Atmospheric Modelling of Mercury in the Southern Hemisphere and Future Research Needs: A Review
Abstract
1. Introduction
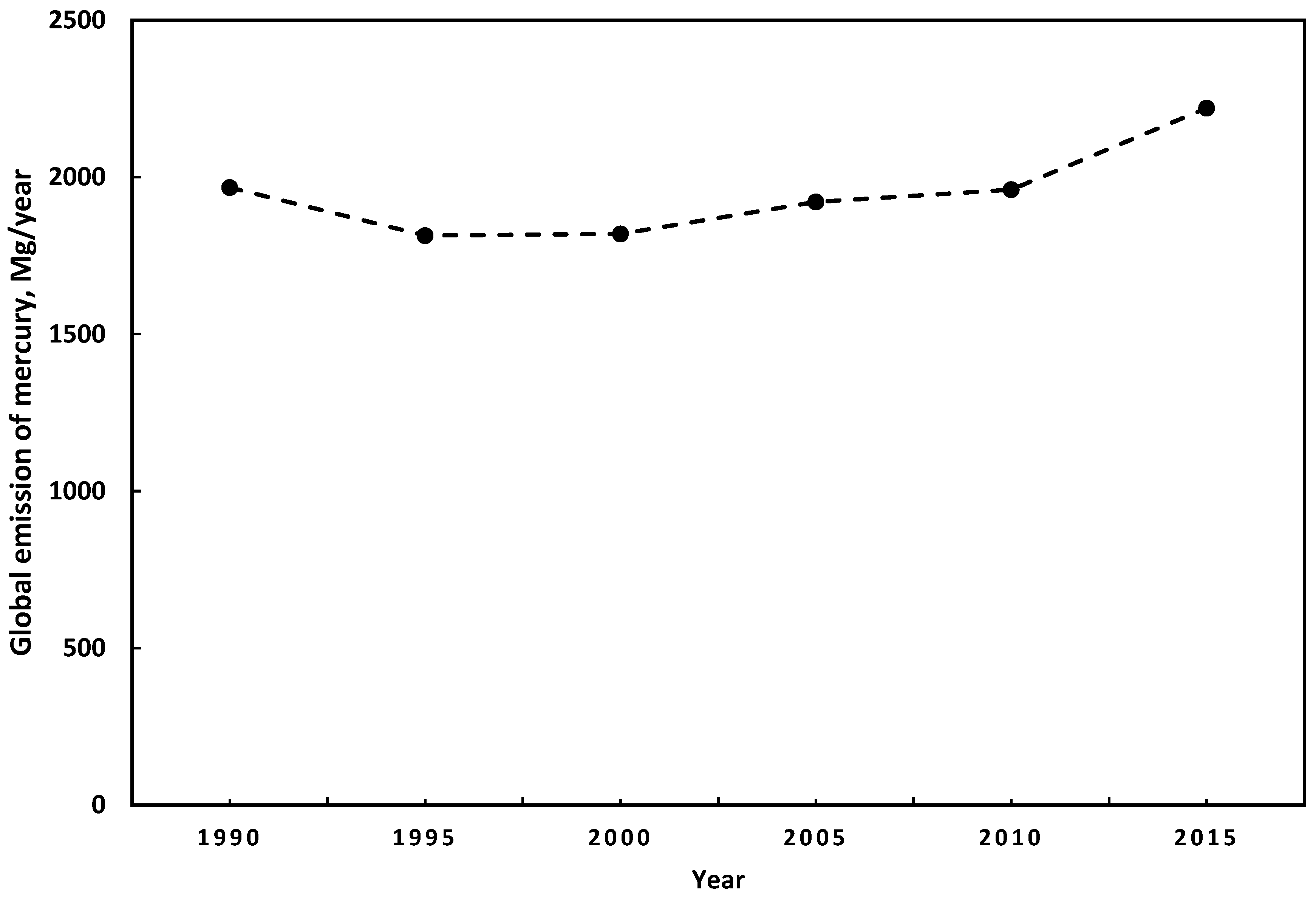
1.1. Emissions of Mercury
1.2. Mercury and Health Effects
2. Modelling Atmospheric Mercury
2.1. General Aspects
2.2. Spatial Distribution of Mercury
2.3. Projections of Mercury Emissions
2.4. Interhemispheric Transport
2.5. Mercury Deposition in the Southern Hemisphere
2.5.1. Mercury Wet Deposition
2.5.2. Mercury Dry Deposition
3. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balakrishnan, K.; Dey, S.; Gupta, T.; Dhaliwal, R.S.; Brauer, M.; Cohen, A.J.; Stanaway, J.D.; Beig, G.; Joshi, T.K.; Aggarwal, A.N.; et al. The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: The Global Burden of Disease Study 2017. Lancet Planet. Health 2019, 3, e26–e39. [Google Scholar] [CrossRef]
- Kim, K.-H.H.; Kabir, E.; Jahan, S.A. A review on the distribution of Hg in the environment and its human health impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Driscoll, C.T.; Eagles-Smith, C.A.; Eckley, C.S.; Gay, D.A.; Hsu-Kim, H.; Keane, S.E.; Kirk, J.L.; Mason, R.P.; Obrist, D.; et al. A Critical Time for Mercury Science to Inform Global Policy. Environ. Sci. Technol. 2018, 52, 9556–9561. [Google Scholar] [CrossRef] [PubMed]
- Toda, E.; Have, C.T.; Pacyna, J.M. The Minamata Convention. Chem. Int. 2020, 42, 10–18. [Google Scholar] [CrossRef]
- Lyman, S.N.; Cheng, I.; Gratz, L.E.; Weiss-Penzias, P.; Zhang, L. An updated review of atmospheric mercury. Sci. Total Environ. 2019, 707, 135575. [Google Scholar] [CrossRef]
- Diéguez, M.C.; Bencardino, M.; García, P.E.; D’Amore, F.; Castagna, J.; De Simone, F.; Soto Cárdenas, C.; Ribeiro Guevara, S.; Pirrone, N.; Sprovieri, F. A multi-year record of atmospheric mercury species at a background mountain station in Andean Patagonia (Argentina): Temporal trends and meteorological influence. Atmos. Environ. 2019, 214, 116819. [Google Scholar] [CrossRef]
- Slemr, F.; Martin, L.; Labuschagne, C.; Mkololo, T.; Angot, H.; Magand, O.; Magand, O.; Garat, P.; Ramonet, M.; Bieser, J. Atmospheric mercury in the Southern Hemisphere—Part 1: Trend and inter-annual variations in atmospheric mercury at Cape Point, South Africa, in 2007–2017, and on Amsterdam Island in 2012–2017. Atmos. Chem. Phys. 2020, 20, 7683–7692. [Google Scholar] [CrossRef]
- Wright, L.; Zhang, L.; Marsik, F.J. Overview of mercury dry deposition, litterfall, and throughfall studies. Atmos. Chem. Phys. 2016, 16, 13399–13416. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska, A.H.; Brągoszewska, P.; Bemowska-Kałabun, O.; Wrzosek-Jakubowska, J. Air Contamination by Mercury, Emissions and Transformations—A Review. Water Air Soil Pollut. 2017, 228, 123. [Google Scholar] [CrossRef]
- Subir, M.; Ariya, P.A.; Dastoor, A.P. A review of uncertainties in atmospheric modeling of mercury chemistry I. Uncertainties in existing kinetic parameters e Fundamental limitations and the importance of heterogeneous chemistry. Atmos. Environ. 2011, 45, 5664–5676. [Google Scholar] [CrossRef]
- Si, L.; Ariya, P.A. Recent Advances in Atmospheric Chemistry of Mercury. Atmosphere 2018, 9, 76. [Google Scholar] [CrossRef]
- Zhang, Y.; Bocquet, M.; Mallet, V.; Seigneur, C.; Baklanov, A. Real-time air quality forecasting, part I: History, techniques, and current status. Atmos. Environ. 2012, 60, 632–655. [Google Scholar] [CrossRef]
- Zaid Abualkishik, A. A comparative study on the software architecture of WRF and other numerical weather prediction models. J. Theor. Appl. Inf. Technol. 2018, 31, 24. [Google Scholar]
- Travnikov, O.; Angot, H.; Artaxo, P.; Bencardino, M.; Bieser, J.; D’Amore, F.; Dastoor, A.; De Simone, F.; DIéguez, M.C.; Dommergue, A.; et al. Multi-model study of mercury dispersion in the atmosphere: Atmospheric processes and model evaluation. Atmos. Chem. Phys. 2017, 17, 5271–5295. [Google Scholar] [CrossRef]
