Optimization of the Efficient Extraction of Organic Components in Atmospheric Particulate Matter by Accelerated Solvent Extraction Technique and Its Application
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Design Approach
2.2. Standards Reference Samples
2.3. Accelerated Solvent Extraction (ASE) and Ultrasonic Solvent Extraction (USE) Methods
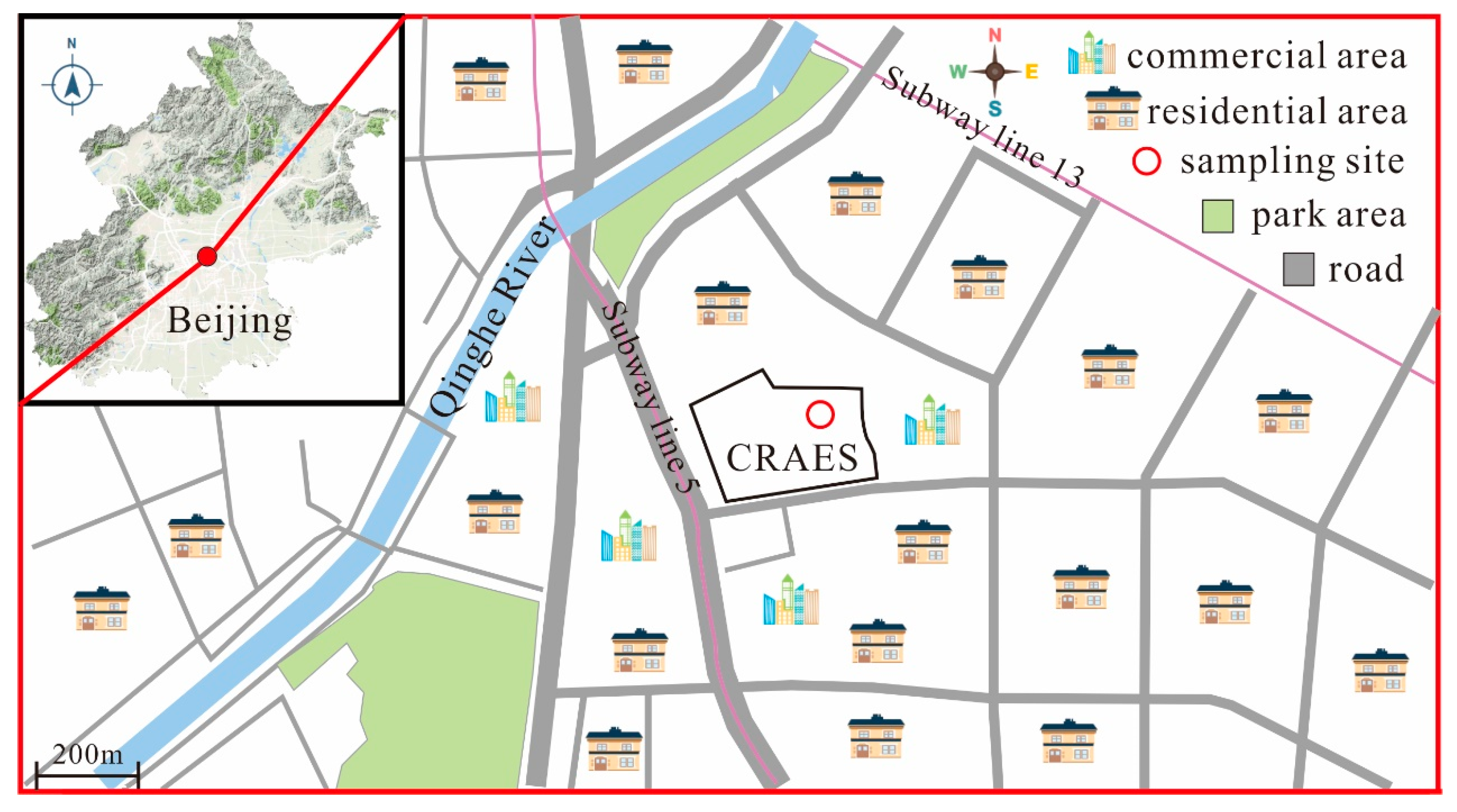
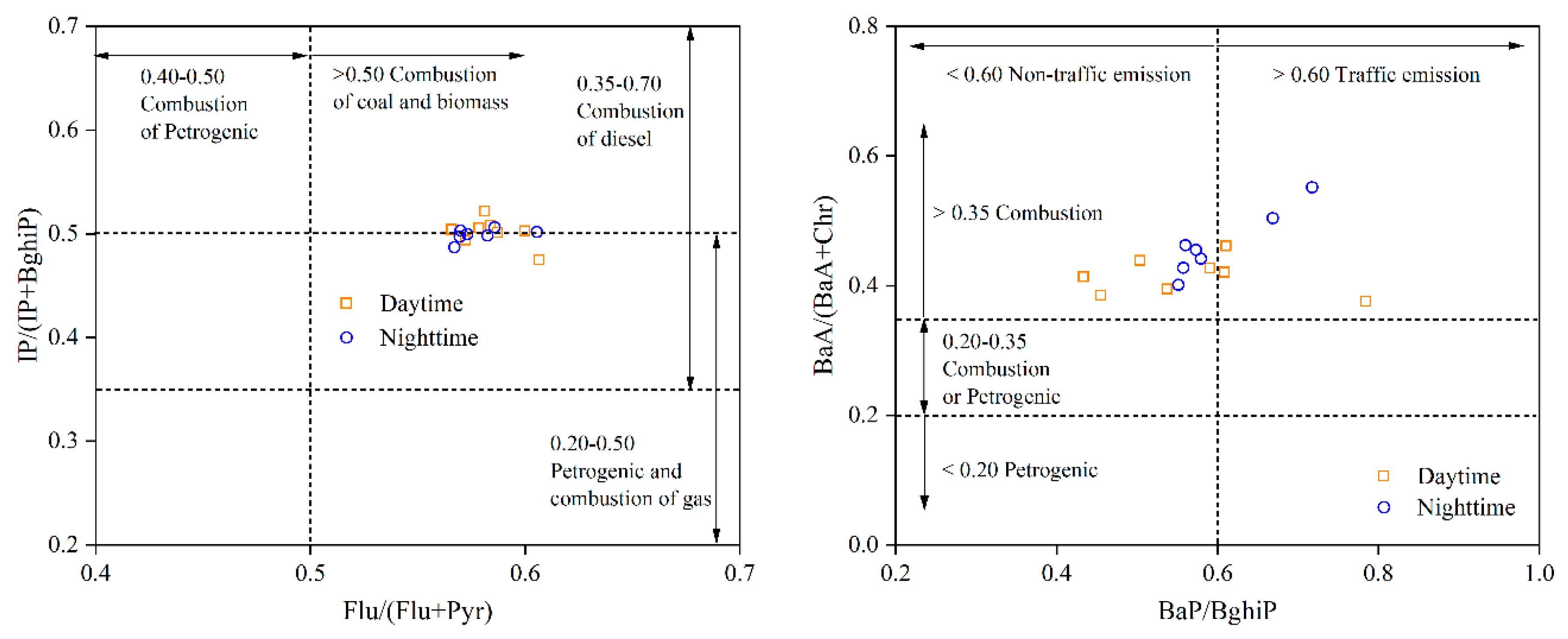
2.4. Case Study
2.5. Quality Assurance and Quality Control (QA/QC)
3. Results and Discussion
3.1. Optimization of ASE Parameters
3.2. Influences of Different Parameters
3.3. Comparison of Extraction Approach
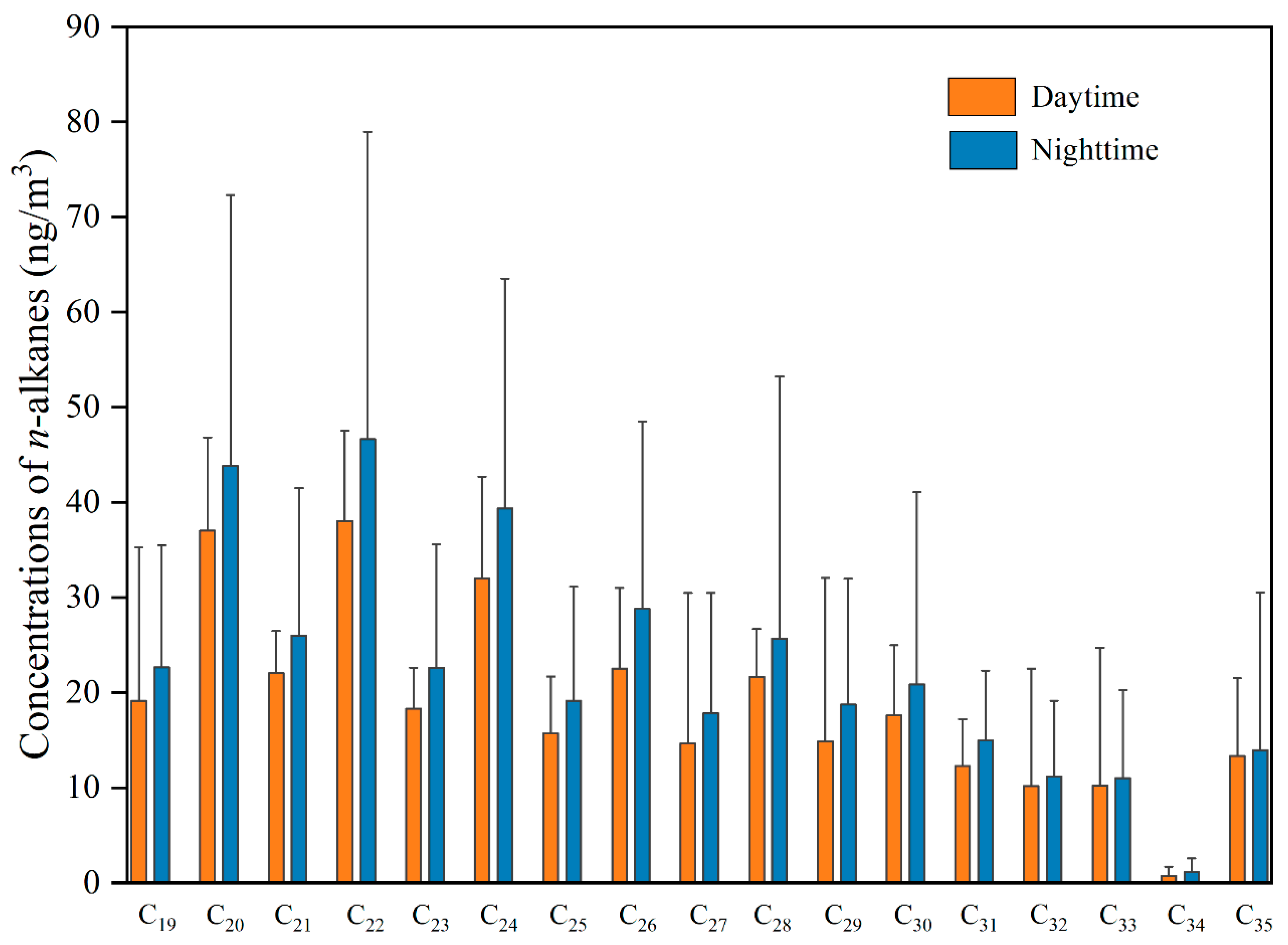
4. Results from Local Sample Collection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, S.; Allen, R.J.; Lim, C.H. A likely increase in fine particulate matter and premature mortality under future climate change. Air Qual. Atmos. Health 2020, 13, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Zhai, S.; Jacob, D.J.; Wang, X.; Shen, L.; Li, K.; Zhang, Y.; Gui, K.; Zhao, T.; Liao, H. Fine particulate matter (PM2.5) trends in China, 2013–2018: Separating contributions from anthropogenic emissions and meteorology. Atmos. Chem. Phys. 2019, 19, 11031–11041. [Google Scholar] [CrossRef] [Green Version]
- Garcia Rivera, P.; Dinkelacker, B.T.; Kioutsioukis, I.; Adams, P.J.; Pandis, S.N. Source-Resolved Variability of Fine Particulate Matter and Human Exposure in an Urban Area. Atmos. Chem. Phys. 2022, 22, 2011–2027. [Google Scholar] [CrossRef]
- Won, W.S.; Oh, R.; Lee, W.; Kim, K.Y.; Ku, S.; Su, P.C.; Yoon, Y. Impact of Fine Particulate Matter on Visibility at Incheon International Airport, South Korea. Aerosol Air Qual. Res. 2020, 20, 1048–1061. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Huang, R.; Cao, J.; Dai, W.; Zhou, J.; Lin, C.; Ni, H.; Duan, J.; Wang, T.; Chen, Y.; et al. Determination of n-alkanes, polycyclic aromatic hydrocarbons and hopanes in atmospheric aerosol: Evaluation and comparison of thermal desorption GC-MS and solvent extraction GC-MS approaches. Atmos. Meas. Tech. 2019, 12, 4779–4789. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Pozzer, A.; Cao, C.X.; Lelieveld, J. Long-term (2001–2012) concentrations of fine particulate matter (PM2.5) and the impact on human health in Beijing, China. Atmos. Chem. Phys. 2015, 15, 5715–5725. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.S.; Choi, M.; Park, M.; Park, M.H.; Kim, Y.H.; Seo, J.; Wang, Y.; Hu, M.; Bae, M.S.; Park, K. Assessment of PM2.5-bound nitrogen-containing organic compounds (NOCs) during winter at urban sites in China and Korea. Environ. Pollut. 2020, 265, 114870. [Google Scholar] [CrossRef]
