable Microbiota Components of Marine Bioaerosols in the North Tropical Atlantic" Atmosphere 14, no. 10: 1470. https://doi.org/10.3390/atmos14101470
APA StyleMoura, G. C. C., Ayres, Y. M., Brito, A. L. d. C., Júnior, E. F. d. S., Rocha, R. d. S., De Sousa, P. M. V., Ferreira, A. G., Sousa, O. V. d., & Veleda, D. (2023). Characterization of the Cultivable Microbiota Components of Marine Bioaerosols in the North Tropical Atlantic. Atmosphere, 14(10), 1470. https://doi.org/10.3390/atmos14101470






