Tropical Air Chemistry in Lagos, Nigeria
Abstract
1. Introduction
2. Methods and Materials
| Measured Parameter | Monitoring Instrument | Averaging Time |
|---|---|---|
| Field Measurement Methods | ||
| CO, CO2, NO, NO2, SO2, | EarthSense Zephyr [8] (electrochemical detectors for gases; optical light scattering for particles; PID for TVOC) | Continuous averaged 5 min avg. |
| O3 | ||
| PM2.5, PM10 | ||
| Trace gases and particles (same as Zephyr) | Aeroqual AQM65 [9] (electrochemical gas detectors; PM light scattering) | Intermittent/Continuous |
| for calib. of Zephyr; 5 min avg. | ||
| PM2.5, PM10 | ARA n-FRM Sampler | Time-integrated, 24 h |
| VOCs, CH4, N2O, CO2, CFCs, HFCs | Entech Silonite Canister | Time-integrated, 24 h |
| Precipitation | Davis Vantage Pro2 Weather Station | As detected |
| Air Temperature and Rel. Humidity | Continuous, averaged 5 min | |
| Wind Speed and Wind Direction | ||
| Laboratory Methods | ||
| PM Gravimetry for PM2.5 and PM10 | RADWAG Microbalance | Post and pre-sampling with laboratory instruments |
| Elements | RIGAKU X-ray Fluorescence (XRF) | |
| Ions | Dionex ICS-3000 | |
| Organic Source Markers | Agilent GC-MS | |
| EC and OC | ANSTO MABI Sunset Analyzer [10] | |
| GHGs | GC/FID/ECD/MS instruments | |
3. Basic Resources for Air Chemistry
3.1. Emissions Sources
3.2. Meteorology
4. Results and Discussion
4.1. Gases
4.1.1. Carbon Monoxide
4.1.2. Sulfur Dioxide
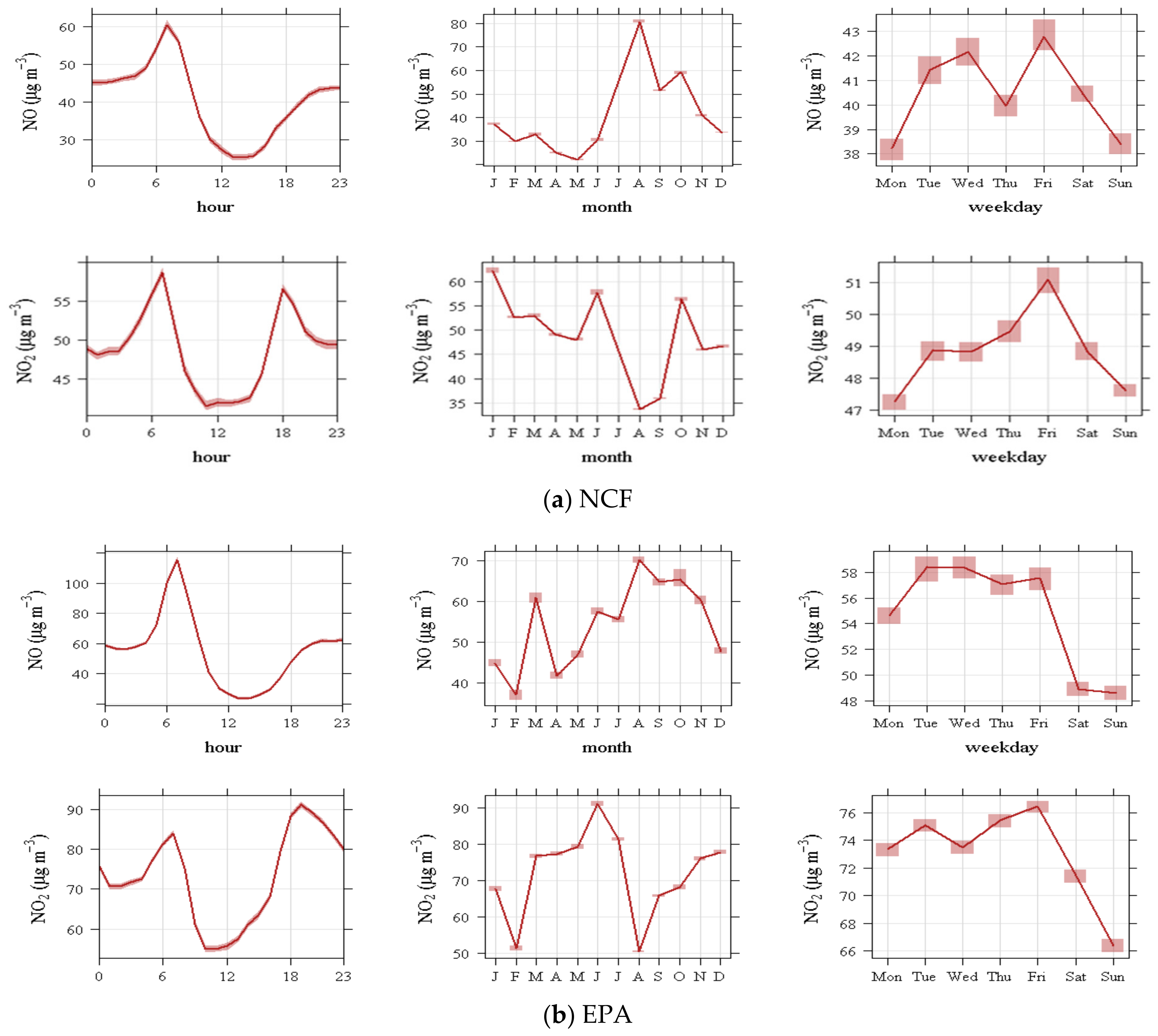
4.1.3. Nitrogen Oxides
4.1.4. Volatile Organic Compounds (VOCs)
4.1.5. Ozone

4.2. Aerosol Particles in Lagos Air
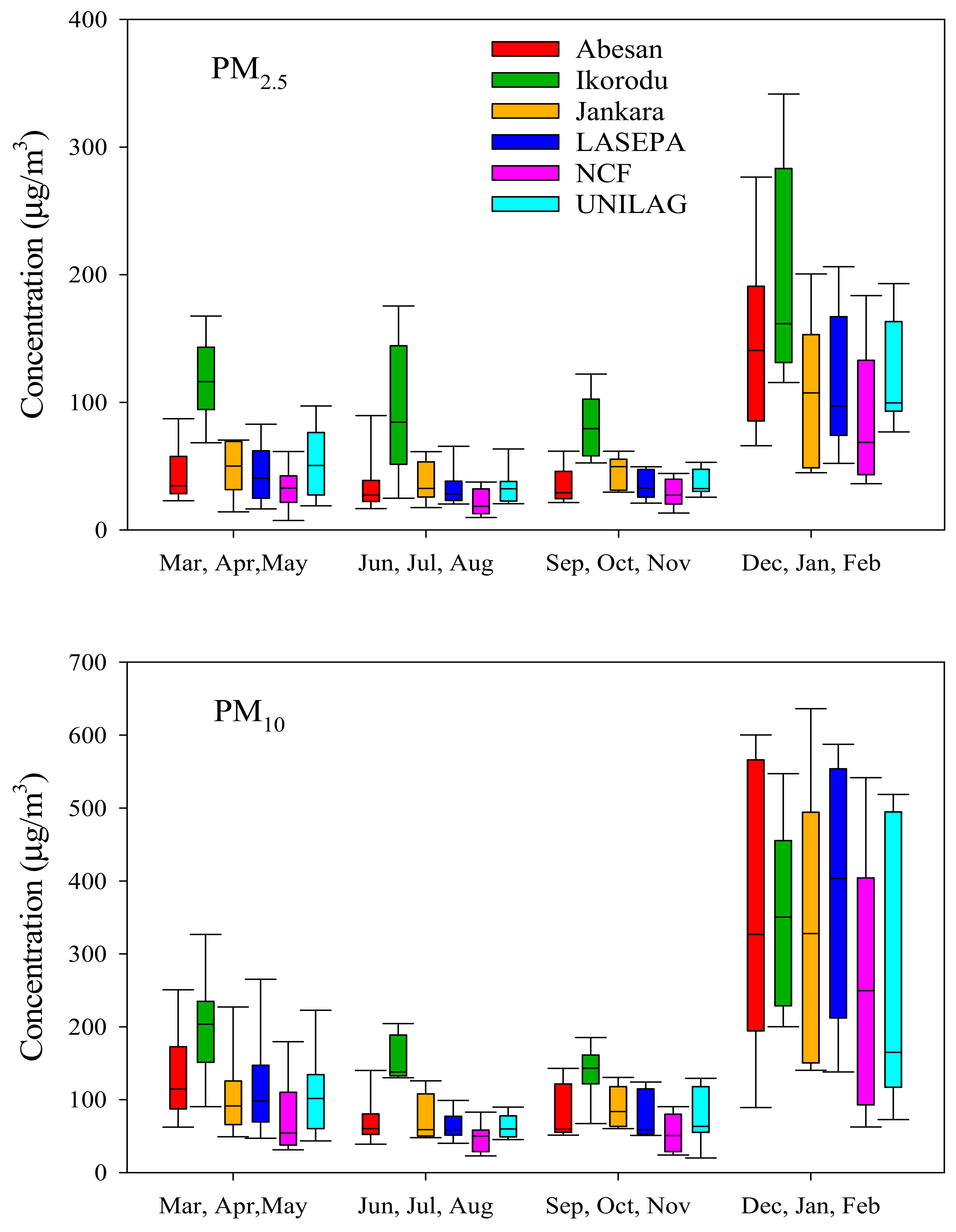
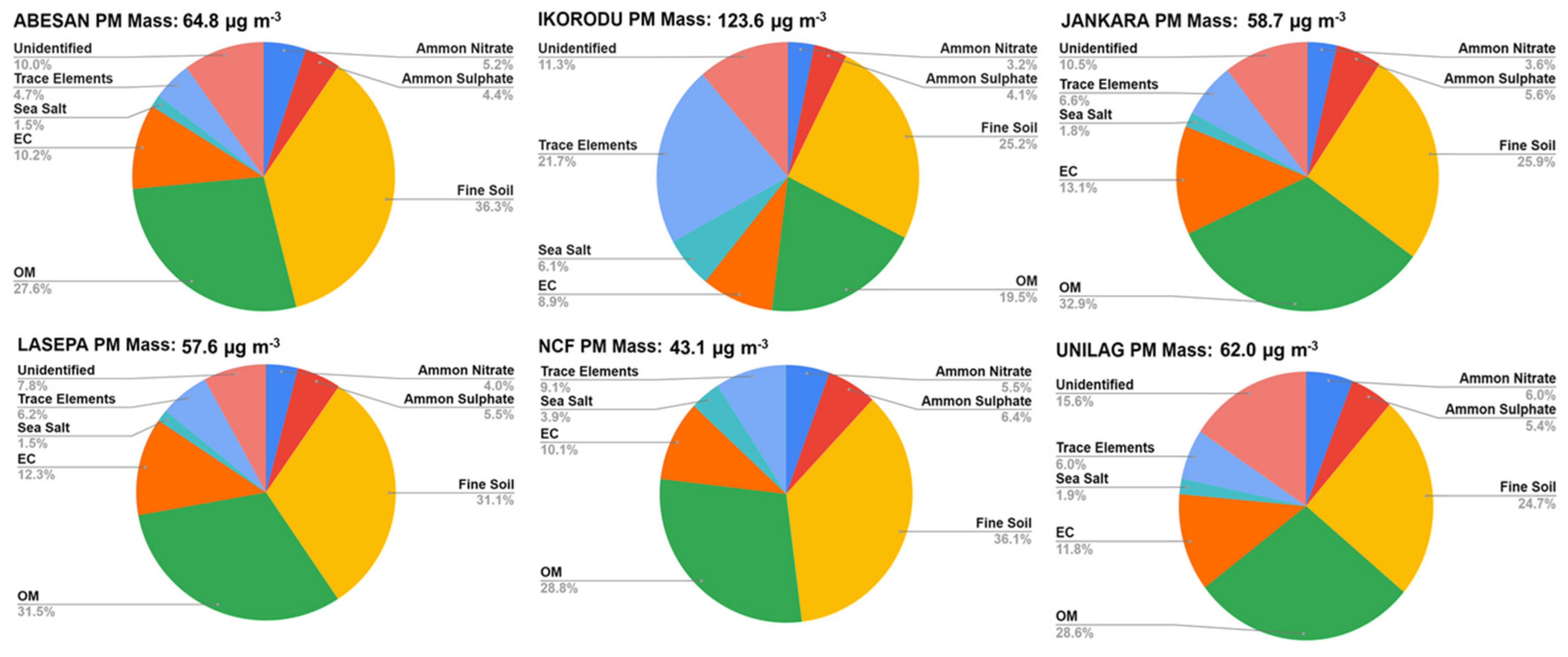
4.2.1. Mass Concentrations
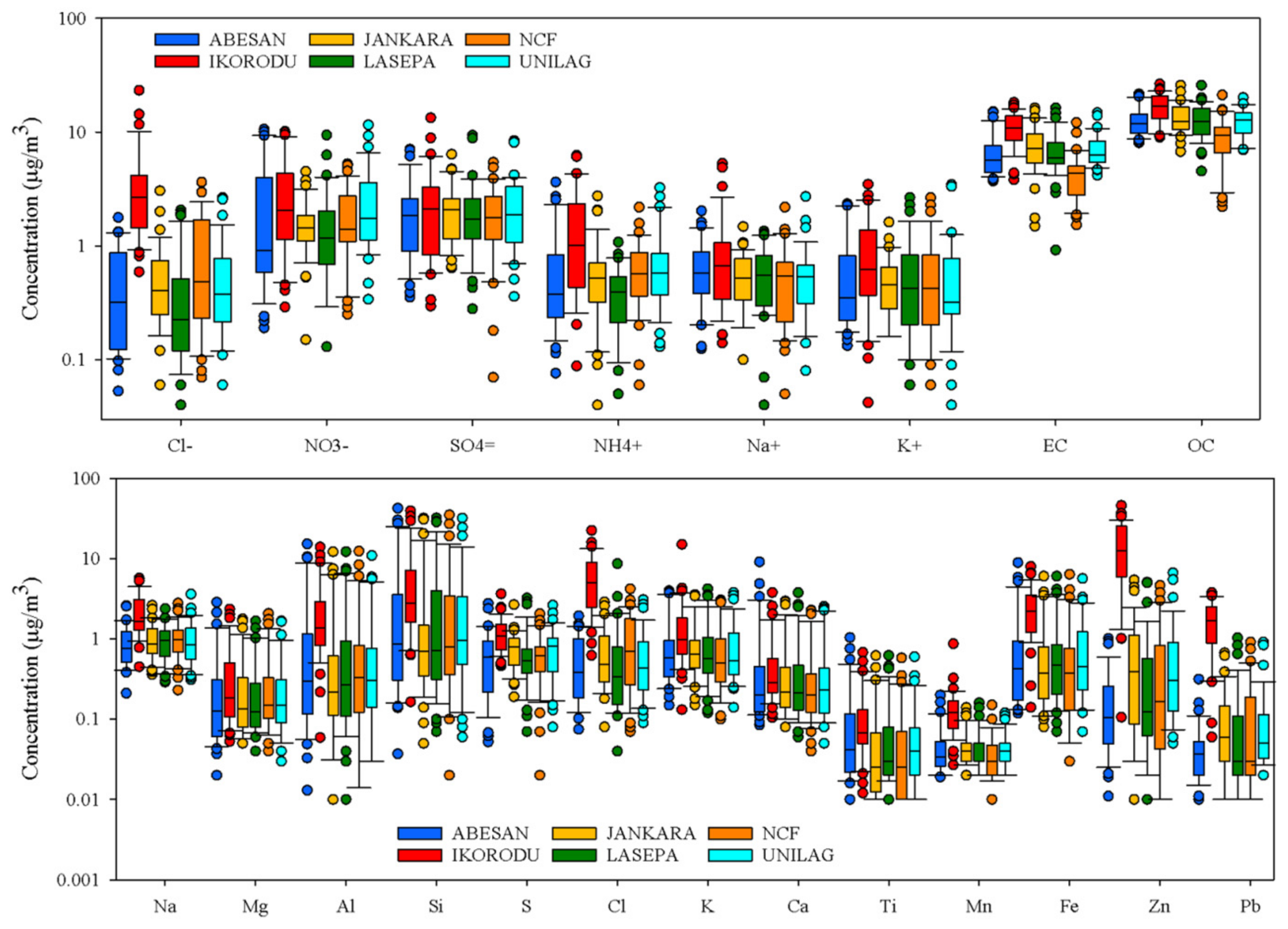
4.2.2. Particle Composition
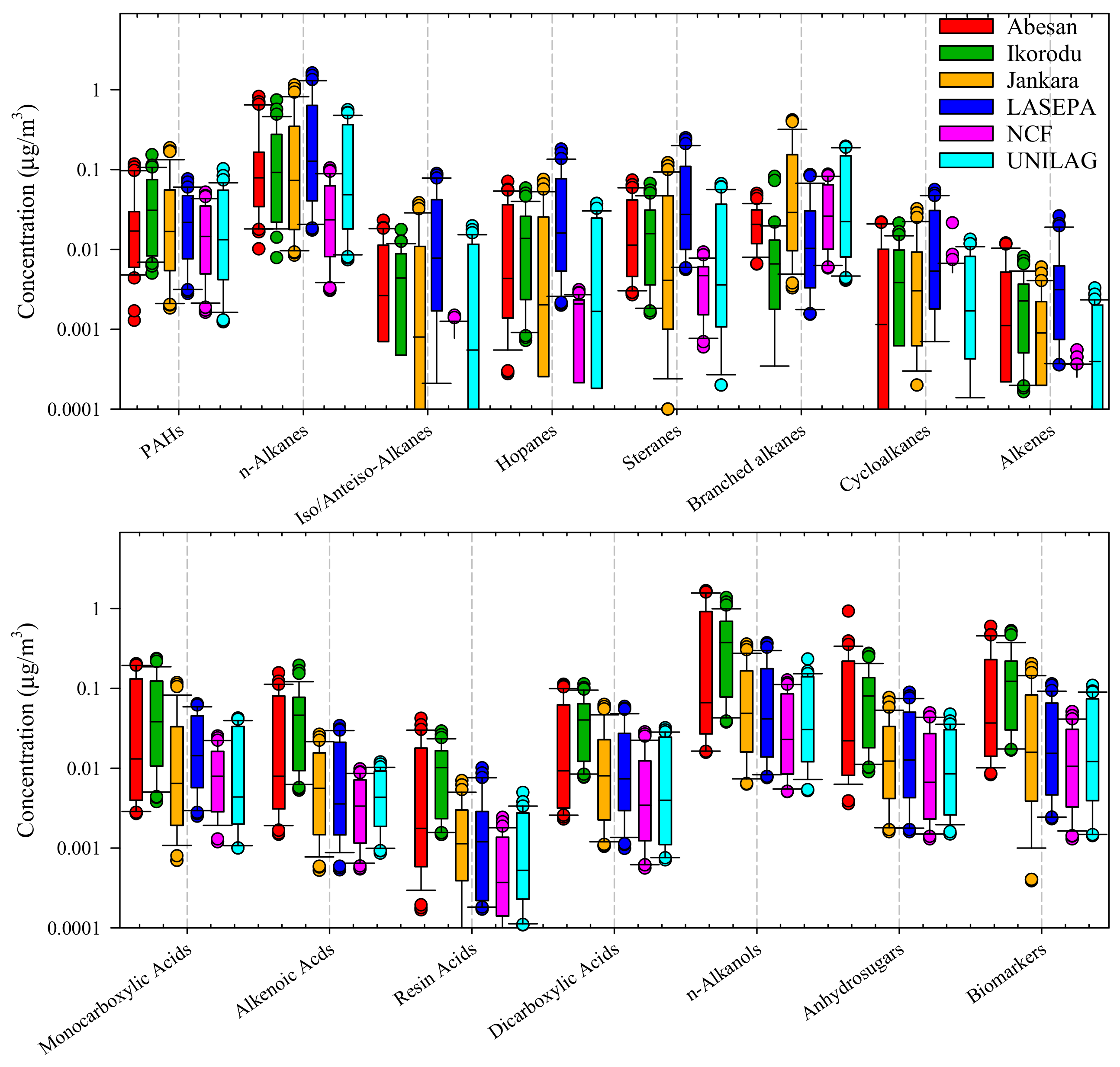
4.2.3. OM Speciation
| Class of Compound | No. of Species | Range (ng m−3); Site Order | Source/Indicator | References for Source Identification |
