Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission
Abstract
:1. Introduction
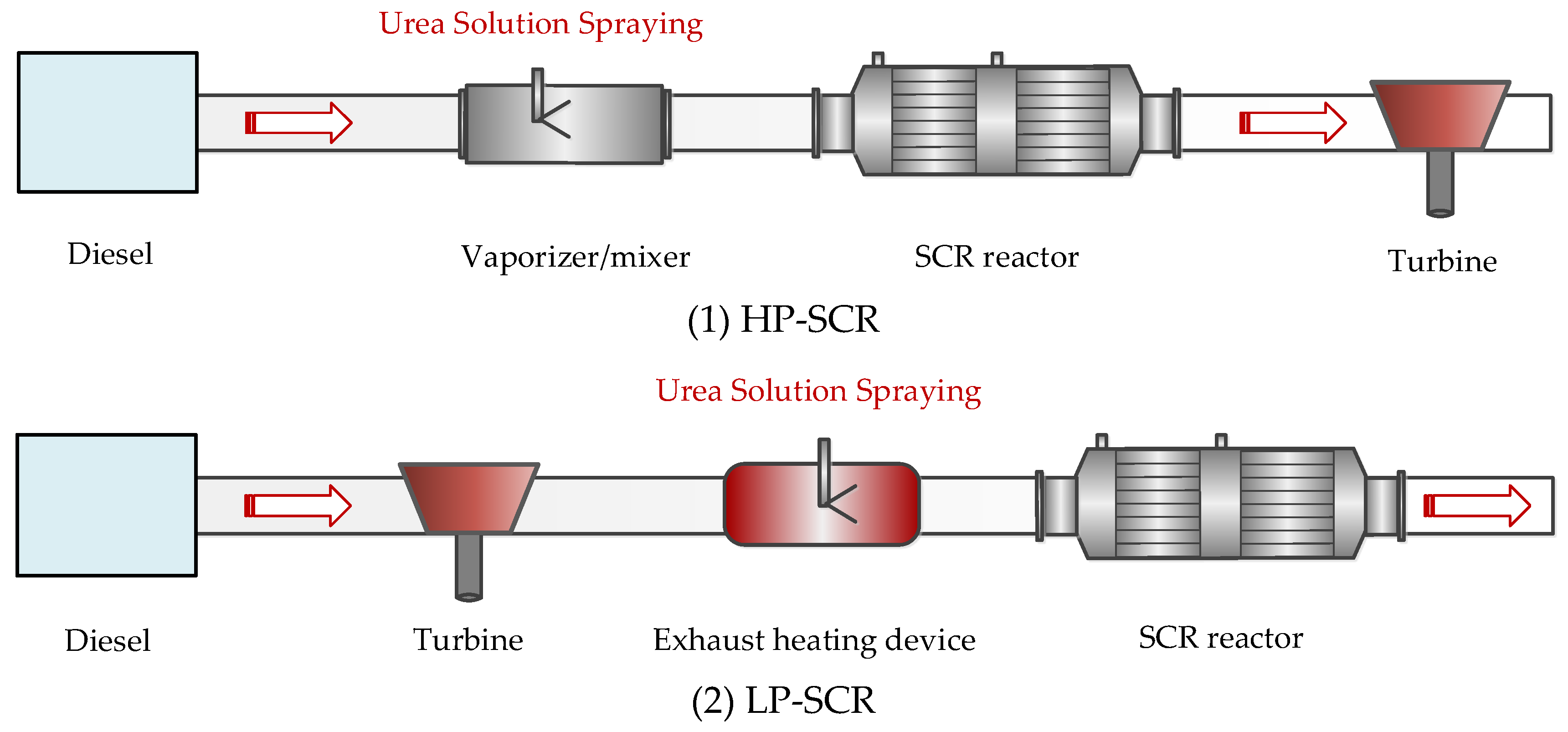
2. Classification of the Marine Selective Catalytic Reduction (SCR) Systems
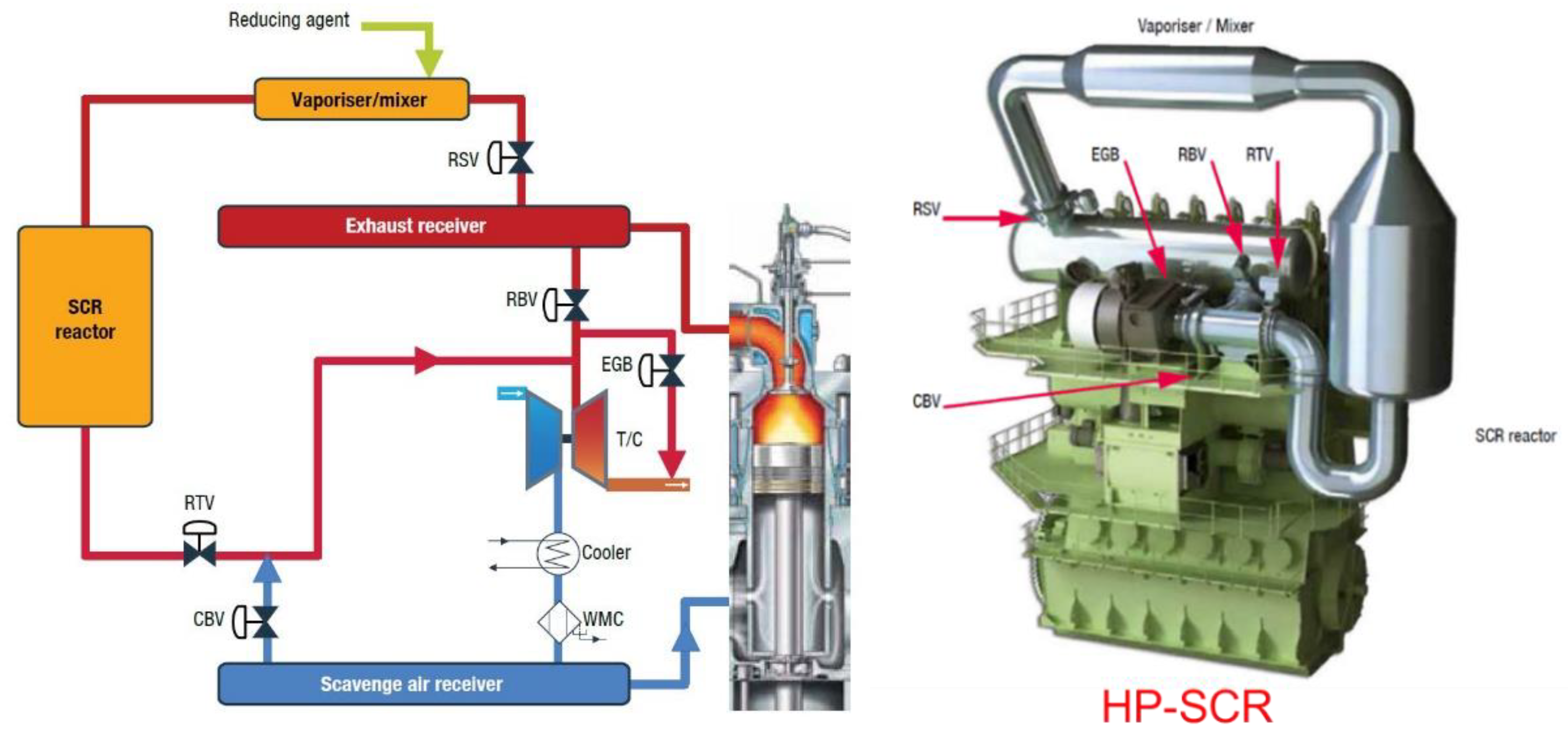
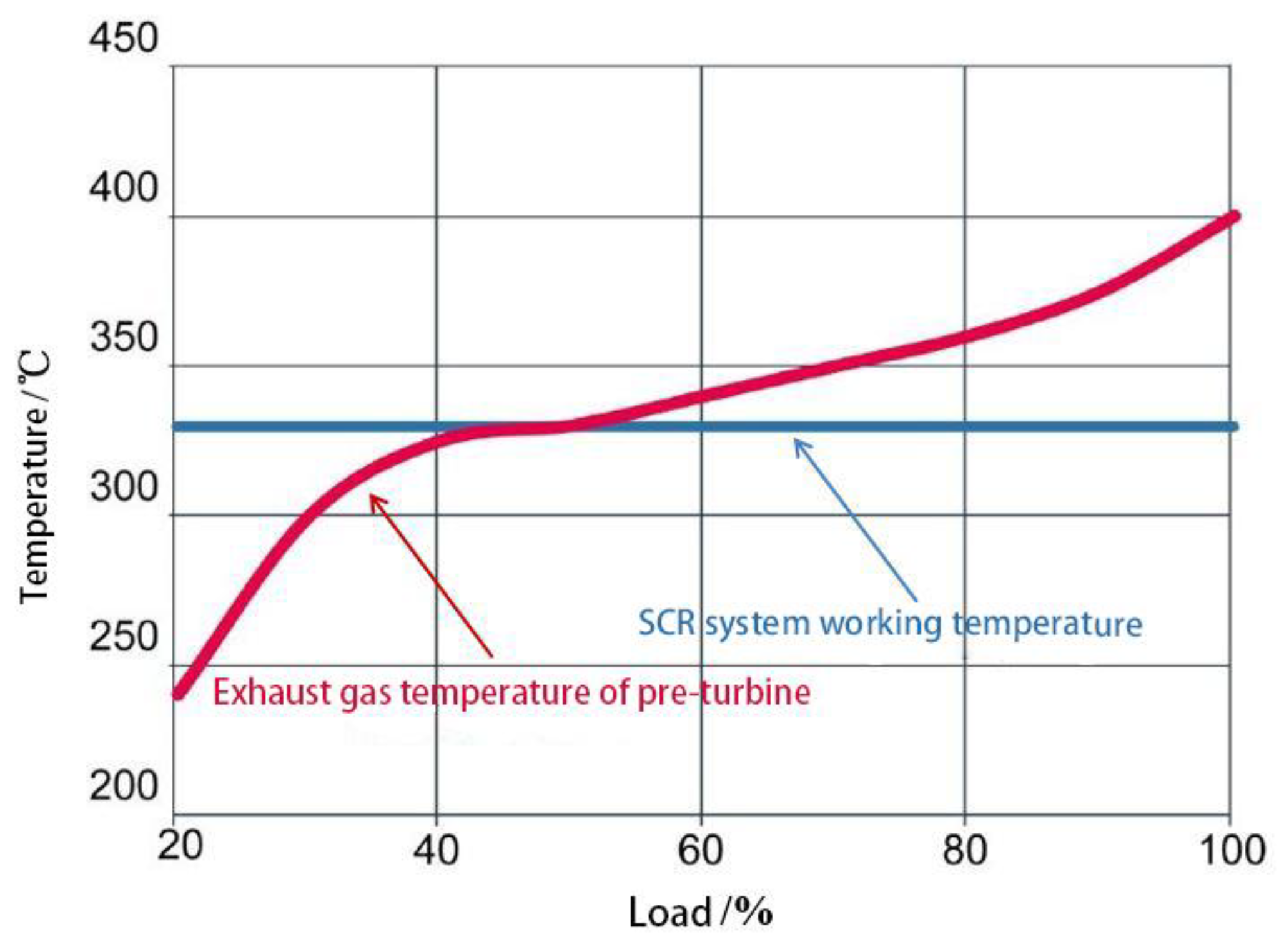
2.1. High-Pressure SCR System
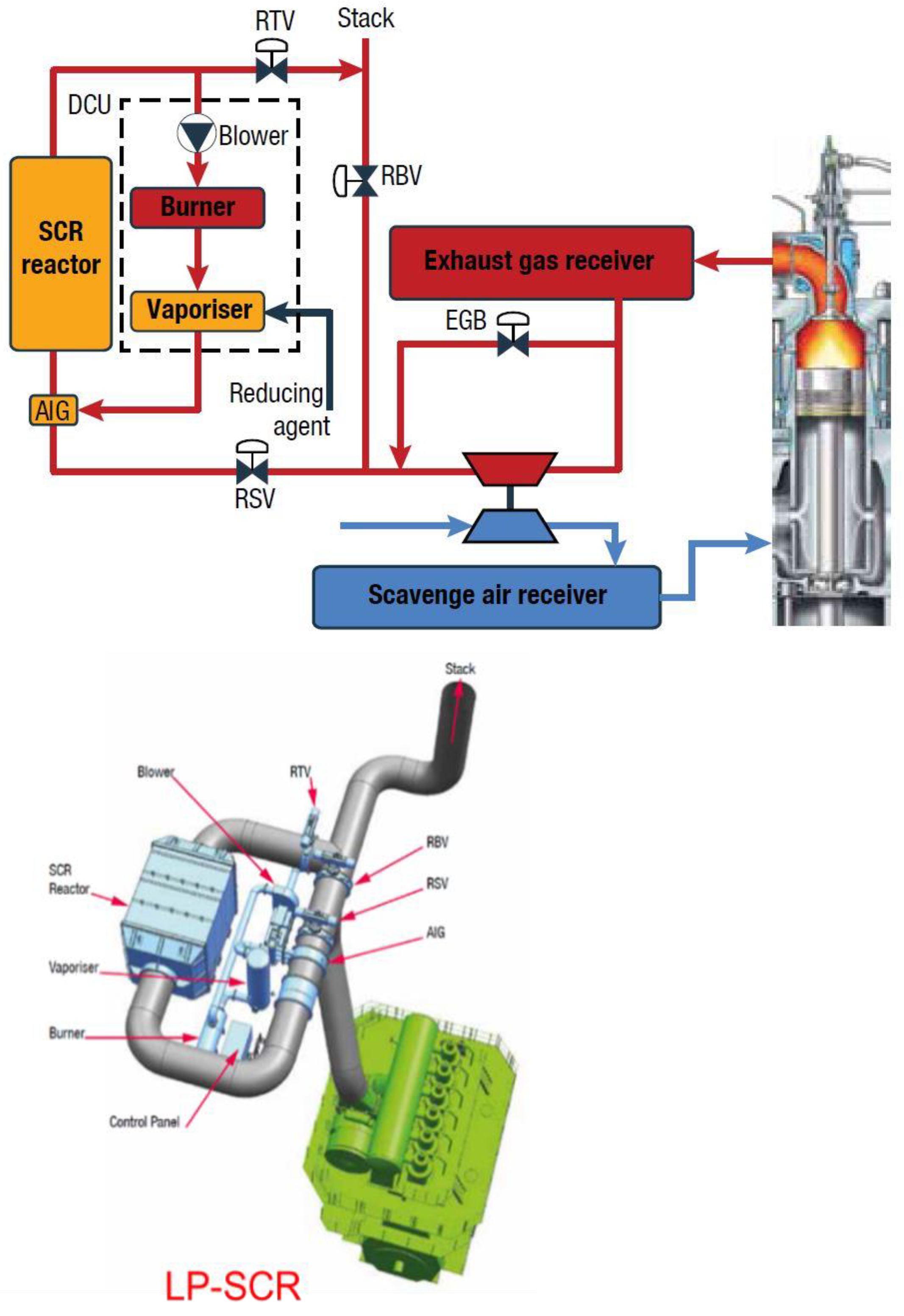
2.2. Low-Pressure SCR System
3. Application Problems of Marine Low-Speed Diesel Engines
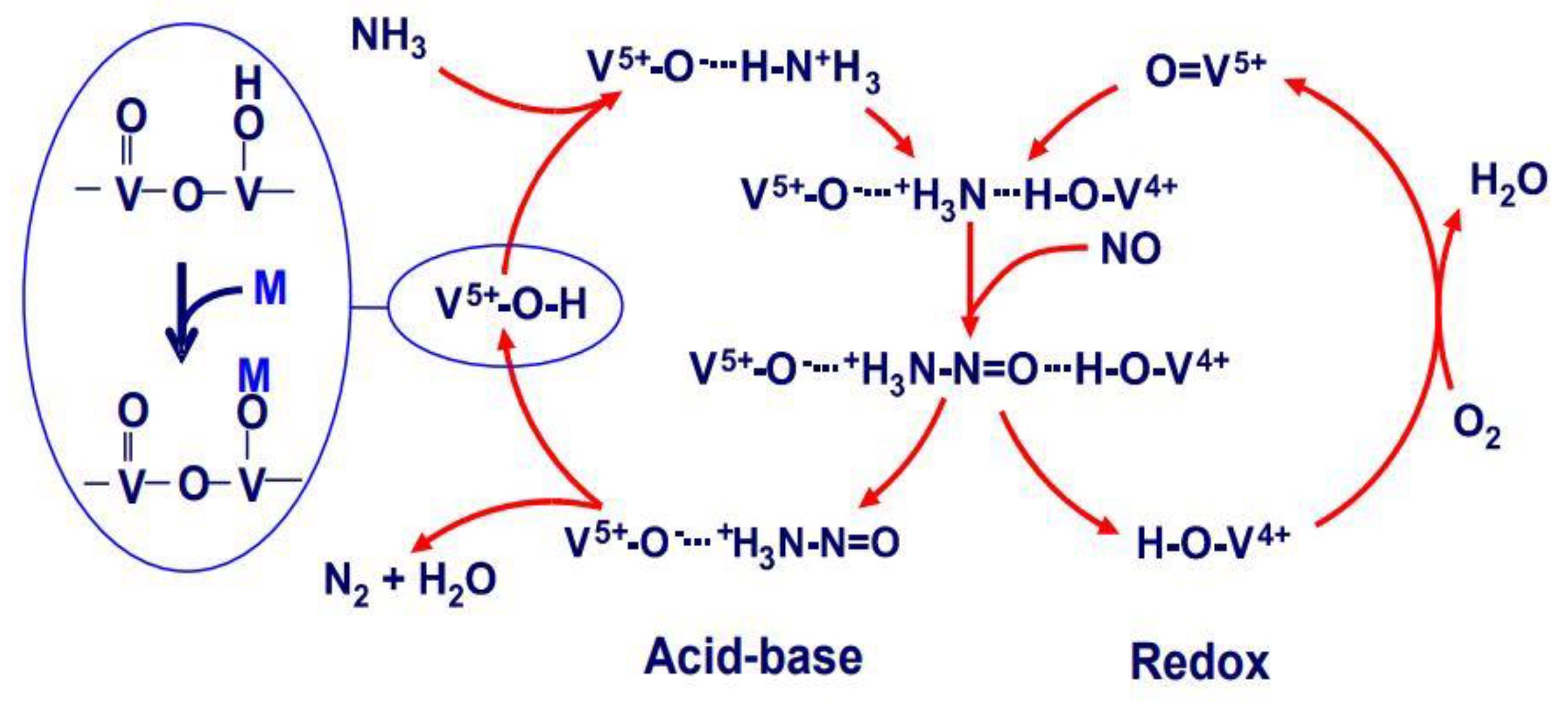
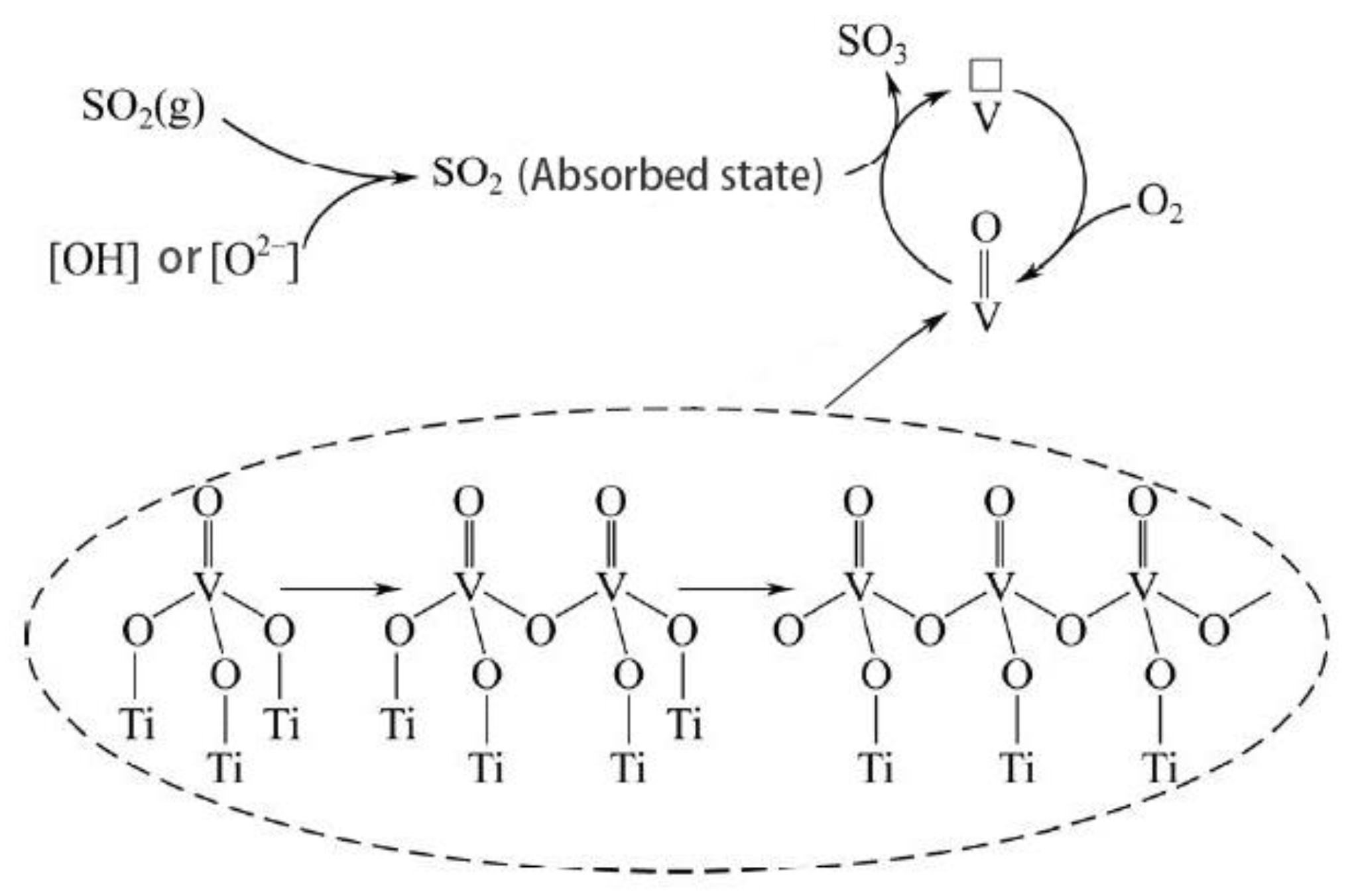
4. Research on Catalytic Reaction Mechanism under the High Sulfur Exhaust Gas Condition
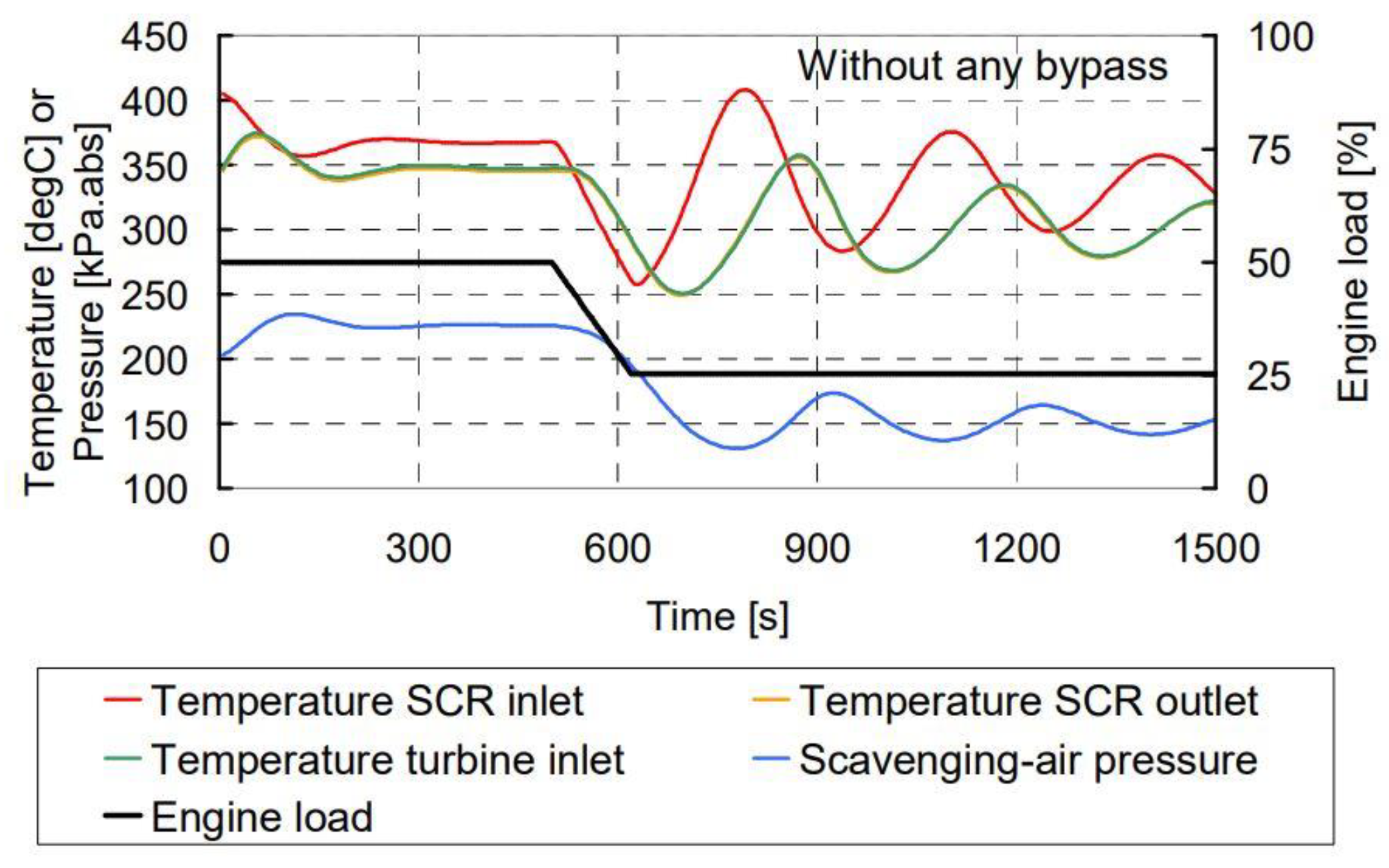
5. Research on Engine Tuning and Control Strategy under Variable Operating Conditions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CBV | Cylinder Bypass Valve |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| DEF | Diesel Exhaust Fluid |
| DPF | Diesel Particulate Filter |
| ECA | Emission Control Areas |
| EGB | Exhaust Gas Bypass Valve |
| EGR | Exhaust Gas Recirculation |
| HCs | Hydrocarbons |
| HFO | Heavy Fuel Oil |
| HP-SCR | High-Pressure SCR |
| H2SO4 | Sulfuric acid vapor |
| IFO | Intermediate Fuel Oil |
| IMO | International Maritime Organization |
| LP-SCR | Low-Pressure SCR |
| MCR | Maximum Continuous Rating |
| MDO | Marine Diesel Oil |
| NH3 | Ammonia |
| NH4HSO3 | Ammonium bisulfite |
| NH4HSO4 | Ammonium bisulfate |
| NH4NO3 | Ammonium nitrate |
| (NH4)2SO3 | Ammonium sulfite |
| (NH4)2SO4 | Ammonium sulfate |
| NOx | Nitrogen Oxides |
| PM | Particulate Matter |
| RBV | Reactor Bypass Valve |
| RO | Residual Oil |
| RSV | Reactor Sealing Valve |
| RTV | Reactor Throttle Valve |
| SCR | Selective Catalytic Reduction |
| SO2 | Sulfur dioxide |
| SOx | Sulphur Oxides |
| UFP | Ultrafine Particles |
| UWS | Urea-Water Solution |
| VOC | Volatile Organic Compounds |
References
- International Maritime Organization (IMO). List of Special Areas, Emission Control Areas and Particularly Sensitive Sea Areas; MEPC.1/Circ.778/Rev.3; International Maritime Organization: London, UK, 2018. [Google Scholar]
- Parthasarathy, M.; Ramkumar, S.; Elumalai, P.V.; Murugu Nachippan, N.; Dhinesh, B. Control Strategies on HCCI Engine Performance and Emission characteristics by Combined Effect of Exhaust Gas Recirculation with Blend of Biodiesel and N-Heptane. Energy Sources Part A Recovery Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Ministry of Transport of the People’s Republic of China. Implementation Plan of the Ship Air Pollutant Emission Control Areas; Ministry of Transport of the People’s Republic of China: Beijing, China, 2018.
