Clustering Analysis on Drivers of O3 Diurnal Pattern and Interactions with Nighttime NO3 and HONO
Abstract
:1. Introduction
2. Measurement and Method
2.1. Measurement Site and Instrument Installation
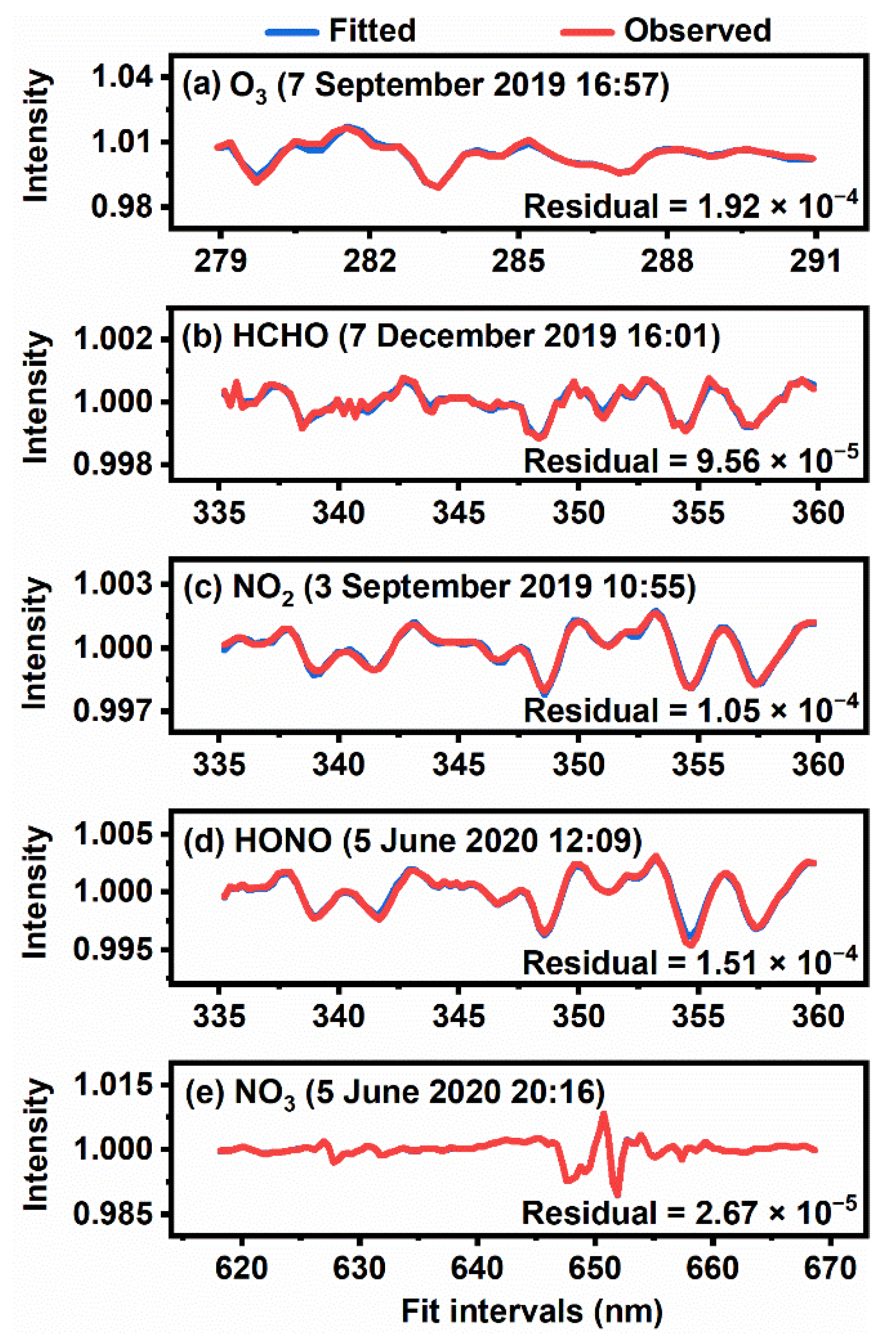