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. Int. 2018, 25, 33901–33910. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mandal, T.K.; Sharma, C.; Kuniyal, J.C.; Joshi, R.; Dhyani, P.P.; Rohtash; Sen, A.; Ghayas, H.; Gupta, N.C.; et al. Measurements of Particulate (PM2.5), BC and Trace Gases Over the Northwestern Himalayan Region of India. Mapan 2014, 29, 243–253. [Google Scholar] [CrossRef]
- Spyros, N.P.; Robert, A. Harley, G.R.; Cass, John, H.S. Secondary organic aerosol formation and transport. Atmos. Environ. 1992, 26, 2269–2282. [Google Scholar]
- Chan, M.N.; Surratt, J.D.; Chan, A.W.H.; Schilling, K.; Offenberg, J.H.; Lewandowski, M.; Edney, E.O.; Kleindienst, T.E.; Jaoui, M.; Edgerton, E.S.; et al. Influence of aerosol acidity on the chemical composition of secondary organic aerosol from β-caryophyllene. Atmospheric Chem. Phys. 2011, 11, 1735–1751. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Aggarwal, S.G.; Chen, J.; Li, J.; Sun, Y.; Wang, Z.; Chen, H.; Liao, H.; Ding, A.; Umarji, G.S.; et al. Molecular Markers of Secondary Organic Aerosol in Mumbai, India. Environ. Sci. Technol. 2016, 50, 4659–4667. [Google Scholar] [CrossRef] [PubMed]
- Surratt, J.D.; Gómez-González, Y.; Chan, A.W.H.; Vermeylen, R.; Shahgholi, M.; Kleindienst, T.E.; Edney, E.O.; Offenberg, J.H.; Lewandowski, M.; Jaoui, M.; et al. Organosulfate Formation in Biogenic Secondary Organic Aerosol. J. Phys. Chem. A 2008, 112, 8345–8378. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Wang, G.; Wu, C.; Wang, J.; Li, J.; Zhang, L.; Han, Y.; Liu, L.; Cao, C.; Cao, J.; et al. Changes in concentration, composition and source contribution of atmospheric organic aerosols by shifting coal to natural gas in Urumqi. Atmos. Environ. 2017, 148, 306–315. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, J.; Wu, Z.; Ji, Y.; Bi, F.; Gao, R.; Wang, Z.; Wang, G.; Li, H. Chemical components and source identification of PM2.5 in non-heating season in Beijing: The influences of biomass burning and dust. Atmos. Res. 2021, 251, 105412. [Google Scholar] [CrossRef]
