Abstract
Atmospheric air quality is a crucial factor in the health of human populations. Suspended particulate matter (SPM) is one of the most dangerous components polluting urban air. The aim of the present article is to study the effect of model suspensions (MS) of SPM that are characteristic of the composition of atmospheric air at locations with various anthropogenic loads on redox processes in alveolar macrophages (AM). Atmospheric air sampling was carried out in the breathing zone according to the method developed by one of the authors. AM were isolated from bronchoalveolar lavage fluid of experimental animals. The MS of SPM were prepared in accordance with the actual air pollution: MS No. 1 corresponded to an area with a low man-made load, and MS No. 2 corresponded to an area with a high man-made load. Load tests with model suspensions were carried out for 2 days. Parameters of oxidant processes and antioxidant system (AOS) were determined in cells and culture media. The proportion of the influence of the qualitative and dispersed composition of MS and the indicator of intra-system tension were calculated based on correlation dependencies. The atmospheric air with a high man-made load was dominated by particles up to 10 µm, whereas air with an insignificant man-made load contained SPM of more than 10 µm in size. Unidirectional changes were observed due to an exposure to both model suspensions, but the most pronounced oxidative modifications of lipids, proteins and genetic structures were caused by the exposure to MS No. 2. When exposed to MS No. 1, the AOS maintained the redox balance at the physiological level, localizing the resulting destruction inside the cells. MS No. 2 caused the redox balance to shift towards oxidants, potentiating the generalization of the destruction process. An increase in the content or a longer stress-inducing effect of PM2.5 causes a depletion in the reserve capacity of the AOS and the transition of destruction processes to the systemic level, which contributes to the development of the preconditions for environmentally dependent pathology.
1. Introduction
Atmospheric air pollution is a global (worldwide) environmental threat to public health [1,2]. The International Burden of Disease study shows that atmospheric air pollution was the sixth and the total impact of indoor and outdoor air pollution was the third largest risk factor for global mortality in 2019. According to this study, 4.5 million people died prematurely, which means that atmospheric air pollution caused 7.8% of deaths around the world [3]. The connection of solid particulate matter (SPM) with the development of respiratory diseases (bronchial asthma, chronic obstructive pulmonary disease, lung cancer), as well as nervous and cardiovascular systems diseases, is well established [1,2,4,5,6,7,8,9]. SPM exposure also increases the risk of developing acute cerebrovascular strokes. Diseases of the respiratory and cardiovascular systems are characterized by a heavier course, a significant number of complications, and a decrease in the quality of life [10]. Epidemiological studies have identified an association between exposure to polluted air and respiratory viral infections [11]. A possible SPM effect on the development of the gastrointestinal tract cancer has also been reported [12]. Research on the harmful effects of PM2.5 on kidney function is of considerable interest [13]. The elderly, children and patients with pre-existing respiratory and cardiovascular diseases are most susceptible to the adverse effects of air pollutants.
Transported by air over great distances, fine particles, due to their physical characteristics, contribute to atmospheric pollution far from the source of formation [14]. The risk of adverse effects of suspended solids on the human body is determined by the combination of their characteristics. Studies have found a change in the magnitude of the effects on public health according to location and time of year. The shape, elemental composition, mass concentration and dimensions of particles polluting the atmospheric air vary widely in time and space. Particles of SPM adsorb toxic substances on their surface that enter directly into the bronchopulmonary system together with inhaled particles.
SPM particles that enter the respiratory organs can be deposited on the mucous membrane in the nasal cavity, larynx, trachea, and bronchi. Particles less than 2.5 µm in diameter (PM2.5) are able to penetrate deeply and precipitate in the respiratory compartments [15,16]. PM2.5 are the most dangerous particles for humans as they include particles in the nano-dispersed range which have special physicochemical and biological properties. Nanoparticles deposited in the respiratory tract are able to cross the cell membrane, alveolar septum, penetrate into the systemic circulation and into the brain.
The respiratory system is most susceptible to the penetration of particles suspended in the air. Structural and functional features of the respiratory tract provide the largest area of contact with atmospheric air; alveolar macrophages (AM) localized there are the first cells to contact microparticles, performing a protective function [17]. AM are responsible for the recognition and primary processing of inhaled atmospheric air pathogens, their isolation by phagocytosis, the neutralization of absorbed substances and subsequent removal from the lung tissue. If AM are constantly phagocytosing SPM for long periods of time at high pollution levels, they may be unable to multiply and replenish, which reduces the ability of the lungs to eliminate invading pathogens.
The earliest pathophysiological mechanism developing in response to exposure to polluted atmospheric air is oxidative stress [18,19]. A decrease in the level of antioxidants due to an increased need for them and an increase in the formation of endogenous reactive oxygen species (ROS) lead to a further aggravation of the imbalance in the peroxidation-antioxidant defense system. The activity of the intracellular system and the ability to restore physiological levels of ROS and damaged cellular structures in the extremely unfavorable redox conditions are important factors contributing to the mechanisms of antioxidant protection against the pathological effects of SPM. The thiol-disulfide unit of the antioxidant system plays an important role in cell protection, performing an antioxidant function, restoring the structure of proteins, acting as a key factor in DNA repair, regulating cell signaling, and interacting with transcription factors [20].
Since the composition of air pollutants varies for different regions, it is necessary to study the impact of SPM in a specific territory. Studying the mechanisms of the impact of atmospheric microparticles in an urbanized area under different anthropogenic loads and assessing the response, both at the level of the whole organism and at the cellular-molecular level, are important research problems. Current studies do not consider the complex response of the main homeostatic systems of the body, the investigation of which could clarify the more subtle mechanisms responsible for disruption to the redox processes in the AM of the respiratory tract that occur in the urbanized areas differing in levels of man-made pollution. There is no data on how the thiol-disulfide unit of the AM antioxidant system responds to the effect of SPM with various levels of dispersity. There is no published data concerning the combined effect of the dispersed composition of SPM on the parameters of the peroxidation-antioxidant protection system of alveolar macrophages.