|---|---|---|---|---|
| Polycyclic aromatic hydrocarbons (PAHs) | 10 | 15 (NCF)-33(IKO) | Carbon fuel combustion | [54] |
| n-Alkanes | 23 | 23(NCF)-159 (EPA) | Petroleum; vegetation | [54,55,56,57] |
| Iso/Anteiso-alkanes | 10 | 1.3(NCF)-13(JAN) | Waxes; leaves | [58] |
| Hopanes | 18 | 2(NCF)-19(EPA) | Petrol prod., lub. oil; motor vehicle exhaust | [59,60] |
| Steranes | D13 | 4(NCF)-28(EPA) | Motor vehicle exhaust | [59,60] |
| Branched alkanes | 2 | 2–100 | Diesel fuel; lub. oil | [57,60] |
| Alkyl cyclohexanes | 5 | <0.1–20 | Diesel fuel; lub. oil | [57,60] |
| Alkenes | 2 | 0.1–2 | Squalene a; octadecene b identified | [61] |
| Monocarboxylic acids | 1 | (UNI)-(IKO) | Nonanoic acid; cooking; fuel combustion; biogenic | [55,62,63,64] |
| Alkenoic acids | 4 | (NCF)-(IKO) | Oleic acid; biomass burning | [64] |
| Resin acids | 4 | (NCF)-(IKO) | Biomass burning | [64] |
| Dicarboxylic acids | 2 | (NCF)-(IKO) | Vehicle exhaust, Biomass burning; Meat cooking. Oxalic acid possible marker for SOC | [60,64,65,66] |
| n-Alkanols | 18 | (NCF)-(IKO) | 1-octa;1-tetra;1-hexa-cosanol; charbroiling | [66,67] |
| Anhydro-sugars | 1 | 6(NCF)-80(IKO) | Levoglucosan; biomass burning | [68] |
| Biomarkers | 11 | Stigmasterol—biomass burning; char-broiling | [69,70] |
4.3. Uncertainties and Limitations
5. Discussion and Conclusions
- Unlike in the mid-latitudes, gas and particle chemistry increases with the onset of the dry season during the mid-latitude winter months; chemical processes decrease during the summer wet months.
- The diel variation in NO and NO2 concentrations are maximal before the midday ozone maxima, suggesting a pattern dominated by local processes; a well-known effect is seen with ozone increasing on the weekends with decreased NO emissions from reduced human activity, likely reduced traffic.
