Exposure to Aerosols Emitted from Common Heating Combustion Sources Indoors—The Jordanian Case as an Example for Eastern Mediterranean Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement Setup
2.1.1. Instrumentation
2.1.2. Data Handling
- 10–25 nm (the difference between the CPC and the P-Trak)
- 25–300 nm (the difference between the P-Trak and the Aerotrak)
- Six channels taken directly from the AeroTrak (0.3–0.5 µm, 0.5–1 µm, 1–2.5 µm, 2.5–5 µm, 5–10 µm, and 10–25 µm)
2.2. Heating Combustion Scenarios
2.2.1. Natural Gas Heater
2.2.2. Kerosene Heater
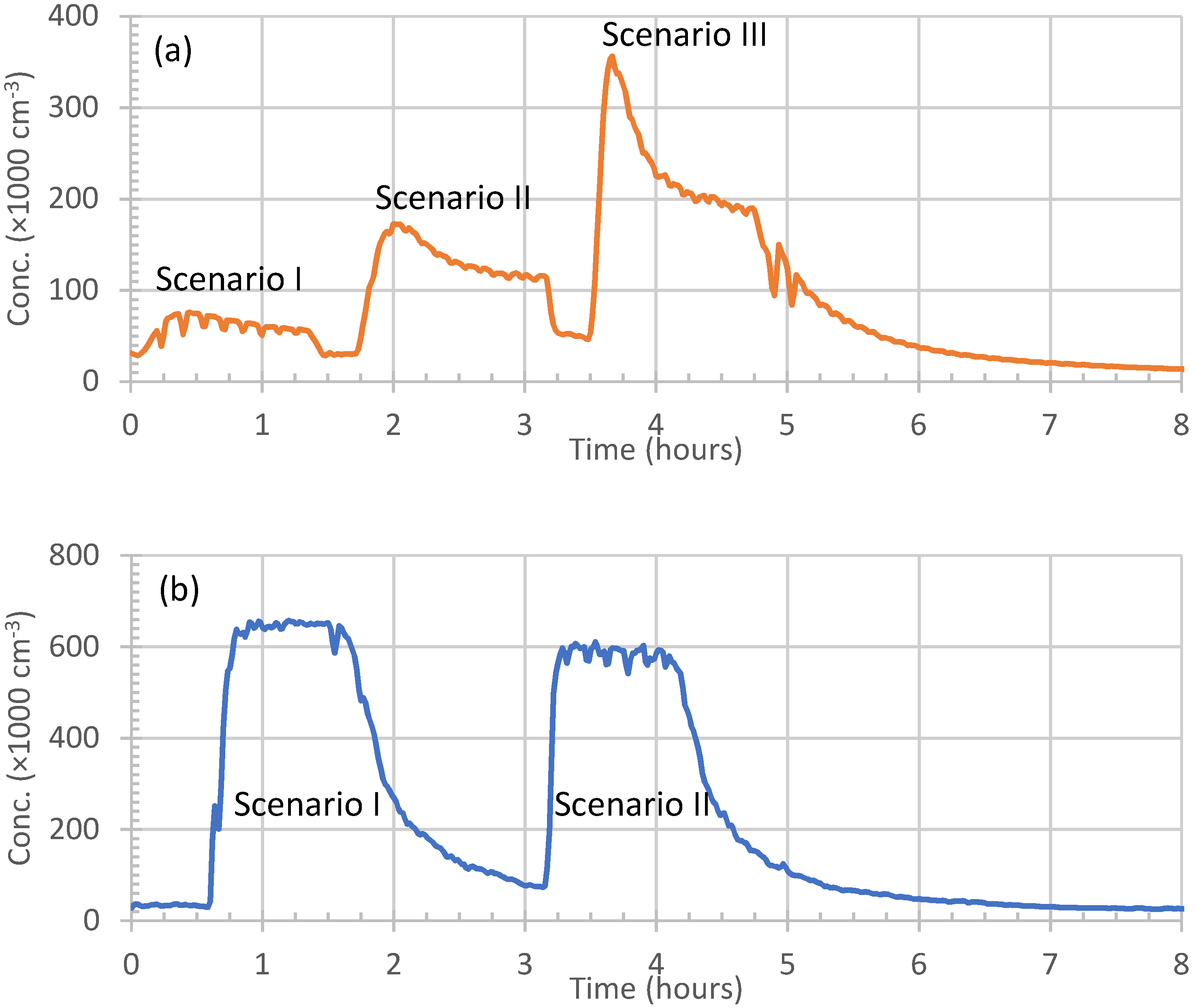
- Scenario 1: the heater was ignited inside the room, and allowed to reach its maximum heat. It operated at its maximum heat for about one hour, and then it was taken out of the room until the indoor aerosols concentrations returned to their pre-Scenario 1 level (this took 1.5 h).
- Scenario 2: the heater was ignited outdoors until it reached its maximum heat, and was then taken inside the room to operate for about one hour. Then, it was taken back outside, and the indoor aerosols concentrations were left to reach their pre-Scenario 2 background level.
