Insight into Source and Evolution of Oxalic Acid: Characterization of Particulate Organic Diacids in a Mega-City, Shanghai from 2008 to 2020
Abstract
:1. Introduction
2. Experimental
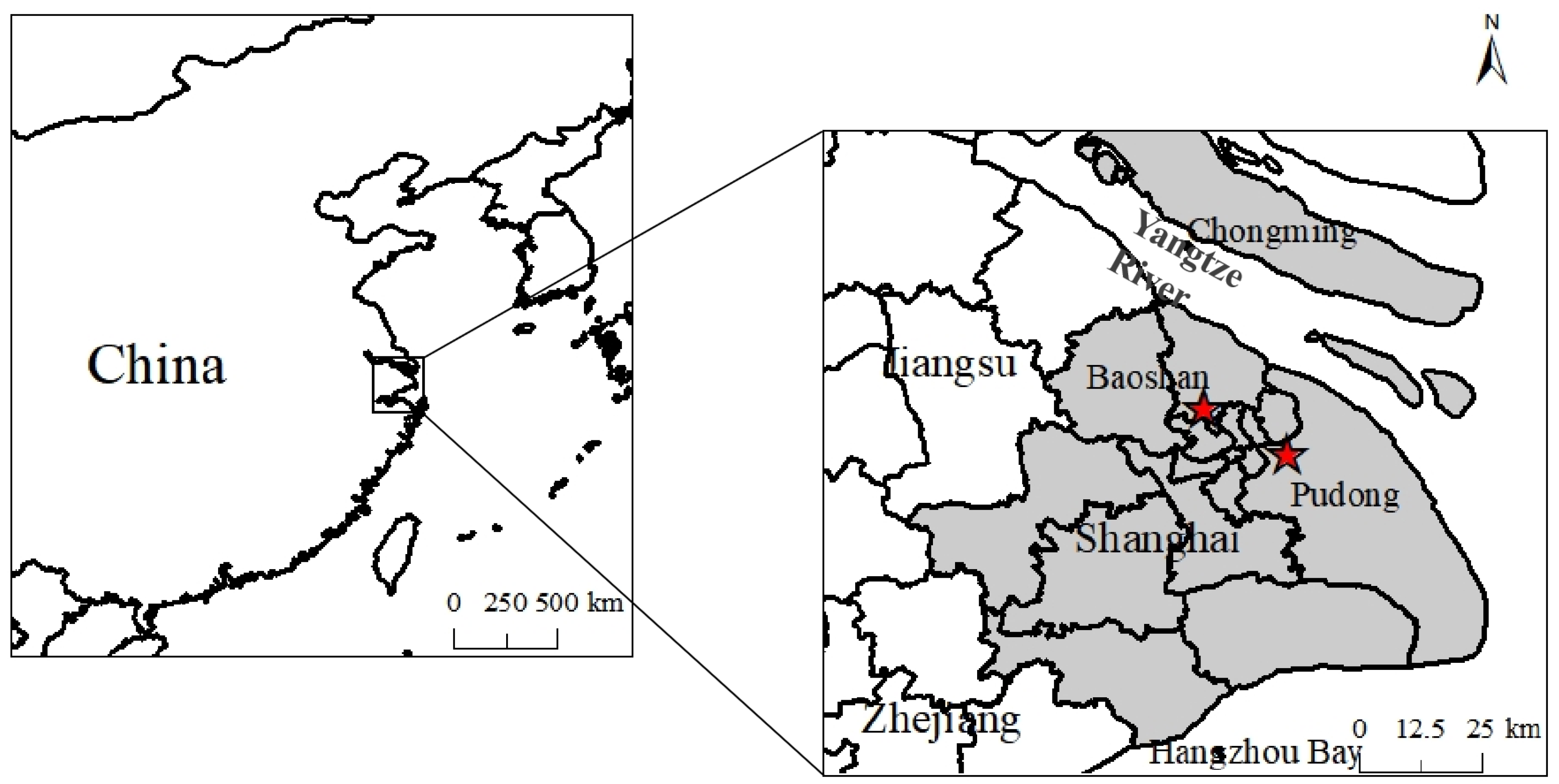
2.1. Sites and Sampling
2.2. Chemical Analysis
2.2.1. Comparison of Extraction Methods for Diacids
2.2.2. OCEC, WSOC and Ion Determination
2.2.3. Air Mass Back Trajectories and PSCF Analysis
3. Results and Discussion
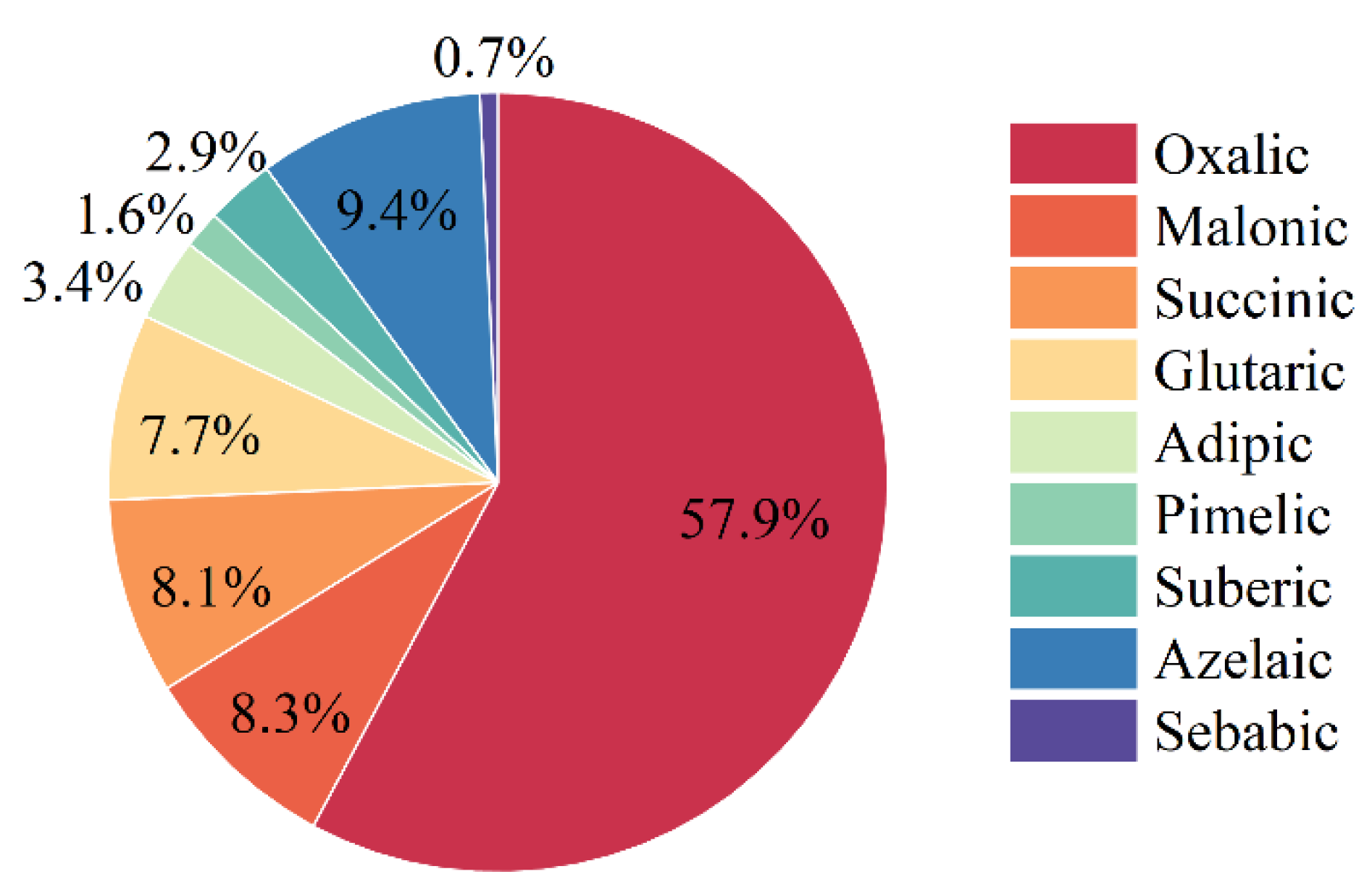
3.1. Characterization of DCA in Shanghai
3.2. Sources of Oxalate
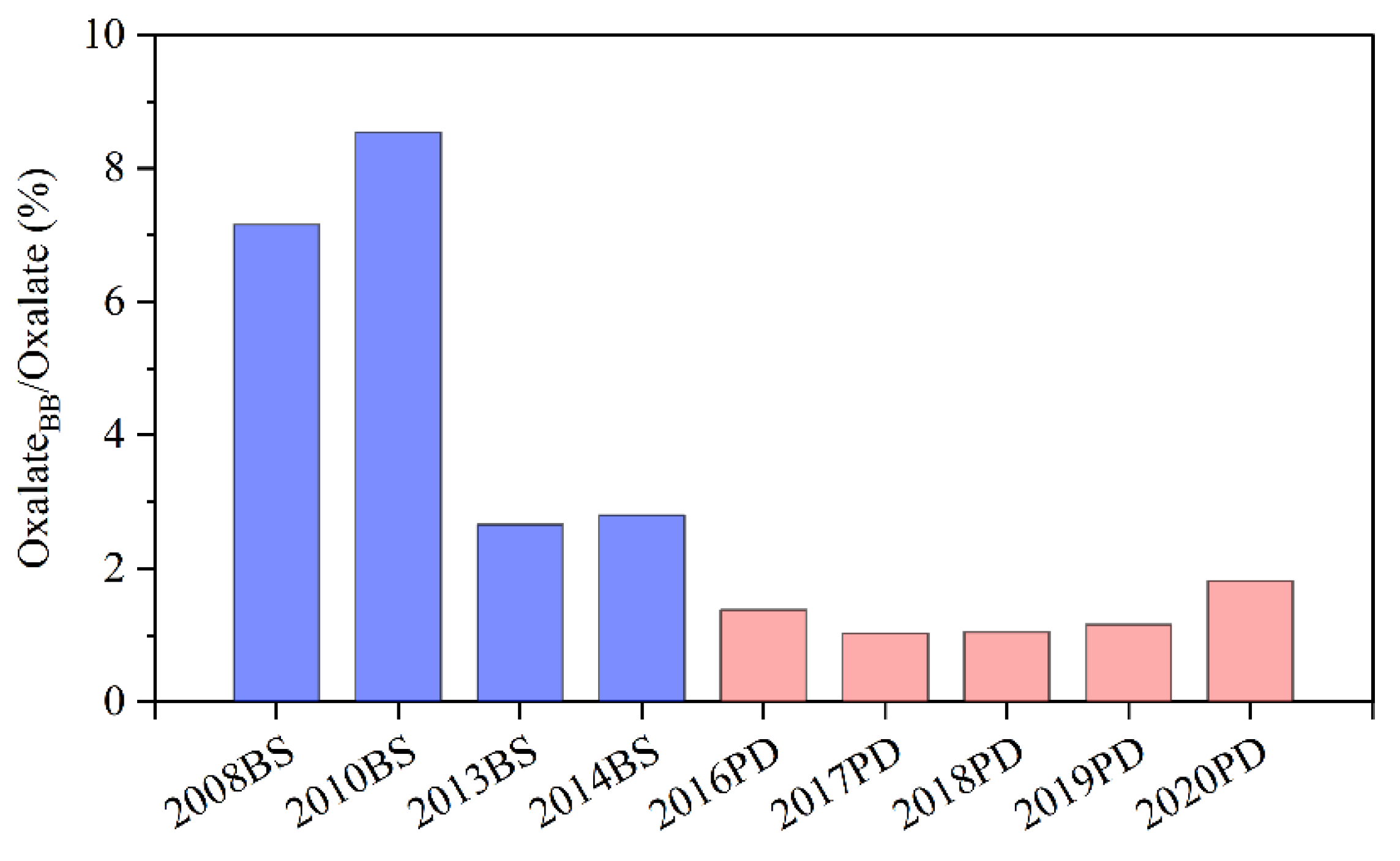
3.2.1. Estimation of Oxalate from BB
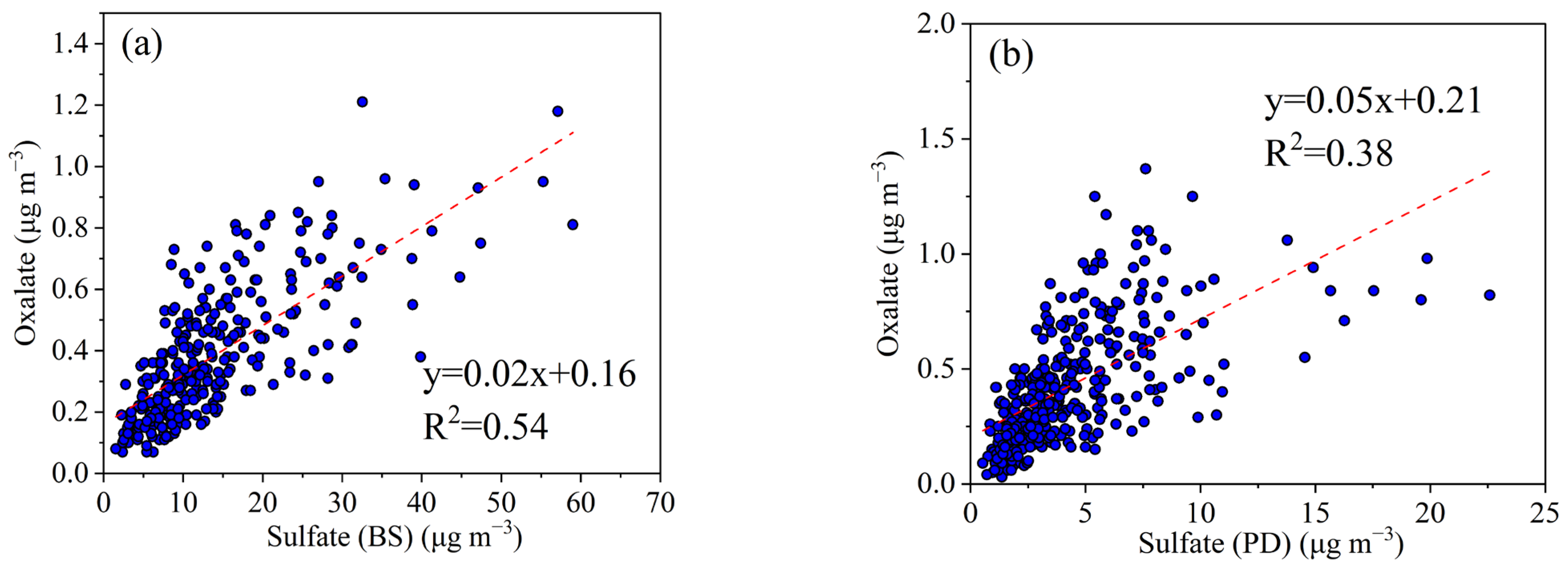
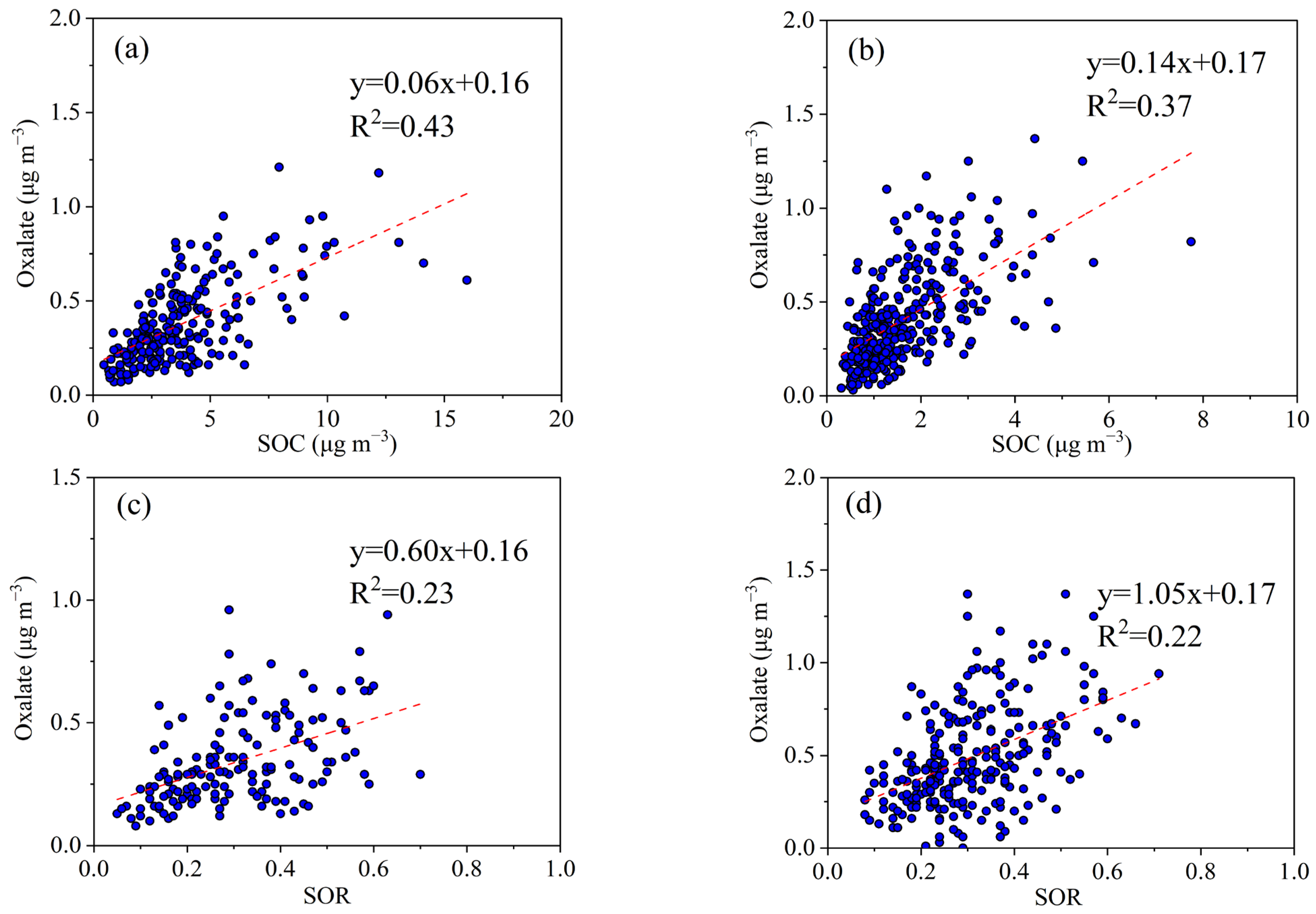
3.2.2. Secondary Formation of Oxalate
3.3. Effects of Meteorological Conditions on the Formation of Particulate Oxalate
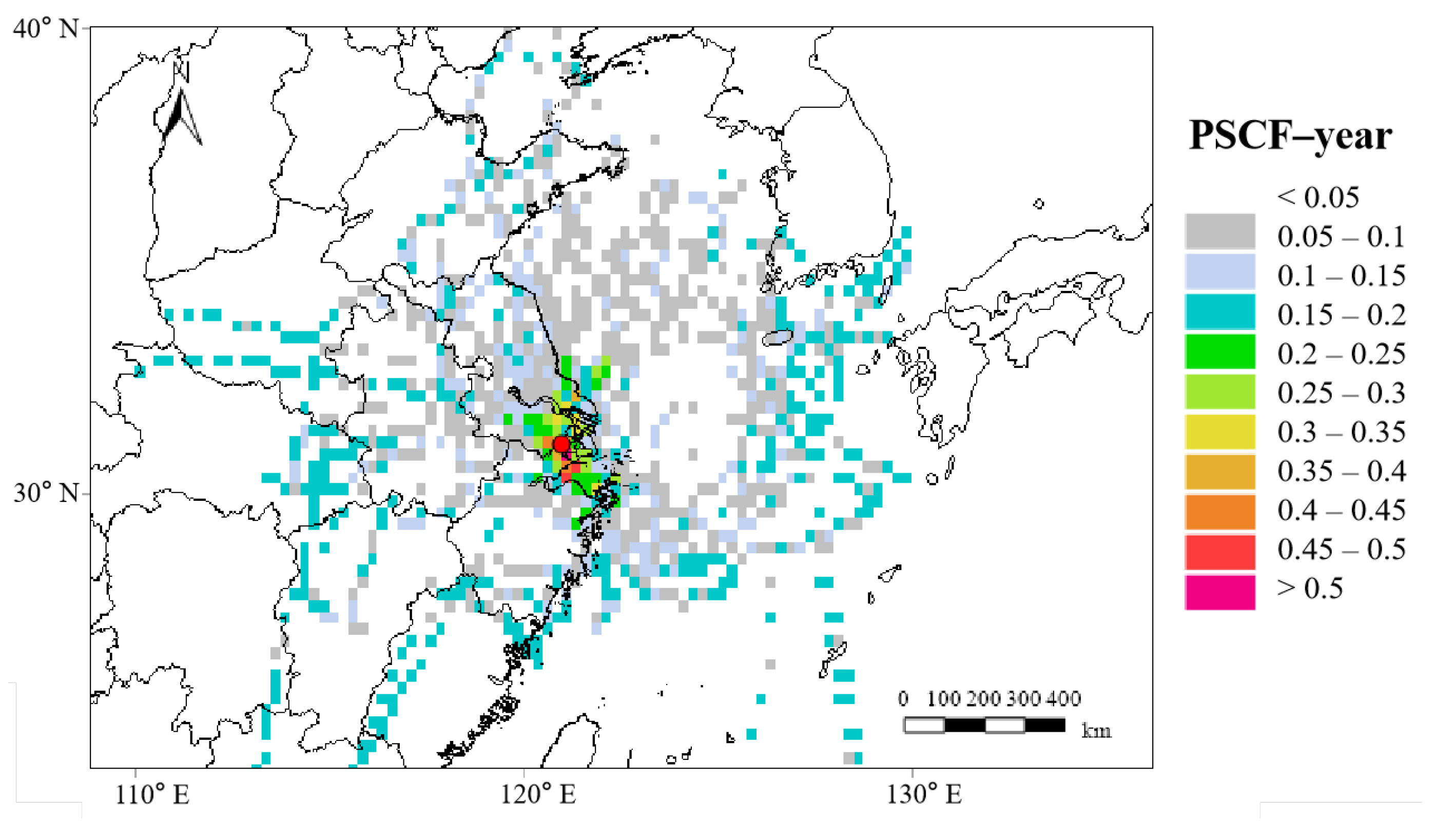
3.4. Regional Transportation of Oxalate in SH
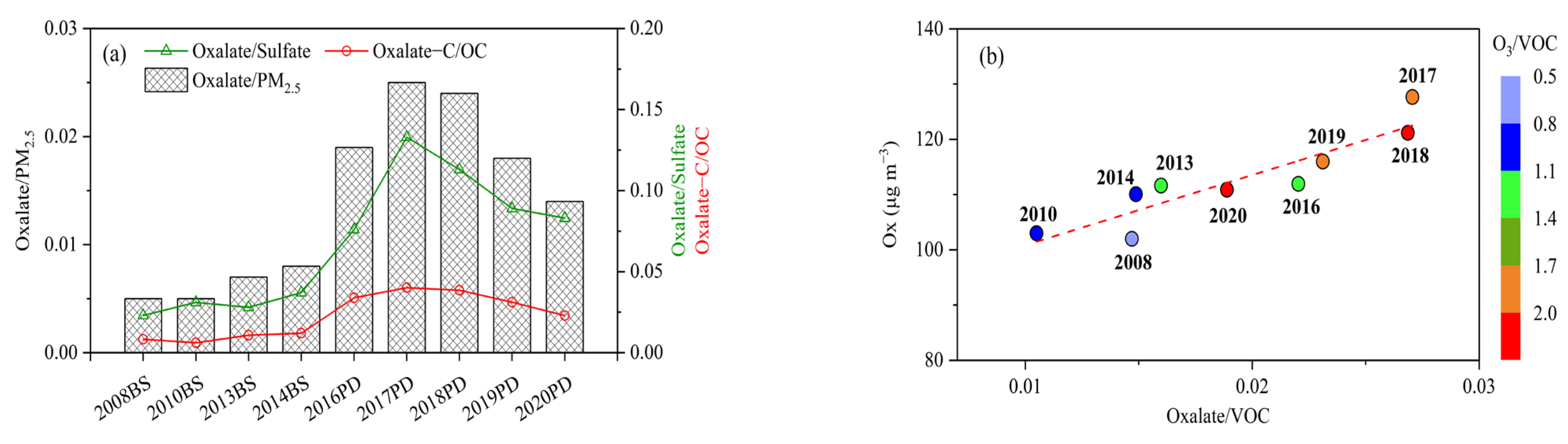
3.5. Interannual Variation of Oxalate in Shanghai during 2008–2020
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timonen, H.; Aurela, M.; Carbone, S.; Saarnio, K.; Hillamo, R. High time-resolution chemical characterization of the water-soluble fraction of ambient aerosols with PILS-TOC-IC and AMS. Atmos. Meas. Tech. 2010, 3, 61–69. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Kawamura, K.; Cao, F.; Lee, M. Stable carbon isotopic compositions of low-molecular-weight dicarboxylic acids, oxocarboxylic acids, α-dicarbonyls, and fatty acids: Implications for atmospheric processing of organic aerosols. J. Geophys. Res. 2015, 121, 3707–3717. [Google Scholar] [CrossRef]
- Kawamura, K.; Bikkina, S. A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources and transformation. Atmos. Res. 2016, 170, 140–160. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Aggarwal, S.G.; Singh, K.; Gupta, P.K.; Kawamura, K. Dicarboxylic acids and water-soluble organic carbon in aerosols in New Delhi, India, in winter: Characteristics and formation processes. J. Geophys. Res. 2009, 114, D19206. [Google Scholar] [CrossRef]