- Wang, Y.; Ge, D.; Che, M.; Gao, S.; Wu, Z. A dual-functional way for regenerating NH3-SCR catalysts while enhancing their poisoning resistance. Catal. Commun. 2018, 117, 69–73. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Yusuf, D.A.; Jie, Z.; Bello, T.Y.; Tambaya, M.; Abdullahi, B.; Muhammed-Dabo, I.A.; Yahuza, I.; Dandakouta, H. Influence of waste oil-biodiesel on toxic pollutants from marine engine coupled with emission reduction measures at various loads. Atmos. Pollut. Res. 2022, 13, 101258. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Inambao, F.L.; Ampah, J.D. Evaluation of biodiesel on speciated PM2.5, organic compound, ultrafine particle and gaseous emissions from a low-speed EPA Tier II marine diesel engine coupled with DPF, DEP and SCR filter at various loads. Energy 2022, 239, 121837. [Google Scholar] [CrossRef]
- Ni, P.; Wang, X.; Li, H. A review on regulations, current status, effects and reduction strategies of emissions for marine diesel engines. Fuel 2020, 279, 118477. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Z.; Shreka, M.; Zhu, Y.; Zhou, S.; Zhang, W. Thermodynamic analysis and performance optimization of the supercritical carbon dioxide Brayton cycle combined with the Kalina cycle for waste heat recovery from a marine low-speed diesel engine. Energy Convers. Manag. 2020, 206, 112483. [Google Scholar] [CrossRef]
- Wartsila Company. Wartsila Environmental Product Guide; Wartsila Company Press: Helsinki, Finland, 2017. [Google Scholar]
- Dirk, K.; Martin, B.; Severin, Z.; Flavio, S. Compact Marine High-Pressure SCR System Technology Development. In Proceedings of the CIMAC Congress, Vancouver, BC, Canada, 10–14 June 2019. [Google Scholar]
- Kaźmierski, B.; Górka, K.; Kapusta, Ł.J. A conceptual design and numerical analysis of the mixerless urea-SCR system. Combust. Engines 2021, 187, 12–20. [Google Scholar] [CrossRef]
- Prabhu, S.S.; Natesan, K.; Shivappa Nayak, N. Effect of UWS spray angle and positioning of injector on ammonia concentration in Urea-SCR system. Mater. Today Proc. 2021, 46, 8051–8055. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lee, S.W.; Choi, W.C.; Yoon, Y.B. Urea-SCR system optimization with various combinations of mixer types and decomposition pipe lengths. Int. J. Automot. Technol. 2014, 15, 723–731. [Google Scholar] [CrossRef]