2.3. Simple Indoor Aerosol Model
2.3.1. Particle Losses
2.3.2. Emission Rates
3. Results and Discussion
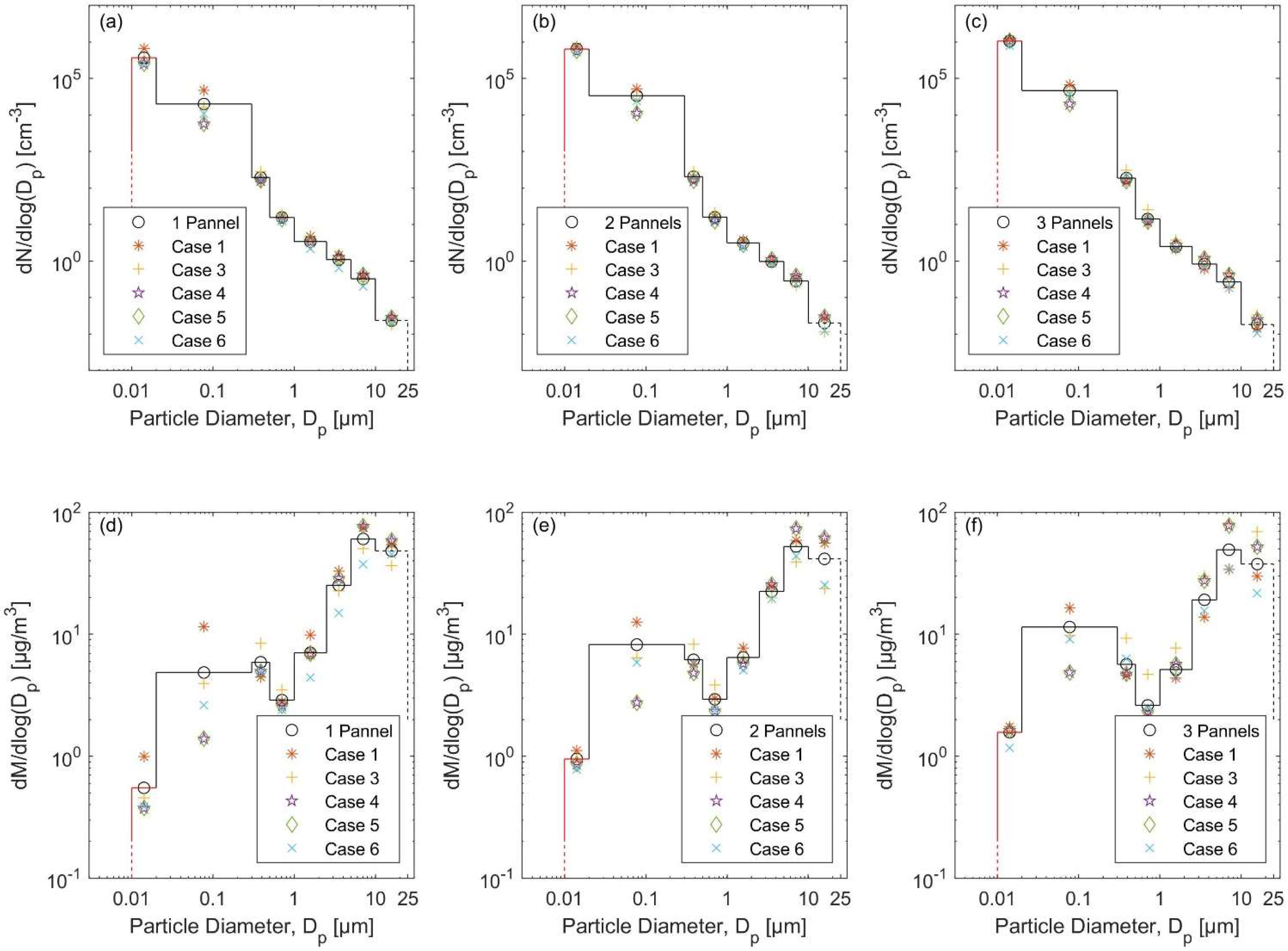
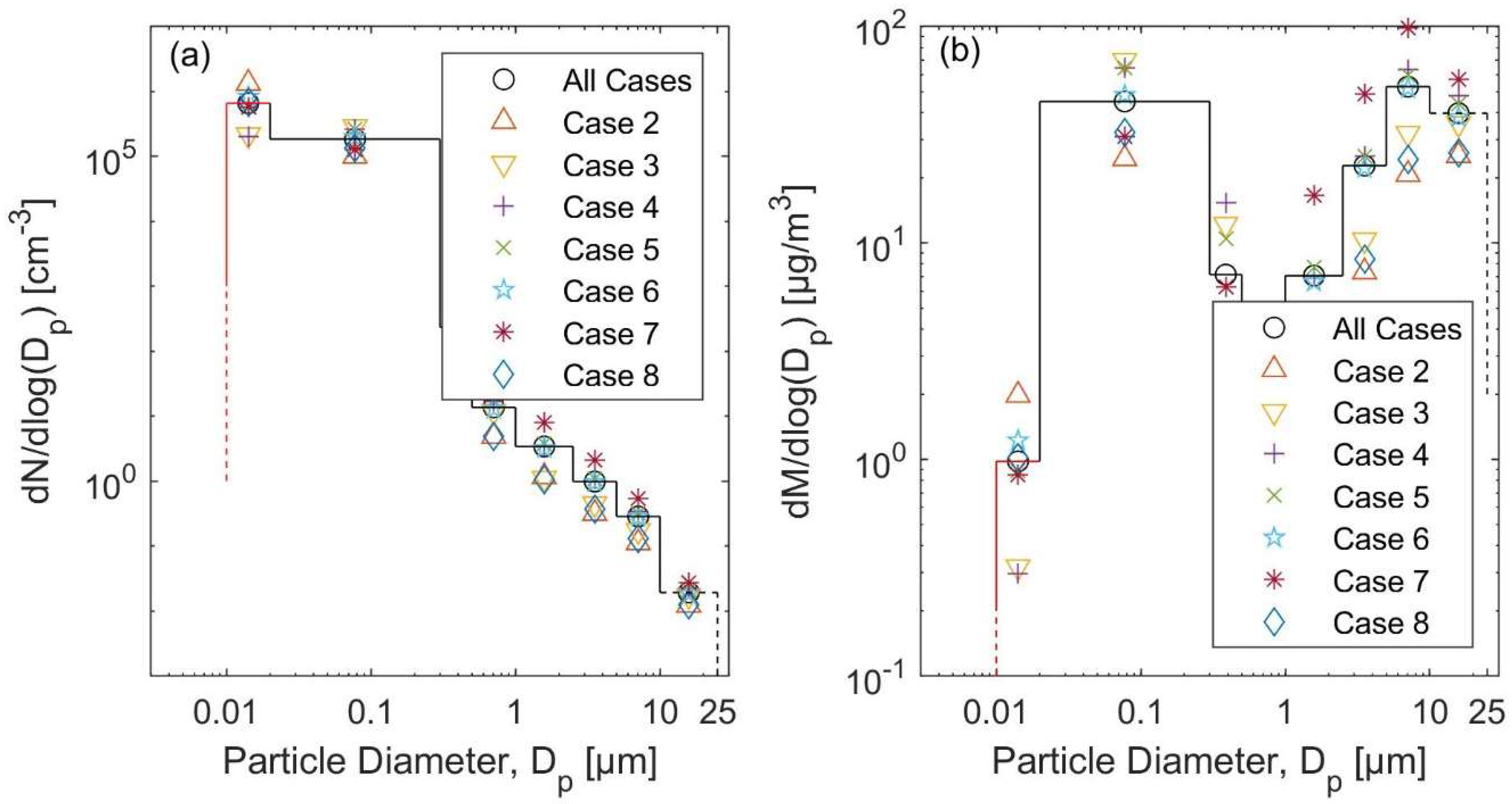
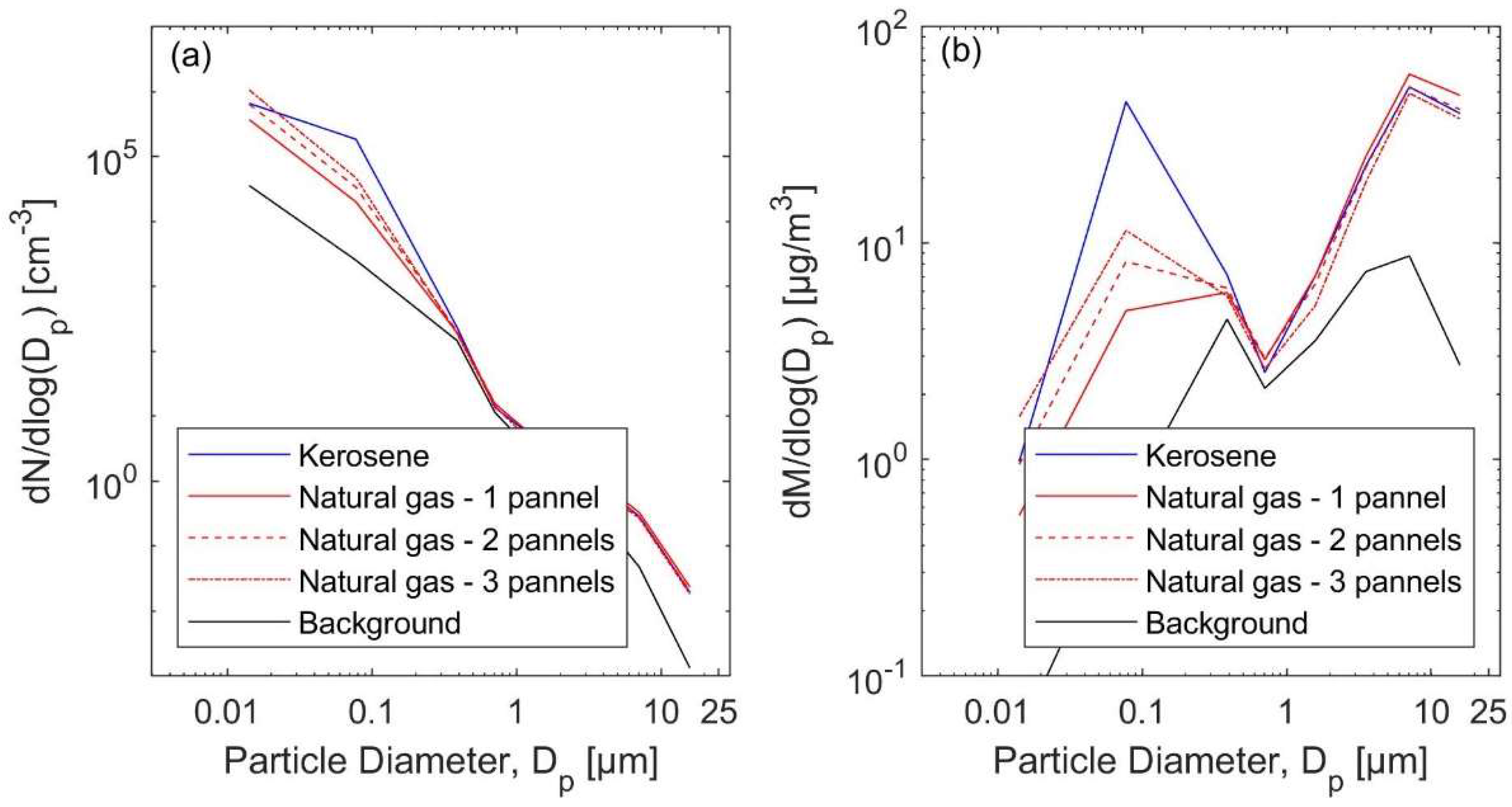
3.1. Total Particle Concentrations and Particle Size Distributions
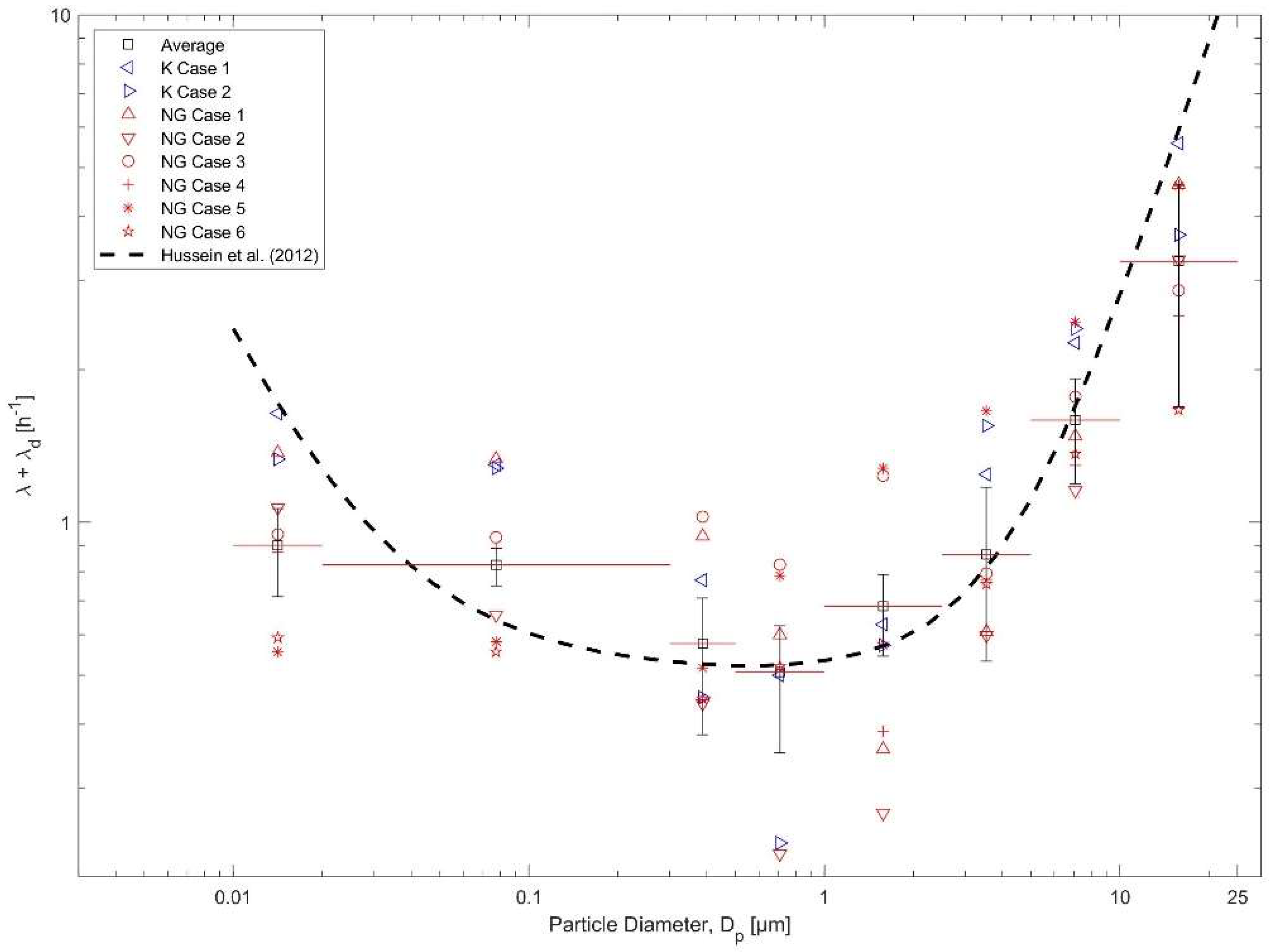
3.2. Particle Losses
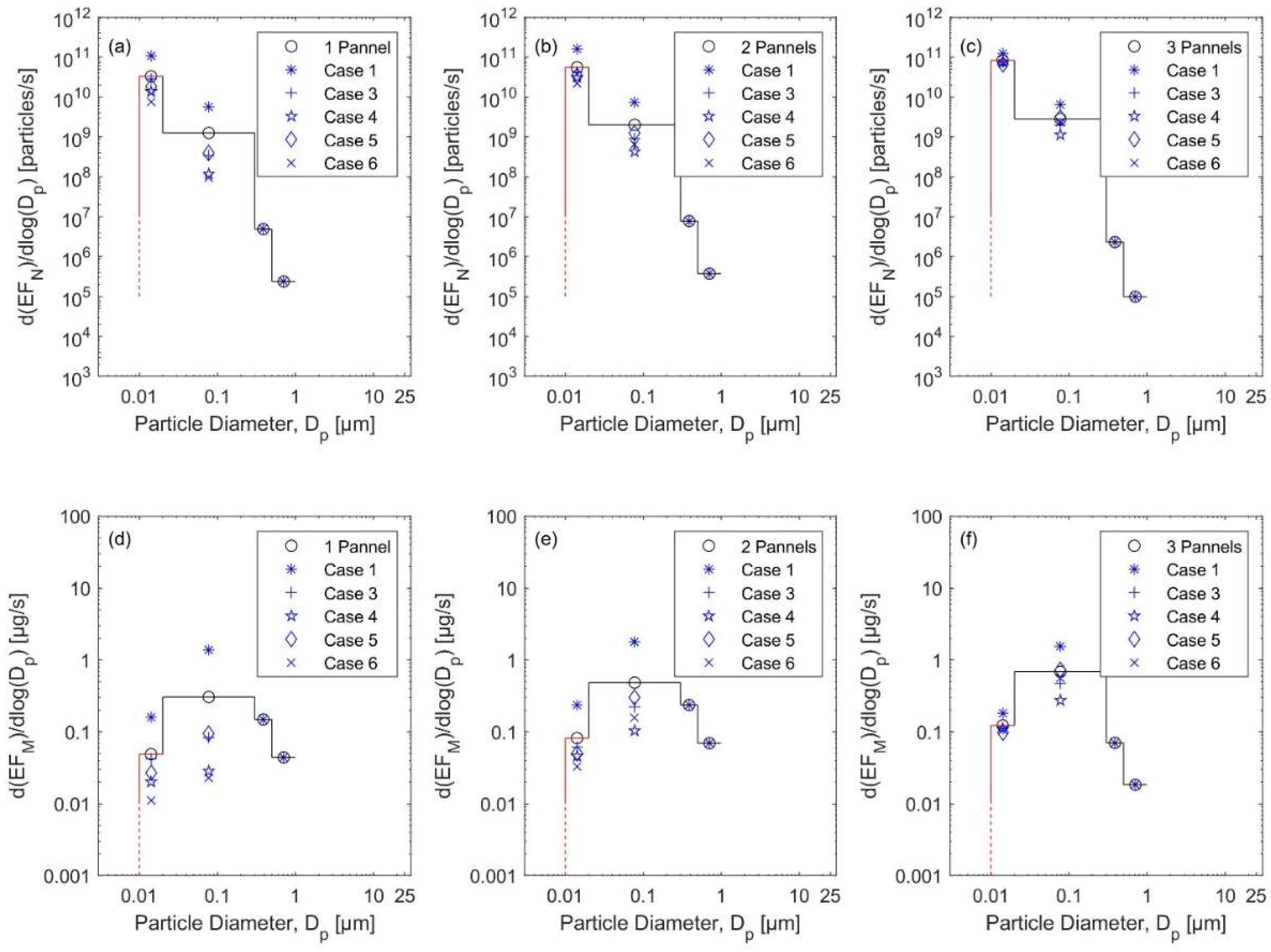
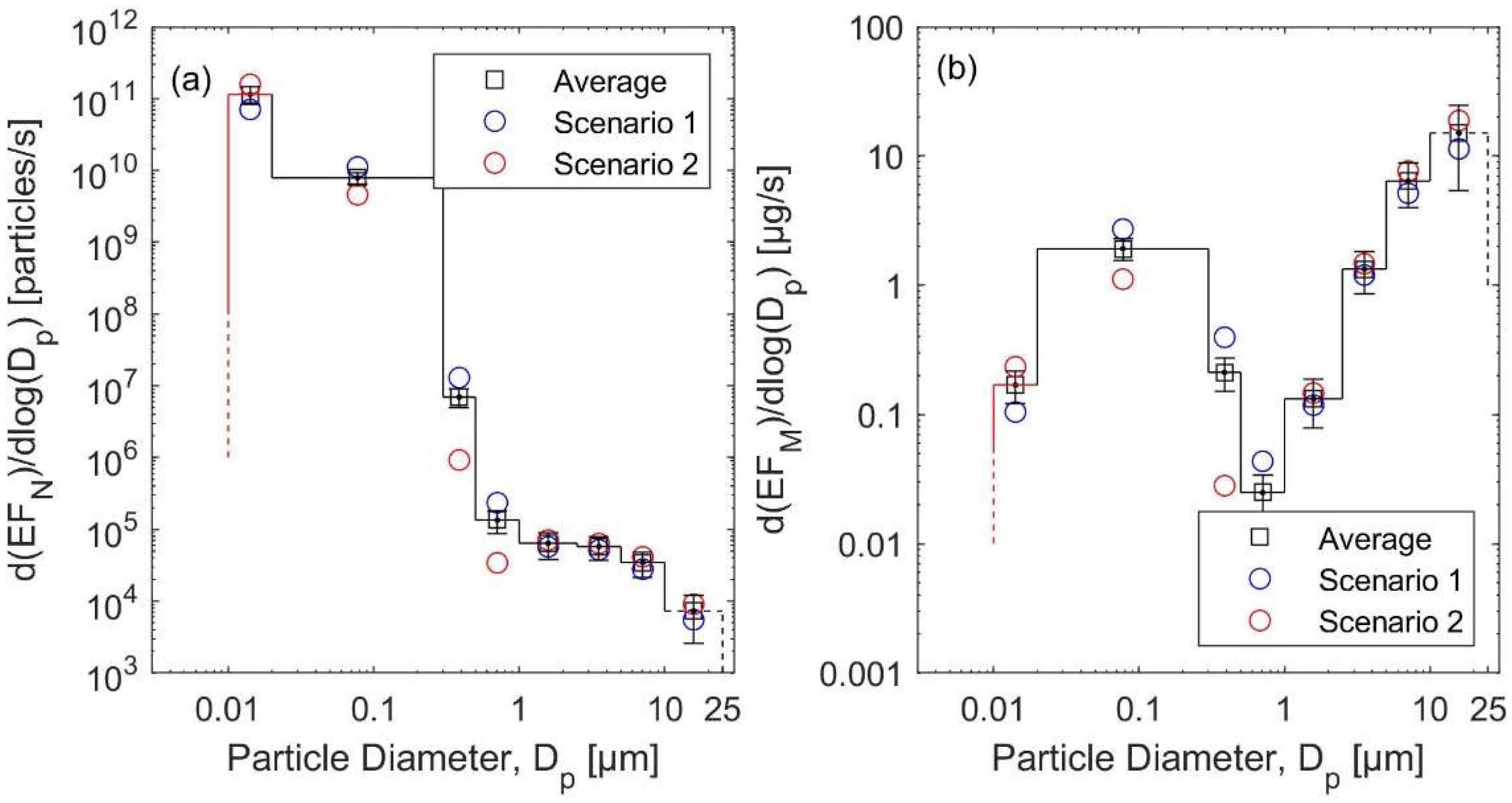
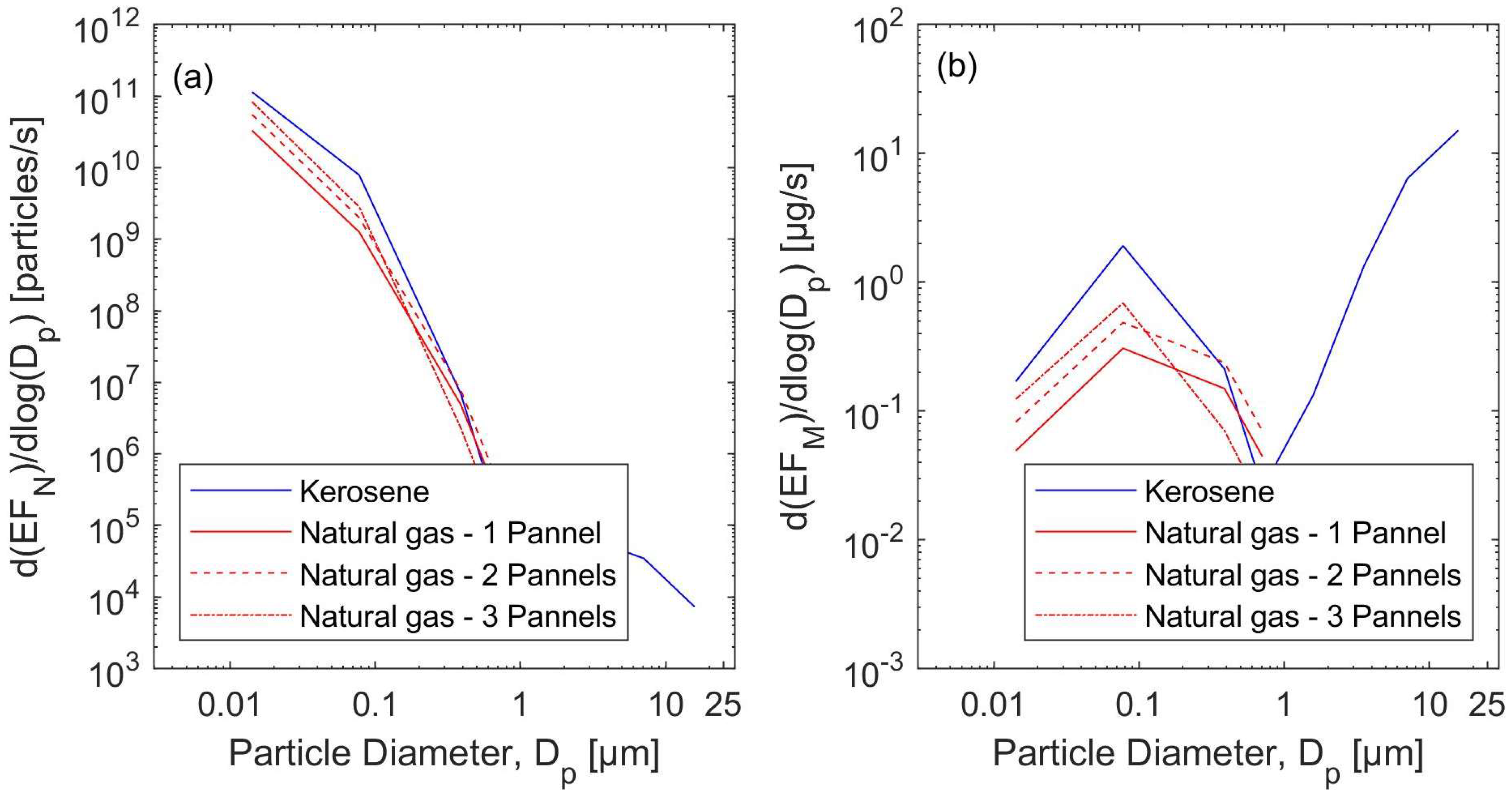
3.3. Emission Rates
3.3.1. The Natural Gas Heater Scenarios
3.3.2. The Kerosene Heater Scenarios
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schilmann, A.; Ruiz-García, V.; Serrano-Medrano, M.; de la Sierra de la Vega, L.A.; Olaya-García, B.; Estevez-García, J.A.; Berrueta, V.; Horacio Riojas-Rodríguez, H.; Masera, O. Just and fair household energy transition in rural Latin American households: Are we moving forward? Environ. Res. Lett. 2021, 16, 105012. [Google Scholar] [CrossRef]
- Vardoulakis, S.; Giagloglou, E.; Steinle, S.; Davis, A.; Sleeuwenhoek, A.; Galea, K.S.; Dixon, K.; Crawford, J.O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. [Google Scholar] [CrossRef]
- Lam, N.L.; Smith, K.R.; Gauthier, A.; Bates, M.N. Kerosene: A review of household uses and their hazards in low- and middle-income countries. J. Toxicol. Environ. Health Part B 2012, 15, 396–432. [Google Scholar] [CrossRef]
- Leaderer, B.P.; Naeher, L.; Jankun, T.; Balenger, K.; Holford, T.R.; Toth, C.; Sullivan, J.; Wolfson, J.M.; Koutrakis, P. Indoor, outdoor, and regional summer and winter concentrations of PM10, PM2.5, SO4(2)−, H+, NH4+, NO3−, NH3, and nitrous acid in homes with and without kerosene space heaters. Environ. Health Perspect. 1999, 107, 223–231. [Google Scholar] [CrossRef]
- Leaderer, B.P. Air pollutant emissions from kerosene space heaters. Science 1982, 218, 1113–1115. [Google Scholar] [CrossRef]
- Leaderer, B.P.; Zagraniski, R.T.; Berwick, M.; Stolwijk, J.A. Assessment of exposure to indoor air contaminants from combustion sources: Methodology and application. Am. J. Epidemiol. 1986, 124, 275–289. [Google Scholar] [CrossRef]
- Ruiz, P.A.; Toro, C.; Caceres, J.; Lopez, G.; Oyola, P.; Koutrakis, P. Effect of gas and kerosene space heaters on indoor air quality: A study in homes of Santiago, Chile. J. Air Waste Manag. Assoc. 2010, 60, 98–108. [Google Scholar] [CrossRef]