Therefore, the study of the mechanisms of the impact of atmospheric microparticles that pollute the surface layer of atmospheric air in urbanized areas with various levels of man-made load, and the assessment of the response to pollution at the cellular and molecular level are important research problems.
The purpose of the present research was to study changes in redox processes in alveolar macrophages occurring under the impact of model SPM suspensions of various compositions.
2. Materials and Methods
Sampling of the air from the atmospheric surface layer was carried out in two areas of Vladivostok, different in terms of the man-made SPM load, for the period from 2013 to 2016. The site with a high man-made load was located in the continental part of Vladivostok, the site with a minor load was in the island part of the city (Russian Island). We collected an average of six samples per month. The sampling method enabled us to determine the entire dispersed composition of SPM (particle-size distribution) in the respiratory zone directly affecting the human organism [21]. The collection of samples was carried out at a height of 1.5 m above the ground with the aim to determine the concentration of small-sized SPM in the atmospheric surface layer. The air samples were collected into a liquid absorbing medium (highly purified water) using a PU-4E electric aspirator (Khimko, Russia) and high-speed Richter’s absorber at a rate of 10 L/min. The particle size analysis of SPM was conducted by laser granulometry (Analysette 22 NanoTec laser analyzer, Fritsch, Germany) immediately after sampling. The data were expressed as mass fractions. At the same time, the samples were collected on the filters (AFA-VP-20, Russia), which were weighted using electronic scales (Shimadzu, Japan). The total mass concentration of suspended particles per unit of air volume (mg/m3) was calculated, and then the mass concentration of fractions (µg/m3) in each sample was calculated.
The averaged data of six consecutive sampling cycles of 30 min (the time of one cycle corresponds to the time of sampling for calculating the maximum allowable concentration) with an interval between cycles of 5–10 min were considered as one air sample. The meteorological parameters (wind speed, wind direction, air temperature, atmospheric pressure, weather conditions and the state of the underlying soil surface) were recorded at the beginning of each cycle.
The assessment of the level of contamination and the dispersed composition of SPM was carried out in the following ranges of particle dimensions: 0–0.1; 0.1–1.0; 1.0–2.5; 2.5–10; 10–100; and 100–2000 µm. Model suspensions prepared according to the results of a preliminary study of the qualitative composition and dispersed composition of suspended microparticles in the air of Vladivostok were provided by the Nanotechnology Scientific and Educational Center at the Engineering School of the Far Eastern Federal University.
In model suspension (MS) No. 1, 78% of the particles were larger than 10 µm (PM > 10) and 22% particles had a size of less than 10 µm (PM10); the proportion of PM2.5 particles was 5% and the proportion of 2.5–10 µm coarse particles was 17%. The qualitative composition of MS No. 1 was as follows: 10% soot and ash particles, 70% minerals, 10% metal particles, and 5% each of particles of synthetic and organic origin. MS No. 1 corresponds to an area with a minor man-made load.
In the MS No. 2, 70% particles were smaller than 10 µm; finely dispersed particles up to 2.5 µm accounted for 34%, and 36% of particles were 2.5–10 µm in size. The share of particles with PM > 10 was 30%. The qualitative composition of MS No. 2 was as follows: 40% soot and ash particles, 40% minerals, 10% metal particles, and 5% each of synthetic and organic particles. MS No. 2 is characteristic of an area with a high man-made load [22].
The study was approved by the Ethics Committee of the Vladivostok branch of the Federal State Budgetary Science Institution “Far Eastern Scientific Center of Physiology and Pathology of Respiration”—Institute of Medical Climatology and Rehabilitative Treatment (protocol No. 08-05/22-11-2016c) and was conducted in accordance with the Europe Convention ETS N 123 for the Protection of Vertebrate Animals used for Experiments or for other Scientific Purposes, Directive 2010/63/EU of the European Parliament and the Council of the European Union of 22 September 2010 on the protection of animals used for scientific purposes, and Recommendation 2007/526/EC. The study used 17 male Wistar white rats, 6 months old, weighing 180–240 g. The animals were euthanized by intramuscular injection of the drug. Prior to autopsy, the surgical site of the animals was treated with a 70% alcohol solution. The skin was dissected with a scalpel, then the chest was opened along the sternum. The trachea was separated from the underlying tissues with scissors and was cut while holding it with a clamp, then a cannula was inserted. Bronchoalveolar lavage (BAL) [23] was performed using DMEM medium containing veal serum, 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 2.5 µg/mL of fungizone and heated to 37 °C. The space of the respiratory tract and alveoli was washed using 15 mL of the medium. The medium was slowly passed through the trachea using a syringe, while the area behind the sternum was simultaneously massaged. The resulting lavage fluid from each animal was collected in a separate sterile tube and stored on ice until further processing.
The cell suspension obtained from BAL was layered on a Percoll solution (with a density of 1.078 g/mL) and centrifuged. Then the macrophage ring at the phase interface was collected. The cells were counted in a Goryaev chamber, and the concentration was brought to 100,000 cells per 1 mL. The AM cultures obtained from each animal were divided into three groups and placed in the wells of tablets. Cultivation was carried out in a DMEM medium containing veal serum, antibiotics (penicillin at a dose of 50 units/mL and streptomycin at a dose of 50 µg/mL) and antimycotic (fungizone at a dose of 2.5 µg/mL), at a temperature of +37 °C, in an incubator where 5% carbon dioxide level was maintained, for 2 days. Before loading, the dry MS samples were disinfected by ultraviolet irradiation, and then 1 µg of the corresponding model suspension was dispersed in 1 mL of DMEM medium with veal serum heated to +37 °C. The resulting suspensions were added to wells with cell culture at a dose of 1 mL of suspension per well (100,000 cells) on the third day of cultivation. The load dose was 1 µg/100 thousand cells. MS No. 1 was added to the culture medium of the Group 2, and MS No. 2 was added to the culture medium of Group 3, and Group 1 was left unaltered as the control group. Load tests were carried out for 2 days [24]. The viability of AM was evaluated before and after the load by staining with trypan blue [25], the proportion of living cells in both experimental groups after the load was at least 85%.