- VOC precursor species are observed to reflect fossil fuel and biomass components typical of large cities with heavy vehicle traffic supplemented with natural emissions and industrial or domestic sources.
- Fine particles and fine plus coarse particle mass concentrations are seasonally variable with maxima in the dry season.
- The particle composition contains a variety of urban-related species dominated by elemental and organic carbon with sulfate produced from SO2 oxidation in the air. Particles also contain significant amounts of nitrate, which may be underestimated from the sampling method used.
- The organic carbon fraction can be attributed to primary sources; there was no direct evidence of significant amounts of organic carbon produced by the oxidation of VOCs.
- The exploration of the organic species in particles indicates the presence of high-molecular-weight material from anthropogenic sources supplemented by biomass contributions from open burning and waxy material from vegetation.
- There is indirect evidence of the occasional influence of regional air mass transport of ozone and dust, especially during the Harmattan wind period in the dry season.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azeez, L.; Oyedeji, A.; Adewuyl, S.; Tijni, K.; Adebisi, S.; Olanaogun, N. Precursors Influencing Tropospheric Ozone Formation and Apportionment in Three Districts of Ilupeju Industrial Estate. Am. J. Chem. 2016, 6, 65–73. [Google Scholar]
- Raheem, A.; Adekola, F.; Obioh, L. The Seasonal Variation of the Concentration of Ozone, Sulfur Dioxide, and Nitrogen Oxides in Two Nigerian Cities. Environ. Model Assess. 2008, 14, 497–509. [Google Scholar] [CrossRef]
- Kemper, K.; Chaudhui, S. Air Pollution: A Silent Killer; The World Bank: Washington, DC, USA, 2022. [Google Scholar]
- Tawari, C.; Abowei, J. Air Pollution in the Niger Delta Area of Nigeria. Int. J. Fish. Aquat. Sci. 2012, 1, 94–117. [Google Scholar]