- Trusz, A.; Ghazal, H.; Piekarska, K. Seasonal variability of chemical composition and mutagenic effect of organic PM2.5 pollutants collected in the urban area of Wroclaw (Poland). Sci. Total Environ. 2020, 733, 138911. [Google Scholar] [CrossRef]
- Andreae, M.O.; Crutzen, P.J. Atmospheric aerosols biogeochemical sources and role in atmospheric chemistry. Science 1997, 276, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cao, J.; Zhao, J.; Xu, H.; Arimoto, R.; Wang, G.; Han, Y.; Shen, Z.; Li, G. n-Alkanes and polycyclic aromatic hydrocarbons in total suspended particulates from the southeastern Tibetan Plateau: Concentrations, seasonal variations, and sources. Sci. Total Environ. 2014, 470–471, 9–18. [Google Scholar]
- Wang, G.; Kawamura, K.; Xie, M.; Gao, S.H.S.; Cao, J.; An, Z.; Wang, Z. Size-distributions of n-alkanes, PAHs and hopanes and their sources in the urban, mountain and marine atmospheres over East Asia. Atmos. Chem. Phys. 2009, 9, 8869–8882. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Kawamura, K.; Lee, S.; Ho, K.; Cao, J. Molecular, Seasonal, and Spatial Distributions of Organic Aerosols from Fourteen Chinese Cities. Environ. Sci. Technol. 2006, 40, 4619–4625. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Ren, Y.; Wang, J.; Wu, C.; Han, Y.; Zhang, L.; Cheng, C.; Meng, J. Identification of chemical compositions and sources of atmospheric aerosols in Xi’an, inland China during two types of haze events. Sci. Total Environ. 2016, 566-567, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Castro-Guijarro, P.A.; Alvarez-Vazquez, E.R.; Fernandez-Espinosa, A.J. A rapid Soxhlet and mini-SPE method for analysis of polycyclic aromatic hydrocarbons in atmospheric particles. Anal. Bioanal. Chem. 2021, 413, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Souza, E.J.; Zapata Mora, C.; Aristizabal Zuluaga, B.H.; Britto do Amaral, C.D.; Grassi, M.T. Multi-elemental analysis of particulate matter PM2.5 and PM10 by ICP OES. Talanta 2021, 221, 121457. [Google Scholar] [CrossRef]
- Han, J.; Tian, J.; Feng, J.; Guo, W.; Dong, S.; Yan, X.; Su, X.; Sun, J. Spatiotemporal distribution and mass loading of organophosphate flame retardants (OPFRs) in the Yellow River of China (Henan segment). Environ. Pollut. 2021, 290, 118000. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Q.; Guo, B.; Zhang, S.; Zhang, S.; Hu, D. Optimization of process parameters for preparation of polystyrene PM2.5 particles by supercritical antisolvent method using BBD-RSM. Sci. Rep. 2020, 10, 11187. [Google Scholar] [CrossRef]