- Kliucininkas, L.; Martuzevicius, D.; Krugly, E.; Prasauskas, T.; Kauneliene, V.; Molnar, P.; Strandberg, B. Indoor and outdoor concentrations of fine particles, particle-bound PAHs and volatile organic compounds in Kaunas, Lithuania. J. Environ. Monit. 2011, 13, 182–191. [Google Scholar] [CrossRef]
- Villanueva, F.; Tapia, A.; Amo-Salas, M.; Notario, A.; Cabañas, B.; Martínez, E. Levels and sources of volatile organic compounds including carbonyls in indoor air of homes of Puertollano, the most industrialized city in central Iberian Peninsula. Estimation of health risk. Int. J. Hyg. Environ. Health 2015, 218, 522–534. [Google Scholar] [CrossRef]
- Alexopoulos, E.C.; Chatzis, C.; Linos, A. An analysis of factors that influence personal exposure to toluene and xylene in residents of Athens, Greece. BMC Public Health 2006, 6, 50. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.S.; Park, M.R.; Lee, S.W.; Kim, E.H.; Cho, J.B.; Kim, J.; Han, Y.; Jung, K.; Cheong, H.K.; et al. Relationship between indoor air pollutant levels and residential environment in children with atopic dermatitis. Allergy Asthma Immunol. Res. 2014, 6, 517–524. [Google Scholar] [CrossRef]
- Ferrero, A.; Esplugues, A.; Estarlich, M.; Llop, S.; Cases, A.; Mantilla, E.; Ballester, F.; Iñiguez, C. Infants’ indoor and outdoor residential exposure to benzene and respiratory health in a Spanish cohort. Environ. Pollut. 2017, 222, 486–494. [Google Scholar] [CrossRef]
- Adgate, J.L.; Church, T.R.; Ryan, A.D.; Ramachandran, G.; Fredrickson, A.L.; Stock, T.H.; Morandi, M.T.; Sexton, K. Outdoor, indoor, and personal exposure to VOCs in children. Environ. Health Perspect. 2004, 112, 1386–1392. [Google Scholar] [CrossRef]
- Phillips, M.L.; Esmen, N.A.; Hall, T.A.; Lynch, R. Determinants of exposure to volatile organic compounds in four Oklahoma cities. J. Expo. Anal. Environ. Epidemiol. 2005, 15, 35–46. [Google Scholar] [CrossRef][Green Version]
- Gillespie-Bennett, J.; Pierse, N.; Wickens, K.; Crane, J.; Nicholls, S.; Shields, D.; Boulic, M.; Viggers, H.; Baker, M.; Woodward, A.; et al. Sources of nitrogen dioxide (NO2) in New Zealand homes: Findings from a community randomized controlled trial of heater substitutions. Indoor Air 2008, 18, 521–528. [Google Scholar] [CrossRef]