- Prospero, J. Long term measurements of transport of African mineral dust to the southeastern United States: Implications for regional air quality. J. Geophys. Res. 1999, 104, 15917–15927. [Google Scholar] [CrossRef]
- Ansmann, A.; Baars, H.; Tesche, M.; Müller, D.; Althausen, D.; Engelmann, R.; Pauliquevis, T.; Artaxo, P. Dust and smoke transport from Africa to South America: Lidar profiling over Cape Verde and the Amazon rain forest. Geophys. Res. Lett. 2009, 36, L111802. [Google Scholar] [CrossRef]
- EnvironQuest Ltd. Lagos Air Quality and PM Source Apportionment Study. Final 12 Month Summary Report; EnvironQuest: Lagos, Nigeria, 2022. [Google Scholar]
- EarthSense. Zephyr Air Quality Sensor (Specifications). 2021. Available online: www.earthsens.cp.uk (accessed on 29 May 2022).
- Aeroqual. AQM 65. Available online: www.aeroqual.com/prioducts/aqm-stations/aqm-65-air-quality-monitoring-station (accessed on 20 February 2022).
- Sunset Laboratory, Inc. Cutting Edge Carbon Aerosol Particulate Analysis Instrument. Available online: www.sunla.com (accessed on 20 February 2022).
- Fakinie, B.; Odekanie, E.; Olalekan, A.; Ije, H.; Oke, D.; Sonibare, J. Air pollutant emissions by anthropogenic combustion processes in Lagos, Nigeria. Cogent Eng. 2020, 7, 1808285. [Google Scholar] [CrossRef]
- Olatunji, S.; Fakanie, B.; Jimoda, L.; Adeniran, J.; Adesanmi, J. Air Emissions of Sulfur Dioxide from Gasoline and Diesel Consumption in the Southwestern States of Nigeria. Pet. Sci. Technol. 2015, 33, 678–685. [Google Scholar] [CrossRef][Green Version]
- Jimoda, L.; Sonivare, J.; Akeredolu, F.A. Emission Inventory of Anthropogenic Activities at Southeastern Lagos. Ife J. Technol. 2009, 18, 35–541. [Google Scholar]
- Oketola, A.; Osibanjo, O. Assessment of Industrial Pollution Load in Lagos, Nigeria by Industrial Pollution Projection System (IPPS) versus Effluent Analysis; Bronicewcz, E., Ed.; Intech Open Book Series; Chap/10; Available online: www.Intechopen.com/books/213 (accessed on 10 February 2022).