- Kim, I.; Lee, S.; Kim, S.D. Determination of toxic organic pollutants in fine particulate matter using selective pressurized liquid extraction and gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1590, 39–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Zhang, X.; Li, J.; Zhao, T.; Gao, Y.; Jiang, P.; Li, Y.; Chen, X.; Wang, W. Characteristics of PM2.5-bound PAHs at an Urban Site and a Suburban Site in Jinan in North China Plain. Aerosol Air Qual. Res. 2019, 19, 871–884. [Google Scholar] [CrossRef]
- He, M.; Ichinose, T.; Yoshida, Y.; Arashidani, K.; Yoshida, S.; Takano, H.; Sun, G.; Shibamoto, T. Urban PM2.5 exacerbates allergic inflammation in the murine lung via a TLR2/TLR4/MyD88-signaling pathway. Sci. Rep. 2017, 7, 11027. [Google Scholar] [CrossRef] [Green Version]
- Roper, C.; Delgado, L.S.; Barrett, D.; Massey Simonich, S.L.; Tanguay, R.L. PM2.5 Filter Extraction Methods: Implications for Chemical and Toxicological Analyses. Environ. Sci. Technol. 2019, 53, 434–442. [Google Scholar] [CrossRef]
- Hubert, A.; Wenzel, K.D.; Manz, M.; Weissflog, L.; Engewald, W.; Schuurmann, G. High Extraction Efficiency for POPs in Real Contaminated Soil Samples Using Accelerated Solvent Extraction. Anal. Chem. 2000, 72, 1294–1300. [Google Scholar] [CrossRef]
- Fisher, J.A.; Scarlett, M.J.; Stott, A.D. Accelerated Solvent Extraction: An Evaluation for Screening of Soils for Selected U.S. EPA Semivolatile Organic Priority Pollutants. Environ. Sci. Technol. 1997, 31, 1120–1127. [Google Scholar] [CrossRef]
- Lau, E.V.; Gan, S.; Ng, H.K. Extraction techniques for polycyclic aromatic hydrocarbons in soils. Int. J. Anal. Chem. 2010, 2010, 398381. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Avila, V.; Young, R.; Beckert, W.F. Microwave-Assisted Extraction of Organic Compounds from Standard Reference Soils and Sediments. Anal. Chem. 1994, 66, 1097–1106. [Google Scholar] [CrossRef]
- Heemken, O.P.; Theobald, N.; Wenclawiak, B.W. Comparison of ASE and SFE with Soxhlet, Sonication, and Methanolic Saponification Extractions for the Determination of Organic Micropollutants in Marine Particulate Matter. Anal. Chem. 1997, 69, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Ge, X.; Lv, Y.; Wang, A. Application of accelerated solvent extraction in the analysis of organic contaminants, bioactive and nutritional compounds in food and feed. J. Chromatogr. A 2012, 1237, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Szulejko, J.E.; Kim, K.H.; Brown, R.J.C.; Bae, M.S. Review of progress in solvent-extraction techniques for the determination of polyaromatic hydrocarbons as airborne pollutants. TrAC Trends Anal. Chem. 2014, 61, 40–48. [Google Scholar] [CrossRef]