- Lim, Y.-H.; Hersoug, L.-G.; Lund, R.; Bruunsgaard, H.; Ketzel, M.; Brandt, J.; Jeanette Jørgensen, T.; Westendorp, R.; Andersen, Z.J.; Loft, S. Inflammatory markers and lung function in relation to indoor and ambient air pollution. Int. J. Hyg. Environ. Health 2022, 241, 113944. [Google Scholar] [CrossRef]
- Belanger, K.; Triche, E.W. Indoor Combustion and Asthma. Immunol. Allergy Clin. N. Am. 2008, 28, 507–519. [Google Scholar] [CrossRef]
- Triche, E.W.; Belanger, K.; Bracken, M.B.; Beckett, W.S.; Holford, T.R.; Gent, J.F.; McSharry, J.-E.; Leaderer, B.P. Indoor Heating Sources and Respiratory Symptoms in Nonsmoking Women. Epidemiology 2005, 16, 377–384. [Google Scholar] [CrossRef]
- Chen, L.C.; Qu, Q.; Gordon, T. Respiratory Effects of Kerosene Space Heater Emissions. Inhal. Toxicol. 1996, 8, 49–64. [Google Scholar] [CrossRef]
- Fullerton, D.G.; Bruce, N.; Gordon, S.B. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 843–851. [Google Scholar] [CrossRef]
- Fullerton, D.G.; Jere, K.; Jambo, K.; Kulkarni, N.S.; Zijlstra, E.E.; Grigg, J.; French, N.; Molyneux, M.E.; Gordon, S.B. Domestic smoke exposure is associated with alveolar macrophage particulate load. Trop. Med. Int. Health 2009, 14, 349–354. [Google Scholar] [CrossRef]
- Apple, J.; Vicente, R.; Yarberry, A.; Lohse, N.; Mills, E.; Jacobson, A.; Poppendieck, D. Characterization of particulate matter size distributions and indoor concentrations from kerosene and diesel lamps. Indoor Air 2010, 20, 399–411. [Google Scholar] [CrossRef]
- Sahu, M.; Peipert, J.; Singhal, V.; Yadama, G.N.; Biswas, P. Evaluation of mass and surface area concentration of particle emissions and development of emissions indices for cookstoves in rural India. Environ. Sci. Technol. 2011, 45, 2428–2434. [Google Scholar] [CrossRef]
- Ahmed, M.; Al-Khashman, O.A.; Al-Shrideh, A.Z. The Optimal Use of Renewable Energy in Residential Buildings Under Jordanian Building Regulation: A Case Study. Int. J. Energy Convers. 2021, 9. [Google Scholar] [CrossRef]
- Younis, A.; Taki, A.; Bhattacharyya, S. Sustainability issues in low-middle income apartments in urban Amman, Jordan: Heating devices and health concerns. WIT Trans. Built Environ. 2020, 193, 27. [Google Scholar]