The redox homeostasis parameters of the cells were identified in the AM culture and in their cultivation medium. The content of lipid hydroperoxides (LOOH, cond. units) was determined in heptane-isopropanol extracts [26,27]. Levels of malondialdehyde (MDA, mmol/L), 8-hydroxy-2’-deoxyguanosine (8-OHDG, ng/mL), thioredoxin (ng/mL), carbonyl protein (PC, nmol/mg) (Northwest Life Science Specialties, LLC, USA), total antioxidant activity (AOA, mmol/L) (Randox Laboratories Ltd., UK), and glutathione (total, oxidized, and reduced, mmol/L) (ArborAssays, USA) were determined according to the relevant instruction manuals.
Statistical processing of the data was performed using the Statistica 8.0 analytics software package. The results of non-parametric descriptive statistics are presented as medians (Me) and quantiles (25% and 75% quantiles). The Mann–Whitney U-test was used to compare the obtained data. The critical significance level was assumed to be 0.05.
The software module providing the selection of paired correlations (Spearman) r was used for calculating interactions at p < 0.05. Correlations r with statistical significance (p < 0.05) were introduced into the calculation of the Dv indicator, whose actual value was summed up and then correlated to the estimated maximum sum of correlations at R = 1.0, as in Equation (1):
where Dv is the indicator showing the intra-system tension (in %), n is the number of correlations (p < 0.05), r is the actual correlation relationship (p < 0.05), N is the maximum number of assumed correlation cells in the matrix, and R is the maximum correlation relationship (equal to 1.0). The Dv parameter indicates the degree of functional stress of the peroxidation–antioxidant protection system under the impact of the model suspensions.
3. Results
The contents of SPM of the air in the atmospheric surface layer in the districts of Vladivostok [28] with varying degrees of man-made load is shown in Figure 1.
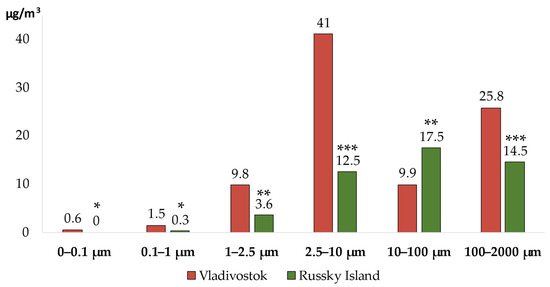
Figure 1.
The level of SPM pollution of the air in the atmospheric surface layer in the districts of Vladivostok with varying degrees of man-made load. Statistical significance relative to Vladivostok: *—p < 0.05; **—p < 0.01; ***—p < 0.001.
It was revealed that the concentration of ultrafine particles (0–0.1 µm) in the area with a significant man-made load was 0.6 (0; 5.5) µg/m3 and that of 0.1–1 µm particles was 1.5 (0; 6.7) µg/m3. In Vladivostok, fine particles ranging from 1 to 2.5 µm were detected at a concentration of 9.8 (3.8; 12.7) µg/m3, and coarse particles from 2.5 to 10 µm were measured at a concentration of 41.0 (21.3; 67.4) µg/m3. The content of particles ranged from 10 to 100 µm (PM > 10) was at the level of 9.9 (0.4; 15.8) µg/m3, with the size from 100 to 2000 µm–at the level of 25.8 (0; 39.2) µg/m3.
Ultrafine particles were not detected in the area with insignificant man-made load. The concentration of particles 0.1–1 µm in size was 0.3 (0; 2.5) µg/m3. The content of fine particles ranged from 1 to 2.5 µm was 3.6 (0.5; 6.0) µg/m3, and particles from 2.5 to 10 µm were detected at 12.5 (2.3; 23.5) µg/m3. On Russian Island, particles with a size from 10 to 100 µm (PM > 10) were detected at a concentration of 17.5 (5.1; 38.9) µg/m3, and particles ranged from 100 to 2000 µm (PM > 10) were detected at a concentration of 14.5 (0; 27.2) µg/m3.
The prooxidant-antioxidant processes in the AM culture (Wistar rats) under the impact of model particle suspensions are shown in Figure 2 and Figure 3. Changes in the content of LOOH and MDA in the cell cultures characterized the processes of lipid peroxidation (LPO) of AM intracellular structures (Figure 2).
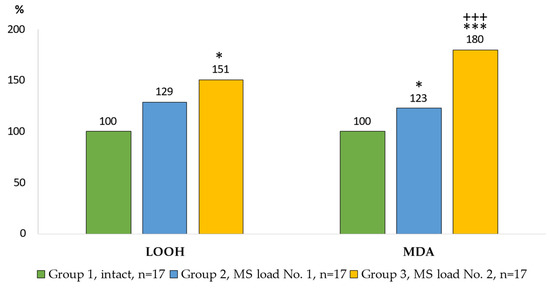
Figure 2.
Indicators of lipid peroxidation in the AM culture. Notes: statistical significance relative to Group 1: *—p < 0.05; ***—p < 0.001; relative to Group 2: +++—p < 0.001.
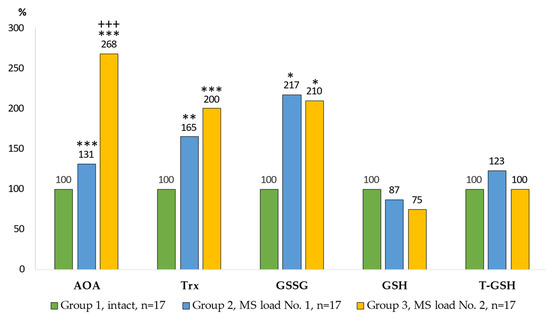
Figure 3.
