Association between Air Pollution Exposure and Daily Outpatient Visits for Dry Eye Disease: A Time-Series Study in Urumqi, China
Abstract
1. Introduction
2. Materials and Methods
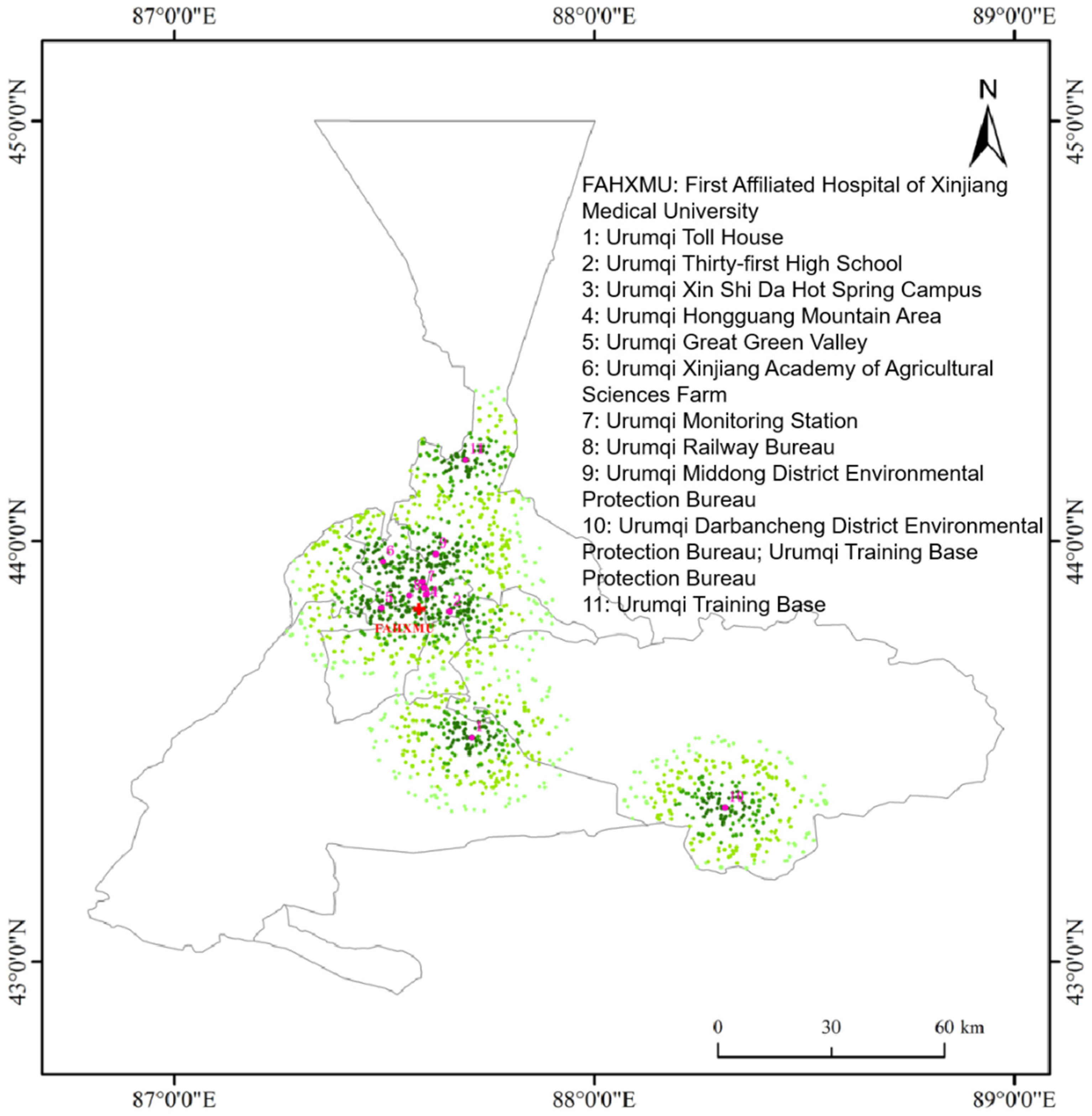
2.1. Study Site
2.2. Study Population
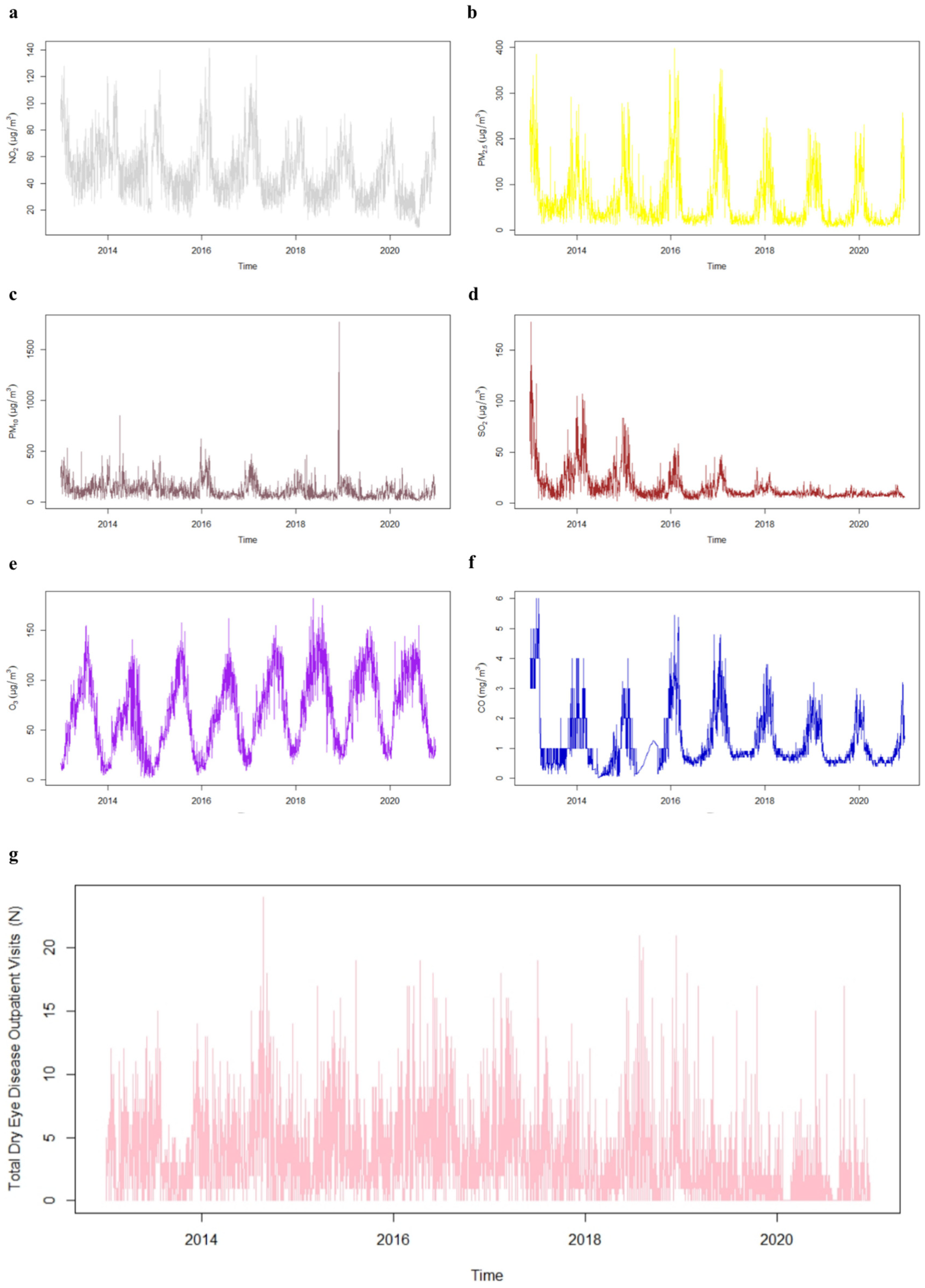
2.3. Atmospheric Pollutants and Meteorological Data
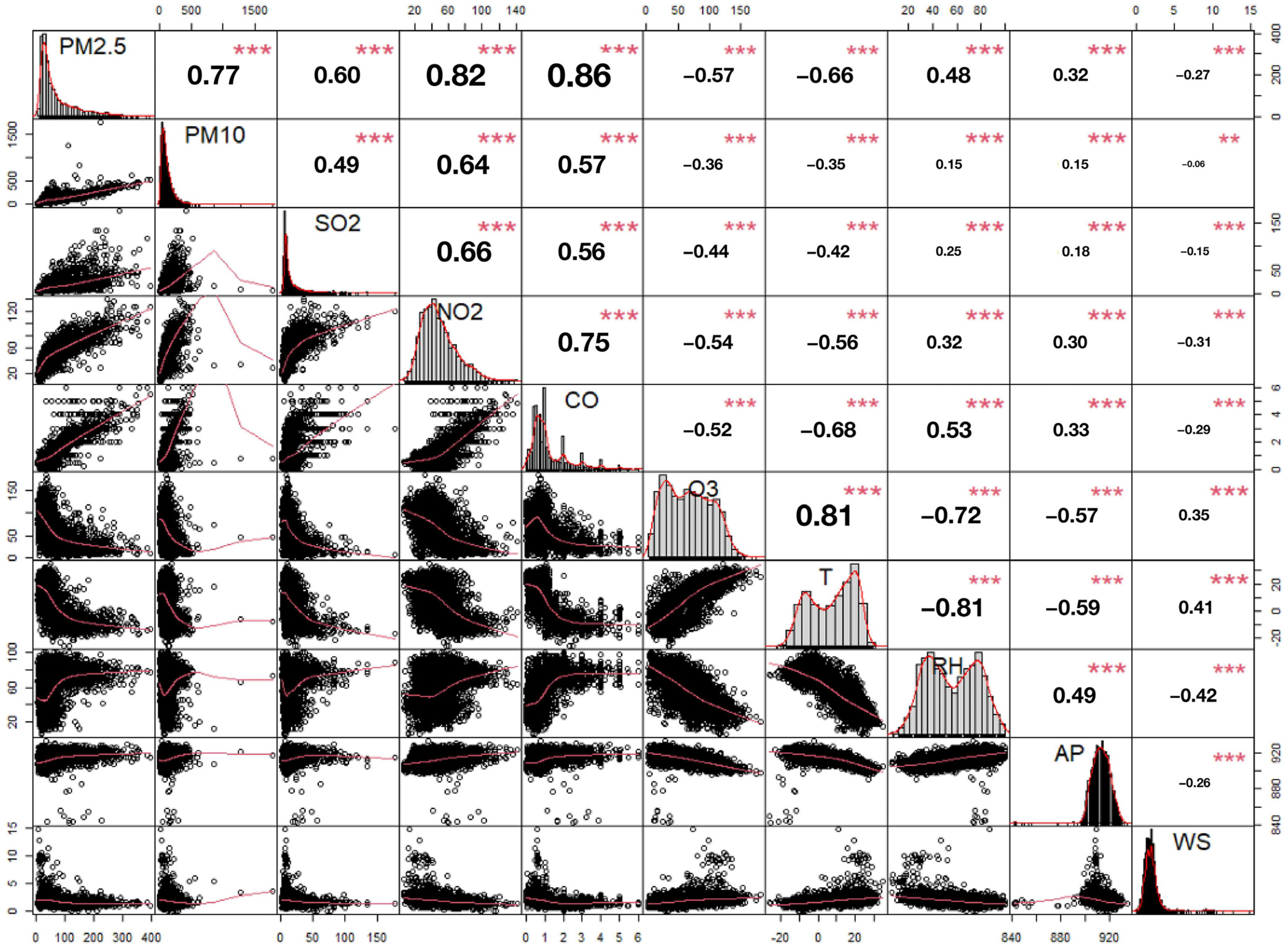
2.4. Statistical Analysis
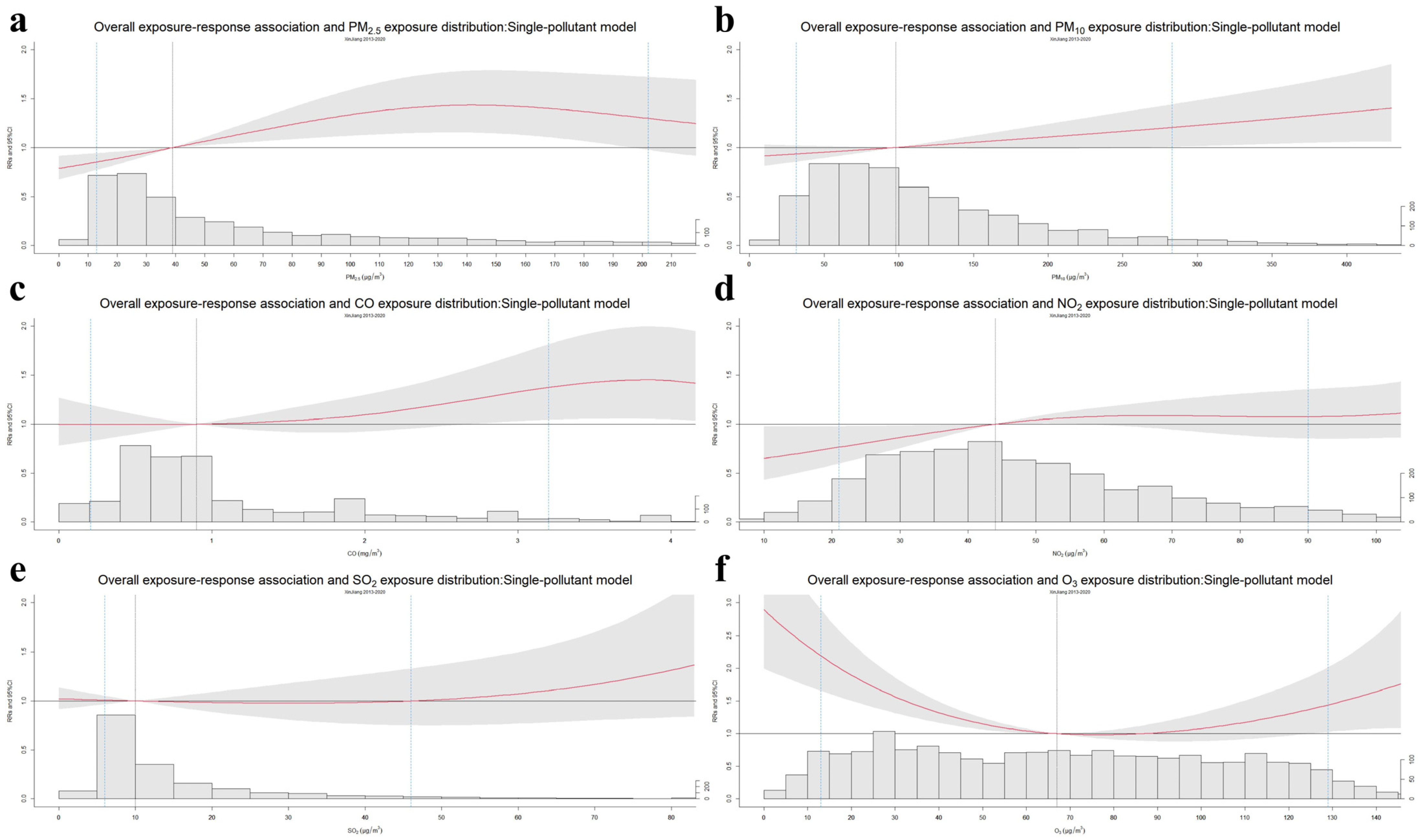
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Y.; Xiang, X.; Wu, Y.; Cao, Y.; Song, J.; Sun, K.; Liu, H.; Hu, Y. Fine Particulate Air Pollution and First Hospital Admissions for Ischemic Stroke in Beijing, China. Sci. Rep. 2017, 7, 3897. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Ding, H.; Jiang, L.N.; Chen, S.W.; Zheng, J.P.; Qiu, M.; Zhou, Y.X.; Chen, Q.; Guan, W.J. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0138146. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Fu, J.; Liu, X.F.; Chen, W.W.; Hao, J.L.; Li, X.L.; Pant, O.P. Air pollution and meteorological conditions significantly contribute to the worsening of allergic conjunctivitis: A regional 20-city, 5-year study in Northeast China. Light Sci. Appl. 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Ichinose, T.; Yamagami, S.; Fujishima, H.; Kamei, Y.; Goto, M.; Takada, S.; Matsubara, M. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci. Total Environ. 2014, 487, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhong, T.; Li, H.; Xu, J.; Ye, X.; Mu, Z.; Lu, Y.; Mashaghi, A.; Zhou, Y.; Tan, M.; et al. Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: A retrospective registry study. Sci. Rep. 2016, 6, 23858. [Google Scholar] [CrossRef] [PubMed]
- Al-Saedi, Z.; Zimmerman, A.; Bachu, R.D.; Dey, S.; Shah, Z.; Baugh, R.; Boddu, S.H. Dry Eye Disease: Present Challenges in the Management and Future Trends. Curr. Pharm. Des. 2016, 22, 4470–4490. [Google Scholar] [CrossRef]
- Um, S.B.; Kim, N.H.; Lee, H.K.; Song, J.S.; Kim, H.C. Spatial epidemiology of dry eye disease: Findings from South Korea. Int. J. Health Geogr. 2014, 13, 31. [Google Scholar] [CrossRef]
- Albdaya, N.A.; Binyousef, F.H.; Alrashid, M.H.; Alajlan, A.A.; Alsharif, F.A.; Alfouzan, S.K.; Alhuthail, R.R. Prevalence of Dry Eye Disease and Its Association With the Frequent Usage of Eye Cosmetics Among Women. Cureus 2022, 14, e27142. [Google Scholar] [CrossRef]
- Kuang, T.M.; Tsai, S.Y.; Liu, C.J.; Lee, S.M.; Chou, P. Association between dry eye and depressive symptoms in an elderly Chinese population in Taiwan: The Shihpai Eye Study. Eye 2021, 35, 2826–2833. [Google Scholar] [CrossRef]
- Caffery, B.E.; Richter, D.; Simpson, T.; Fonn, D.; Doughty, M.; Gordon, K. CANDEES. The Canadian Dry Eye Epidemiology Study. Adv. Exp. Med. Biol. 1998, 438, 805–806. [Google Scholar]