- Angot, H.; Hoffman, N.; Giang, A.; Thackray, C.P.; Hendricks, A.N.; Urban, N.R.; Selin, N.E. Global and Local Impacts of Delayed Mercury Mitigation Efforts. Environ. Sci. Technol. 2018, 52, 12968–12977. [Google Scholar] [CrossRef] [PubMed]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, P.; Cao, S.; Zhao, Y. Atmospheric mercury deposition over the land surfaces and the associated uncertainties in observations and simulations: A critical review. Atmos. Chem. Phys. 2019, 19, 15587–15608. [Google Scholar] [CrossRef]
- Zhou, J.; Obrist, D.; Dastoor, A.; Jiskra, M.; Ryjkov, A. Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2021, 2, 269–284. [Google Scholar] [CrossRef]
- Falakdin, P.; Terzaghi, E.; Di Guardo, A. Spatially resolved environmental fate models: A review. Chemosphere 2022, 290, 133394. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Y. Earth system modeling of mercury using CESM2—Part 1: Atmospheric model CAM6-Chem/Hg v1.0. Geosci. Model Dev. 2022, 15, 3587–3601. [Google Scholar] [CrossRef]
- AMAP; UN Environment. Technical Background Report for the Global Mercury Assessment 2018; Arctic Monitoring and Assessment Programme: Oslo, Norway; UN Environment Programme, Chemicals and Health Branch: Geneva, Switzerland, 2019; pp. viii + 426. [Google Scholar]
- Kos, G.; Ryzhkov, A.; Dastoor, A.; Narayan, J.; Steffen, A.; Ariya, P.A.; Zhang, L. Evaluation of discrepancy between measured and modelled oxidized mercury species mercury species. Atmos. Chem. Phys 2013, 13, 4839–4863. [Google Scholar] [CrossRef]
- De Simone, F.; D’Amore, F.; Bencardino, M.; Carbone, F.; Hedgecock, I.M.; Sprovieri, F.; Cinnirella, S.; Pirrone, N. The GOS4M Knowledge Hub: A web-based effectiveness evaluation platform in support of the Minamata Convention on Mercury. Environ. Sci. Policy 2021, 124, 235–246. [Google Scholar] [CrossRef]
- Charv At, P.; Klime, L.; Pospíšil, J.; Kleme, J.; Varbanov, S. An overview of mercury emissions in the energy industry-A step to mercury footprint assessment. J. Clean. Prod. 2020, 267, 122087. [Google Scholar] [CrossRef]
- Steenhuisen, F.; Wilson, S.J. Development and application of an updated geospatial distribution model for gridding 2015 global mercury emissions. Atmos. Environ. 2019, 211, 138–150. [Google Scholar] [CrossRef]
- De Simone, F.; Hedgecock, I.M.; Carbone, F.; Cinnirella, S.; Sprovieri, F.; Pirrone, N. Estimating Uncertainty in Global Mercury Emission Source and Deposition Receptor Relationships. Atmosphere 2017, 8, 236. [Google Scholar] [CrossRef]
- AMAP; UN Environment. Technical Background Report for the Global Mercury Assessment; United Nations Environment Programme: Nairobi, Kenya, 2013. [Google Scholar]
- Pirrone, N.; Cinnirella, S.; Feng, X.; Finkelman, R.B.; Friedli, H.R.; Leaner, J.; Mason, R.; Mukherjee, A.B.; Stracher, G.B.; Streets, D.G.; et al. Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos. Chem. Phys. 2010, 10, 5951–5964. [Google Scholar] [CrossRef]
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global Sources and Pathways of Mercury in the Context of Human Health. Int. J. Environ. Res. Public Health 2017, 14, 105. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, A.; Matsunaga, T.; Yamaguchi, Y.; Zang, S.; Li, Z.; Yu, T.; Gu, X. High-resolution inventory of mercury emissions from biomass burning in tropical continents during 2001–2017. Sci. Total Environ. 2019, 653, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Friedli, H.R.; Arellano, A.F.; Cinnirella, S.; Pirrone, N. Mercury emissions from global biomass burning: Spatial and temporal distribution. In Mercury Fate and Transport in the Global Atmosphere; Springer: Boston, MA, USA, 2009; pp. 193–220. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a Global Pollutant: Sources, Pathways, and Effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Z.; Huang, S.; Zhang, P.; Peng, Y.; Wu, P.; Gu, J.; Dutkiewicz, S.; Zhang, H.; Wu, S.; et al. Global health effects of future atmospheric mercury emissions. Nat. Commun. 2021, 12, 3035. [Google Scholar] [CrossRef]