- Tischer, S.; Börnhorst, M.; Amsler, J.; Schoch, G.; Deutschmann, O. Thermodynamics and reaction mechanism of urea decomposition. Phys. Chem. Chem. Phys. 2019, 21, 16785–16797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Chen, Y.; Li, Z.; Wang, H.; Hao, B.; Chen, Y.; Lei, H.; Guo, X. Analysis of deposit formation mechanism and structure optimization in urea-SCR system of diesel engine. Fuel 2020, 265, 116941. [Google Scholar] [CrossRef]
- Yu, J.; Mo, C.; Huang, W.; Duan, L.; Long, H.; Mo, Y. Detailed Reaction Pathways of Urea Deposit in Diesel Engine with Selective Catalytic Reduction System. Trans. CSICE 2020, 38, 169–177. [Google Scholar]
- Qian, F.; Lv, L.; Yang, D. Simulation of Deposit Formation Inside the Exhaust Pipe on a Diesel Engine with SCR. Trans. CSICE 2018, 36, 144–152. [Google Scholar]
- MAN B&W. MAN Emission Project Guide: MAN B&W Two-Stroke Marine Engines; MAN B&W Press: Augsburg, Germany, 2019. [Google Scholar]
- Ryu, C.; Hwang, J.; Cheon, J. The world’s first commercialized low pressure SCR system on 2-stroke engine DeNOx system. In Proceedings of the Helsinki: 28th CIMAC Congress, Helsinki, Finland, 6–10 June 2016. No. 305. [Google Scholar]
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective Catalytic Reduction of NOx with NH3 by Using Novel Catalysts: State of the Art and Future Prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Liu, X.; Zhang, Q.; Li, Z. Numerical investigation on the urea deposit formation process in a selective catalytic reduction system of a diesel engine based on a fluid–solid coupling method. ACS Omega 2021, 6, 5921–5932. [Google Scholar] [CrossRef]
- Tan, L.; Feng, P.; Yang, S.; Guo, Y.; Liu, S.; Li, Z. CFD studies on effects of SCR mixers on the performance of urea conversion and mixing of the reducing agent. Chem. Eng. Process. 2018, 123, 82–88. [Google Scholar] [CrossRef]
- Christensen, H. Tier III SCR for large 2-stroke MAN B&W diesel engines. In Proceedings of the International Symposium on Marine Engineering, Kobe, Japan, 17–21 October 2011. [Google Scholar]
- Zhu, Y.; Li, T.; Xia, C.; Feng, Y.; Zhou, S. Simulation analysis on vaporizer/mixer performance of the high-pressure SCR system in a marine diesel. Chem. Eng. Process. 2020, 148, 107819. [Google Scholar] [CrossRef]
- Han, B.; Shen, Y.; Zhu, S.; Liu, Y.; Shen, S. Promotional effect of phosphorylation on CeSn0.8W0.6Ox/TiAl0.2Si0.1Oy for NH3-SCR of NO from marine diesel exhaust. J. Rare Earths 2016, 34, 1010–1016. [Google Scholar] [CrossRef]