Indicators of the antioxidant system in the AM culture. Notes: statistical significance relative to Group 1: *—p < 0.05; **—p < 0.01; ***—p < 0.001; relative to Group 2: +++—p < 0.001.
The level of LOOH in the Group 2 did not differ from the control group. The content of LOOH in the Group 3 was 51% higher than in the control group. The greatest increase in the level of MDA production in comparison with the control group was noted in the Group 3 of AM (80%), and it was 46% higher (p = 0.003) compared with the Group 2.
The characteristics of the antioxidant processes and reparative mechanisms are shown in Figure 3.
An integral indicator was used to assess the activity of AOS. Statistically significant differences in total AOA between all groups were observed in the cell cultures. The highest total AOA was noted in the Group 3 where the indicator was increased 2.7 times (p = 0.0001) compared with the control group and 2.1 times (p = 0.0002) compared with the Group 2. The content of the main components of the thiol-disulfide unit of AOS was characterized by a twofold increase in oxidized glutathione in the AM culture of the Group 2 and Group 3. No statistically significant differences in the content of reduced and total glutathione were detected. The content of thioredoxin in the cell cultures differed between the control and experimental groups: it increased by 65% (p = 0.0021) for the Group 2 and doubled (p = 0.0006) for the Group 3.
The prooxidant-antioxidant processes in the culture medium of AM (Wistar rats) exposed to model particle suspensions are shown in Figure 4 and Figure 5.
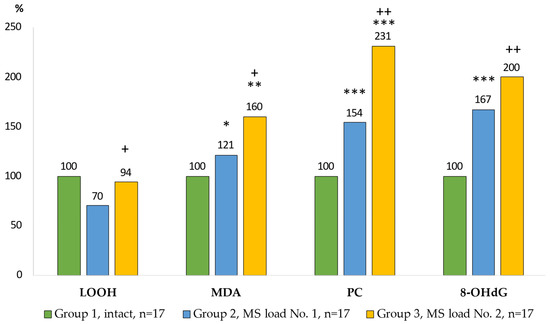
Figure 4.
Indicators of peroxidation in the culture medium of AM. Notes: statistical significance relative to Group 1: *—p < 0.05; **—p < 0.01; ***—p < 0.001; relative to Group 2: +—p < 0.05; ++—p < 0.01.
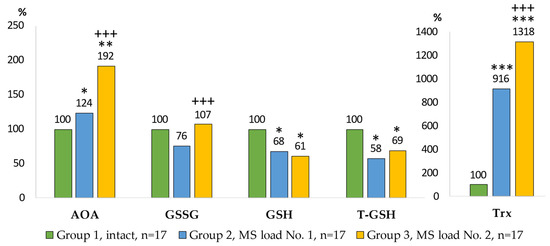
Figure 5.
Indicators of the antioxidant system in the AM culture medium. Notes: statistical significance relative to Group 1: *—p < 0.05; **—p < 0.01; ***—p < 0.001; relative to Group 2: +++—p < 0.001.
Due to exposure to MS No. 2, the level of LOOH was increased by more than a third compared to the Group 2. The content of MDA in the Group 3 was 33% higher compared with the Group 2. The largest increase in protein carbonyl (PC) levels (by 2.3 times) was found in the Group 3 (p = 0.0001). The content of 8-OHdG compared with the control group was 67% higher (p = 0.0001) in the Group 2 of AM, and in the Group 3, the content of 8-OHdG increased two-fold (p = 0.0001) compared with the control group and by 20% (p = 0.0001) compared with the Group 2.
In the AM culture medium, the greatest increase in total AOA was observed in the Group 3, and was higher by 92% (p = 0.0002) compared with the control group, and by 54% (p = 0.0066) compared with the Group 2 (Figure 5).
The content of oxidized glutathione was not statistically significant different from the control group. The level of reduced glutathione in the Group 2 was 32% lower (p = 0.034) and 39% lower (p = 0.0495) in the Group 3 compared with the control. The level of total glutathione in the Group 2 was reduced by 42% (p = 0.0495) and in the Group 3 by 31% (p = 0.0495) compared with the control. The content of thioredoxin in the Group 2 was 9 times (p = 0.00004) higher than in the Group 1. In the Group 3, the level of thioredoxin was 13 times (p = 0.00002) higher than in the control group, and 44% (p = 0.0045) higher than that in the Group 2.
The relationship between the prooxidant-antioxidant homeostasis indicators of alveolar macrophages and the characteristics of model particle suspensions is shown in Figure 6 and Figure 7. We have analyzed the dependence of the redox homeostasis of the cell on the qualitative and dispersed composition of the suspensions. To determine the contribution of each group of factors to the cell response, their specific weight was calculated (Figure 6).
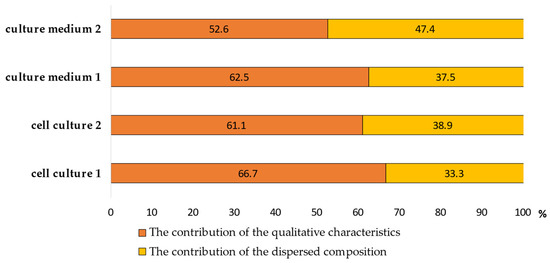
Figure 6.
The proportion of the contribution of qualitative and dispersed composition to the cell response.