2.2. Spectral Analysis
2.3. Auxiliary Data
3. Results and Discussion
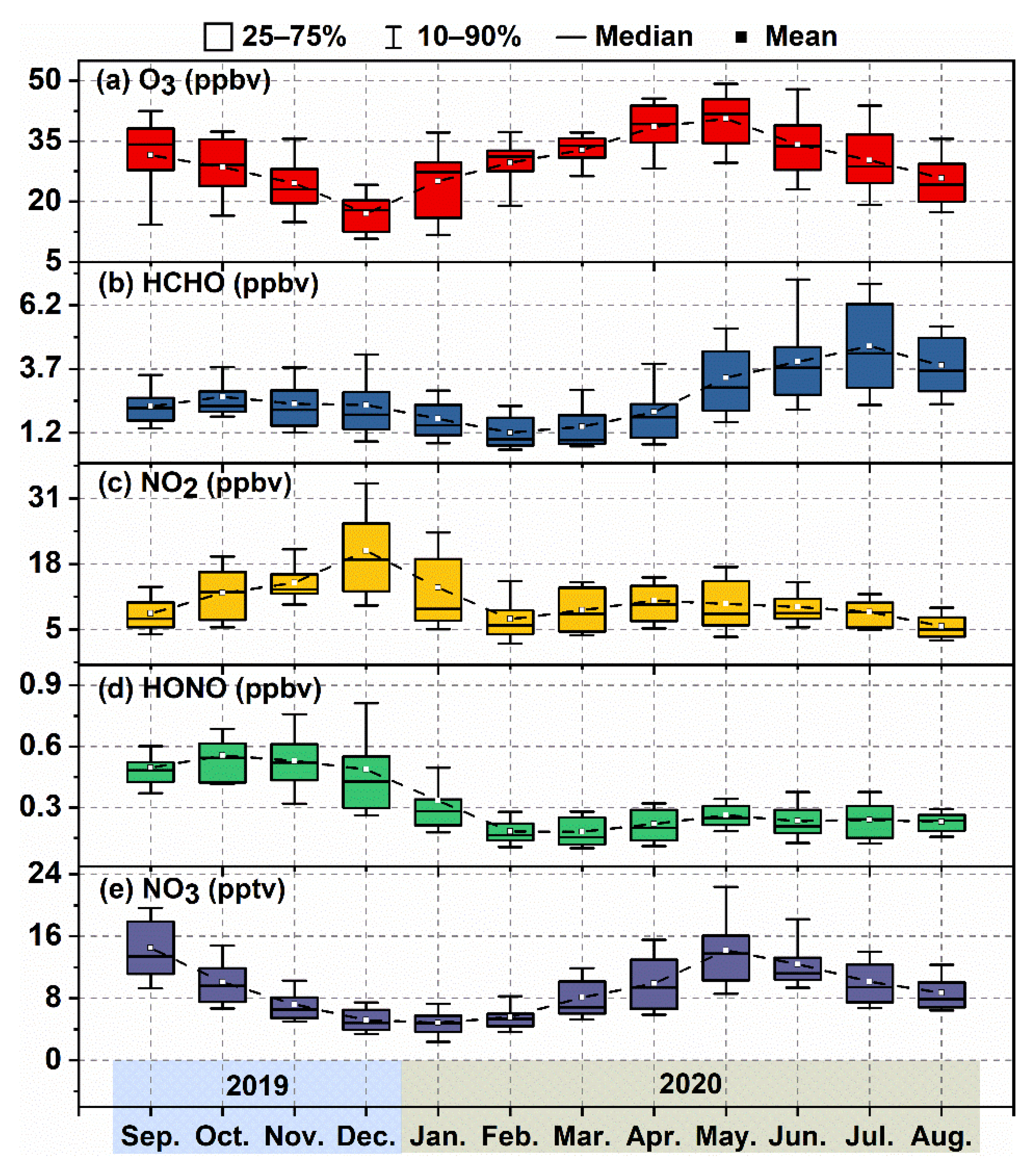
3.1. Overview of Observation