- Marais, E.; Jacob, D.; Wecht, K.; Lerot, C.; Zhang, L.; Yu, K.; Kurosu, T.P.; Chance, K.; Sauvage, B. Anthropogenic emissions in Nigeria and implications for atmospheric ozone pollution: A view from space. Atmos. Environ. 2014, 99, 22–40. [Google Scholar] [CrossRef]
- Amadiou, C.; Omotosho, B.; Coulialy, A.; Ballo, A. Characteristics of land and sea breezes along the Guinea Coast of West Africa. Theor. Appl. Climatol. 2019, 188, 953–971. [Google Scholar]
- Gbambie, A.; Steyn, G. Sea breezes at Cotonou and their interaction with the West African Monsoon. Intl. J. Clim. 2012, 33, 2889–2899. [Google Scholar] [CrossRef]
- Climate Data for Lagos, Nigeria. Available online: www.lagos.climatemps.com/sunlight.php (accessed on 20 February 2022).
- US Environmental Protection Agency. Carbon Monoxide Trends. Available online: www.epa.gov/air-trends/carbon-monoxide-trends (accessed on 11 February 2022).
- Njoku, K.; Rumide, T.; Akinola, M.; Adesuyi, A.; Jolaoso, A. Ambient Air Quality Monitoring in Metropolitan City of Lagos, Nigeria. J. Appl. Sci. Environ. Manag. 2016, 20, 178–185. [Google Scholar] [CrossRef]
- Olajire, A.; Azeez, L.; Oluyemi, E. Exposure to hazardous air pollutants along Oba Akran Road, Lagos-Nigeria. Chemosphere 2011, 84, 1044–1051. [Google Scholar] [CrossRef]
- Obanya, H.; Ameeze, N.; Otitoloju, A. Air Pollution Monitoring Around Residential and Transportation Sector Locations in Lagos, Mainland. J. Health Poll. 2018, 8, 9180903. [Google Scholar] [CrossRef]
- Abulude, F.; Damodharen, U.; Acha, S.; Adamu, A.; Arifalo, K. Preliminary Assessment of Air Pollution Quality Levels of Lagos. Aerosol Sci. Eng. 2021, 5, 275–284. [Google Scholar] [CrossRef]
- George, L. Nigeria to Cut Sulfur in Fuels a Year after U.N. Deadline. 2018. Available online: www.reuters.com/article/us-nigeria-idUSKNGP1HQ (accessed on 15 February 2022).
- Ogundairo, F. Nigeria: Petrol, Diesel Imported from Europe Dirtier Than Fuel from Illegal Refineries—Report. 2020. Available online: http://allafrica.com/stories/202007030199.html (accessed on 20 February 2022).
- Yao, X.; Lau, N.; Chan, C.; Fang, M. The use of tunnel concentration profile data to determine the ratio of NO2/NOx directly emitted from vehicles. Atmos. Chem. Phys. Discuss. 2005, 5, 12373–12740. [Google Scholar]
- Sillman, S.; He, D.; Cardelino, C.; Imhoff, R.E. The use of photochemical indicators to evaluate ozone-NOx-hydrocarbon sensitivity: Case studies from Atlanta, New York, and Los Angeles. J. Air Waste Manag. Assoc. 1997, 47, 1030–1040. [Google Scholar] [CrossRef]
- Blanchard, C.; Hidy, G.; Tanenbaum, S.; Rasmussen, R.; Watkins, R.; Edgerton, E. NMOC, ozone, and organic aerosol in the southeastern United States 1999–2007: 1. Spatial and temporal variations of NMOC concentrations and composition in Atlanta, Georgia. Atmos. Environ. 2010, 44, 4840–4849. [Google Scholar] [CrossRef]
- Olajire, A.; Azeez, L. Source apportionment and ozone formation potential of volatile organic compounds in Lagos, Nigeria. Chem. Ecol. 2014, 30, 156–168. [Google Scholar] [CrossRef]
- Drozd, G.T.; Zhao, Y.; Saliba, G.; Frodin, B.; Maddox, C.; Weber, R.J.; Chang, M.-C.O.; Maldonado, H.; Sardar, S.; Robinson, A.L.; et al. Time resolved measurements of speciated tail pipe emissions from motor vehicles: Trends with emission control technology, cold start effects, speciation. Environ. Sci. Technol. 2016, 50, 13592–13599. [Google Scholar] [CrossRef]
- Hidy, G.M.; Blanchard, C.L.; Baumann, K.; Edgerton, E.; Tanenbaum, S.; Shaw, S.; Knipping, E.; Tombach, I.; Jansen, J.; Walters, J. Chemical climatology of the southeastern United States, 1999–2013. Atmos. Chem. Phys. 2014, 14, 11823–11914. [Google Scholar] [CrossRef]
- Sauvage, B.; Gheusi, F.; Thouret, V.; Cammas, J.-P.; Duron, J.; Escobar, J.; Mari, C.; Mascart, P.; Pont, V. Medium-range mid-tropospheric transport of ozone and precursors over Africa: Two numerical case studies in dry and wet seasons. Atmos. Chem. Phys. 2007, 7, 53757–53770. [Google Scholar] [CrossRef]
- Minga, A.; Thouet, V.; Saunola, M.; Dlon, C.; Serca, D.; Mari, C.; Sauvage, B.; Mariscal, A.; Leriche, M.; Cros, B. What caused extreme ozone concentrations over Coutonou in December 2005? Atmos. Chem. Phys. 2010, 10, 895–907. [Google Scholar] [CrossRef]
- Menut, L.; Flamant, C.; Turquety, S.; Derioubaix, A.; Chazette, P.; Meynadier, R. Impact of biomass burning on pollutant surface concentrations in mega-cities of the Gulf of Guinea. Atmos. Chem. Phys. 2018, 18, 2687–2707. [Google Scholar] [CrossRef]
- Offor, H.; Adie, G.; Ana, G. Review of Particulate Matter and Elemental Composition of Aerosols at Selected Locations in Nigeria from 1985–2015. J. Health Pollut. 2016, 6, 1–18. [Google Scholar] [CrossRef]
- Ezeh, G.; Obioh, I.; Asubiojo, O. Trace Matals and Source Identification of Air-Borne Particulate Matter Pollution in a Nigerian Megacity. J. Environ. Toxicol. 2017, 7, 3. [Google Scholar] [CrossRef]
- Taiwo, A.; Arowolo, T.; Abdullah, K.; Taiwo, O. Particulate Matter Pollution in Nigeria. In Proceedings of the 14th Conference on the Environmental Influence of Atmospheric Science and Technology, Rhodes, Greece, 3–5 September 2015; Available online: https://cest2015.gnest.org/papers/cest2015_poster_paper.pdf (accessed on 25 January 2022).