- Liu, N.N.; Liu, L.; Li, J.; Sun, Y.Z. Prevalence of and risk factors for dry eye symptom in mainland china: A systematic review and meta-analysis. J. Ophthalmol. 2014, 2014, 748654. [Google Scholar] [CrossRef]
- Yu, D.; Deng, Q.; Wang, J.; Chang, X.; Wang, S.; Yang, R.; Yu, J.; Yu, J. Air Pollutants are associated with Dry Eye Disease in Urban Ophthalmic Outpatients: A Prevalence Study in China. J. Transl. Med. 2019, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Ehret, M.; Sauer, A.; Speeg-Schatz, C.; Bourcier, T. Ocular surface and outdoor air pollution: A systematic review. J. Fr. Ophtalmol. 2022, 45, 784–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Zheng, Y.; Deng, L.; Huang, X. Prevalence of dry eye disease in the elderly: A protocol of systematic review and meta-analysis. Medicine (Baltimore) 2020, 99, e22234. [Google Scholar] [CrossRef]
- Najafi, L.; Malek, M.; Valojerdi, A.E.; Aghili, R.; Khamseh, M.E.; Fallah, A.E.; Tokhmehchi, M.R.; Behrouz, M.J. Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. J. Diabetes Complicat. 2013, 27, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Wei, W. Identified risk factors for dry eye syndrome: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0271267. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.Y.; Lee, Y.C.; Hsieh, C.J.; Tseng, C.C.; Yiin, L.M. Association between Dry Eye Disease, Air Pollution and Weather Changes in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 2269. [Google Scholar] [CrossRef]
- Mo, Z.; Fu, Q.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Wang, X.; et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: A case-crossover study. Environ. Pollut. 2019, 246, 183–189. [Google Scholar] [CrossRef]
- An, Y.; Kim, H. Sleep disorders, mental health, and dry eye disease in South Korea. Sci. Rep. 2022, 12, 11046. [Google Scholar] [CrossRef]
- Mu, J.; Zeng, D.; Fan, J.; Liu, M.; Yu, S.; Ding, W.; Zhang, S. Associations Between Air Pollution Exposure and Daily Pediatric Outpatient Visits for Dry Eye Disease: A Time-Series Study in Shenzhen, China. Int. J. Public Health 2021, 66, 1604235. [Google Scholar] [CrossRef]
- Hwang, S.H.; Choi, Y.H.; Paik, H.J.; Wee, W.R.; Kim, M.K.; Kim, D.H. Potential Importance of Ozone in the Association Between Outdoor Air Pollution and Dry Eye Disease in South Korea. JAMA Ophthalmol. 2016, 134, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Lu, Y.; Huang, K.; Gao, X.; Gui, S.Y.; Hu, C.Y.; Jiang, Z.X. Association between short-term exposure to ambient nitrogen dioxide and the risk of conjunctivitis in Hefei, China: A time-series analysis. Environ. Res. 2021, 195, 110807. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yang, J.; Chen, D.; Liu, W.J.; Zhang, C.; Wang, H.; Li, B.; Xiong, P.; Wang, B.; Wang, Y.; et al. Air pollution and hospital outpatient visits for conjunctivitis: A time-series analysis in Tai’an, China. Environ. Sci. Pollut. Res. Int. 2021, 28, 15453–15461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Liu, C.; Tu, B.; Zhang, X.; Chen, F.; Xu, J.; Qian, D.; Wang, X.; Zhou, W. Short-Term effects of ambient ozone on the risk of conjunctivitis outpatient visits: A time-series analysis in Pudong New Area, Shanghai. Int. J. Environ. Health Res. 2022, 27, 1–10. [Google Scholar] [CrossRef]
- Goshua, A.; Akdis, C.A.; Nadeau, K.C. World Health Organization global air quality guideline recommendations: Executive summary. Allergy 2022, 77, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, Y.H.; Kim, M.K.; Paik, H.J.; Kim, D.H. Different adverse effects of air pollutants on dry eye disease: Ozone, PM2.5, and PM10. Environ. Pollut 2020, 265, 115039. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.T.; Idarraga, M.; Kumar, N.; Galor, A. Impact of Air Pollution and Weather on Dry Eye. J. Clin. Med. 2020, 9, 3710. [Google Scholar] [CrossRef]
- Yin, P.; Brauer, M.; Cohen, A.J.; Wang, H.; Li, J.; Burnett, R.T.; Stanaway, J.D.; Causey, K.; Larson, S.; Godwin, W.; et al. The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990–2017: An analysis for the Global Burden of Disease Study 2017. Lancet Planet Health 2020, 4, e386–e398. [Google Scholar] [CrossRef]
- Rupakheti, D.; Yin, X.; Rupakheti, M.; Zhang, Q.; Li, P.; Rai, M.; Kang, S. Spatio-temporal characteristics of air pollutants over Xinjiang, northwestern China. Environ. Pollut. 2021, 268, 115907. [Google Scholar] [CrossRef]
- Yin, P.; He, G.; Fan, M.; Chiu, K.Y.; Fan, M.; Liu, C.; Xue, A.; Liu, T.; Pan, Y.; Mu, Q.; et al. Particulate air pollution and mortality in 38 of China’s largest cities: Time series analysis. BMJ 2017, 356, j667. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W.; Jiskani, I.M.; Ding, X.; Luo, H. Dust pollution in cold region Surface Mines and its prevention and control. Environ. Pollut. 2022, 292, 118293. [Google Scholar] [CrossRef]
- Moryani, H.T.; Kong, S.; Du, J.; Bao, J. Health Risk Assessment of Heavy Metals Accumulated on PM2.5 Fractioned Road Dust from Two Cities of Pakistan. Int. J. Environ. Res. Public Health 2020, 17, 7124. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Iizuka, Y. Effect and underlying mechanisms of airborne particulate matter 2.5 (PM2.5) on cultured human corneal epithelial cells. Sci. Rep. 2020, 10, 19516. [Google Scholar] [CrossRef]
- Xiang, P.; Liu, R.Y.; Sun, H.J.; Han, Y.H.; He, R.W.; Cui, X.Y.; Ma, L.Q. Molecular mechanisms of dust-induced toxicity in human corneal epithelial cells: Water and organic extract of office and house dust. Environ. Int. 2016, 92, 348–356. [Google Scholar] [CrossRef]