- Peplow, D.; Augustine, S. Neurological abnormalities in a mercury exposed population among indigenous Wayana in Southeast Suriname. Environ. Sci. Process. Impacts 2014, 16, 2415–2422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury—Current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Guallar, E.; Sanz-Gallardo, M.I.; Veer, P.V.T.; Bode, P.; Aro, A.; Gómez-Aracena, J.; Kark, J.D.; Riemersma, R.A.; Martín-Moreno, J.M.; Kok, F.J. Mercury, Fish Oils, and the Risk of Myocardial Infarction. N. Engl. J. Med. 2009, 347, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Nagafuchi, O.; Kawakami, T.; Inoue, T.; Elvince, R.; Kanefuji, K.; Nur, I.; Napitupulu, M.; Basir-Cyio, M.; Kinoshita, H.; et al. Human health risk assessment of atmospheric mercury inhalation around three artisanal small-scale gold mining areas in Indonesia. Environ. Sci. Atmos. 2021, 1, 423–433. [Google Scholar] [CrossRef]
- Pavilonis, B.; Grassman, J.; Johnson, G.; Diaz, Y.; Caravanos, J. Characterization and risk of exposure to elements from artisanal gold mining operations in the Bolivian Andes. Environ. Res. 2017, 154, 1–9. [Google Scholar] [CrossRef]
- Bose-O’reilly, S.; Drasch, G.; Beinhoff, C.; Tesha, A.; Drasch, K.; Roider, G.; Taylor, H.; Appleton, D.; Siebert, U. Health assessment of artisanal gold miners in Tanzania. Sci. Total Environ. 2009, 408, 796–805. [Google Scholar] [CrossRef]
- Ohlander, J.; Huber, S.M.; Schomaker, M.; Heumann, C.; Schierl, R.; Michalke, B.; Jenni, O.G.; Caflisch, J.; Muñoz, D.M.; von Ehrenstein, O.S.; et al. Mercury and neuromotor function among children in a rural town in Chile. Int. J. Occup. Environ. Health 2016, 22, 27–35. [Google Scholar] [CrossRef][Green Version]
- Leiva González, J.; Onederra, I. Environmental Management Strategies in the Copper Mining Industry in Chile to Address Water and Energy Challenges-Review. Mining 2022, 2, 197–232. [Google Scholar] [CrossRef]
- Hrubá, F.; Strömberg, U.; Černá, M.; Chen, C.; Harari, F.; Harari, R.; Horvat, M.; Koppová, K.; Kos, A.; Krsková, A.; et al. Blood cadmium, mercury, and lead in children: An international comparison of cities in six European countries, and China, Ecuador, and Morocco. Environ. Int. 2012, 41, 29–34. [Google Scholar] [CrossRef]
- Laborde, A.; Tomasina, F.; Bianchi, F.; Bruné, M.N.; Buka, I.; Comba, P.; Corra, L.; Cori, L.; Duffert, C.M.; Harari, R.; et al. Children’s health in Latin America: The infuence of environmental exposures. Environ. Health Perspect. 2015, 123, 201–209. [Google Scholar] [CrossRef]
- González-Carrasco, V.; Velasquez-Lopez, P.C.; Olivero-Verbel, J.; Pájaro-Castro, N. Air Mercury Contamination in the Gold Mining Town of Portovelo, Ecuador. Bull. Environ. Contam. Toxicol. 2011, 87, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jaeglé, L.; Thompson, L.A.; Streets, D.G. Six centuries of changing oceanic mercury. Glob. Biogeochem. Cycles 2014, 28, 1251–1261. [Google Scholar] [CrossRef]
- Muntean, M.; Janssens-Maenhout, G.; Song, S.; Giang, A.; Selin, N.E.; Zhong, H.; Zhao, Y.; Olivier, J.G.J.; Guizzardi, D.; Crippa, M.; et al. Evaluating EDGARv4.tox2 speciated mercury emissions ex-post scenarios and their impacts on modelled global and regional wet deposition patterns. Atmos. Environ. 2018, 184, 56–68. [Google Scholar] [CrossRef]
- Shah, V.; Jacob, D.J.; Thackray, C.P.; Wang, X.; Sunderland, E.M.; Dibble, T.S.; Saiz-Lopez, A.; Černušák, I.; Kellö, V.; Castro, P.J.; et al. Improved Mechanistic Model of the Atmospheric Redox Chemistry of Mercury. Environ. Sci. Technol. 2021, 55, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, S.; Leibensperger, E.M. Source-receptor relationships for atmospheric mercury deposition in the context of global change. Atmos. Environ. 2021, 254, 118349. [Google Scholar] [CrossRef]
- Zhang, H.; Holmes, C.D.; Wu, S. Impacts of changes in climate, land use and land cover on atmospheric mercury. Atmos. Environ. 2016, 141, 230–244. [Google Scholar] [CrossRef]
- Kumar, A.; Wu, S. Mercury Pollution in the Arctic from Wildfires: Source Attribution for the 2000s. Environ. Sci. Technol. 2019, 53, 11269–11275. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, Y. Interannual Variability of Air-Sea Exchange of Mercury in the Global Ocean: The “seesaw Effect” in the Equatorial Pacific and Contributions to the Atmosphere. Environ. Sci. Technol. 2021, 55, 7145–7156. [Google Scholar] [CrossRef]