- Owoade, O.K.; Fawole, O.G.; Olise, F.S.; Ogundele, L.T.; Olaniyi, H.B.; Almeida, M.S.; Ho, M.D.; Hopke, P.K. Characterization and source identification of airborne particulate loadings at receptor site-classes of Lagos Mega-City, Nigeria. J. Air Waste Manag. Assoc. 2013, 63, 1026–1035. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change; John Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Interagency Monitoring of Protected Visual Environments (IMPROVE). Available online: http://vista.cira.colostate.edu/Improve/ (accessed on 10 February 2022).
- Landis, M.; Lewis, C.; Stevens, R.; Keeler, R.; Dvonch, J.; Trembly, R.T. Ft. McHenry tunnel study: Source profiles and mercury emissions from diesel and gasoline powered vehicles. Atmos. Environ. 2007, 41, 8711–8724. [Google Scholar] [CrossRef]
- Leck, C.; Engardt, M.; Heintzenburg, J. A meridional profile of the chemical composition of submicrometre particles over the East Atlantic Ocean: Regional and hemispheric variabilities. Tellus 2002, 54B, 377–394. [Google Scholar] [CrossRef]
- Oluleye, A.; Jimoh, O. Influence of atmospheric circulation patterns on dust transport during the Harmattan Period in West Africa. Pollution 2018, 4, 9–27. [Google Scholar]
- Chukwuemeka, M.; Adenola, E. Variability of Harmattan Dust Haze Over Northern Nigeria. J. Pollut. 2018, 1, 1000107. [Google Scholar]
- Oluyemi, E.; Asubojo, O. Ambient Air Particulate Matter in Lagos, Nigeria: A Study Using Receptor Modeling with X-ray Fluorescent Analysis. Bull. Chem. Soc. Ethiop. 2001, 15, 97–108. [Google Scholar]
- Hidy, G.M. Worldwide aerosol chemistry: From hemispheric distributions to megacity sources. J. Air Waste Manag. Assoc. 2009, 59, 770–789. [Google Scholar] [CrossRef]
- Shaw, R., Jr.; Stevens, R.; Bowermaster, J.; Tesch, J.; Tew, E. Measurements of atmospheric nitrate and nitric acid: The Denuder Difference Experiment. Atmos. Environ. 1982, 16, 845–855. [Google Scholar] [CrossRef]
- Ashbaugh, L.; Eldred, R. Loss of Particle Nitrate from Teflon Sampling filters. Effects on Measured Gravimetric mass in California and in the IMPROVE network. J. Air Waste Manag. Assoc. 2002, 54, 93–104. [Google Scholar] [CrossRef]
- Lim, H.; Turpin, B. Origins of primary and secondary organic aerosol in Atlanta: Results of time-resolved measurements during the Atlanta supersite experiment. Environ. Sci. Technol. 2002, 36, 4489–4490. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Kuhns, H.; Etyemezian, V.; Lowenthal, D.H.; Crow, D.; Kohl, S.D.; Engelbrecht, J.P.; Green, M.C. Source profiles for industrial, mobile, and additional sources in Big Bend Regional Visibility and Observation Study. Chemosphere 2014, 554, 185–208. [Google Scholar]
- Atiku, F.; Mitchell, E.; Le-Langton, A.; Jones, J.; Williams, A.; Bartle, K. The Impact of Fuel Properties on the Composition of Soot Produced by the Combustion of Residential Solid Fuels in a Domestic Stove. Fuel Process. Technol. 2016, 151, 117–125. [Google Scholar] [CrossRef]
- Sirignano, C.; Riccio, A.; Chianese, E.; Ni, H.; Zenker, K.; D’Onofrio, A.; Meijer, H.A.J.; Dusek, U. High Contribution of Biomass Combustion to PM2.5 in the City Centre of Naples (Italy). Atmosphere 2019, 10, 451. [Google Scholar] [CrossRef]
- Robinson, A.L.; Donahue, N.M.; Shrivastava, M.K.; Weitkamp, E.A.; Sage, A.M.; Grieshop, A.P.; Lane, T.E.; Pierce, J.R.; Pandis, S.N. Rethinking organic aerosols: Semi-volatile emissions and photochemical aging. Science 2007, 315, 1259–1262. [Google Scholar] [CrossRef]
- Wang, M.; Huang, R.-J.; Cao, J.; Dai, W.; Zhou, J.; Lin, C.; Ni, H.; Duan, J.; Wang, T.; Chen, Y.; et al. Determination of n-alkanes, polycyclic aromatic hydrocarbons and hopanes in atmospheric aerosol: Evaluation and comparison of thermal desorption GC-MS approaches. Atmos. Meas. Tech. 2019, 12, 4779–4789. [Google Scholar] [CrossRef]
- Brown, S.G.; Herckes, P.; Ashbaugh, L.; Hannigan, M.P.; Kreidenweis, S.M.; Collett, J.L., Jr. Characterization of organic aerosol in Big Bend National Park, Texas. Atmos. Environ. 2002, 36, 5807–5813. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. Characterization of organic constituents in aerosols in relation to their origin and transport: A review. Int. J. Environ. Analyt. Chem. 1986, 23, 207–237. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Li, H.; Wang, X.; Wang, W.; Wang, K.; Ge, M. Long-chain alkanes in the atmosphere: A review. J. Environ. Sci. 2022, 114, 37–52. [Google Scholar] [CrossRef]
- He, D.; Simoneit, B.; Jara, B.; Jaffe, R. Composition and isotopic differences of iso- and anteiso-alkanes in black mangroves (Avicennia germinans) across a salinity gradient in a subtropical estuary. Environ. Chem. 2015, 13, 6230630. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 5. C1–C32 organic compounds from gasoline-powered motor vehicles. Environ. Sci. Technol. 2002, 36, 1169–1180. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 2. C1 through C30 organic compounds from medium duty diesel trucks. Environ. Sci. Technol. 1999, 33, 1578–1587. [Google Scholar] [CrossRef]
- Popa, O.; Babeano, N.; Popa, I.; Nita, S.; Dinu-Parvu, C. Methods for obtaining and determination of squalene from natural sources. Biomed. Res. Int. 2015, 2015, 367202. [Google Scholar] [CrossRef]