- Hao, R.; Zhang, M.; Zhao, L.; Liu, Y.; Sun, M.; Dong, J.; Xu, Y.; Wu, F.; Wei, J.; Xin, X.; et al. Impact of Air Pollution on the Ocular Surface and Tear Cytokine Levels: A Multicenter Prospective Cohort Study. Front. Med. 2022, 9, 909330. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Chang, Y.; Liu, X.; Li, K.; Gong, Y.; He, G.; Wang, X.; Christie, P.; Zheng, M.; Dore, A.J.; et al. A multiyear assessment of air quality benefits from China’s emerging shale gas revolution: Urumqi as a case study. Environ. Sci. Technol. 2015, 49, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Zeng, D.; Zeng, H. Effects of nitrogen dioxide exposure on the risk of eye and adnexa diseases among children in Shenzhen, China: An assessment using the generalized additive modeling approach. Int. J. Environ. Health Res. 2022, 32, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Alassane, S.; Binquet, C.; Bretillon, L.; Acar, N.; Arnould, L.; Muselier-Mathieu, A.; Delcourt, C.; Bron, A.M.; Creuzot-Garcher, C. Dry eye disease in the elderly in a French population-based study (the Montrachet study: Maculopathy, Optic Nerve, nuTRition, neurovAsCular and HEarT diseases): Prevalence and associated factors. Ocul. Surf. 2018, 16, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Davis, A.F. Sex/gender disparities and women’s eye health. Curr. Eye Res. 2015, 40, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Korpole, N.R.; Kurada, P.; Korpole, M.R. Gender Difference in Ocular Diseases, Risk Factors and Management with Specific Reference to Role of Sex Steroid Hormones. J. Midlife Health 2022, 13, 20–25. [Google Scholar] [CrossRef]
- Ezzati, M.; Saleh, H.; Kammen, D.M. The contributions of emissions and spatial microenvironments to exposure to indoor air pollution from biomass combustion in Kenya. Environ. Health Perspect 2000, 108, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Man, R.E.K.; Veerappan, A.R.; Tan, S.P.; Fenwick, E.K.; Sabanayagam, C.; Chua, J.; Leong, Y.Y.; Wong, T.Y.; Lamoureux, E.L.; Cheng, C.Y.; et al. Incidence and risk factors of symptomatic dry eye disease in Asian Malays from the Singapore Malay Eye Study. Ocul. Surf. 2017, 15, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, M.M.; Shihadeh, W.A.; Haddad, M.F.; Khader, Y.S. Epidemiology of symptoms of dry eye disease (DED) in Jordan: A cross-sectional non-clinical population-based study. Cont. Lens. Anterior. Eye 2016, 39, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Krewski, D.; Jerrett, M.; Shi, Y.; Calle, E.E.; Thun, M.J. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: Shape of the exposure-response relationship. Circulation 2009, 120, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Lanier, B. Urban eye allergy syndrome: A new clinical entity? Curr. Med. Res. Opin. 2008, 24, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Asbell, P.A. The core mechanism of dry eye disease is inflammation. Eye Contact Lens. 2014, 40, 248–256. [Google Scholar] [CrossRef]