- Ruiz-Mercado, I.; Masera, O.; Zamora, H.; Smith, K.R. Adoption and sustained use of improved cookstoves. Energy Policy 2011, 39, 7557–7566. [Google Scholar] [CrossRef]
- Kawamoto, T.; Matsuno, K.; Arashidani, K.; Yoshikawa, M.; Kayama, F.; Kodama, Y. Personal Exposure to Nitrogen Dioxide from Indoor Heaters and Cooking Stoves. Arch. Environ. Contam. Toxicol. 1993, 25, 534–538. [Google Scholar]
- Changa, W.R.; Chenga, C.L. Carbon monoxide transport in an enclosed room with sources from a water heater in the adjacent balcony. Build. Environ. 2008, 43, 861–870. [Google Scholar] [CrossRef]
- Carteret, M.; Pauwels, J.-F.; Hanoune, B. Emission factors of gaseous pollutants from recent kerosene space heaters and fuels available in France in 2010. Indoor Air 2012, 22, 299–308. [Google Scholar] [CrossRef]
- Arashidanii, K.; Yoshikawa, M.; Kawamoto, T.; Matsuno, K.; Kayama, F.; Kodama, Y. Indoor Pollution from Heating. Ind. Health 1996, 34, 205–215. [Google Scholar]
- Bozzelli, J.W.; Kebbekus, B.; Bobenhausen, C. Analysis of selected volatile organic compounds associated with residential kerosene heater use. Int. J. Environ. Stud. 1995, 49, 125–131. [Google Scholar] [CrossRef]
- Chambon, C.; Schadkowski, C. Impact of Kerosene Portable Heaters on Indoor Carbon Monoxide Concentrations. Pollut. Atmos. 2005, 1, 103–106. [Google Scholar]
- Ristovski, Z.D.; Tas, I.; Morawska, L.; Saxby, W. Investigation into the emission of fine particles, formaldehyde, oxides of nitrogen and carbon monoxide from natural gas heaters. J. Aerosol Sci. 2000, 31 (Suppl. Sl), S490–S491. [Google Scholar] [CrossRef]
- Uchiyama, S.; Tomizawa, T.; Tokoro, A.; Aoki, M.; Hishiki, M.; Yamada, T.; Tanaka, R.; Sakamoto, H.; Yoshida, T.; Bekki, K.; et al. Gaseous chemical compounds in indoor and outdoor air of 602 houses throughout Japan in winter and summer. Environ. Res. 2015, 137, 364–372. [Google Scholar] [CrossRef]
- Hansel, N.; McCormack, M.; Belli, A.; Matsui, E.; Peng, R.; Aloe, C.; Paulin, L.; Williams, D.A.; Diette, G.; Breysse, P. In-home air pollution is linked to respiratory morbidity in former smokers with COPD. Am. J. Respir. Crit. Care Med. 2013, 187, 1085–1090. [Google Scholar] [CrossRef]
- Dong, J.I.; Banerjee, K.; Bozzelli, J.W. Total hydrocarbon pollutants from a non-vented radiant kerosene heater. Int. J. Environ. Stud. 1988, 32, 75–83. [Google Scholar] [CrossRef]