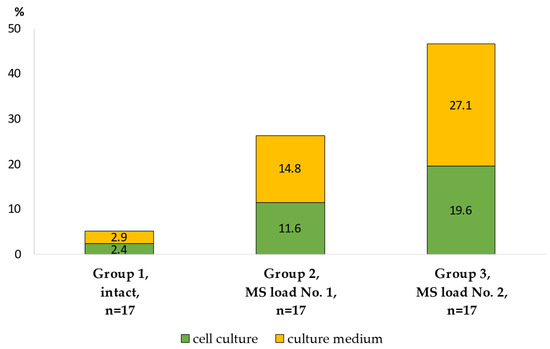
Figure 7.
Integral response (Dv, %, p < 0.05) of the redox homeostasis parameters of alveolar macrophages to the model suspensions exposure.
The contribution of the dispersed composition of MS No. 1 was 33.3% in the cell culture and 37.5% in the culture medium, and when exposed to MS No. 2 it was 38.9% in the cell culture and 47.4% in the culture medium. The contribution of the qualitative characteristics of SPM when exposed to MS No. 1 was 66.7% in the cell culture and 62.5% in the culture medium, and when exposed to MS No. 2: it was 61.1% in the cell culture, and 52.6% in the culture medium.
The integral intra-system response (Dv, p < 0.05) of the redox homeostasis parameters in the experimental AM groups is shown in Figure 7.
The power of integrated intra-system connections in the cell culture of the Group 2 was 4.8 times higher than that in the intact group, while in the cell culture of the Group 3 it was 8.2 times higher than in the control group, and 1.7 times higher than in the Group 2. The culture medium of the Group 3 reacted the most: the indicator was 9.3 times higher than in the control group, and 1.8 times higher than in the Group 2.
4. Discussion
The results obtained in the atmospheric air pollution assessment for the districts of Vladivostok indicate differences in the content of SPM fractions depending on the man-made load. The atmospheric air of an urbanized area with a high man-made load is dominated by particles in the micro-dimensional range, which have the most pathogenic effect on the human body. Nano-dispersed suspended particles were detected only in an area with a significant man-made load, which is due to the presence of a constant source of their generation. The area with a negligible man-made load is characterized by the predominance of a coarse fraction of particles with dimensions of more than 10 µm.
Exposure to fine and ultrafine particles causes ROS-mediated oxidative stress. The main free radical in the lungs is hydrogen peroxide. SPM interaction with hydrogen peroxide results in the formation of highly reactive hydroxyl radicals via the Fenton reaction. Additionally, the formation of hydroxyl radicals via the Haber–Weiss reaction is caused by superoxide induced by particles with a size of less than 2.5 µm. Along with this, the alveolar macrophages themselves generate oxidants during the phagocytosis of fine particles, caused by the leakage of superoxide during the destruction of the mitochondrial matrix. Thus, the increase in peroxidation products is due to the fact that SPM up to 1 µm and with a size from 1 to 2.5 µm increased the amount of ROS and free radicals to the greatest extent and contributed to the intensification of oxidative reactions (Figure 8).
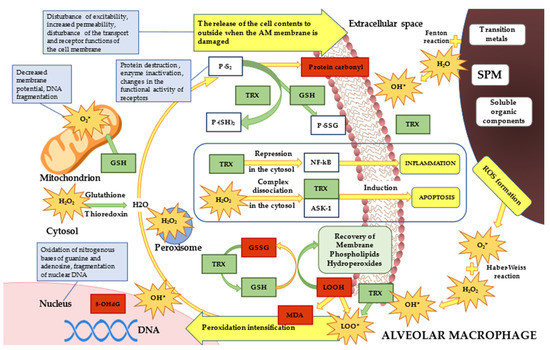
Figure 8.
Pathophysiological mechanisms of redox processes violation in AM of Wistar rats when exposed to SPM. Note: LOOH—lipid hydroperoxides, MDA—malonic dialdehyde, DNA—deoxyribonucleic acid, SPM—suspended particulate matter, TRX—thioredoxin, P-S2—protein in oxidized form, P-(SH)2—protein in reduced form, P-SSG—protein in glutathionylated form, ASK-1—apoptosis-regulating kinase-1, NF-kB—nuclear factor kappaB, H2O2—hydrogen peroxide, GSH—glutathione in oxidized form, GSSG—glutathione in reduced form, 8-OHdG—8-hydroxy-2’-deoxyguanosine, LOO*—lipoperoxyl radical, O2*—superoxide anion radical, HO*—hydroxyl radical.
The intracellular level of LOOH of the Group 2 did not differ from the control group since MS No. 1 contained 78% of particles larger than 10 µm. These particles were not subjected to phagocytosis due to their size. The proportion of absorbed particles did not cause statistically significant changes in the content of LOOH compared with the control group. At the same time, the content of LOOH in the Group 3 was increased by 51% compared with the control group, which indicated the increase in LPO due to the predominance of particles of the phagocytized fractions in MS No. 2. The formation of the final metabolites of LPO-MDA characterized the intensification of the oxidative process in the cells. The greatest increase in the level of MDA production (by 80%) was observed in the AM of the Group 3. These defining characteristics of the LOOH and MDA content in the AM culture indicate the increase in lipid peroxidation processes under the impact of both model SPM suspensions. However, the level of intensification of LPO was most pronounced under the impact of MS No. 2, which is identical in terms of microparticles content to the atmospheric air of an area with a high man-made load.
To maintain the redox balance and structural integrity of the cells during formation of a large number of peroxidation metabolites and highly reactive compounds constantly produced under the impact of micro-sized SPM, a compensatory enhancement of antioxidant processes was activated, as well as reparative mechanisms. The presence of particles of fine fractions of both model suspensions in the cells increased the response from the AOS, as evidenced by the increase in the total AOA. The presence of even a small fraction (5%) of particles up to 2.5 µm in MS No. 1 activated the cells.