- Li, J.; Tan, G.; Ding, X.; Wang, Y.; Wu, A.; Yang, Q.; Ye, L.; Shao, Y. A mouse dry eye model induced by topical administration of the air pollutant particulate matter 10. Biomed Pharm. 2017, 96, 524–534. [Google Scholar] [CrossRef]
- Malerbi, F.K.; Martins, L.C.; Saldiva, P.H.; Braga, A.L. Ambient levels of air pollution induce clinical worsening of blepharitis. Environ. Res. 2012, 112, 199–203. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Novaes, P.; Matsuda, M.; Braga, A.; Saldiva, P.H.; Alves, M.R.; Monteiro, M.L. Correlation between signs and symptoms of ocular surface dysfunction and tear osmolarity with ambient levels of air pollution in a large metropolitan area. Cornea 2013, 32, e11–e15. [Google Scholar] [CrossRef]
- Wolkoff, P. Ocular discomfort by environmental and personal risk factors altering the precorneal tear film. Toxicol. Lett. 2010, 199, 203–212. [Google Scholar] [CrossRef]
- Lee, H.; Kim, E.K.; Kim, H.Y.; Kim, T.I. Effects of Exposure to Ozone on the Ocular Surface in an Experimental Model of Allergic Conjunctivitis. PLoS ONE 2017, 12, e0169209. [Google Scholar] [CrossRef]
- Torricelli, A.A.; Matsuda, M.; Novaes, P.; Braga, A.L.; Saldiva, P.H.; Alves, M.R.; Monteiro, M.L. Effects of ambient levels of traffic-derived air pollution on the ocular surface: Analysis of symptoms, conjunctival goblet cell count and mucin 5AC gene expression. Environ. Res. 2014, 131, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef] [PubMed]
| Variables | Number of Measurements | Mean ± SD | Min | P25 | Median | P75 | Max |
|---|---|---|---|---|---|---|---|
| Air pollutant concentration | |||||||
| PM2.5 (μg/m3) | 2921 | 64.5 ± 61.9 | 6 | 23 | 39 | 84 | 397 |
| PM10 (μg/m3) | 2921 | 120.2 ± 90.8 | 10 | 62 | 98 | 153 | 1766 |
| SO2 (μg/m3) | 2921 | 16 ± 15.2 | 2 | 8 | 10 | 17 | 177 |
| NO2 (μg/m3) | 2921 | 48.5 ± 21 | 7 | 33 | 44 | 60 | 141 |
| CO (mg/m3) | 2921 | 1.2 ± 1 | 0.01 | 0.6 | 0.9 | 1.5 | 6 |
| O3 (μg/m3) | 2921 | 68.2 ± 37.7 | 2 | 35 | 67 | 99 | 182 |
| Meteorological factors | |||||||
| Mean temperature (°C) | 2921 | 8.4 ± 13.7 | −26 | −4.5 | 10.7 | 20.6 | 35.1 |
| Relative humidity (%) | 2921 | 55.5 ± 21.3 | 6 | 37 | 54 | 74 | 100 |
| Wind speed (m/s) | 2921 | 2 ± 1.1 | 0 | 1.4 | 1.9 | 2.4 | 14.8 |
| Atmospheric pressure (hpa) | 2921 | 912.7 ± 7.8 | 842 | 908 | 913 | 918 | 934 |
| Number of dry eye disease outpatient visits (n) | |||||||
| Total | 9970 | 3.4 ± 3.6 | 0 | 1 | 2 | 5 | 24 |