- Apte, M.G.; Traynor, G.W.; Froehlich, D.A.; Sokol, H.A.; Porter, W.K. The impact of add-on catalytic devices on pollutant emissions from unvented kerosene heaters. J. Air Pollut. Control Assoc. 1989, 39, 1228–1230. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.-S.; Zhou, Y.; Chow, J.; Watson, J.; Frazier, C. Chemical composition of aerosols from kerosene heaters burning jet fuels. Aerosol Sci. Technol. 2001, 35, 949–957. [Google Scholar] [CrossRef]
- Lionel, T.; Martin, R.J.; Brown, N.J. A comparative study of combustion in kerosine heaters. Environ. Sci. Technol. 1986, 20, 78–85. [Google Scholar] [CrossRef]
- Traynor, G.W.; Allen, J.R.; Apte, M.G.; Girman, J.R.; Hollowell, C.D. Pollutant emissions from portable kerosene fired space heaters. Environ. Sci. Technol. 1983, 17, 369–371. [Google Scholar] [CrossRef]
- Traynor, G.W.; Apte, M.G.; Carruthers, A.R.; Dillworth, J.F.; Grimsrud, D.T.; Thompson, W.T. Indoor air pollution and inter-room pollutant transport due to unvented kerosene-fired space heaters. Environ. Int. 1987, 13, 159–166. [Google Scholar] [CrossRef]
- Traynor, G.W.; Apte, M.G.; Sokol, H.A.; Chuang, J.C.; Tucker, W.G.; Mumford, J.L. Selected organic pollutant emissions from unvented kerosene space heaters. Environ. Sci. Technol. 1990, 24, 1265–1270. [Google Scholar] [CrossRef]
- Yamanaka, S.; Hirose, H.; Takada, S. Nitrogen oxide emissions from domestic kerosene-fired and gas-fired appliances. Atmos. Environ. 1979, 13, 407–412. [Google Scholar] [CrossRef]
- Yamanaka, S. Decay rates of nitrogen oxides in a typical Japanese living room. Environ. Sci. Technol. 1984, 18, 566–570. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y. Characterization of emissions from kerosene heaters in an unvented tent. Aerosol Sci. Technol. 2000, 33, 510–524. [Google Scholar] [CrossRef]
- Adgate, J.; Reid, H.F.; Helms, R.W.; Berg, R.A. Nitrogen dioxide and urinary excretion of hydroxyproline and desmosine. Arch. Environ. Health 1992, 47, 376–384. [Google Scholar] [CrossRef]
- Mumford, J.L.; Williams, R.W.; Walsh, D.B.; Burton, R.M.; Svendsgaard, D.J.; Chuang, J.C.; Houk, V.S.; Lewtas, J. Indoor air pollutants from unvented kerosene heater emissions in mobile homes: Studies on particles, semivolatile organics, carbon monoxide, and mutagenicity. Environ. Sci. Technol. 1991, 25, 1732–1738. [Google Scholar] [CrossRef]