- Toubane, A.; Rezzoug, S.A.; Besombes, C.; Daoud, K. Optimization of Accelerated Solvent Extraction of Carthamus Caeruleus L. Evaluation of antioxidant and anti-inflammatory activity of extracts. Ind. Crops Prod. 2017, 97, 620–631. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Peng, X.; Tang, X.; Deng, X. Optimization of sample pretreatment for determination of polycyclic aromatic hydrocarbons in estuarine sediments by gas chromatography. J. Ocean. Univ. China 2012, 11, 159–164. [Google Scholar] [CrossRef]
- Ji, L.; Si, Y.; Liu, H.; Song, X.; Zhu, W.; Zhu, A. Application of orthogonal experimental design in synthesis of mesoporous bioactive glass. Microporous Mesoporous Mater. 2014, 184, 122–126. [Google Scholar] [CrossRef]
- Ren, Y.; Wei, J.; Ji, Y.; Wu, Z.; Bi, F.; Gao, R.; Wang, X.; Li, H. Chemical composition of fine organic aerosols during a moderate pollution event in summertime in Beijing: Combined effect of primary emission and secondary formation. Atmos. Environ. 2021, 246, 118167. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, G.; Li, J.J.; Wu, C.; Cao, C.; Li, J.; Wang, J.Y.; Ge, S.; Xie, Y.; Li, X.; et al. Evolution of aerosol chemistry in Xi’an during the spring dust storm periods: Implications for heterogeneous formation of secondary organic aerosols on the dust surface. Chemosphere 2019, 215, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Wu, C.; Cao, C.; Ren, Y.; Wang, J.; Li, J.; Cao, J.; Zeng, L.; Zhu, T. Characterization of isoprene-derived secondary organic aerosols at a rural site in North China Plain with implications for anthropogenic pollution effects. Sci. Rep. 2018, 8, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Wei, J.; Wang, G.; Wu, Z.; Ji, Y.; Li, H. Evolution of aerosol chemistry in Beijing under strong influence of anthropogenic pollutants: Composition, sources, and secondary formation of fine particulate nitrated aromatic compounds. Environ. Res. 2021, 204, 111982. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Y.; Wu, Z.; Peng, L.; Bao, J.; Peng, Z.; Li, H. Atmospheric volatile halogenated hydrocarbons in air pollution episodes in an urban area of Beijing: Characterization, health risk assessment and sources apportionment. Sci. Total Environ. 2022, 806, 150283. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Janssen, J.G.M.; Cramers, C.A. Parameters Affecting the Accelerated Solvent Extraction of Polymeric Samples. Anal. Chem. 1997, 69, 1598–1603. [Google Scholar] [CrossRef] [Green Version]