- Zhang, R.; Suh, I.; Zhao, J.; Zhang, D.; Fortner, E.C.; Tie, X.; Molina, L.T.; Molina, M.J. Atmospheric new particle formation enhanced by organic acids. Science 2004, 304, 1487–1490. [Google Scholar] [CrossRef]
- Yu, S. Role of organic acids (formic, acetic, pyruvic and oxalic) in the formation of cloud condensation nuclei (CCN): A review. Atmos. Res. 2000, 53, 185–217. [Google Scholar] [CrossRef]
- Feng, J.L.; Guo, Z.G.; Zhang, T.R.; Yao, X.H.; Chan, C.K.; Fang, M. Source and formation of secondary particulate matter in PM2.5 in Asian continental outflow. J. Geophys. Res. 2012, 117, D03302. [Google Scholar] [CrossRef]
- Huang, X.F.; Chen, D.L.; Lan, Z.J.; Feng, N.; He, L.Y.; Yu, G.H.; Luan, S.J. Characterization of organic aerosol in fine particles in a mega-city of South China: Molecular composition, seasonal variation, and size distribution. Atmos. Res. 2012, 114–115, 28–37. [Google Scholar] [CrossRef]
- Huang, X.F.; Yu, J.Z.; He, L.Y.; Yuan, Z. Water-soluble organic carbon and oxalate in aerosols at a coastal urban site in China: Size distribution characteristics, sources, and formation mechanisms. J. Geophys. Res. 2006, 111, D22212. [Google Scholar] [CrossRef]
- Sunder Raman, R.; Hopke, P.K.; Holsen, T.M. Carbonaceous aerosol at two rural locations in New York State: Characterization and behavior. J. Geophys. Res. 2008, 113, D12202. [Google Scholar] [CrossRef]
- Sempére, R.; Kawamura, K. Comparative distributions of dicarboxylic acids and related polar compounds in snow, rain and aerosols from urban atmosphere. Atmos. Environ. 1994, 28, 449–459. [Google Scholar] [CrossRef]
- Sempéré, R.; Kawamura, K. Low molecular weight dicarboxylic acids and related polar compounds in the remote marine rain samples collected from Western Pacific. Atmos. Environ. 1996, 30, 1609–1619. [Google Scholar] [CrossRef]
- Van Pinxteren, D.; Neusüß, C.; Herrmann, H. On the abundance and source contributions of dicarboxylic acids in size-resolved aerosol particles at continental sites in central Europe. Atmos. Chem. Phys. 2014, 13, 32093–32131. [Google Scholar] [CrossRef]