- Hussein, T.; Alameer, A.; Jaghbeir, O.; Albeitshaweesh, K.; Malkawi, M.; Boor, B.E.; Koivisto, A.J.; Löndahl, J.; Alrifai, O.; Al-Hunaiti, A. Indoor Particle Concentrations, Size Distributions, and Exposures in Middle Eastern Microenvironments. Atmosphere 2020, 11, 41. [Google Scholar] [CrossRef]
- Khumsaeng, T.; Kanabkaew, T. Measurement of Indoor Air Pollution in Bhutanese Households during Winter: An Implication of Different Fuel Uses. Sustainability 2021, 13, 9601. [Google Scholar] [CrossRef]
- Wang, X.; Chancellor, G.; Evenstad, J.; Farnsworth, J.; Hase, A.; Olson, G.; Sreenath, A.; Agarwal, J. A novel optical instrument for estimating size segregated aerosol mass concentration in real time. Aerosol Sci. Technol. 2009, 43, 939–950. [Google Scholar] [CrossRef]
- Maricq, M.M. Monitoring Motor Vehicle PM Emissions: An Evaluation of Three Portable Low-Cost Aerosol Instruments. Aerosol Sci. Technol. 2013, 47, 564–573. [Google Scholar] [CrossRef]
- Chung, A.; Chang, D.P.Y.; Kleeman, M.J.; Perry, K.; Cahill, T.A.; Dutcher, D.; McDougal, E.M.; Stroud, K. Comparison of real-time instruments used to monitor airborne particulate matter. J. Air Waste Manag. Assoc. 2001, 51, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Nyarku, M.; Mazaheri, M.; Jayaratne, R.; Dunbabin, M.; Rahman, M.M.; Uhde, E.; Morawska, L. Mobile phones as monitors of personal exposure to air pollution: Is this the future? PLoS ONE 2018, 13, e0193150. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Lin, M.H. Real-time performance of the micro-aeth AE51 and the effects of aerosol loading on its measurement results at a traffic site. Aerosol Air Qual. Res. 2013, 13, 1853–1863. [Google Scholar] [CrossRef]
- Cai, J.; Yan, B.; Ross, J.; Zhang, D.; Kinney, P.L.; Perzanowski, M.S.; Jung, K.; Miller, R. and Chillrud, S.N. Validation of MicroAeth® as a black carbon monitor for fixed-site measurement and optimization for personal exposure characterization. Aerosol Air Qual. Res. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Hämeri, K.; Koponen, I.K.; Aalto, P.P.; Kulmala, M. The particle detection efficiency of the TSI3007 condensation particle counter. Aerosol Sci. 2002, 33, 1463–1469. [Google Scholar] [CrossRef]