- Börnhorst, M.; Deutschmann, O. Advances and challenges of ammonia delivery by urea-water sprays in SCR systems. Prog. Energ. Combust. 2021, 87, 100949. [Google Scholar] [CrossRef]
- Cimino, S.; Lisi, L.; Tortorelli, M. Low temperature SCR on supported MnOx catalysts for marine exhaust gas cleaning: Effect of KCl poisoning. Chem. Eng. J. 2016, 283, 223–230. [Google Scholar] [CrossRef]
- Murakami, M.; Nakao, T. Test Results of Marine SCR System for Large Diesel Engines during Long Term Service Test. J. JIME 2015, 50, AP3. [Google Scholar] [CrossRef]
- Xiao, L.; Lyu, L.; Zhu, N. Research and Application on the Structural Design of SCR Catalystin Marine Diesel Engine. Intern. Combust. Engines 2019, 4, 1–6. [Google Scholar]
- Yang, Z.; Jiang, G.; Zhang, X.; Wei, H. Application of selective reduction catalyst to marine diesel engine. J. Harbin Eng. Univ. 2017, 38, 1539–1544. [Google Scholar]
- Liang, R. Quantum Chemistry Research on DeNOX and Poisoning Mechanism of SCR Catalyst. Master’s Thesis, North China Electric Power University, Beijing, China, 2017. [Google Scholar]
- Qu, J.; Feng, Y.; Xu, G.; Zhang, M.; Zhu, Y.; Zhou, S. Design and thermodynamics analysis of marine dual fuel low speed engine with methane reforming integrated high pressure exhaust gas recirculation system. Fuel 2022, 319, 123747. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Zhou, S.; Feng, Y. Reaction mechanism and chemical kinetics of NH3-NO/NO2-SCR system with vanadium-based catalyst under marine diesel exhaust conditions. Proc. Inst. Mech. Eng. Part A J. Power Energy 2020, 234, 342–352. [Google Scholar] [CrossRef]
- Zhang, L. The Injecting Structural Parameters Optimization and Matching Performance of SCR System for Marine Diesel Engine. Master’s Thesis, Jimei University, Xiamen, China, 2016. [Google Scholar]
- Ji, P. Research of SO2 Oxidation over SCR Catalyst and Regulatory Mechanism. Master’s Thesis, Zhejiang University, Hangzhou, China, 2016. [Google Scholar]
- Clair, S.; de Oteyza, D.G. Controlling a Chemical Coupling Reaction on a Surface: Tools and Strategies for On-Surface Synthesis. Chem. Rev. 2019, 119, 4717–4776. [Google Scholar] [CrossRef]
- Qu, J.; Feng, Y.; Zhu, Y.; Zhou, S.; Zhang, W. Design and thermodynamic analysis of a combined system including steam Rankine cycle, organic Rankine cycle, and power turbine for marine low-speed diesel engine waste heat recovery. Energy Convers. Manag. 2021, 245, 114580. [Google Scholar] [CrossRef]