- Horowitz, H.; Jacob, D.; Zhang, Y.; DIbble, T.S.; Slemr, F.; Amos, H.M.; Schmidt, J.A.; Corbitt, E.S.; Marais, E.A.; Sunderland, E.M. A new mechanism for atmospheric mercury redox chemistry: Implications for the global mercury budget. Atmos. Chem. Phys. 2017, 17, 6353–6371. [Google Scholar] [CrossRef]
- Howard, D.; Nelson, P.F.; Edwards, G.C.; Morrison, A.L.; Fisher, J.A.; Ward, J.; Harnwell, J.; Van Der Schoot, M.; Atkinson, B.; Chambers, S.D.; et al. Atmospheric mercury in the Southern Hemisphere tropics: Seasonal and diurnal variations and influence of inter-hemispheric transport. Atmos. Chem. Phys. 2017, 17, 11623–11636. [Google Scholar] [CrossRef]
- Holmes, C.D.; Jacob, D.J.; Corbitt, E.S.; Mao, J.; Yang, X.; Talbot, R.; Slemr, F. Global atmospheric model for mercury including oxidation by bromine atoms. Atmos. Chem. Phys. 2010, 10, 12037–12057. [Google Scholar] [CrossRef]
- Dibble, T.S.; Tetu, H.L.; Jiao, Y.; Thackray, C.P.; Jacob, D.J. Modeling the OH-Initiated Oxidation of Mercury in the Global Atmosphere without Violating Physical Laws. J. Phys. Chem. A 2020, 124, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Horowitz, H.; Wang, J.; Xie, Z.; Kuss, J.; Soerensen, A.L. A Coupled Global Atmosphere-Ocean Model for Air-Sea Exchange of Mercury: Insights into Wet Deposition and Atmospheric Redox Chemistry. Environ. Sci. Technol. 2019, 53, 5052–5061. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Jaeglé, L. Subtropical subsidence and surface deposition of oxidized mercury produced in the free troposphere. Atmos. Chem. Phys. 2017, 17, 8999–9017. [Google Scholar] [CrossRef]
- Strode, S.A.; Jaeglé, L.; Selin, N.E.; Jacob, D.J.; Park, R.J.; Yantosca, R.M.; Mason, R.P.; Slemr, F. Air-sea exchange in the global mercury cycle. Glob. Biogeochem. Cycles 2007, 21, 1017. [Google Scholar] [CrossRef]
- Schofield, R.; Utembe, S.; Gionfriddo, C.; Tate, M.; Krabbenhoft, D.; Adeloju, S.; Keywood, M.; Dargaville, R.; Sandiford, M. Atmospheric mercury in the Latrobe Valley, Australia: Case study June 2013. Elementa 2021, 9, 00072. [Google Scholar] [CrossRef]
- Bieser, J.; Slemr, F.; Ambrose, J.; Brenninkmeijer, C.; Brooks, S.; Dastoor, A.; Desimone, F.; Ebinghaus, R.; Gencarelli, C.N.; Geyer, B.; et al. Multi-model study of mercury dispersion in the atmosphere: Vertical and interhemispheric distribution of mercury species. Atmos. Chem. Phys. 2017, 17, 6925–6955. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Travnikov, O.; De Simone, F.; Hedgecock, I.M.; Sundseth, K.; Pacyna, E.G.; Steenhuisen, F.; Pirrone, N.; Munthe, J.; Kindbom, K. Current and future levels of mercury atmospheric pollution on a global scale. Atmos. Chem. Phys. 2016, 16, 12495–12511. [Google Scholar] [CrossRef]
- De Simone, F.; Gencarelli, C.N.; Hedgecock, I.M.; Pirrone, N. A Modeling Comparison of Mercury Deposition from Current Anthropogenic Mercury Emission Inventories. Environ. Sci. Technol. 2016, 50, 5154–5162. [Google Scholar] [CrossRef] [PubMed]
- De Simone, F.; Artaxo, P.; Bencardino, M.; Cinnirella, S.; Carbone, F.; D’Amore, F.; Dommergue, A.; Bin Feng, X.; Gencarelli, C.N.; Hedgecock, I.M.; et al. Particulate-phase mercury emissions from biomass burning and impact on resulting deposition: A modelling assessment. Atmos. Chem. Phys. 2017, 17, 1881–1899. [Google Scholar] [CrossRef]
- De Simone, F.; Gencarelli, C.N.; Hedgecock, I.M.; Pirrone, N. Global atmospheric cycle of mercury: A model study on the impact of oxidation mechanisms. Environ. Sci. Pollut. Res. 2014, 21, 4110–4123. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.M.; Magand, O.; Laj, P.; Andrade, M.; Moreno, I.; Velarde, F.; Salvatierra, G.; Gutierrez, R.; Blacutt, L.; Aliaga, D.; et al. Seasonal patterns of atmospheric mercury in tropical South America as inferred by a continuous total gaseous mercury record at Chacaltaya station (5240 m) in Bolivia. Atmos. Chem. Phys 2021, 21, 3447–3472. [Google Scholar] [CrossRef]