| Gender | |||||||
| Male | 2729 | 1 ± 1.3 | 0 | 0 | 0 | 1 | 9 |
| Female | 7241 | 2.5 ± 2.7 | 0 | 0 | 2 | 4 | 20 |
| Age (years) | |||||||
| 0–5 | 68 | 0 ± 0.2 | 0 | 0 | 0 | 0 | 2 |
| 6–18 | 211 | 0 ± 0.3 | 0 | 0 | 0 | 0 | 3 |
| 19–64 | 7790 | 2.7 ± 2.8 | 0 | 0 | 2 | 4 | 20 |
| ≥65 | 1901 | 0.7 ± 1.1 | 0 | 0 | 0 | 1 | 16 |
| Season | |||||||
| Warm (April to September) | 5069 | 3.5 ± 3.7 | 0 | 1 | 2 | 5 | 24 |
| Cold (October to March) | 4901 | 3.4 ± 3.4 | 0 | 1 | 2 | 5 | 21 |
| Lag Effects | PM2.5 (μg/m3) | PM10 (μg/m3) | CO (mg/m3) | SO2 (μg/m3) | NO2 (μg/m3) | O3 (μg/m3) |
|---|---|---|---|---|---|---|
| Single lag effects RRs (95% CI) | ||||||
| Lag 0 | 1.02 (0.99–1.04) | 1.01 (1.00–1.02) * | 1.08 (0.93–1.25) | 1.09 (1.01–1.18) * | 1.08 (1.04–1.13) ** | 1.00 (0.96–1.04) |
| Lag 1 | 1.01 (0.98–1.04) | 1.00 (0.99–1.01) | 1.04 (0.89–1.22) | 1.00 (0.92–1.08) | 0.99 (0.95–1.03) | 0.99 (0.96–1.03) |
| Lag 2 | 1.00 (0.98–1.02) | 1.00 (0.99–1.00) | 0.96 (0.86–1.08) | 1.01 (0.95–1.06) | 0.99 (0.97–1.02) | 1.00 (0.97–1.02) |
| Lag 3 | 1.01 (0.99–1.02) | 1.00 (0.99–1.00) | 0.99 (0.92–1.06) | 0.99 (0.95–1.02) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) |
| Lag 4 | 1.01 (1.00–1.02) | 1.00 (1.00–1.01) | 1.03 (0.95–1.1) | 0.97 (0.94–1.01) | 1.00 (0.98–1.02) | 1.00 (0.98–1.02) |
| Lag 5 | 1.01 (1.00–1.02) | 1.00 (0.99–1.01) | 1.03 (0.96–1.10) | 0.97 (0.94–1.00) | 1.00 (0.99–1.02) | 1.00 (0.98–1.02) |
| Lag 6 | 1.01 (1.00–1.02) | 1.00 (0.99–1.00) | 1.00 (0.96–1.05) | 0.98 (0.95–1.00) | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) |
| Lag 7 | 1.00 (0.98–1.02) | 1.00 (0.99–1.01) | 0.97 (0.87–1.08) | 0.99 (0.94–1.05) | 1.00 (0.97–1.03) | 1.00 (0.97–1.02) |
| Cumulative lag effects RRs (95% CI) | ||||||
| Lag 0–1 | 1.02 (1.00–1.05) | 1.01 (1.00–1.02) | 1.12 (0.96–1.32) | 1.09 (1.00–1.18) | 1.07 (1.02–1.11) ** | 0.99 (0.94–1.03) |
| Lag 0–2 | 1.02 (1.00–1.05) | 1.01 (1.00–1.01) | 1.08 (0.93–1.26) | 1.09 (1.01–1.19) * | 1.06 (1.02–1.11) ** | 0.98 (0.94–1.03) |
| Lag 0–3 | 1.03 (1.00–1.06) | 1.01 (1.00–1.02) | 1.07 (0.91–1.26) | 1.08 (0.99–1.18) | 1.06 (1.01–1.11) * | 0.98 (0.93–1.03) |
| Lag 0–4 | 1.04 (1.01–1.07) * | 1.01 (1.00–1.02) | 1.10 (0.93–1.3) | 1.05 (0.96–1.15) | 1.06 (1.01–1.11) * | 0.98 (0.93–1.04) |
| Lag 0–5 | 1.05 (1.02–1.09) ** | 1.01 (1.00–1.02) | 1.13 (0.94–1.35) | 1.02 (0.92–1.12) | 1.06 (1.01–1.12) * | 0.99 (0.93–1.04) |
| Lag 0–6 | 1.06 (1.02–1.09) ** | 1.01 (1.00–1.02) | 1.13 (0.95–1.35) | 0.99 (0.90–1.10) | 1.06 (1.01–1.12) * | 0.99 (0.93–1.04) |
| Lag 0–7 | 1.06 (1.02–1.10) ** | 1.01 (1.00–1.02) | 1.10 (0.92–1.30) | 0.98 (0.89–1.09) | 1.06 (1.01–1.13) * | 1.06 (1.01–1.13) * |
| . | PM2.5 (μg/m3) | PM10 (μg/m3) | CO (mg/m3) | NO2 (μg/m3) | SO2 (μg/m3) | O3 (μg/m3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lag effect | Lag effect | Lag effect | Lag effect | Lag effect | Lag effect | |||||||