- Hussein, T.; Kulmala, M. Indoor aerosol modeling: Basic principles and practical applications. Water Air Soil Pollut. Focus 2007, 8, 23–34. [Google Scholar] [CrossRef]
- Nazaroff, W.W. Indoor Particle Dynamics. Indoor Air 2004, 14, 175–183. [Google Scholar] [CrossRef]
- Hussein, T.; Smolik, J.; Kerminen, V.-M.; Kulmala, M. Modeling dry deposition of aerosol particles onto rough surfaces. Aerosol Sci. Technol. 2012, 46, 44–59. [Google Scholar] [CrossRef]
- Alsbou, E.M.; Omari, K.W. BTEX indoor air characteristic values in rural areas of Jordan: Heaters and health risk assessment consequences in winter season. Environ. Pollut. 2020, 267, 115464. [Google Scholar] [CrossRef]
- Jodeh, S.; Hasan, A.R.; Amarah, J.; Judeh, F.; Salghi, R.; Lgaz, H.; Jodeh, W. Indoor and outdoor air quality analysis for the city of Nablus in Palestine: Seasonal trends of PM10, PM5.0, PM2.5, and PM1.0 of residential homes. Air Qual. Atmos. Health 2018, 11, 229–237. [Google Scholar] [CrossRef]
- Jaber, J.O.; Mohsen, M.S.; Al-Sarkhi, A.; Akash, B. Energy analysis of Jordan’s commercial sector. Energy Policy 2003, 31, 887–894. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, T.; Al-Jaghbeer, O.; Bqour, N.; Zidan, B.; Lahlouh, B. Exposure to Aerosols Emitted from Common Heating Combustion Sources Indoors—The Jordanian Case as an Example for Eastern Mediterranean Conditions. Atmosphere 2022, 13, 870. https://doi.org/10.3390/atmos13060870
Hussein T, Al-Jaghbeer O, Bqour N, Zidan B, Lahlouh B. Exposure to Aerosols Emitted from Common Heating Combustion Sources Indoors—The Jordanian Case as an Example for Eastern Mediterranean Conditions. Atmosphere. 2022; 13(6):870. https://doi.org/10.3390/atmos13060870
Chicago/Turabian StyleHussein, Tareq, Omar Al-Jaghbeer, Nizar Bqour, Bilal Zidan, and Bashar Lahlouh. 2022. "Exposure to Aerosols Emitted from Common Heating Combustion Sources Indoors—The Jordanian Case as an Example for Eastern Mediterranean Conditions" Atmosphere 13, no. 6: 870. https://doi.org/10.3390/atmos13060870
APA StyleHussein, T., Al-Jaghbeer, O., Bqour, N., Zidan, B., & Lahlouh, B. (2022). Exposure to Aerosols Emitted from Common Heating Combustion Sources Indoors—The Jordanian Case as an Example for Eastern Mediterranean Conditions. Atmosphere, 13(6), 870. https://doi.org/10.3390/atmos13060870