The content of the main components of the thiol-disulfide unit of AOS was characterized by the twofold increase in oxidized glutathione in the AM culture of the Group 2 and Group 3 due to enhanced detoxification of an excessive amount of hydroperoxides. No statistically significant differences in the content of reduced and total glutathione were detected, which indicates the ability of macrophages to maintain the redox potential of cells at a stationary level due to partial reduction in oxidized glutathione. The main line of action of glutathione AOS is associated with the protection of lipids and membrane proteins against oxidation and destruction [29]. Being the most abundant antioxidant, glutathione directly neutralizes ROS formed due to exposure to microparticles of atmospheric air and interrupts the chain reaction of lipid oxidation, thereby restraining the stress-induced avalanche accumulation of peroxidation products and ensuring the restoration of membrane phospholipid hydroperoxides. Glutathione plays a major role in maintaining the level of mitochondrial membrane potential, which ensures the normal energy state of cells [30]. Glutathione affects the functions of redox-sensitive proteins by reversible S-glutathionylation, causing structural and functional changes and, therefore, protecting them from destruction [31].
The content of thioredoxin in cell culture differs between the control group and the groups loaded with model suspensions. The greatest increase in the level of thioredoxin in AM of the Group 3 indirectly indicates the increase in oxidative reactions associated with the phagocytosis of fine particles. The reduction in the level of oxidative damage to proteins, including oxidized glutathione, due to the restoration of disulfide bonds largely depends on the activity of thioredoxin AOS [29,32]. Thioredoxin restores key enzymes necessary for DNA synthesis and provides protection to the DNA structure by maintaining the redox potential of cells and transmitting a signal to the rest of the AOS participants, and also is engaged in the repair of DNA molecule damage [33]. Thioredoxin binds to signaling proteins and controls their activity and functioning. The Trx complex with apoptosis-signaling kinase-1 (Apoptosis-regulating Kinase 1–ASK-1) inhibits its activity (Figure 8). Oxidative stress leads to the physical dissociation of this complex and, consequently, to the activation of ASK-1, stimulating an apoptotic signaling cascade leading to controllable cell death. Cytosolic thioredoxin activates the transcription factor NF-kB, which regulates the cell cycle, apoptosis, and immune response [34].
A portion of the cells that phagocytized a large number of fine particles collapsed, which contributed to the release of the contents of the cell into the culture medium. When exposed to MS No. 2, this caused an increase in the level of both LOOH and MDA in the cultivation medium of the Group 3 by more than a third compared with the Group 2. Along with lipids, protein structures were also involved in the oxidation process, which caused an irreversible biodegradation of molecules and their loss of functional activity. Under the influence of ROS, free carboxyl groups of proteins relatively early transformed into carbonyl groups forming a stable compound, protein carbonyl (PC). The greatest increase in the content of PC (2.3 times) was found in the culture medium of the Group 3.
The oxidized nitrogenous bases of nucleic acids were also detected in the culture medium. Their formation was caused by both the LPO-MDA products and the very ROS. The formation of 8-OHdG indicates a further increase in oxidative stress and damage to genetic structures. The significant increase in the level of this indicator was observed when exposed to MS No. 2, which contains a greater proportion of fine particle fractions.
The increase in the content of lipid, protein, and DNA peroxidation products led to a significant compensatory increase in total AOA. Evaluation of the parameters of the glutathione unit of AOS in the AM culture medium showed that the content of oxidized glutathione was not significantly different from that of the control group, since its exocytosis by macrophages did not occur. The reverse pattern was observed for the level of reduced glutathione. The decrease in the content of reduced and total glutathione in the culture medium of the Group 2 and Group 3 may be associated with decreased exocytosis of reduced glutathione, since this compound is more necessary for implementing antioxidant protection of the intracellular space [31]. The decrease in the levels of total and reduced glutathione and the increase in the level of oxidized glutathione resulted from the increase in the LOOH concentration when exposed to fine fractions of SPM.
The maximum response of the AOS parameters in the culture medium was observed for the thioredoxin unit. This is due to increased protein production in response to the pro-oxidant action of fine fraction particles, the ones most represented in MS No. 2. These particles coincided in size with the bacterial cell and were more often phagocytized by AM, which caused their greater pathogenicity. AM actively synthesized and released thioredoxin via exocytosis in response to the impact. Having chemotactic activity, thioredoxin attracted cells to particle clusters that need to be disposed of. As a result, an increase in the synthesis of thioredoxin involved in macrophage chemotaxis and inactivating the start of apoptosis in AM was observed.
Recent studies indicate differences in the pathogenicity of SPM, determined by the condition of the air environment in different regions. One of the most important factors in this phenomenon is the dispersion of microparticles. The analysis of correlations between the AM response indicators and the characteristics of MS shows the dependence of the redox homeostasis of the cell on both the qualitative and dispersed composition of SPM. The qualitative characteristics of SPM made a greater contribution, both for the cell culture and the culture medium, when exposed to both MS. The proportion of these factors ranged from 52.6 to 66.7%. However, the proportion of the contribution of the dispersed composition of SPM that is characteristic of atmospheric air pollution in the area with a high man-made load is higher than after exposure to MS No. 1, both for the cell culture (38.9 vs. 33.3%) and the AM culture medium (47.4 vs. 37.5%). Thus, the contribution of the dispersed composition increases with an increase in the proportion of fine particles in the model suspension.
The integral intra-system response (Dv, p < 0.05) of the redox homeostasis parameters in the experimental AM groups is characterized by the increase in intra-system tension compared with the control group, with the greatest stress observed as a result of exposure to MS No. 2 (Figure 8). The response from the culture medium was more pronounced than the AM response. Thus, the resulting intra-system response of cells to the impact of the model suspensions characterizes the functional stress of redox homeostasis. In addition, a more pronounced imbalance was developed in the cell culture exposed to MS No. 2, represented to a greater extent by particles of phagocytized fractions. The presence of a large number of fine-fraction particles not phagocytized by alveolar macrophages in the culture medium of the Group 3 leads to the significant increase in the functional tension of cells.