- Cong, Z.; Kawamura, K.; Kang, S.; Fu, P. Penetration of biomass-burning emissions from South Asia through the Himalayas: New insights from atmospheric organic acids. Sci. Rep. 2015, 5, 9580. [Google Scholar] [CrossRef]
- Dabek-Zlotorzynska, E.; Celo, V. Measurement of low-molecular-weight carboxylic acids in ambient air and vehicle emission by capillary electrophoresis. Methods Mol. Biol. 2008, 384, 43. [Google Scholar]
- Claeys, M.; Graham, B.; Vas, G.; Wu, W.; Vermeylen, R.; Pashynska, V.; Cafmeyer, J.; Cuyon, P.; Andreae, M.O.; Artaxo, P. Formation of secondary organic aerosols through photooxidation of isoprene. Science 2004, 303, 1173–1176. [Google Scholar] [CrossRef]
- Yang, F.; Chen, H.; Wang, X.; Yang, X.; Du, J.; Chen, J. Single particle mass spectrometry of oxalic acid in ambient aerosols in Shanghai: Mixing state and formation mechanism. Atmos. Environ. 2009, 43, 3876–3882. [Google Scholar] [CrossRef]
- Li, L.; Wu, W.; Feng, J.; Zhang, D.; Li, H.; Gu, Z.; Wang, B.; Sheng, G.; Fu, J. Composition, source, mass closure of PM2.5 aerosols for four forests in eastern China. Environ. Sci. 2010, 022, 405–412. [Google Scholar] [CrossRef]
- Ren, G.; Yan, X.; Ma, Y.; Qiao, L.; Li, L. Characteristics and source apportionment of PM2.5-bound saccharides and carboxylic acids in Central Shanghai, China. Atmos. Res. 2019, 237, 104817. [Google Scholar] [CrossRef]
- Thepnuan, D.; Chantara, S.; Lee, C.T.; Lin, N.H.; Tsai, Y.I. Molecular markers for biomass burning associated with the characterization of PM2.5 and component sources during dry season haze episodes in Upper South East Asia. Sci. Total Environ. 2019, 658, 708–722. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.H.; Bian, Q.; Griffith, S.M.; Louie, P.K.K.; Yu, J.Z. Sources and atmospheric processes impacting oxalate at a suburban coastal site in hong kong: Insights inferred from 1year hourly measurements. J. Geophys. Res. 2015, 120, 9772–9788. [Google Scholar] [CrossRef]