- Jiskra, M.; Sonke, J.E.; Obrist, D.; Bieser, J.; Ebinghaus, R.; Myhre, C.L.; Pfaffhuber, K.A.; Wängberg, I.; Kyllönen, K.; Worthy, D.; et al. A vegetation control on seasonal variations in global atmospheric mercury concentrations. Nat. Geosci. 2018, 11, 244–250. [Google Scholar] [CrossRef]
- Sprovieri, F.; Pirrone, N.; Bencardino, M.; D’Amore, F.; Carbone, F.; Cinnirella, S.; Mannarino, V.; Landis, M.; Ebinghaus, R.; Weigelt, A.; et al. Atmospheric mercury concentrations observed at ground-based monitoring sites globally distributed in the framework of the GMOS network. Atmos. Chem. Phys. 2016, 16, 11915–11935. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Selin, N.E.; Soerensen, A.L.; Angot, H.; Artz, R.; Brooks, S.; Brunke, E.-G.; Conley, G.; Dommergue, A.; Ebinghaus, R.; et al. Top-down constraints on atmospheric mercury emissions and implications for global biogeochemical cycling. Atmos. Chem. Phys. 2015, 15, 7103–7125. [Google Scholar] [CrossRef]
- Fisher, J.A.; Nelson, P.F. Atmospheric mercury in Australia: Recent findings and future research needs. Elem. Sci. Anthr. 2020, 8, 070. [Google Scholar] [CrossRef]
- Sprovieri, F.; Pirrone, N.; Bencardino, M.; D’Amore, F.; Angot, H.; Barbante, C.; Brunke, E.G.; Arcega-Cabrera, F.; Cairns, W.; Comero, S.; et al. Five-year records of mercury wet deposition flux at GMOS sites in the Northern and Southern hemispheres. Atmos. Chem. Phys. 2017, 17, 2689–2708. [Google Scholar] [CrossRef]
- Slemr, F.; Angot, H.; Dommergue, A.; Magand, O.; Barret, M.; Weigelt, A.; Ebinghaus, R.; Brunke, E.G.; Pfaffhuber, K.A.; Edwards, G.; et al. Comparison of mercury concentrations measured at several sites in the Southern Hemisphere. Atmos. Chem. Phys. 2015, 15, 3125–3133. [Google Scholar] [CrossRef]
- Soerensen, A.L.; Skov, H.; Jacob, D.J.; Soerensen, B.T.; Johnson, M.S. Global concentrations of gaseous elemental mercury and reactive gaseous mercury in the marine boundary layer. Environ. Sci. Technol. 2010, 44, 7425–7430. [Google Scholar] [CrossRef]
- Angot, H.; Magand, O.; Helmig, D.; Ricaud, P.; Quennehen, B.; Gallée, H.; Del Guasta, M.; Sprovieri, F.; Pirrone, N.; Savarino, J.; et al. New insights into the atmospheric mercury cycling in central Antarctica and implications on a continental scale. Atmos. Chem. Phys. 2016, 16, 8249–8264. [Google Scholar] [CrossRef]
- Miller, M.B.; Howard, D.A.; Pierce, A.M.; Cook, K.R.; Keywood, M.; Powell, J.; Gustin, M.S.; Edwards, G.C. Atmospheric reactive mercury concentrations in coastal Australia and the Southern Ocean. Sci. Total Environ. 2021, 751, 141681. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Wuebbles, D.J.; Liang, X.-Z.; Tao, Z.; Olsen, S.; Artz, R.; Ren, X.; Cohen, M. Atmospheric Chemistry and Physics Projections of atmospheric mercury levels and their effect on air quality in the United States. Atmos. Chem. Phys 2014, 14, 783–795. [Google Scholar] [CrossRef]
- Corbitt, E.S.; Jacob, D.J.; Holmes, C.D.; Streets, D.G.; Sunderland, E.M. Global source-receptor relationships for mercury deposition under present-day and 2050 emissions scenarios. Environ. Sci. Technol. 2011, 45, 10477–10484. [Google Scholar] [CrossRef]
- Hamilton, J.F.; Allen, G.; Watson, N.M.; Lee, J.D.; Saxton, J.E.; Lewis, A.C.; Vaughan, G.; Bower, K.N.; Flynn, M.J.; Crosier, J.; et al. Observations of an atmospheric chemical equator and its implications for the tropical warm pool region. J. Geophys. Res. Atmos. 2008, 113, D20313. [Google Scholar] [CrossRef]
- Li, C.; Sonke, J.E.; Roux, L.; Piotrowska, N.; Van der Putten, N.; Roberts, S.J.; Daley, T.; Rice, E.; Gehrels, R.; Enrico, M.; et al. Unequal Anthropogenic Enrichment of Mercury in Earth’s Northern and Southern Hemispheres. ACS Earth Sp. Chem. 2020, 4, 2073–2081. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; Zhang, P. Mercury Deposition, Climate Change and Anthropogenic Activities: A Review. Front. Earth Sci. 2020, 8, 316. [Google Scholar] [CrossRef]