- Peng, H.; Chen, W. The analysis and countermeasures of the system problems in SCR flue gas denitrification ammonia zone. Green Pet. Petrochem. 2019, 4, 45–48. [Google Scholar]
- Ou, S.; Min, E.; Zhang, L.; Deng, Z.; Yin, Z. Experimental Analysis on Matching Performance of SCR System for a Marine Diesel Engine. Ship Eng. 2017, 39, 66–70. [Google Scholar]
- Zheng, C. Numerical Simulation and Test Verification of the whole Process of SCR System for Marine Low Speed Diesel Engine. Master’s Thesis, China Ship Research Institute, Beijing, China, 2019. [Google Scholar]
- Gao, C.; Shi, J.; Fan, Z.; Wang, B.; Wang, Y.; He, C.; Wang, X.; Li, J.; Niu, C. “Fast SCR” reaction over Sm-modified MnOx–TiO2 for promoting reduction of NOx with NH3. Appl. Catal. A Gen. 2018, 564, 102–112. [Google Scholar] [CrossRef]
- Xie, P.; Gao, M.; Zhang, H.; Niu, Y.; Wang, X. Dynamic modeling for NOx emission sequence prediction of SCR system outlet based on sequence to sequence long short-term memory network. Energy 2020, 190, 116482. [Google Scholar] [CrossRef]
- Liu, G.; Cui, Y.; Ji, J.; Shen, D.; Wang, Q.; Li, C.; Luo, K. A technical method to improve NOx/NH3 mixing ratio in CR systemand its engineering applications. J. Energy Inst. 2019, 92, 1757–1764. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, R.; Zhou, S.; Huang, C.; Feng, Y.; Zhang, C. Performance Optimization of High-pressure SCR System in a Marine Diesel Engine. Part I: Flow Optimization and Analysis. Top. Catal. 2019, 62, 27–39. [Google Scholar] [CrossRef]
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent Advances in the Catalytic Oxidation of Volatile Organic Compounds: A Review Based on Pollutant Sorts and Sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Won, J.M.; Hong, S.C. Selective Catalytic Reduction (SCR) Technology Trend for the Removal of Nitrogen Oxide from Ship Flue Gas. KIC News 2019, 22, 25–40. [Google Scholar]
- Lenka, S.C. Evaluation of Selective Catalytic Reduction for Marine Two-Stroke Diesel Engines. Master’s Thesis, Aalborg University, Aalborg, Denmark, 2010. [Google Scholar]
- Andreas, D.; Mirko, B.; Joachim, H.; Bader, I.; Struckmeier, D.; Baydak, M.; Losher, R.; Stiesch, G. The MAN SCR System-More Than Just Fulfilling IMO Tier III. In Proceedings of the CIMAC Congress 2016, Helsinki, Finland, 6–10 January 2016; Ppaer No. 26. Available online: https://www.researchgate.net/publication/304525249_The_MAN_SCR_System_-_More_Than_Just_Fulfilling_IMO_Tier_III (accessed on 26 April 2022).