- Yu, j.; Huang, x.; Xu, j.; Hu, m. When aerosol sulfate goes up, so does oxalate: Implication for the formation mechanisms of oxalate. Environ. Sci. Technol. 2005, 39, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Cheng, C.; Li, M.; Yang, S.; Zhou, Q.; Zhong, Q.E.; Zhang, Y.; Xie, Y.; Zhou, Z. The enhanced mixing states of oxalate with metals in single particles in Guangzhou, China. Sci. Total Environ. 2021, 783, 146962. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, Q.; Peng, L.; Yang, Y.; Jiang, F.; Liu, F.; Song, W.; Chen, D.; Cai, Z.; Bi, X.; et al. Oxalate formation enhanced by Fe-containing particles and environmental implications. Environ. Sci. Technol. 2019, 53, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Li, Z.; Zhou, R.; Chen, M.; Li, Y.; Yi, Y.; Ding, Z.; Li, H.; Yan, L.; Hou, Z.; et al. Enhanced photochemical formation of secondary organic aerosols during the COVID-19 lockdown in Northern China. Sci. Total Environ. 2021, 758, 143709. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Murphy, J.G. The mechanisms responsible for the interactions among oxalate, pH, and Fe dissolution in PM2.5. ACS Earth Space Chem. 2019, 3, 2259–2265. [Google Scholar] [CrossRef]
- Ma, Q.; He, H.; Liu, C. Hygroscopic properties of oxalic acid and atmospherically relevant oxalates. Atmos. Environ. 2013, 69, 281–288. [Google Scholar] [CrossRef]
- Feng, J.; Li, M.; Zhang, P.; Gong, S.; Zhong, M.; Wu, M.; Zheng, M.; Chen, C.; Wang, H.; Lou, S. Investigation of the sources and seasonal variations of secondary organic aerosols in PM2.5 in Shanghai with organic tracers. Atmos. Environ. 2013, 79, 614–622. [Google Scholar] [CrossRef]
- Bikkina, S.; Kawamura, K.; Miyazaki, Y.; Fu, P. High abundances of oxalic, azelaic, and glyoxylic acids and methylglyoxal in the open ocean with high biological activity: Implication for secondary OA formation from isoprene. Geophys. Res. Lett. 2014, 41, 3649–3657. [Google Scholar] [CrossRef]
- Bi, X.; Sheng, G.; Chen, Y.; Fu, J. Size distribution of n-alkanes and polycyclic aromatic hydrocarbons (PAHs) in urban and rural atmospheres of Guangzhou, China. Atmos. Environ. 2005, 39, 477–487. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Pritchett, L.C.; Pierson, W.R.; Frazier, C.A.; Purcell, R.G. The dri thermal/optical reflectance carbon analysis system: Description, evaluation and applications in U.S. Air quality studies. Atmos. Environ. 1993, 27, 1185–1201. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikushima, K. Seasonal changes in the distribution of dicarboxylic acids in the urban atmosphere. Environ. Sci. Technol. 1993, 27, 2227–2235. [Google Scholar] [CrossRef]