- Saiz-Lopez, A.; Travnikov, O.; Sonke, J.E.; Thackray, C.P.; Jacob, D.J.; Carmona-García, J.; Francés-Monerris, A.; Roca-Sanjuán, D.; Acuña, A.U.; Dávalos, J.Z.; et al. Photochemistry of oxidized Hg(I) and Hg(II) species suggests missing mercury oxidation in the troposphere. Proc. Natl. Acad. Sci. USA 2020, 117, 30949–30956. [Google Scholar] [CrossRef]
- Cossa, D.; Knoery, J.; Bănaru, D.; Harmelin-Vivien, M.; Sonke, J.E.; Hedgecock, I.M.; Bravo, A.G.; Rosati, G.; Canu, D.; Horvat, M.; et al. Mediterranean Mercury Assessment 2022: An Updated Budget, Health Consequences. Environ. Sci. Technol. 2022, 56, 3840–3862. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.P.; Zhang, L.; Cheng, I.; Aherne, J.; Wentworth, G.R. Impacts and Effects Indicators of Atmospheric Deposition of Major Pollutants to Various Ecosystems—A Review. Aerosol Air Qual. Res. 2018, 18, 1953–1992. [Google Scholar] [CrossRef]
- MacSween, K.; Edwards, G.C.; Beggs, P.J. Seasonal gaseous elemental mercury fluxes at a terrestrial background site in south-eastern Australia. Elem. Sci. Anthr. 2020, 8, 27. [Google Scholar] [CrossRef]
- Illuminati, S.; Annibaldi, A.; Bau, S.; Scarchilli, C.; Ciardini, V.; Grigioni, P.; Girolametti, F.; Vagnoni, F.; Scarponi, G.; Truzzi, C. Seasonal Evolution of Size-Segregated Particulate Mercury in the Atmospheric Aerosol Over Terra Nova Bay, Antarctica. Molecules 2020, 25, 3971. [Google Scholar] [CrossRef] [PubMed]
| Source | Type | Sector | Emissions Mg/Year | Contribution % |
|---|---|---|---|---|
| Artisanal gold production | A | Artisanal and small-scale gold mining | 838 | 11.31% |
| Combustion of fuels (energy, industry, and domestic/residential uses) | A | Stationary combustion of coal for transportation, domestic, and residential. | 55.8 | 0.75% |
| Stationary combustion of gas for transportation, domestic, and residential. | 0.165 | 0.00% | ||
| Stationary combustion of oil for transportation, domestic, and residential. | 2.7 | 0.04% | ||
| Industrial stationary combustion of coal. | 126 | 1.70% | ||
| Industrial stationary combustion of gas. | 0.123 | 0.002% | ||
| Industrial stationary combustion of oil. | 1.4 | 0.02% | ||
| Power plants’ stationary combustion of coal | 292 | 3.94% | ||
| Power plants’ stationary combustion of gas | 0.349 | 0.002% | ||
| Power plants’ stationary combustion of oil | 2.45 | 0.03% | ||
| Diverse industrial sectors | A | Raw materials and fuel for cement production (excluding coal) | 233 | 3.15% |
| Production of non-ferrous metals, including Al, Cu, Pb and Zn | 228 | 3.08% | ||
| Gold production on a large scale | 84.5 | 1.14% | ||
| Production of mercury | 13.8 | 0.19% | ||
| Refining of oil | 14.4 | 0.19% | ||
| Production of steel and pig iron (primary) | 29.8 | 0.40% | ||
| Secondary production of steel | 10.1 | 0.14% | ||
| Biomass burning | A | Biomass burning from domestic, industrial, and power plants | 51.9 | 0.70% |
| Waste | A | Other waste | 147 | 1.98% |
| Vinyl-chloride monomer | A | Vinyl-chloride monomer production (mercury catalyst) and vinyl-chloride monomer recycling | 58.2 | 0.79% |
| Oceans | N | Re-emissions from the ocean | 2682 | 36.21% |
| Biomass Burning | N | Emissions from accumulated mercury in biomass | 675 | 9.11% |
| Arid areas | N | Deserts and metalliferous and non-vegetated zones | 546 | 7.37% |
| Tundra/Grassland/Savannah | N | Tundra/Grassland/Savannah | 448 | 6.05% |
| Forests | N | Forests | 342 | 4.62% |
| Evasion after mercury depletion events | N | Evasion after mercury depletion events | 200 | 2.70% |
| Agricultural areas | N | Areas with agricultural processes | 128 | 1.73% |
| Lakes | N | Lakes | 96 | 1.30% |
| Geothermal activities | N | Volcanoes and geothermal activities | 90 | 1.22% |
| Location | Lat (°) | Long (°) | Period | TGM, ng/m3 | GEM ng/m3 | Studied Years | Used Model | Reference |
|---|---|---|---|---|---|---|---|---|
| Amsterdam Island | −37.80 | 77.55 | Summer | 0.93 | 2009–2011 | GEOS–Chem | [69] * | |
| Autumn | 1.15 | |||||||
| Winter | 1.31 | |||||||
| Spring | 0.99 | |||||||
| Summer | 0.97 | 0.95–1.0 | 2015 | GEM–MACH–Hg model Uptake of Hg by vegetation is considered. | [18] * | |||