- Federico, C. Selective Catalytic Reduction System for Marine Applications: Dynamic Modelling and System Integration. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2018. [Google Scholar]
- Langeslay, R.R.; Kaphan, D.M.; Marshall, C.L.; Stair, P.C.; Sattelberger, A.P.; Delferro, M. Catalytic Applications of Vanadium: A Mechanistic Perspective. Chem. Rev. 2019, 119, 2128–2191. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z.; Zeng, Y. Research progresses of low temperature SCR denitration catalyst. Environ. Prot. Chem. Ind. 2019, 39, 1–5. [Google Scholar]
- Jensen-Holm, H.; Castellino, F.; White, N.T. SCR DeNOx catalyst considerations when using biomass in power generation. In Proceedings of the Power Plant Air Pollutant Control “MEGA” Symposium, Washington, DC, USA, 30 August–2 September 2010. [Google Scholar]
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Zhou, Z.; Guan, Y. Application of the selective catalytic reduction system in heavy oil catalytic cracking flue gas denitrification. Pet. Processing Petrochem. 2019, 50, 103–107. [Google Scholar]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2019, 119, 2611–2680. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Shi, C.; Li, M.; Zhang, T.; Wang, C.; Jiang, L.; Wang, X. The effect of cations (NH4+, Na+, K+, and Ca2+) on chemical deactivation of commercial SCR catalyst by bromides. J. Catal. 2018, 39, 710–717. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, R.; Zhou, S.; Huang, C.; Feng, Y.; Shreka, M.; Zhang, C. Performance Optimization of High-pressure SCR System in a Marine Diesel Engine. Part II: Catalytic Reduction and Process. Top. Catal. 2019, 62, 40–48. [Google Scholar] [CrossRef]
- MAN Energy Solutions. MAN B&W Two-Stroke Marine Engines Emission Project Guide for Marpol Annex VI Regulations, 9th ed.; MAN Energy Solutions: Augsburg, Germany, 2018. [Google Scholar]
- Liu, B.; Lyu, L.; Zhang, R. A nonlinear active disturbance rejection control system for the Urea-SCR device in a diesel engine. J. Harbin Eng. Univ. 2017, 38, 385–391. [Google Scholar]
- Zhu, Y.; Xia, C.; Shreka, M.; Wang, Z.; Yuan, L.; Zhou, S.; Feng, Y.; Hou, Q.; Ahmed, S.A. Combustion and Emission Characteristics for a Marine Low-speed Diesel Engine with High-pressure SCR System. Environ. Sci. Pollut. R. 2020, 27, 12851–12865. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, Y.; Peng, H.; Yan, H.; Li, T.; Zhang, J.; Zhou, S. Modeling and simulation of marine SCR system based on Modelica. Int. J. Engine Res. 2022. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, G.; Li, C. Ammonia storage characteristics simulation of SCR catalyst for marine diesel engine based on AVL BOOST. J. Dalian Marit. Univ. 2016, 42, 68–74. [Google Scholar]
- Yang, Y.; Li, X.; Wang, Z.; Zhong, Q.; Chen, Q.; Wang, J.; Shen, F. Research on Performance Test and Verification Technology of Low-pressure SCR System for Marine Low-speed Diesel Engine. Intern. Combust. Engine Parts 2018, 10–13. [Google Scholar]
- Zhao, Y.; Hu, J.; Hua, L.; Chen, Z.; Shuai, S.; Wang, J. Experimental Study of Dynamic Reaction and Ammonia Storage Characteristics of Vanadium-Based SCR Catalyst. Chin. Intern. Combust. Engine Eng. 2011, 32, 1–6. [Google Scholar]
- Guo, M.; Li, X.; Zhu, X.; Kong, W.; Wang, M.; Wang, Z. Flow Simulation and Optimization Design of the High-pressure SCR System for Marine High-power Low-speed Machine. Intern. Combust. Engine Parts 2019, 19–22. [Google Scholar]
- Zhang, H.; Jin, Y.; Zhang, D.; Liu, R.; Ping, T.; Wang, F. SCR System Matching and Experimental Study for Medium Speed Marine Diesel Engine. Diesel Engine 2016, 38, 15–19. [Google Scholar]
- Peng, J.; Huang, F.; Wan, M. Current Research and Future Technical Challenges of Diesel Engine SCR Control. Agric. Equip. Veh. Eng. 2019, 57, 50–55. [Google Scholar]
- Wang, H.; Huang, B.; Yu, C.; Lu, M.; Huang, H.; Zhou, Y. Research progress, challenges and perspectives on the sulfur and water resistance of catalysts for low temperature selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2019, 588, 117207. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, Q. Surface characterization studies on the interaction of V2O5-WO3/TiO2 catalyst for low temperature SCR of NO with NH3. J. Solid. State. Chem. 2015, 221, 49–56. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, S.; Feng, Y.; Wang, Z. Influence of NH4NO3 formation on the NOx reduction pathways over vanadium-based catalyst under diesel exhaust conditions. Russ. J. Phys. Chem. A 2018, 92, 1473–1480. [Google Scholar]
- Dun, J.; Du, H.; Wu, X.; Wang, P.; Xu, B. Urea deposition characteristics of the diesel selective catalytic reduction after treatment system. J. Henan Univ. Sci. Technol. 2020, 41, 24–29. [Google Scholar]
- Shi, Y.; Wang, X.; Li, S.; Li, W.; Shan, S.; Zhuang, H.; Wang, Q. Review on catalysts for NH3-SCR of NOx from diesel vehicle. J. Chem. Eng. Chin. Univ. 2019, 33, 10–20. [Google Scholar]
- Bai, Y.; Teng, Q.; Ma, B. A Urea injection control system for the SCR catalyst performance evaluation. Intern. Combust. Engine Powerpl. 2019, 36, 5–14. [Google Scholar]
- Luo, S. Simulation Study on Matching Performance of Marine High Pressure SCR Diesel Engine. Master’s Thesis, Harbin Engineering University, Harbin, China, 2017. [Google Scholar]
- Zhu, M.; Hu, Z.; Xia, S.; Ju, Y.; Lyu, J. Evaluation and Prediction About the Risk of Urea Deposits in the SCR System. Trans. Csice 2020, 38, 90–95. [Google Scholar]
- Xia, C.; Zhu, Y.; Zhou, S.; Peng, H.; Feng, Y.; Zhou, W.; Shi, J.; Zhang, J. Simulation study on transient performance of a marine engine matched with high-pressure SCR system. Int. J. Engine Res. 2022. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.; Gao, Y. Diesel Engine SCR Control: Current Development and Future Challenges. Emiss. Control Sci. Technol. 2015, 1, 121–133. [Google Scholar] [CrossRef]
- Muzio, L.; Bogseth, S.; Himes, R.; Chien, Y.C.; Dunn-Rankin, D. Ammonium bisulfate formation and reduced load SCR operation. Fuel 2017, 206, 180–189. [Google Scholar] [CrossRef]
- Lietti, L.; Nova, I.; Camurri, S.; Tronconi, E.; Forzatti, P. Dynamics of the SCR-DeNOx reaction by the transient-response method. AIChE J. 1997, 43, 2559–2570. [Google Scholar] [CrossRef]
- Liu, H.; Fu, M.; Jin, X.; Shang, Y.; Shindell, D.; Faluvegi, G.; Shindell, C.; He, K. Health and climate impacts of ocean-going vessels in East Asia. Nat. Clim. Chang. 2016, 6, 1037–1041. [Google Scholar] [CrossRef]
- Corbett, J.J.; Winebrake, J.J.; Green, E.H.; Kasibhatla, P.; Eyring, V.; Lauer, A. Mortality from ship emissions: A global assessment. Environ. Sci. Technol. 2007, 41, 8512–8518. [Google Scholar] [CrossRef]
- Vareide, K. An Outlook for the Maritime Industry Towards 2020—Future Development in Maritime Shipping; Det Norske Veritas A.S.: Bærum, Norway, 2013. [Google Scholar]
- MAN B&W. Exhaust Gas Emission Control Today and Tomorrow, Application on MAN B&W Two-Stroke Marine Diesel Engines; MAN Diesel: Copenhagen, Denmark, 2010. [Google Scholar]
- Marco, P.; Yolanda, G.C.; Andrés, A.; de la Rosa, J.D.; Mantilla, E.; de la Campa, A.S.; Querol, X.; Pey, J.; Amato, F.; Moreno, T. Source apportionment of PM (10) and PM (2.5) at multiple sites in the strait of Gibraltar by PMF: Impact of shipping emissions. Environ. Sci. Pollut. R. 2011, 18, 260–269. [Google Scholar]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Xiong, S. The Construction and Application of Steady-State Kinetic Study over Several Typical NH3-SCR Catalysts. Master’s Thesis, Nanjing University of Science & Technology, Nanjing, China, 2017. [Google Scholar]
- Federal Marine Comperssion-Ingnition (CI) Engines: Exhaust Emission Standards; U.S. Environmental Protection Agency: Washington, DC, USA, 2016.