- Kawamura, K.; Sakaguchi, F. Molecular distributions of water soluble dicarboxylic acids in marine aerosols over the Pacific Ocean including tropics. J. Geophys. Res. 1999, 104, 3501–3509. [Google Scholar] [CrossRef]
- Yang, F.; Gu, Z.; Feng, J.; Liu, X.; Yao, X. Biogenic and anthropogenic sources of oxalate in PM2.5 in a mega city, Shanghai. Atmos. Res. 2014, 138, 356–363. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, J.; Zhao, Q.; Zhang, N.; Feng, J.; Fu, Q. Temporal trends of the concentration and sources of secondary organic aerosols in PM2.5 in Shanghai during 2012 and 2018. Atmos. Environ. 2021, 261, 118596. [Google Scholar] [CrossRef]
- Ye, C.; Lu, K.; Song, H.; Mu, Y.; Chen, J.; Zhang, Y. A critical review of sulfate aerosol formation mechanisms during winter polluted periods. J. Environ. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Hilario, M.; Crosbie, E.; Baaga, P.A.; Betito, G.; Braun, R.A.; Cambaliza, M.O. Particulate oxalate-to-sulfate ratio as an aqueous processing marker: Similarity across field campaigns and limitations. Geophys. Res. Lett. 2021, 48, e2021GL096520. [Google Scholar] [CrossRef]
- Yao, X.; Lau, A.P.S.; Fang, M.; Chan, C.K.; Hu, M. Size distributions and formation of ionic species in atmospheric particulate pollutants in Beijing, China: 2—Dicarboxylic acids. Atmos. Environ. 2003, 37, 3001–3007. [Google Scholar] [CrossRef]
- Yao, X.; Ming, F.; Chan, C.K. Size distributions and formation of dicarboxylic acids in atmospheric particles. Atmos. Environ. 2002, 36, 2099–2107. [Google Scholar] [CrossRef]
- Weber, R.J.; Sullivan, A.P.; Peltier, R.E.; Russell, A.; Yan, B.; Zheng, M.; De Gouw, J.; Warneke, C.; Brock, C.; Holloway, J.S. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J. Geophys. Res.-Atmos. 2007, 112, D13302. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, Y.; Takegawa, N.; Miyakawa, T.; Weber, R.J.; Jimenez, J.L.; Zhang, Q.; Worsnop, D.R. Oxygenated and water-soluble organic aerosols in Tokyo. J. Geophys. Res.-Atmos. 2007, 112, D01203. [Google Scholar] [CrossRef]