- Budsaereechai, S.; Hunt, A.; Ngernyen, Y. Catalytic pyrolysis of plastic waste for the production of liquid fuels in engines. RSC Adv. 2019, 9, 5844–5857. [Google Scholar] [CrossRef]
- Rogge, W.; Hildemann, L.; Mazurek, M.; Cass, G.; Simoneit, B. Sources of fine organic aerosol. 1. Charbroilers and meat cooking operations. Environ. Sci. Technol. 1991, 25, 1112–1125. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 3. C1–C29 organic compounds from fireplace combustion of wood. Environ. Sci. Technol. 2001, 35, 1716–1728. [Google Scholar] [CrossRef]
- Kawamura, K.; Bikkina, S. A review of dicarboxylic acids and related compounds in atmospheric aerosols: Molecular distributions, sources and transformation. Atmos. Res. 2016, 170, 140–160. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, M.; Zhang, Y.; Wei, Y.; Hu, M.; Guo, S. Source apportionment of fine organic aerosols in Beijing. Atmos. Chem. Phys. 2009, 9, 8573–8585. [Google Scholar] [CrossRef]
- Tao, S.; Yin, X.; Jiao, L.; Zhao, S.; Chen, L. Temporal Variability of Source-Specific Solvent-Extractable Organic Compounds in Coastal Aerosols over Xiamen, China. Atmosphere 2017, 8, 33. [Google Scholar] [CrossRef]
- Simoneit, B.; Schauer, J.; Nolte, C.; Oros, D.; Elias, V.; Fraser, M.; Rogge, W.; Cass, G. Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles. Atmos. Environ. 1999, 33, 173–182. [Google Scholar] [CrossRef]
- Kemmo, S.; Ollilainen, V.; Lamopi, A.-M.; Piiromem, V. Determination of sigmasterol and cholesterol using atmospheric pressure chemical ionization liquid chromatography/mass spectroscopy. Food Chem. 2007, 10, 1438–1445. [Google Scholar] [CrossRef]
- Lee, A.; Lau, A.; Cheng, J.; Fang, M.; Chan, C. Source identification analysis for the airborne bacteria and fungi using a biomarker approach. Atmos. Environ. 2007, 41, 2831–2843. [Google Scholar] [CrossRef]
- Lim, Y.; Ziemann, P. Chemistry of Secondary Organic Aerosol Formation from OH Radical-initiated Reactions of Linear, Branched, and Cyclic alkanes in the presence of NO. Aerosol Sci. Technol. 2009, 43, 604–619. [Google Scholar] [CrossRef]
- Tkacik, D.; Presto, A.; Donohue, N.; Robinson, A. Secondary organic aerosol formation from intermediate-volatility organic compounds: Cyclic, linear and branched alkanes. Environ. Sci. Technol. 2012, 46, 8773–8781. [Google Scholar] [CrossRef]
- Fehsenfeld, F.; Hastie, D.; Chow, J.; Solomon, P. Particle and Gas Measurements. In Particulate Matter Science for Policy Makers; McMurry, P., Shepherd, M., Vickery, J., Eds.; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Jiminez, O.; Perez-Pastor, R.; Garcia-Alonso, S. Assessment uncertainty associated to the analysis of organic compounds linked to particulate matter of atmospheric aerosols. Talanta 2010, 80, 1121–1128. [Google Scholar] [CrossRef]
- Yttri, K.E.; Schnelle-Kreis, J.; Maenhaut, W.; Abbaszade, G.; Alves, C.; Bjerke, A.; Bonnier, N.; Bossi, R.; Claeys, M.; Dye, C.; et al. An intercomparison study of analytical methods used for quantification of levoglucosan in ambient filter samplers. Atmos. Meas. Tech. 2015, 8, 125–147. [Google Scholar] [CrossRef]
- Schantz, M.; Cleveland, D.; Heckert, N.; Kucklick, J.; Leigh, S.; Long, S.; Lynch, J.; Murphy, K.; Olfaz, R.; Pintar, A.; et al. Development of two particulate matte standard reference materials (<4 µm and <10 µm) for the determination of organic and inorganic constituents. Anal. Bioanal. Chem. 2016, 408, 4257–4266. [Google Scholar] [CrossRef]
- Mancilla, Y.; Medina, G.; Gonzalez, L.; Herckes, P.; Fraser, M.; Mendoza, A. Determination and Similarity Analysis of PM2.5 Emission Source Profiles Based on Organic Markers for Monterrey, Mexico. Atmosphere 2021, 12, 554. [Google Scholar] [CrossRef]
- Parshintsev, J.; Hyotylainen, T. Methods for characterization of organic compounds in atmospheric aerosol particles. Anal. Bioanal. Chem. 2015, 407, 5877–5897. [Google Scholar] [CrossRef] [PubMed]
- Rogge, W.; Ondov, J.; Bernardo-Bricker, A.; Sevimogu, O. Baltimore PM2.5 Supersite: Highly time-resolved organic compounds-sampling duration and phase distribution-implications for health effects studies. Anal. Bioanal. Chem. 2011, 401, 3069–3082. [Google Scholar] [CrossRef] [PubMed]
- Cretescu, I.; Lutic, D.; Manea, L. Electrochemical Sensors for Monitoring of Indoor and Outdoor Air Pollution. In Electrochemical Sensors Technology; Muzibu, R., Asiri, A., Eds.; Intechopin: London, UK, 2017. [Google Scholar] [CrossRef]
- Popoola, O. Studies of Urban Air Quality Using Electrochemical Based Sensor Instruments. Ph.D. Dissertation, Queens College, University of Cambridge, Cambridge, UK, 2012. [Google Scholar]
- Mead, M.I.; Popoola, O.A.M.; Stewart, G.B.; Landshoff, P.; Calleja, M.; Hayes, M.; Baldovi, J.J.; McLeod, M.W.; Hodgson, T.F.; Dicks, J.; et al. The use of electrochemical sensors for monitoring urban air quality in low-cost, high density networks. Atmos. Environ. 2013, 70, 186–203. [Google Scholar] [CrossRef]
- Jiao, W.; Hagler, G.; Williams, R.; Sharpe, R.; Brown, R.; Garver, D.; Judge, R.; Caudill, M.; Rickard, J.; Davis, M.; et al. Community Air Sensor Network (CAIRSENSE) project: Evaluation of low-cost sensor performance in a suburban environment in the southeastern United States. Atmos. Meas. Tech. 2016, 9, 5281–5292. [Google Scholar] [CrossRef]
- Taylor, J.; Henshaw, G.; Aeroqual, Aukland, New Zealand. AQM 65 Colocation Field Study Hartford, CT, USA; Unpublished Report. 2017.