| Single | Cumulative | Single | Cumulative | Single | Cumulative | Single | Cumulative | Single | Cumulative | Single | Cumulative | |
| Adjusted for PM2.5 | 1.00 (1.00–1.01) | 1.01 (0.99–1.02) | 1.03 (0.96–1.10) | 1.04 (0.85–1.28) | 1.08 (1.03–1.13) ** | 1.06 (1.01–1.12) * | 0.97 (0.94–1.01) | 1.05 (0.96–1.15) | 1.00 (0.97–1.02) | 0.98 (0.94–1.03) | ||
| Adjusted for PM10 | 1.01 (1.00–1.02) | 1.04 (1.00–1.08) | 1.02 (0.96–1.10) | 1.06 (0.88–1.27) | 1.07 (1.02–1.12) ** | 1.05 (1.00–1.10) * | 0.97 (0.94–1.00) | 1.05 (0.96–1.15) | 0.99 (0.95–1.03) | 0.98 (0.94–1.03) | ||
| Adjusted for CO | 1.01 (1.00–1.02) | 1.05 (1.01–1.09) ** | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | 1.09 (1.04–1.15) ** | 1.08 (1.03–1.14) ** | 0.97 (0.93–1.00) | 1.06 (0.97–1.15) | 0.99 (0.96–1.03) | 0.98 (0.92–1.03) | ||
| Adjusted for NO2 | 1.01 (1.00–1.02) | 1.04 (1.00–1.08) * | 1.00 (1.00–1.01) | 1.00 (0.99–1.02) | 0.96 (0.87–1.07) | 0.95 (0.77–1.15) | 0.97 (0.93–1.00) | 0.91 (0.81–1.02) | 0.99 (0.97–1.02) | 0.99 (0.94–1.04) | ||
| Adjusted for SO2 | 1.01 (1.00–1.02) | 1.05 (1.02–1.09) ** | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | 1.03 (0.96–1.10) | 1.09 (0.91–1.31) | 1.07 (1.02–1.12) | 1.05 (1.00–1.11) * | 0.99 (0.95–1.03) | 0.98 (0.94–1.03) | ||
| Adjusted for O3 | 1.01 (1.00–1.02) | 1.06 (1.02–1.10) ** | 1.00 (1.00–1.01) | 1.01 (1.00–1.02) | 1.03 (0.96–1.11) | 1.10 (0.92–1.31) | 1.07 (1.02–1.11) | 1.08 (1.02–1.15) ** | 1.07 (0.99–1.16) | 1.11 (1.02–1.20)* | ||
| Adjusted for the other 5 pollutants | 1.01 (1.00–1.02) | 0.97 (0.94–1.01) | 1.00 (1.00–1.01) | 1.01 (1.00–1.03) | 0.84 (0.70–1.01) | 0.84 (0.70–1.01) | 1.07 (1.02–1.11) ** | 1.10 (1.03–1.18) ** | 0.97 (0.94–1.01) | 1.06 (0.96–1.17) | 0.99 (0.95–1.03) | 0.98 (0.92–1.04) |
| Characteristics | PM2.5 (μg/m3) | PM10 (μg/m3) | CO (mg/m3) | NO2 (μg/m3) | SO2 (μg/m3) | O3 (μg/m3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lag effect | Lag effect | Lag effect | Lag effect | Lag effect | Lag effect | |||||||
| Single | Cumulative | Single | Cumulative | Single | Cumulative | Single | Cumulative | Single | Cumulative | Single | Cumulative | |
| Sex | ||||||||||||
| Male | 1.04 (1.00–1.08) | 1.03 (0.97–1.10) | 1.01 (1.00–1.02) | 1.02 (1.00–1.05) | 1.14 (0.91–1.42) | 0.93 (0.69–1.24) | 1.01 (0.99–1.03) | 1.15 (1.05–1.27) ** | 0.98 (0.93–1.03) | 0.96 (0.81–1.13) | 0.98 (0.94–1.02) | 0.98 (0.92–1.05) |
| Female | 1.01 (1.00–1.03) | 1.03 (0.98–1.07) | 1.00 (1.00–1.01) | 1.01 (0.99–1.03) | 1.04 (0.96–1.12) | 0.83 (0.67–1.02) | 1.09 (1.03–1.15) ** | 1.08 (1.01–1.16) * | 0.97 (0.94–1.01) | 1.08 (0.97–1.20) | 0.99 (0.95–1.03) | 0.98 (0.92–1.04) |
| Age (years) | ||||||||||||