- Mayol-Bracero, O.L.; Guyon, P.; Graham, B.; Roberts, G.; Andreae, M.O.; Decesari, S.; Facchini, M.C.; Fuzzi, S.; Artaxo, P. Water-soluble organic compounds in biomass burning aerosols over Amazonia 2. Apportionment of the chemical composition and importance of the polyacidic fraction. J. Geophys. Res.-Atmos. 2002, 107, LBA-59. [Google Scholar] [CrossRef]
- Yuan-xun, Z.; Min, S.; Yuan-hang, Z.; Li-min, Z.; Ling-yan, H.; Bin, Z.; Yong-jie, W.; Xian-lei, Z. Source profiles of particulate organic matters emitted from cereal straw burnings. J. Environ. Sci. (China) 2007, 19, 167–175. [Google Scholar]
- Kundu, S.; Kawamura, K.; Andreae, T.W.; Hoffer, A.; Andreae, M.O. Molecular distributions of dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in biomass burning aerosols: Implications for photochemical production and degradation in smoke layers. Atmos. Chem. Phys. 2010, 10, 2209–2225. [Google Scholar]
- Meng, J.; Wang, G.; Hou, Z.; Liu, X.; Wei, B.; Wu, C.; Cao, C.; Wang, J.; Li, J.; Cao, J. Molecular distribution and stable carbon isotopic compositions of dicarboxylic acids and related SOA from biogenic sources in the summertime atmosphere of Mt. Tai in the North China Plain. Atmos. Chem. Phys. 2018, 18, 15069–15086. [Google Scholar] [CrossRef]
- Fu, Z.; Cheng, L.; Ye, X.; Ma, Z.; Wang, R.; Duan, Y.; Juntao, H.; Chen, J. Characteristics of aerosol chemistry and acidity in Shanghai after PM2.5 satisfied national guideline: Insight into future emission control. Sci. Total Environ. 2022, 827, 154319. [Google Scholar] [CrossRef]
- Martinelango, P.K.; Dasgupta, P.K.; Al-Horr, R.S. Atmospheric production of oxalic acid/oxalate and nitric acid/nitrate in the Tampa Bay airshed: Parallel pathways. Atmos. Environ. 2007, 41, 4258–4269. [Google Scholar] [CrossRef]
- Meng, J.; Wang, G.; Li, J.; Cheng, C.; Ren, Y.; Yao, H.; Cheng, Y.; Cao, J.; Zhang, T. Seasonal characteristics of oxalic acid and related SOA in the free troposphere of Mt. Hua, central China: Implications for sources and formation mechanisms. Sci. Total Environ. 2014, 493, 1088–1097. [Google Scholar] [CrossRef]