- Saim, N.; Dean, J.R.; Abdullah, M.P.; Zakari, Z. An Experimental Design Approach for the Determination of Polycyclic Aromatic Hydrocarbons from Highly Contaminated Soil Using Accelerated Solvent Extraction. Anal. Chem. 1998, 70, 420–424. [Google Scholar] [CrossRef]
- Wang, G.; Lee, A.S.; Lewis, M.; Kamath, B.; Archer, R.K. Accelerated Solvent Extraction and Gas Chromatography/Mass Spectrometry for Determination of Polycyclic Aromatic Hydrocarbons in Smoked Food Samples. J. Agric. Food Chem. 1999, 47, 1062–1066. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Duan, F.; He, K.; Liu, X. Characteristics and source identification of fine particulate n-alkanes in Beijing, China. J. Environ. Sci. 2010, 22, 998–1005. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, M.; Zhang, Y.; Wei, Y.; Hu, M.; Guo, S. Source apportionment of fine organic aerosols in Beijing. Atmos. Chem. Phys. 2009, 9, 8573–8585. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cao, J.; Li, J.; Zhou, J.; Xu, H.; Zhang, R.; Ouyang, Z. Molecular distribution and seasonal variation of hydrocarbons in PM2.5 from Beijing during 2006. Particuology 2013, 11, 78–85. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Guo, Q.; Zhang, X.; Schauer, J.J.; Zhang, R. Quantitative evaluation of emission controls on primary and secondary organic aerosol sources during Beijing 2008 Olympics. Atmos. Chem. Phys. 2013, 13, 8303–8314. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lu, J.; Feng, J.; Yu, Y.; Xia, F.; Ren, L. Distribution characteristics and source identification of n-alkanes in PM2.5 in typical urban area of Beijing. Res. Environ. Sci. 2015, 28, 691–696. [Google Scholar]
- Wu, Y.; Yang, L.; Zheng, X.; Zhang, S.; Song, S.; Li, J.; Hao, J. Characterization and source apportionment of particulate PAHs in the roadside environment in Beijing. Sci. Total Environ. 2014, 470–471, 76–83. [Google Scholar] [CrossRef]
- Gao, Y.; Ji, H. Characteristics of polycyclic aromatic hydrocarbons components in fine particle during heavy polluting phase of each season in urban Beijing. Chemosphere 2018, 212, 346–357. [Google Scholar] [CrossRef]