This study details the mechanisms of the response by the peroxidation-antioxidant protection system of AM to the impact of SPM. For the first time, our work shows that the increase in the proportion of finely dispersed fractions increases the influence of the fractional composition. A comparative analysis of the effect of SPM on cell culture and culture medium was carried out. The features of the integral effect of SPM on the alveolar macrophages are highlighted. The key components of the process responsible for the destruction and protection of alveolar macrophages under the influence of model suspensions, identical to the real pollution of the surface layer of atmospheric air, have been identified.
5. Conclusions
In this study, unidirectional changes were observed in the AM culture under the influence of both model suspensions of particles; however, the most pronounced oxidative modification of lipids, proteins and genetic structures of BAL macrophages of experimental animals was caused by exposure to MS No. 2, which was identical in particle composition to the atmospheric air of the region with a high man-made load. Under the influence of MS No. 1 which had a lower specific gravity of particles up to 2.5 µm in size (6.9 times lower compared with MS No. 2), AOS coped with highly reactive compounds and maintained the redox balance at the physiological level, localizing the resulting destruction within the cells. MS No. 2 caused a shift in the redox balance towards oxidants, potentiating the generalization of the destruction process with the release of products into the extracellular space. The irreversible damage to the cells as a result of exposure to fine fractions of SPM is evidenced by increased levels of protein carbonyl and 8-hydroxy-2’-deoxyguanosine in the culture medium. The most significant participant in thiol-disulfide-dependent antioxidant processes is thioredoxin, which plays a key multi-component role in the protection of cells from microtoxicants due to its reparative action and is involved in redox regulation, prevention of apoptosis, and chemotaxis of macrophages. An increase in the level of thioredoxin in response to an increase in the number of fine particles is necessary for the protection of cells under conditions of intensified oxidative stress.
An increase in the proportion of finely dispersed fractions also increases the influence of the dispersed composition of SPM on the response of the peroxidation-antioxidant protection system. Another aspect of the impact of finely dispersed fractions is a significant increase in the integral intra-system response (Dv) of the parameters of AM redox homeostasis. Further strengthening of these processes can cause the formation of an inflammatory reaction. Thus, an increase in the content or a longer stress-inducing effect of PM2.5 causes the depletion of the reserve capacity of the cell and the transition of destruction processes to the systemic level, which contributes to the development of the preconditions for environmentally dependent pathology.
Author Contributions
Conceptualization, L.S.B.; methodology, T.I.V., L.S.B. and L.V.V.; validation, L.S.B. and L.V.V.; formal analysis, T.A.G.; investigation, L.V.V., T.I.V., E.V.K. and T.A.G.; writing—original draft preparation, L.S.B.; writing—review and editing, E.V.K.; supervision, T.I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the Vladivostok branch of the Federal State Budgetary Science Institution “Far Eastern Scientific Center of Physiology and Pathology of Respiration”—Institute of Medical Climatology and Rehabilitative Treatment (protocol No. 08-05/22-11-2016c of approval).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data set available on request to corresponding authors.
Acknowledgments
The authors are grateful to K.S. Golokhvast, Department of Life Safety in the Technosphere, Scientific Advisor of the Nanotechnology Scientific and Educational Center at Engineering School of the Far Eastern Federal University for the model particle suspensions provided. The authors express their gratitude to N.E. Zyumchenko and N.P. Tokmakova (Department of Cell Biology and Genetics at the Natural Sciences School of the Far Eastern Federal University) for their invaluable assistance in conducting the experimental part of the study and discussing the results obtained.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, M.; Chandel, J.; Malik, J.; Naura, A.S. Particulate matter in COPD pathogenesis: An overview. Inflamm. Res. 2022, 71, 797–815. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Outdoor Air Pollution. 2019. Available online: https://ourworldindata.org/outdoor-air-pollution (accessed on 22 February 2022).
- Hansel, N.N.; Paulin, L.M.; Gassett, A.J.; Peng, R.D.; Alexis, N.; Fan, V.S.; Bleecker, E.; Bowler, R.; Comellas, A.P.; Dransfield, M.; et al. Design of the Subpopulations and Intermediate Outcome Measures in COPD (SPIROMICS) AIR Study. BMJ Open Respir. Res. 2017, 4, e000186. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Beelen, R.; Wang, M.; Hoek, G.; Andersen, Z.J.; Hoffmann, B.; Stafoggia, M.; Samoli, E.; Weinmayr, G.; Dimakopoulou, K.; et al. Particulate matter air pollution components and risk for lung cancer. Environ. Int. 2016, 87, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Villamizar, L.A.; Magico, A.; Osornio-Vargas, A.; Rowe, B.H. The effects of outdoor air pollution on the respiratory health of Canadian children: A systematic review of epidemiological studies. Can. Respir. J. 2015, 22, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, Q.; Liu, Z. Ambient particulate matter exposure and cardiovascular diseases: A focus on progenitor and stem cells. J. Cell Mol. Med. 2016, 20, 782–793. [Google Scholar] [CrossRef]