- Yang, C.; Zhou, S.; Zhang, C.; Yu, M.; Cao, F.; Zhang, Y. Atmospheric Chemistry of Oxalate: Insight into the role of relative humidity and aerosol acidity from high-resolution observation. J. Geophys. Res.-Atmos. 2022, 127, e2021JD035364. [Google Scholar] [CrossRef]
- Ye, B.; Ji, X.; Yang, H.; Yao, X.; Chan, C.K.; Cadle, S.H.; Chan, T.; Mulawa, P.A. Concentration and chemical composition of PM2.5 in Shanghai for a 1-year period. Atmos. Environ. 2003, 37, 499–510. [Google Scholar] [CrossRef]
- Fu, X.; Wang, S.; Zhao, B.; Xing, J.; Cheng, Z.; Liu, H.; Hao, J. Emission inventory of primary pollutants and chemical speciation in 2010 for the Yangtze River Delta region, China. Atmos. Environ. 2013, 70, 39–50. [Google Scholar] [CrossRef]
- Huang, C.; Chen, C.H.; Li, L.; Cheng, Z.; Wang, H.L.; Huang, H.Y.; Streets, D.G.; Wang, Y.J.; Zhang, G.F.; Chen, Y.R. Emission inventory of anthropogenic air pollutants and VOC species in the Yangtze River Delta region, China. Atmos. Chem. Phys. 2011, 11, 4105–4120. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Zheng, B.; Tong, D.; He, K. Persistent growth of anthropogenic non-methane volatile organic compound (NMVOC) emissions in China during 1990-2017: Drivers, speciation and ozone formation potential. Atmos. Chem. Phys. 2019, 19, 8897–8913. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, H.; Wang, Q.; Jing, S.; An, J.; Gao, Y.; Huang, C.; Yan, R.; Dai, H.; Cheng, T. Observation-based sources evolution of non-methane hydrocarbons (NMHCs) in a megacity of China—Science Direct. J. Environ. Sci. (China) 2022, 124, 794–805. [Google Scholar] [CrossRef]
- Xu, J.; Tie, X.; Gao, W.; Lin, Y.; Fu, Q. Measurement and model analyses of the ozone variation during 2006 to 2015 and its response to emission change in megacity Shanghai, China. Atmos. Chem. Phys. 2019, 19, 9017–9035. [Google Scholar] [CrossRef]