- Luo, M.; Ji, Y.; Ren, Y.; Gao, F.; Zhang, H.; Zhang, L.; Yu, Y.; Li, H. Characteristics and Health Risk Assessment of PM2.5-Bound PAHs during Heavy Air Pollution Episodes in Winter in Urban Area of Beijing, China. Atmosphere 2021, 12, 323. [Google Scholar] [CrossRef]
- Yao, L.; Li, X.; Liu, X.; Wang, Y. Pollution Characteristics of n-alkanes in Atmospheric Fine Particles During Spring Festival of 2007 in Beijing. Environ. Sci. 2009, 30, 549–593. [Google Scholar]
- Li, X.; Guo, X.; Liu, X.; Liu, C.; Zhang, S.; Wang, Y. Distribution and sources of solvent extractable organic compounds in PM2.5 during 2007 Chinese Spring Festival in Beijing. J. Environ. Sci. 2009, 21, 142–149. [Google Scholar] [CrossRef]
- Rogge, W.F.; Hildemann, L.M.; Mazurek, M.A.; Cass, G.R.; Simoneit, B.R.T. Sources of fine organic aerosol. 4. Particulate abrasion products from leaf surfaces of urban plants. Environ. Sci. Technol. 1993, 27, 2700–2711. [Google Scholar] [CrossRef]
- Fernández, P.G.; Grimalt, J.O.; Vilanova, R.M. Atmospheric Gas-Particle Partitioning of Polycyclic Aromatic Hydrocarbons in High Mountain Regions of Europe. Environ. Sci. Technol. 2002, 36, 1162–1168. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.J.; Lee, W.Y.; Campos-Diaz, S.I. Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: Analysis of a potential problem in the United States/Mexico border region. J. Hazard. Mater. 2009, 163, 946–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, B.; Li, L.; Xu, H.; Wang, T.; Wu, R.; Chen, J.; Zhang, Y.; Liu, S.; Ho, S.S.H.; Cao, J.; et al. PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) in Beijing: Seasonal variations, sources, and risk assessment. J. Environ. Sci. 2019, 77, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, Q.; Yibing, Y.; Tong, L.; Qin, W.; Yaxi, W.; Jianlong, F. Characterization of ambient PM2.5 and PAHs during 2017 Spring Festival in urban and suburb areas of Beijing. Environ. Chem. 2018, 37, 2191–2198. [Google Scholar]

| ASE Parameters | First Group | Second Group | Third Group | Fourth Group |
|---|---|---|---|---|
| Temperature (°C) | 100 | 100 | 120 | 150 |
| Static time (minutes) | 3 | 2 | 5 | 5 |
| Cycle (times) | 2 | 2 | 2 | 2 |
| Flush volume (%) | 50 | 40 | 60 | 60 |
| Purge time (seconds) | 60 | 50 | 90 | 60 |
| Species | First Group | Second Group | Third Group | Fourth Group |
|---|---|---|---|---|
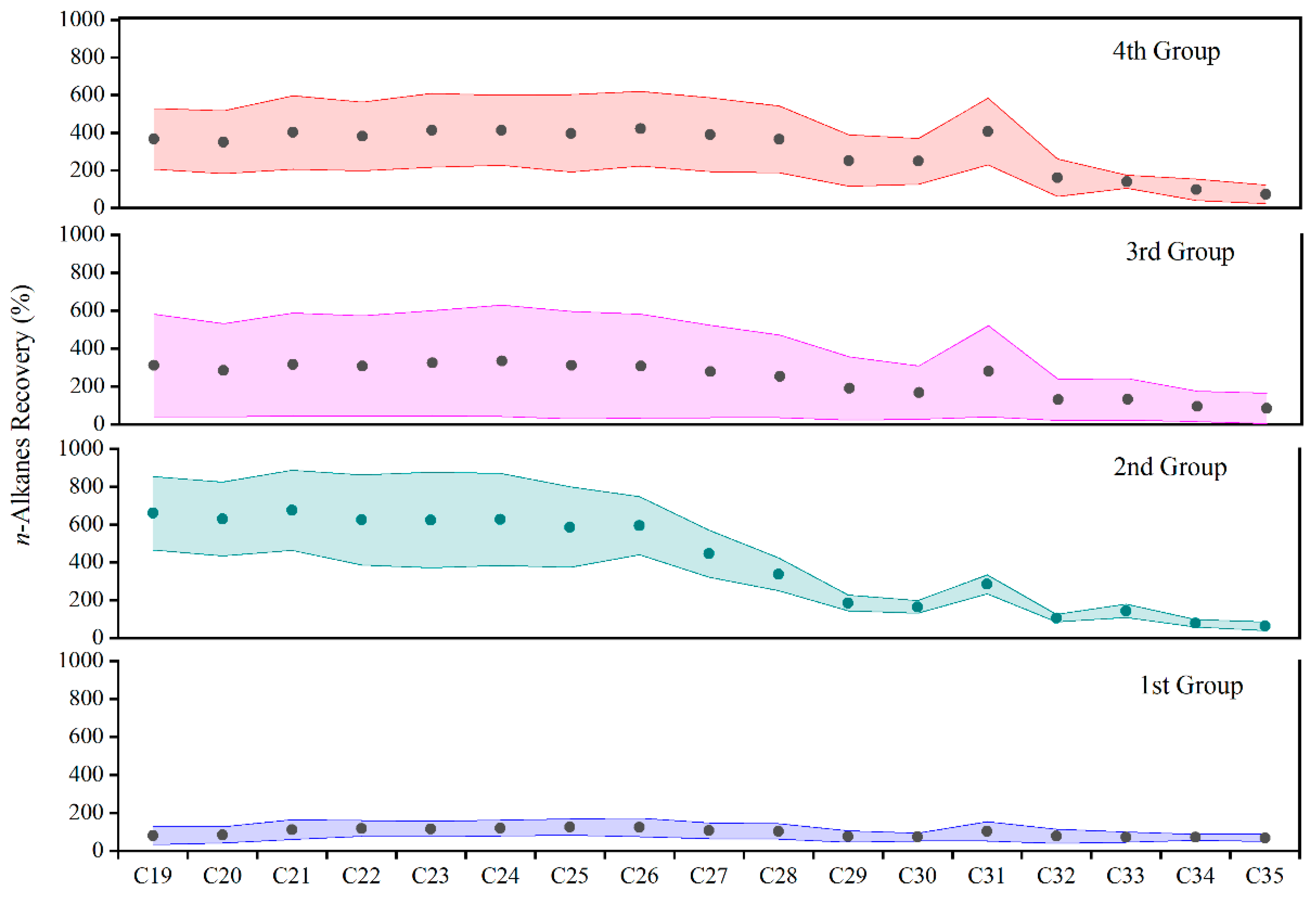