- Tronconi, E.; Nova, I.; Ciardelli, C.; Chatterjee, D.; Weibel, M. Redox features in the catalytic mechanism of the “standard” and “fast” NH3-SCR of NOx over a V-based catalyst investigated by dynamic methods. J. Catal. 2007, 245, 1–10. [Google Scholar] [CrossRef]
- Codan, E.; Bernasconi, S.; Born, H. IMO III Emission Regulation: Impact on the Turbocharging System. In Proceedings of the CIMAC Congress, Bergen, Norway, 14–17 June 2010. Paper No. 139. [Google Scholar]
- Viana, M.; Hammingh, P.; Colette, A.; Querol, X.; Degraeuwe, B.; de Vlieger, I.; van Aardenne, J. Impact of maritime transport emissions on coastal air quality in Europe. Atmos. Environ. 2014, 90, 96–105. [Google Scholar] [CrossRef]
- Ciardelli, C.; Nova, I.; Tronconi, E.; Chatterjee, D.; Burkhardt, T.; Weibel, M. NH3 SCR of NOx for diesel exhausts aftertreatment: Role of NO2 in catalytic mechanism, unsteady kinetics and monolith converter modelling. Chem. Eng. Sci. 2007, 62, 5001–5006. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Z.; Sun, Q.; Xu, W.; Li, Y.; Wang, B.; Zhu, T.; Lin, D.; Ji, G.; Ma, J. Formation mechanism, effects and prevention of NH4HSO4 formed on the surface of V2O5 based catalysts. Chem. Ind. Eng. Prog. 2018, 37, 2635–2643. [Google Scholar]
- Omidvarborna, H.; Kumar, A.; Kim, D.S. Recent studies on soot modeling for diesel combustion. Renew. Sust. Energ. Rev. 2015, 48, 635–647. [Google Scholar] [CrossRef]
- Nova, I.; Ciardelli, C.; Tronconi, E.; Chatterjee, D.; Bandl-Konrad, B. NH3-NO/NO2 chemistry over V-based catalysts and its role in the mechanism of the fast SCR reaction. Catal. Today 2006, 114, 3–12. [Google Scholar] [CrossRef]
- Zhu, Y. Experimental and Numerical Study on the Catalytic Kinetics Characteristics of Marine Diesel SCR System. Ph.D. Thesis, Harbin Engineering University, Harbin, China, 2014. [Google Scholar]
- Ono, K.; Matsukawa, Y.; Dewa, K.; Watanabe, A.; Takahashi, K.; Saito, Y.; Matsushita, Y.; Aoki, H.; Era, K.; Aoki, T.; et al. Formation mechanisms of soot from high-molecular-weight polycyclic aromatic hydrocarbons. Combust. Flame 2015, 162, 2670–2678. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E.; Chatterjee, D.; Weibel, M. The chemistry of the NO/NO2-NH3 “fast” SCR reaction over Fe-ZSM5 investigated by transient reaction analysis. J. Catal. 2008, 256, 312–322. [Google Scholar] [CrossRef]
- Shi, X.; Luo, H.; Ni, J.; Peng, H.; Wang, Q.; Liu, Y. Model-Based Closed-Loop Control Strategy of Diesel Urea-SCR System Simulation. Trans. CSICE 2017, 35, 346–353. [Google Scholar]
- Kubota, Y.; Kawahara, N.; Tsuboi, K. Evaluation of Combustion Characteristics of Ultra Low Sulphur Fuel Oil by Using OCA (Optical Combustion Analyzer) and a Two Stroke Test Diesel Engine. In Proceedings of the 29th CIMAC World Congress on Combustion Engines, Vancouver, BC, Canada, 10–14 June 2019. [Google Scholar]
- Prikhodko, V.Y.; Parks, J.E.; Pihl, J.A.; Toops, T.J. Passive SCR for lean gasoline NOx control: Engine-based strategies to minimize fuel penalty associated with catalytic NH3 generation. Catal. Today 2016, 267, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Qiu, T.; Li, X.; Lei, Y.; Liu, X.; Zhang, C.; Feng, X.; Xu, H. The prediction of fuel injection quality using a NOx sensor for the on-board diagnosis of heavy-duty diesel engines with SCR systems. Fuel 2015, 141, 192–199. [Google Scholar] [CrossRef]
- Choi, C.; Sung, Y.; Choi, G.M.; Kim, D.J. Numerical analysis of NOx reduction for compact design in marine urea-SCR system. Int. J. Nav. Arch. Ocean 2015, 7, 1020–1034. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Yan, F.; Miao, Y.; Hou, J.; Liu, C. Control Model of Urea-SCR System for Diesel Engine. Trans. CSICE 2013, 31, 148–153. [Google Scholar]
- Anderson, M.; Salo, K.; Hallquist, Å.M.; Fridell, E. Characterization of particles from a marine engine operating at low loads. Atmos. Environ. 2015, 101, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Solaimuthu, C.; Ganesan, V.; Senthilkumar, D.; Ramasamy, K.K. Emission reductions studies of a biodiesel engine using EGR and SCR for agriculture operations in developing countries. Appl. Energ. 2015, 138, 91–98. [Google Scholar] [CrossRef]
- Park, T.; Sung, Y.; Kim, T.; Lee, I.; Choi, G.; Kim, D. Effect of static mixer geometry on flow mixing and pressure drop in marine SCR applications. Int. J. Nav. Arch. Ocean 2014, 6, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.; Yang, G.; Xiang, H.; Hu, Z.; Cui, Y.; Tian, S. DeNOx performance of a compact SCR catalyst on marine diesel engine. Chin. J. Environ. Eng. 2016, 10, 3130–3134. [Google Scholar]
- Zetterdahl, M.; Moldanová, J.; Pei, X.; Pathak, R.K.; Demirdjian, B. Impact of the 0.1% fuel sulfur content limit in SECA on particle and gaseous emissions from marine vessels. Atmos. Environ. 2016, 145, 338–345. [Google Scholar] [CrossRef]
- Wang, W.; Herreros, J.M.; Tsolakis, A.; York, A.P.E. Increased NO2 concentration in the diesel engine exhaust for improved Ag/Al2O3 catalyst NH3-SCR activity. Chem. Eng. J. 2015, 270, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.; Min, E.; Zhang, L.; Deng, Z.; Yin, Z. Simulation Optimization of Urea Injection Structure Parameters for SCR System of a Marine Diesel Engine. Ship Eng. 2017, 39, 10–14. [Google Scholar]
- Chen, N.; Zhang, Z.; Li, Y.; Meng, M. Design and experimental study of the marine engine’s SCR system. J. Jiangsu Univ. Sci. Technol. 2016, 30, 33–38. [Google Scholar]
- Verschaeren, R.; Schaepdryver, W.; Serruys, T.; Bastiaen, M.; Vervaeke, L.; Verhelst, S. Experimental study of NOx reduction on a medium speed heavy duty diesel engine by the application of EGR (exhaust gas recirculation) and Miller timing. Energy 2014, 76, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Capetillo, A.; Ibarra, F. Multiphase injector modelling for automotive SCR systems: A full factorial design of experiment and optimization. Comput. Math. Appl. 2017, 74, 188–200. [Google Scholar] [CrossRef]
- Jiang, L.; Ge, Y.; Li, P.; Liu, Z. Study on Emission Characteristics of Urea-SCR Aftertreatment System of Diesel Engine. Chin. Intern. Combust. Engine Eng. 2010, 31, 30–35. [Google Scholar]
- Koder, A.; Schwanzer, P.; Zacherl, F.; Rabl, H.P.; Mayer, W.; Gruber, G.; Dotzer, T. Combustion and emission characteristics of a 2.2L common-rail diesel engine fueled with jatropha oil, soybean oil, and diesel fuel at various EGR-rates. Fuel 2018, 228, 23–29. [Google Scholar] [CrossRef]