| 0–5 | 1.09 (1.02–1.17)* | 1.17 (0.89–1.56) | 1.02 (1.00–1.05) | 1.03 (0.92–1.15) | 1.77 (0.59–5.34) | 0.75 (0.15–3.65) | 1.16 (1.04–1.30) ** | 1.56 (0.95–2.54) | 2.08 (1.00–4.33) | 2.57 (1.17–5.65) * | 0.94 (0.83–1.06) | 0.77 (0.52–1.16) |
| 6–18 | 0.95 (0.90–1.01) | 0.77 (0.63–0.95) * | - | - | 0.84 (0.59–1.18) | 0.75 (0.26–2.20) | 1.29 (1.01–1.64) * | 1.50 (1.06–2.13) * | 0.83 (0.54–1.28) | 0.72 (0.45–1.15) | 1.09 (1.02–1.15)** | 0.86 (0.70–1.07) |
| 19–64 | 1.01 (1.00–1.03) * | 1.04 (0.99–1.08) | 1.00 (1.00–1.01) | 1.01 (0.99–1.03) | 0.83 (0.69–0.99) * | 0.80 (0.66–0.97)* | 1.08 (1.02–1.14) ** | 1.10 (1.02–1.17) ** | 0.98 (0.95–1.01) | 1.05 (0.95–1.16) | 0.99 (0.96–1.02) | 0.97 (0.92–1.03) |
| ≥65 | 1.04 (1.00–1.07) | 1.04 (0.96–1.12) | 1.01 (1.00–1.01) | 1.02 (0.99–1.05) | 1.18 (0.86–1.61) | 1.20 (0.79–1.82) | 1.06 (0.96–1.17) | 1.10 (0.98–1.24) | 0.93 (0.87–1.00) | 1.14 (0.93–1.38) | 0.96 (0.90–1.03) | 0.97 (0.89–1.05) |
| Season | ||||||||||||
| Warm (April to September) | 0.98 (0.95–1.01) | 1.03 (0.92–1.15) | 1.03 (0.99–1.06) | 1.05 (1.00–1.11) * | 1.19 (1.01–1.4)* | 1.75 (1.01–3.03) * | 0.98 (0.94–1.01) | 1.03 (0.92–1.15) | 0.95 (0.89–1.02) | 0.84 (0.62–1.14) | 0.98 (0.95–1.02) | 1.02 (0.95–1.11) |
| Cold (October to March) | 1.01 (1.00–1.02) ** | 1.03 (0.99–1.07) | 1.00 (1.00–1.01) * | 1.02 (1.00–1.04) * | 1.07 (1.02–1.12) *** | 0.87 (0.74–1.02) | 1.09 (1.02–1.17) * | 1.16 (1.07–1.26) ** | 1.05 (1.01–1.09) | 0.91 (0.81–1.01) | 0.92 (0.86–0.98) ** | 0.87 (0.81–0.93)** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.; Gui, S.-Y.; Qiao, J.-C.; Wang, X.-C.; Yang, F.; Tao, F.-B.; Yi, X.-L.; Jiang, Z.-X. Association between Air Pollution Exposure and Daily Outpatient Visits for Dry Eye Disease: A Time-Series Study in Urumqi, China. Atmosphere 2023, 14, 90. https://doi.org/10.3390/atmos14010090
Liang K, Gui S-Y, Qiao J-C, Wang X-C, Yang F, Tao F-B, Yi X-L, Jiang Z-X. Association between Air Pollution Exposure and Daily Outpatient Visits for Dry Eye Disease: A Time-Series Study in Urumqi, China. Atmosphere. 2023; 14(1):90. https://doi.org/10.3390/atmos14010090
Chicago/Turabian StyleLiang, Kun, Si-Yu Gui, Jian-Chao Qiao, Xin-Chen Wang, Fan Yang, Fang-Biao Tao, Xiang-Long Yi, and Zheng-Xuan Jiang. 2023. "Association between Air Pollution Exposure and Daily Outpatient Visits for Dry Eye Disease: A Time-Series Study in Urumqi, China" Atmosphere 14, no. 1: 90. https://doi.org/10.3390/atmos14010090
APA StyleLiang, K., Gui, S.-Y., Qiao, J.-C., Wang, X.-C., Yang, F., Tao, F.-B., Yi, X.-L., & Jiang, Z.-X. (2023). Association between Air Pollution Exposure and Daily Outpatient Visits for Dry Eye Disease: A Time-Series Study in Urumqi, China. Atmosphere, 14(1), 90. https://doi.org/10.3390/atmos14010090