- Analytical Methods Committee. What Should Be Done with Results below the Detection Limit? Mentioning the Unmentionable; Tech. Brief No. 5; Royal Society of Chemistry: London, UK, 2001. [Google Scholar]
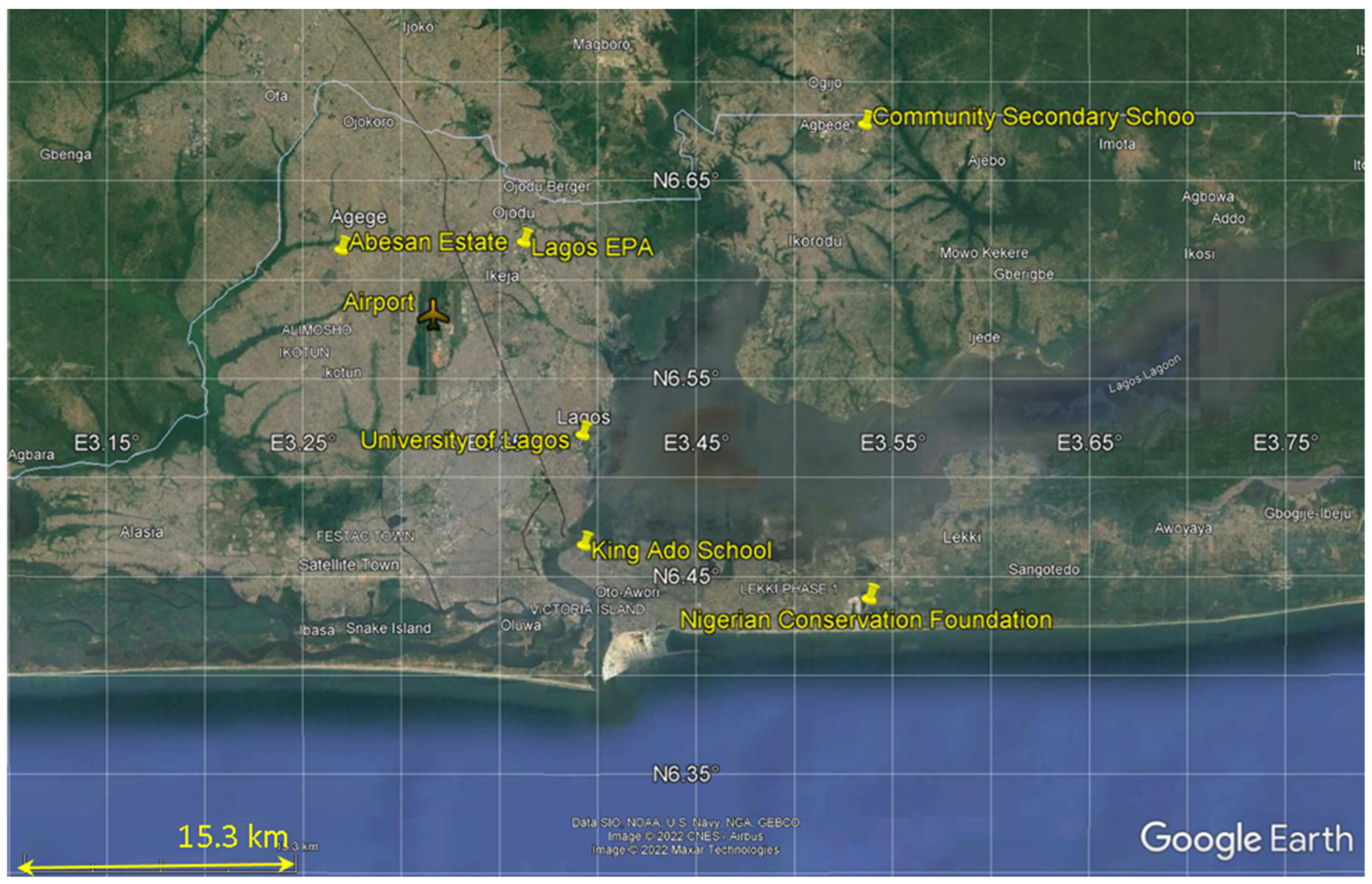




| No. | Key: Name and District | Latitude | Longitude | Site Characteristics |
|---|---|---|---|---|
| 1. | University of Lagos (UNI), Akoka | 6.515500° N | 3.390450° E | Urban scale: Business/residential/institutional |
| 2. | Nigerian Conservation Foundation (NCF), Lekki | 6.432200° N | 3.535917° E | Regional scale: low-density residential; near coast |
| 3. | Abesan Estate (ABE), Ipaia | 6.609062° N | 3.267706° E | Neighborhood scale: High-density residential, school environment |
| 4. | King Ado School (JAN), Jankara | 6.459800° N | 3.391100° E | Commercial scale near seaport: High vehicular traffic and high-density residential |
| 5. | Community Secondary School (IKO), Ikorodu | 6.672360° N | 3.534030° E | Neighborhood scale: Industrial low-density residential |
| 6. | Lagos State EPA (EPA), Alausa | 6.612873° N | 3.360596° E | Urban scale: Institutional, business district with moderate traffic |
| Location | Air Temperature (°C) | Relative Humidity (%) | Wind Speed (ms−1) | |||
|---|---|---|---|---|---|---|
| Range | Mean | Range | Mean | Range | Mean | |
| ABE | 22.9–29.6 | 26.9 | 51.0–95.0 | 87.7 | 0.0–1.8 | 0.8 |
| IKO | 21.9–31.9 | 26.7 | 56.0–96.0 | 87.6 | 0.3–3.7 | 1.7 |
| JAN | 23.4–31.4 | 27.9 | 49.0–91.0 | 83.6 | 0.1–3.4 | 1.2 |
| EPA | 22.8–35.0 | 27.5 | 50.0–95.0 | 84.8 | 0.0–3.4 | 1.4 |
| NCF | 23.4–29.8 | 27.3 | 69.0–96.0 | 88.3 | 0.1–3.9 | 1.7 |
| UNI | 23.1–30.0 | 27.1 | 54.0–94.0 | 86.6 | 0.1–4.1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odu-Onikosi, A.; Herckes, P.; Fraser, M.; Hopke, P.; Ondov, J.; Solomon, P.A.; Popoola, O.; Hidy, G.M. Tropical Air Chemistry in Lagos, Nigeria. Atmosphere 2022, 13, 1059. https://doi.org/10.3390/atmos13071059
Odu-Onikosi A, Herckes P, Fraser M, Hopke P, Ondov J, Solomon PA, Popoola O, Hidy GM. Tropical Air Chemistry in Lagos, Nigeria. Atmosphere. 2022; 13(7):1059. https://doi.org/10.3390/atmos13071059
Chicago/Turabian StyleOdu-Onikosi, Adebola, Pierre Herckes, Matthew Fraser, Philip Hopke, John Ondov, Paul A. Solomon, Olalekan Popoola, and George M. Hidy. 2022. "Tropical Air Chemistry in Lagos, Nigeria" Atmosphere 13, no. 7: 1059. https://doi.org/10.3390/atmos13071059
APA StyleOdu-Onikosi, A., Herckes, P., Fraser, M., Hopke, P., Ondov, J., Solomon, P. A., Popoola, O., & Hidy, G. M. (2022). Tropical Air Chemistry in Lagos, Nigeria. Atmosphere, 13(7), 1059. https://doi.org/10.3390/atmos13071059