- Weichenthal, S.A.; Lavigne, E.; Evans, G.J.; Godri Pollitt, K.J.; Burnett, R.T. Fine Particulate Matter and Emergency Room Visits for Respiratory Illness. Effect Modification by Oxidative Potential. Am. J. Respir. Crit. Care Med. 2016, 194, 577–586. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roque, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Pinault, L.; Tjepkema, M.; Crouse, D.L.; Weichenthal, S.; van Donkelaar, A.; Martin, R.V.; Brauer, M.; Chen, H.; Burnett, R.T. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ. Health 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Pritchett, N.; Spangler, E.C.; Gray, G.M.; Livinski, A.A.; Sampson, J.N.; Dawsey, S.M.; Jones, R.R. Exposure to Outdoor Particulate Matter Air Pollution and Risk of Gastrointestinal Cancers in Adults: A Systematic Review and Meta-Analysis of Epidemiologic Evidence. Environ. Health Perspect. 2022, 130, 36001. [Google Scholar] [CrossRef]
- Rasking, L.; Vanbrabant, K.; Bove, H.; Plusquin, M.; De Vusser, K.; Roels, H.A.; Nawrot, T.S. Adverse Effects of fine particulate matter on human kidney functioning: A systematic review. Environ. Health 2022, 21, 24. [Google Scholar] [CrossRef]
- Baek, J.I.; Ban, Y.U. The Impacts of Urban Air Pollution Emission Density on Air Pollutant Concentration Based on a Panel Model. Sustainability 2020, 12, 8401. [Google Scholar] [CrossRef]
- Lakhdar, R.; Mumby, S.; Abubakar-Waziri, H.; Porter, A.; Adcock, I.M.; Chung, K.F. Lung tox-icity of particulates and gaseous pollutants using ex-vivo airway epithelial cell culture systems. Environ. Pollut. 2022, 305, 119323. [Google Scholar] [CrossRef]
- Pryor, J.T.; Cowley, L.O.; Simonds, S.E. The Physiological Effects of Air Pollution: Particulate Matter, Physiology and Disease. Front. Public Health 2022, 10, 882569. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Y.; Ma, L.; Liu, Y.; Qi, Y.; Liu, S. Ageing Significantly Alters the Physicochemical Properties and Associated Cytotoxicity Profiles of Ultrafine Particulate Matters towards Macrophages. Antioxidants 2022, 11, 754. [Google Scholar] [CrossRef]
- Chen, H.; Oliver, B.G.; Pant, A.; Olivera, A.; Poronnik, P.; Pollock, C.A.; Saad, S. Effects of air pollution on human health-Mechanistic evidence suggested by in vitro and in vivo modelling. Environ. Res. 2022, 212 Pt C, 113378. [Google Scholar] [CrossRef]
- Carraro, S.; Ferraro, V.A.; Zanconato, S. Impact of air pollution exposure on lung function and exhaled breath biomarkers in children and adolescents. J. Breath Res. 2022, 16, 044002. [Google Scholar] [CrossRef]
- Vitkina, T.I.; Barskova, L.S.; Zyumchenko, N.E.; Tokmakova, N.P.; Gvozdenko, T.A.; Golokhvast, K.S. Balance of glutathione-related processes in alveolar macrophages under exposure to suspended particulate matter of atmospheric air in of wistar rats. Hyg. Sanit. 2020, 99, 200–205. (In Russian) [Google Scholar] [CrossRef]
- Veremchuk, L.V.; Vitkina, T.I.; Barskova, L.S.; Gvozdenko, T.A.; Mineeva, E.E. Estimation of the size distribution of suspended particulate matters in the urban atmospheric surface layer and its influence on bronchopulmonary pathology. Atmosphere 2021, 12, 1010. [Google Scholar] [CrossRef]
- Golokhvast, K.S.; Panichev, A.M.; Gul’kov, A.N.; Chaika, V.V. Method of Preparation of Aerosol Standard Samples. Patent 2525427 RU, 10 August 2014. (In Russian). [Google Scholar]
- Tseluyko, S.S.; Zinoviev, S.V.; Ogorodnikova, T.L. Alveolar macrophages culture micromethod of–a new technique for bronchial asthma diagnostics. Bull. Physiol. Pathol. Respir. 2001, 9, 15–16. (In Russian) [Google Scholar]
- Vitkina, T.I.; Golokhvast, K.S.; Barskova, L.S.; Ziumchenko, N.E.; Tokmakova, N.P.; Gvozdenko, T.A. Methodological approaches to the experimental study of the effects of micro-dimensional air suspensions. Bull. Physiol. Pathol. Respir. 2019, 73, 80–86. (In Russian) [Google Scholar] [CrossRef]
- Pogorelov, V.M.; Medovyi, V.S.; Khazem, G.M.; Kozinets, G.I. Analysis of cell image. Klin. Lab. Diagn. 1995, 3, 40–43. (In Russian) [Google Scholar]
- Galaktionova, L.P.; Yelchaninova, S.A.; Molchanov, A.V.; Varshavskiy, B.Y. The state of peroxidation in patients with peptic ulcer of the stomach and 12 duodenal ulcer. Klin. Lab. Diagn. 1998, 10–14. (In Russian) [Google Scholar]
- Novgorodtseva, T.P.; Endakova, E.A.; Yankova, V.I. Manual Methods Studies Parameter Systems «Lipid Peroxidation-Antioxidant Protection» in Biological Liquid; Far Eastern University Publishment: Vladivostok, Russia, 2003; 80p. (In Russian) [Google Scholar]
- Barskova, L.S.; Vitkina, T.I.; Gvozdenko, T.A.; Veremchuk, L.V.; Golokhvast, K.S. Assessment of air pollution by small-sized suspended particulate matter in urbanized territories with various technogenic load (on the example of Vladivostok, Russia). Russ. Open Med. J. 2019, 8, 304. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]
- Golokhvast, K.; Vitkina, T.; Gvozdenko, T.; Kolosov, V.; Yankova, V.; Kondratieva, E.; Gorkavaya, A.; Nazarenko, A.; Chaika, V.; Romanova, T.; et al. Impact of Atmospheric Microparticles on the Development of Oxidative Stress in Healthy City/Industrial Seaport Residents. Oxidative Med. Cell. Longev. 2015, 2015, 412173. [Google Scholar] [CrossRef]
- Dominko, K.; Dikic, D. Glutathionylation: A regulatory role of glutathione in physiological processes. Arh. Hig. Rada Toksikol. 2018, 69, 1–24. [Google Scholar] [CrossRef]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Netto, L.E.; Antunes, F. The Roles of Peroxiredoxin and Thioredoxin in Hydrogen Peroxide Sensing and in Signal Transduction. Mol. Cells 2016, 39, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).