| Autumn | 1.07 | 1.0–1.1 | ||||||
| Winter | 1.05 | 1.1–1.05 | ||||||
| Spring | 0.93 | |||||||
| Cape Point | −34.35 | 18.49 | Summer | 0.95 | 2009–2011 | GEOS–Chem | [69] * | |
| Autumn | 1.15 | |||||||
| Winter | 1.3 | |||||||
| Spring | 0.98 | |||||||
| Summer | 1.08 | 2007–2008 | GEOS–Chem (Hg + Br) | [55] * | ||||
| Autumn | 1.03 | |||||||
| Winter | 1.03 | |||||||
| Spring | 1.03 | |||||||
| Summer | 1.33 | 2007–2008 | GEOS–Chem (Hg + OH/O3) | [55] * | ||||
| Autumn | 1.23 | |||||||
| Winter | 1.17 | |||||||
| Spring | 1.27 | |||||||
| Summer | 1 | 0.95–1.05 | 2015 | GEM–MACH–Hg model Uptake of Hg by vegetation is considered. | [18] * | |||
| Autumn | 1.08 | 1.05–1.1 | ||||||
| Winter | 1.05 | 1.1–1.05 | ||||||
| Spring | 0.96 | |||||||
| Amsterdam Island and Cape Point | Summer | 0.67 | 2009–2011 | GEOS–Chem–MITgcm | [59] * | |||
| Autumn | 0.82 | |||||||
| Winter | 1 | |||||||
| Spring | 0.88 | |||||||
| Summer | 0.98 | 2015 | GEM–MACH–Hg model Uptake of Hg by vegetation is considered. | [18] * | ||||
| Autumn | 1.08 | |||||||
| Winter | 1.04 | |||||||
| Spring | 0.94 | |||||||
| Troll Research Station, Antarctica | −72 | 3 | Summer | 0.82 | 2009–2011 | GEOS-Chem | [69] * | |
| Autumn | 1.07 | |||||||
| Winter | 1.23 | |||||||
| Spring | 0.86 | |||||||
| Summer | 0.84 | 0.8–0.9 | 2015 | GEM–MACH–Hg model Uptake of Hg by vegetation is considered. | [18] * | |||
| Autumn | 0.96 | 0.9–1.0 | ||||||
| Winter | 0.96 | 1.0–0.95 | ||||||
| Spring | 0.83 | 0.95–0.8 | ||||||
| Amsterdam Island, Cape Point and Troll Research Station | Summer | 0.93 | 2009–2011 | GEOS–Chem | [69] * | |||
| Autumn | 1.13 | |||||||
| Winter | 1.28 | |||||||
| Spring | 0.99 | |||||||
| Neumayer, Antarctica | −70.68 | −8.27 | Summer | 1.15 | 2007–2008 | GEOS–Chem (Hg + Br) | [55] * | |
| Autumn | 0.99 | |||||||
| Winter | 0.97 | |||||||
| Spring | 0.99 | |||||||
| Summer | 1.36 | 2007–2008 | GEOS–Chem (Hg + OH/O3) | [55] * | ||||
| Autumn | 1.18 | |||||||
| Winter | 1.13 | |||||||
| Spring | 1.26 | |||||||
| ATARS, Gunn Point, Australia | 12.25 | 131 | Wet season (December–March) | 0.90 ± 0.10 | 2013–2014 | GEOS–Chem y HYSPLIT | [46] | |
| Dry Season (Jun–September) | 0.97 ± 0.13 | |||||||
| Summer | 1 | 0.90–0.95 | 2015 | GEM–MACH–Hg model Uptake of Hg by vegetation is considered. | [18] * | |||
| Autumn | 0.91 | 0.95–0.95 | ||||||
| Winter | 0.99 | 0.95–1.0 | ||||||
| Spring | 0.99 | |||||||
| Antarctica | - | - | Annual Average | 1.03 | 2015 | Hybrid Single-Particle Lagrangian Trajectory model (HYSPLIT) | [71] | |
| Southern Ocean | - | - | Spatial concentration pattern | 1.0 ± 0.22 | 2014–2015 | GEOS–Chem–MITgcm | [49] | |
| Spatial concentration pattern | 0.9 ± 0.38 | GEOS–Chem–MITgcm–model with improved CMF (cloud mass flux) | ||||||
| South America | - | - | Annual average | 1024 | 2015 | Hybrid Single-Particle Lagrangian Trajectory model (HYSPLIT) | [71] | |
| The Southern Hemisphere | - | - | Spatial concentration pattern | 1.27 ± 0.21 | 2007–2008 | GEOS–Chem | [55] | |
| Spatial concentration pattern | 1.1–1.3 | 2008–2009 | ECHMERIT | [65] | ||||
| Annual average | 0.986 | 2015 | Hybrid Single-Particle Lagrangian Trajectory model (HYSPLIT) | [71] | ||||
| Spatial concentration pattern | 1.16 ± 0.03 | 2000 | GEOS–Chem | [55] | ||||
| Spatial concentration pattern | 0.9–1.1 | 2013 | GLEMOS GEOS–Chem GEM–MACH–Hg ECHMERIT | [14] | ||||
| Location | Lat (°) | Long (°) | Period | TGM, ng/m3 | GEM ng/m3 | Years Studied | Reference |
|---|---|---|---|---|---|---|---|
| Amsterdam Island | −37.8 | 77.55 | Summer | - | 1.00 | 2013 | [68] |
| Autumn | - | 0.98 | |||||
| Winter | - | 1.10 | |||||
| Spring | - | 1.03 | |||||
| Summer | 1.02 | 2012–2017 | [7] * | ||||
| Autumn | 1.05 | ||||||
| Winter | 1.07 | ||||||
| Spring | 1.01 | ||||||
| Annual Average | 1.025 ± 0.065 | 2012 | [72] | ||||
| 1.028 ± 0.096 | 2013 | ||||||
| Summer | 1.02 | 2012-2013 | [72] * | ||||
| Autumn | 1 | ||||||
| Winter | 1.07 | ||||||
| Spring | 0.99 | ||||||
| Annual mean | 1.03 ± 0.1 | 2013–2014 | [73] | ||||