- Kaario, O.T.; Vuorinen, V.; Zhu, L.; Larmi, M.; Liu, R. Mixing and evaporation analysis of a high-pressure SCR system using a hybrid LES-RANS approach. Energy 2017, 120, 827–841. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Ge, Y.; Ding, Y.; Liu, Z.; Tan, J.; You, K. Study on Steady and Transient Characteristics of Urea-SCR System for Diesel Engine. Chin. Intern. Combust. Engine Eng. 2010, 31, 38–42. [Google Scholar]
- Tarabet, L.; Loubar, K.; Lounici, M.S.; Khiari, K.; Belmrabet, T.; Tazerout, M. Experimental investigation of DI diesel engine operating with eucalyptus biodiesel/natural gas under dual fuel mode. Fuel 2014, 133, 129–138. [Google Scholar] [CrossRef]
- Ebrahimian, V.; Nicolle, A.; Habchi, C. Detailed modeling of the evaporation and thermal decomposition of urea-water solution in SCR systems. Aiche. J. 2011, 58, 1998–2009. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tu, S.; Li, X.; Zhong, Q.; Wang, Z. The Bench Experiment Research of Low Pressure SCR Systems for Low Speed Marine Diesel Engines. Diesel Engine 2018, 40, 23–26. [Google Scholar]
- Zhou, S.; Zhou, J.; Zhu, Y. Chemical composition and size distribution of particulate matters from marine diesel engines with different fuel oils. Fuel 2019, 235, 972–983. [Google Scholar] [CrossRef]
- Xu, T.; Wu, X.; Gao, Y.; Lin, Q.; Hu, J.; Weng, D. Comparative study on sulfur poisoning of V2O5-Sb2O3/TiO2 and V2O5-WO3/TiO2 monolithic catalysts for low-temperature NH3-SCR. Catal. Commun. 2017, 93, 33–36. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, X. Research on High Pressure SCR System for Large Marine Diesel Engine. Diesel Engine 2018, 40, 22–26. [Google Scholar]
- Li, Z.; Meng, M.; Zha, Y.; Dai, F.; Hu, T.; Xie, Y.; Zhang, J. Highly efficient multifunctional dually-substituted perovskite catalysts La1−xKxCo1−yCuyO3−δ used for soot combustion, NOx storage and simultaneous NOx-soot removal. Appl. Catal. B-Environ. 2012, 121-122, 65–74. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, J.; Zhao, Z.; Song, W.; Wei, Y. Highly efficient and simultaneously catalytic removal of PM and NOx from diesel engines with 3DOM Ce0.8M0.1Zr0.1O2 (M= Mn, Co, Ni) catalysts. Chem. Eng. Sci. 2017, 167, 219–228. [Google Scholar] [CrossRef]
- Tan, L.; He, L.; Kou, C.; Jiang, J.; Gong, J. Numerical simulation on spray atomization of diesel engine Urea-SCR system. Chin. J. Environ. Eng. 2014, 8, 1554–1560. [Google Scholar]
- Chen, Y.; Lv, L. Design and evaluation of an Integrated SCR and exhaust Muffler from marine diesels. J. Mar. Sci. Technol 2015, 20, 505–519. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Li, G.; Guo, S. Emission reduction effect and carbon market efficiency of carbon emissions trading policy in China. Energy 2020, 196, 117117. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Geng, P.; Li, Q. Recent advances in selective hydrogenation of cinnamaldehyde over supported metal-based catalysts. ACS Catal. 2020, 10, 2395–2412. [Google Scholar] [CrossRef]
- Mahmoud, N.; Yan, F.; Zhou, M.; Xu, L.; Wang, Y. Coupled effects of carbon dioxide and water vapor addition on soot formation in ethylene diffusion flames. Energ. Fuel 2019, 33, 5582–5596. [Google Scholar] [CrossRef]
- Lehtoranta, K.; Aakko-Saksa, P.; Murtonen, T.; Vesala, H.; Ntziachristos, L.; Rönkko, T.; Karjalainen, P.; Kuittinen, N.; Timonen, H. Particulate Mass and Nonvolatile Particle Number Emissions from Marine Engines Using Low-Sulfur Fuels, Natural Gas, or Scrubbers. Environ. Sci. Technol. 2019, 53, 3315–3322. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Li, X.; Peng, Y.; Li, J. A neutral and coordination regeneration method of Ca-poisoned V2O5-WO3/TiO2 SCR catalyst. Catal. Commun. 2017, 100, 112–116. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, S.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn-Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 199. [Google Scholar] [CrossRef]
- Magnusson, M.; Fridell, E.; Ingelsten, H.H. The influence of sulfur dioxide and water on the performance of a marine SCR catalyst. Appl. Catal. B Environ. 2012, 111–112, 20–26. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yang, R.T.; Mo, J.; Li, J.; Hao, J. The poisoning effects of calcium on V2O5-WO3/TiO2 catalyst for the SCR reaction: Comparison of different forms of calcium. Mol. Catal. 2017, 434, 16–24. [Google Scholar] [CrossRef]
- Takahiro, F.; Shinji, B.; Hironaka, T. Development of Marine SCR System for Large Two-stroke Diesel Engines Complying with IMO NOx Tier III. In Proceedings of the Shanghai: CIMAC Congress, Shanghai, China, 13–17 May 2013. No. 29. [Google Scholar]
- WinGD Global. Proceedings of the Marine Engine Manufacturer Conference; WinGD: Winterthur, Switzerland, 2018. [Google Scholar]
- Foteinos, M.I.; Konstantinidis, S.K.; Kyrtatos, N.P.; Busk, K.V. Simulation of the Transient Thermal Response of a High Pressure Selective Catalytic Reduction Aftertreatment System for a Tier III Two-Stroke Marine Diesel Engine. J. Eng. Gas. Turb. Power. 2019, 141, 071001. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Hou, Q.; Shreka, M.; Yuan, L.; Zhou, S.; Feng, Y.; Xia, C. Ammonium-Salt Formation and Catalyst Deactivation in the SCR System for a Marine Diesel Engine. Catalysts 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Tzannatos, E. Ship emissions and their externalities for Greece. Atmos. Environ. 2010, 44, 2194–2202. [Google Scholar] [CrossRef]





| Operating Mode | NOx | SOx | CO2 | PM | VOC |
|---|---|---|---|---|---|
| Normal sailing | 17 | 10.5 | 620 | 1.7 | 0.6 |
| Idle or berthing state | 13.6 | 10.6 | 682 | 2.4 | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhou, W.; Xia, C.; Hou, Q. Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission. Atmosphere 2022, 13, 731. https://doi.org/10.3390/atmos13050731
Zhu Y, Zhou W, Xia C, Hou Q. Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission. Atmosphere. 2022; 13(5):731. https://doi.org/10.3390/atmos13050731
Chicago/Turabian StyleZhu, Yuanqing, Weihao Zhou, Chong Xia, and Qichen Hou. 2022. "Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission" Atmosphere 13, no. 5: 731. https://doi.org/10.3390/atmos13050731
APA StyleZhu, Y., Zhou, W., Xia, C., & Hou, Q. (2022). Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission. Atmosphere, 13(5), 731. https://doi.org/10.3390/atmos13050731