- Wu, J.; Kong, S.; Yan, Y.; Yao, L.; Yan, Q.; Liu, D.; Shen, G.; Zhang, X.; Qi, S. Neglected biomass burning emissions of air pollutants in China-views from the corncob burning test, emission estimation, and simulations. Atmos. Environ. 2022, 278, 119082. [Google Scholar] [CrossRef]
- Ni, H.; Tian, J.; Wang, X.; Wang, Q.; Han, Y.; Cao, J.; Long, X.; Chen, L.; Chow, J.C.; Watson, J.G. PM2.5 emissions and source profiles from open burning of crop residues. Atmos. Environ. 2017, 169, 229–237. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, J.; Qi, Y.; Li, C.; Chen, J.; Wang, X.; He, J.; Wang, S.; Hao, J.; Zhang, L. Emission characterization, environmental impact, and control measure of PM2.5 emitted from agricultural crop residue burning in China. J. Cleaner Prod. 2017, 149, 629–635. [Google Scholar] [CrossRef]
- Tian, J. Study on Emission Characteristics of PM2.5 from Crop Residue Burning and Residential Coal Combustion in China. Ph.D. Thesis, University of Chinese Academy of Sciences, Xi’an, China, October 2016. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Fan, F.; Feng, Y.; Hu, M.; Fu, Q.; Chen, J.; Wang, S.; Feng, J. Insight into Source and Evolution of Oxalic Acid: Characterization of Particulate Organic Diacids in a Mega-City, Shanghai from 2008 to 2020. Atmosphere 2022, 13, 1347. https://doi.org/10.3390/atmos13091347
Zhang N, Fan F, Feng Y, Hu M, Fu Q, Chen J, Wang S, Feng J. Insight into Source and Evolution of Oxalic Acid: Characterization of Particulate Organic Diacids in a Mega-City, Shanghai from 2008 to 2020. Atmosphere. 2022; 13(9):1347. https://doi.org/10.3390/atmos13091347
Chicago/Turabian StyleZhang, Ning, Fan Fan, Yi Feng, Ming Hu, Qingyan Fu, Jing Chen, Shunyao Wang, and Jialiang Feng. 2022. "Insight into Source and Evolution of Oxalic Acid: Characterization of Particulate Organic Diacids in a Mega-City, Shanghai from 2008 to 2020" Atmosphere 13, no. 9: 1347. https://doi.org/10.3390/atmos13091347
APA StyleZhang, N., Fan, F., Feng, Y., Hu, M., Fu, Q., Chen, J., Wang, S., & Feng, J. (2022). Insight into Source and Evolution of Oxalic Acid: Characterization of Particulate Organic Diacids in a Mega-City, Shanghai from 2008 to 2020. Atmosphere, 13(9), 1347. https://doi.org/10.3390/atmos13091347