| Cape Point | −34.35 | 18.49 | Summer | - | 0.71 | 2013 | [68] |
| Autumn | - | 1.01 | |||||
| Winter | - | 1.00 | |||||
| Spring | - | 1.03 | |||||
| Summer | 1.04 | 2012–2017 | [7] * | ||||
| Autumn | 1.02 | ||||||
| Winter | 1.05 | ||||||
| Spring | 1.06 | ||||||
| Annual Average | 1.017 ± 0.095 | 2012 | [72] | ||||
| 1.052 ± 0.160 | 2013 | ||||||
| Summer | 0.99 | 2011–2013 | [72] * | ||||
| Autumn | 1.01 | ||||||
| Winter | 0.99 | ||||||
| Spring | 0.97 | ||||||
| ATARS, Gunn Point, Australia | 12.25 | 131 | Annual mean | 0.95 ± 0.12 | 2014–2016 | [46] | |
| Summer | 0.82 | 2015 | |||||
| Autumn | 0.89 | ||||||
| Winter | 0.98 | ||||||
| Spring | 0.99 | ||||||
| Antarctica–Dumont d’Urville | −66.66 | 140 | Annual Average | 0.87 ± 0.23 | 2012–2015 | [74] | |
| Summer | - | 0.83 | 2013 | [68] | |||
| Autumn | - | 0.89 | |||||
| Winter | - | 0.74 | |||||
| Spring | - | 0.33 | |||||
| Antarctica–Concordia Station | −75.1 | 123.34 | Annual Average | 0.76 ± 0.24 | 2012 | [74] | |
| 0.81 ± 0.28 | 2013 | ||||||
| Summer | - | 0.84 | 2013 | [68] | |||
| Autumn | - | 1.03 | |||||
| Winter | - | 0.83 | |||||
| Spring | - | 0.75 | |||||
| Troll Research Station, Antarctica | −72 | 3 | Annual Average | 1.052 ± 0.160 | 2012 | [74] | |
| 0.970 ± 0.162 | 2013 | ||||||
| Summer | 1.07 | 2011–2013 | [72] * | ||||
| Autumn | 1.03 | ||||||
| Winter | 1.03 | ||||||
| Spring | 0.92 | ||||||
| Cape Grim | −40.68 | 144.69 | Annual Average | 0.872 ± 0.130 | 2012 | [72] | |
| 0.848 ± 0.112 | 2013 | ||||||
| Summer | 0.89 | 2011–2013 | [72] * | ||||
| Autumn | 0.86 | ||||||
| Winter | 0.89 | ||||||
| Spring | 0.85 | ||||||
| Annual Average | 0.9 ± 0.35 | 2015–2017 | [75] | ||||
| Overall Mean–November 3 to November 6 | 1.03 (±0.16) | 2006 | |||||
| Bariloche | −41.13 | −71.42 | Summer | - | 0.88 | 2013 | [68] |
| Autumn | - | 0.89 | |||||
| Winter | - | 0.93 | |||||
| Spring | - | 0.86 | |||||
| Annual mean | 0.9 ± 0.14 | 2014–2015 | [73] | ||||
| Annual Average | 0.86 ±0.16 | 2012–2017 | [6] | ||||
| Latrobe Valley, Australia | 38.18 | 146.25 | Overnight–6 PM–6 AM (May to July) | 1.6–1.8 | 2013 | [60] | |
| 1.2–1.3 | |||||||
| The Macquarie University Weather Station, Australia | −33.765 | 151.117 | Annual Average | 0.65 ± 0.24 | 2016–2017 | [75] | |
| Chacaltaya Station, Bolivia | 16.35 | 68.13 | Overall Mean–normal condition | 0.89 ± 0.01 | July 2014–May 2015 | [66] | |
| Overall Mean–unusual condition because of the Niño phenomena | 1.34 ± 0.01 | Jun 2015–February 2016 | |||||
| Amsterdam Island, Cape Point, Bariloche, Dumont d’Urville, and Concordia Stations | Annual mean | - | 0.93 | 2013 | [68] | ||
| Summer | - | 0.91 | 2013 | ||||
| Autumn | - | 0.96 | |||||
| Winter | - | 0.92 | |||||
| Spring | - | 0.92 | |||||
| Amsterdam Island, Cape Point, Bariloche, and Dumont d’Urville Stations | Annual mean | - | 0.97 | 2014 | [68] | ||
| Summer | - | 0.92 | 2014 | ||||
| Autumn | - | 0.96 | |||||
| Winter | - | 1.03 | |||||
| Spring | - | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiva González, J.; Diaz-Robles, L.A.; Cereceda-Balic, F.; Pino-Cortés, E.; Campos, V. Atmospheric Modelling of Mercury in the Southern Hemisphere and Future Research Needs: A Review. Atmosphere 2022, 13, 1226. https://doi.org/10.3390/atmos13081226
Leiva González J, Diaz-Robles LA, Cereceda-Balic F, Pino-Cortés E, Campos V. Atmospheric Modelling of Mercury in the Southern Hemisphere and Future Research Needs: A Review. Atmosphere. 2022; 13(8):1226. https://doi.org/10.3390/atmos13081226
Chicago/Turabian StyleLeiva González, Jorge, Luis A. Diaz-Robles, Francisco Cereceda-Balic, Ernesto Pino-Cortés, and Valeria Campos. 2022. "Atmospheric Modelling of Mercury in the Southern Hemisphere and Future Research Needs: A Review" Atmosphere 13, no. 8: 1226. https://doi.org/10.3390/atmos13081226
APA StyleLeiva González, J., Diaz-Robles, L. A., Cereceda-Balic, F., Pino-Cortés, E., & Campos, V. (2022). Atmospheric Modelling of Mercury in the Southern Hemisphere and Future Research Needs: A Review. Atmosphere, 13(8), 1226. https://doi.org/10.3390/atmos13081226