| n−alkanes | 50.7–123 | 302–531 | 197–505 | 218–483 |
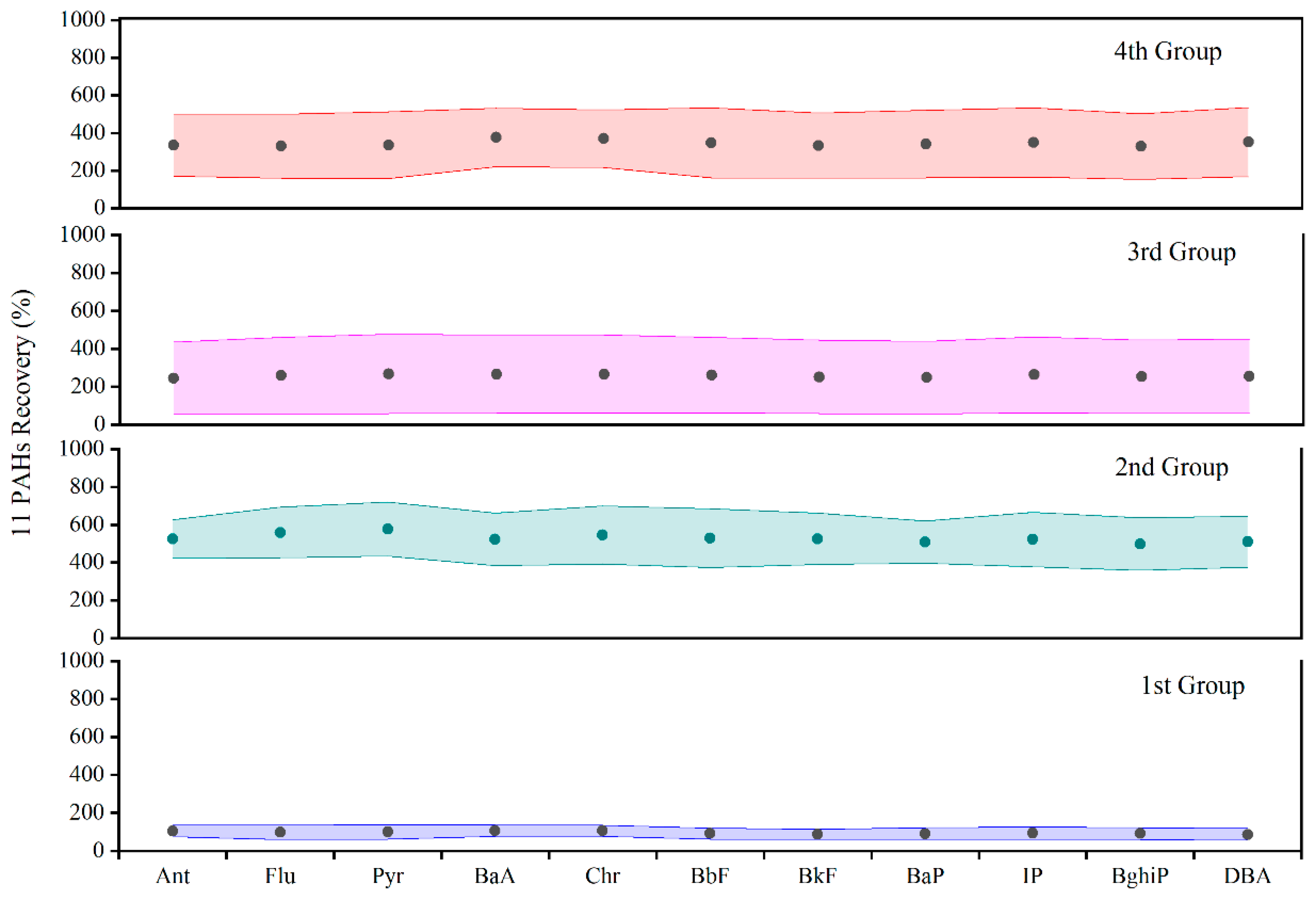
| PAHs | 55.3–122 | 371–667 | 255–484 | 177–587 |
| Pretreatment Methods | ASE | USE | ||
|---|---|---|---|---|
| 17 n−Alkanes | 11 PAHs | 17 n−Alkanes | 11 PAHs | |
| First group | 50.7 ± 13.0 | 55.3 ± 15.1 | 71.2 ± 36.8 | 35.5 ± 87.7 |
| Second group | 123 ± 36.0 | 118 ± 30.2 | 85.8 ± 42.2 | 45.3 ± 112 |
| Third group | 87.7 ± 19.5 | 90.2 ± 22.1 | 74.8 ± 34.2 | 39.1 ± 90.3 |
| Fourth group | 121 ± 33.7 | 122 ± 31.0 | 76.4 ± 31.8 | 35.2 ± 91.0 |
| Average recovery rate | 95.7 ± 21.1 | 96.4 ± 23.1 | 77.0 ± 35.4 | 38.4 ± 95.2 |
| Sampling Year | Species | Mass Concentration | Reference |
|---|---|---|---|
| 2004 | C11–C34 | 105.38 | [50] |
| 2005 | C18–C36 | 48.00 | [51] |
| 2006 | C19–C36 | 179.00 | [52] |
| 2008 | C12–C36 | 71.60 | [53] |
| 2013 | C14–C36 | 80.52 | [54] |
| 2017 | C19–C35 | 350.02 | This study |
| 2008–2009 | BaP | 3.25 | [55] |
| 2015–2016 | 2.77 | [56] | |
| 2017 | 0.69 | This study | |
| 2018 | 0.82 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Ren, Y.; Wei, J.; Ji, Y.; Bai, X.; Shao, Y.; Li, H.; Gao, R.; Wu, Z.; Peng, Z.; et al. Optimization of the Efficient Extraction of Organic Components in Atmospheric Particulate Matter by Accelerated Solvent Extraction Technique and Its Application. Atmosphere 2022, 13, 818. https://doi.org/10.3390/atmos13050818
Zhang H, Ren Y, Wei J, Ji Y, Bai X, Shao Y, Li H, Gao R, Wu Z, Peng Z, et al. Optimization of the Efficient Extraction of Organic Components in Atmospheric Particulate Matter by Accelerated Solvent Extraction Technique and Its Application. Atmosphere. 2022; 13(5):818. https://doi.org/10.3390/atmos13050818
Chicago/Turabian StyleZhang, Hao, Yanqin Ren, Jie Wei, Yuanyuan Ji, Xurong Bai, Yanqiu Shao, Hong Li, Rui Gao, Zhenhai Wu, Zhijian Peng, and et al. 2022. "Optimization of the Efficient Extraction of Organic Components in Atmospheric Particulate Matter by Accelerated Solvent Extraction Technique and Its Application" Atmosphere 13, no. 5: 818. https://doi.org/10.3390/atmos13050818
APA StyleZhang, H., Ren, Y., Wei, J., Ji, Y., Bai, X., Shao, Y., Li, H., Gao, R., Wu, Z., Peng, Z., & Xue, F. (2022). Optimization of the Efficient Extraction of Organic Components in Atmospheric Particulate Matter by Accelerated Solvent Extraction Technique and Its Application. Atmosphere, 13(5), 818. https://doi.org/10.3390/atmos13050818