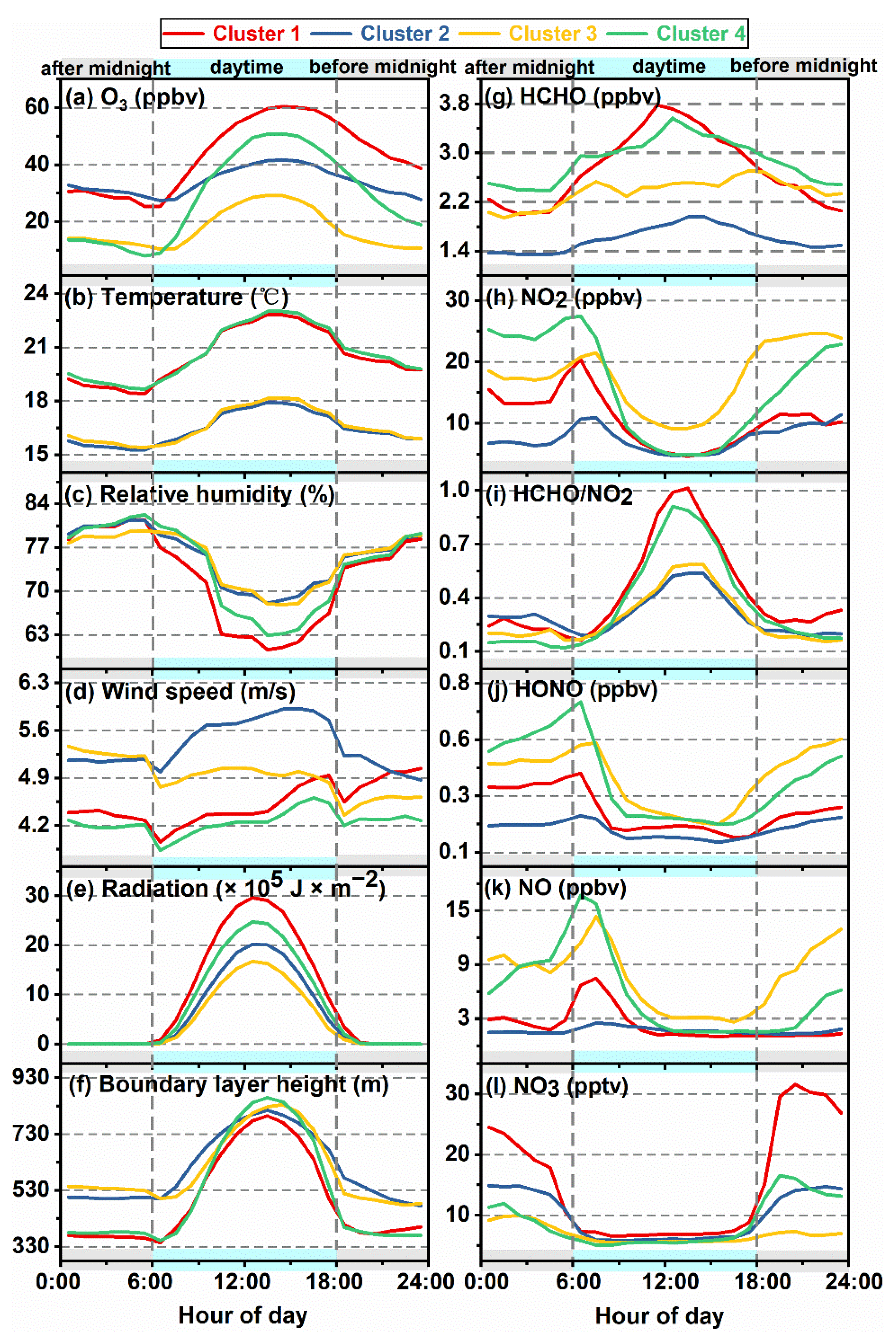
3.2. Clustering of O3 Diurnal Patterns
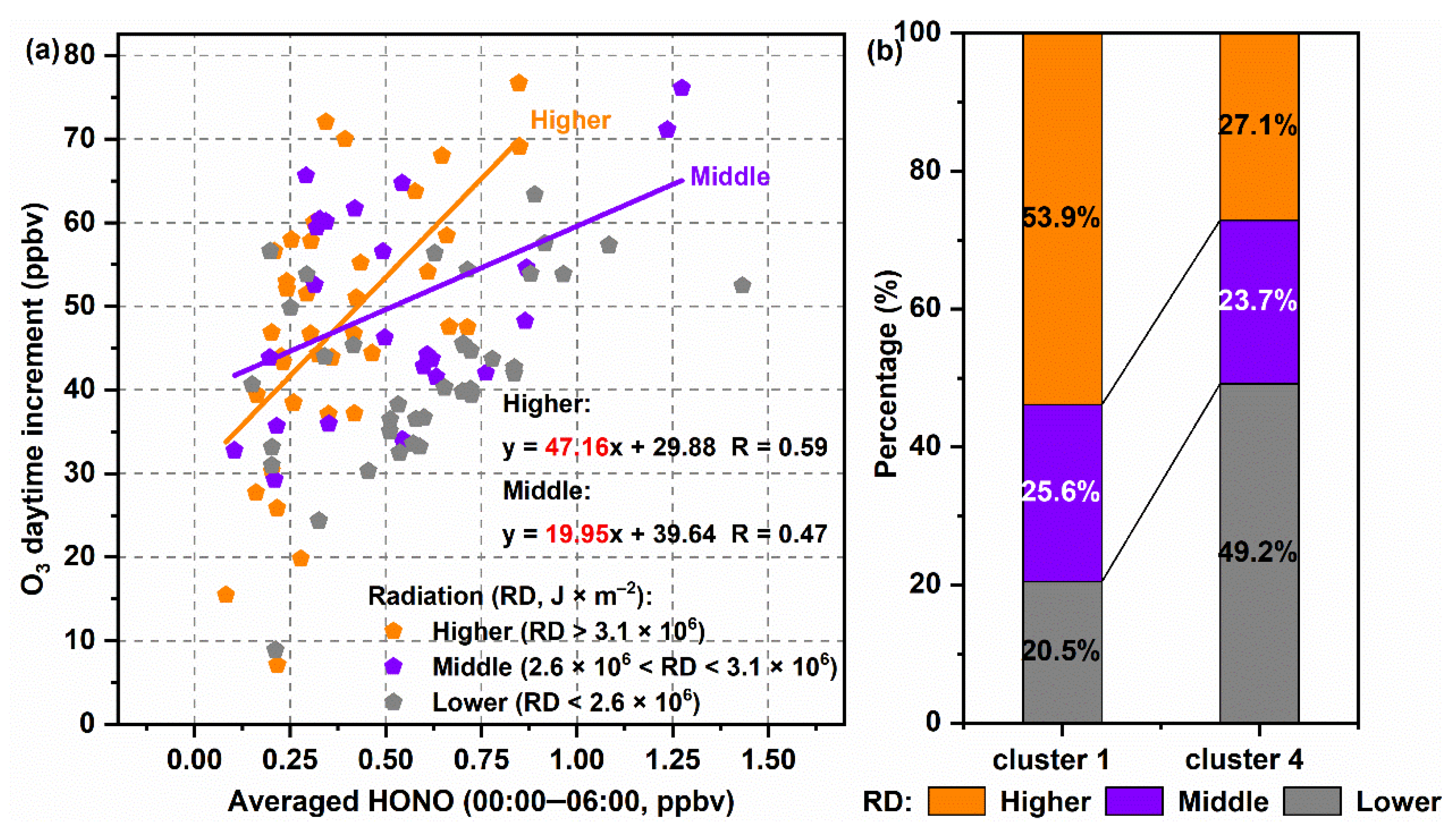
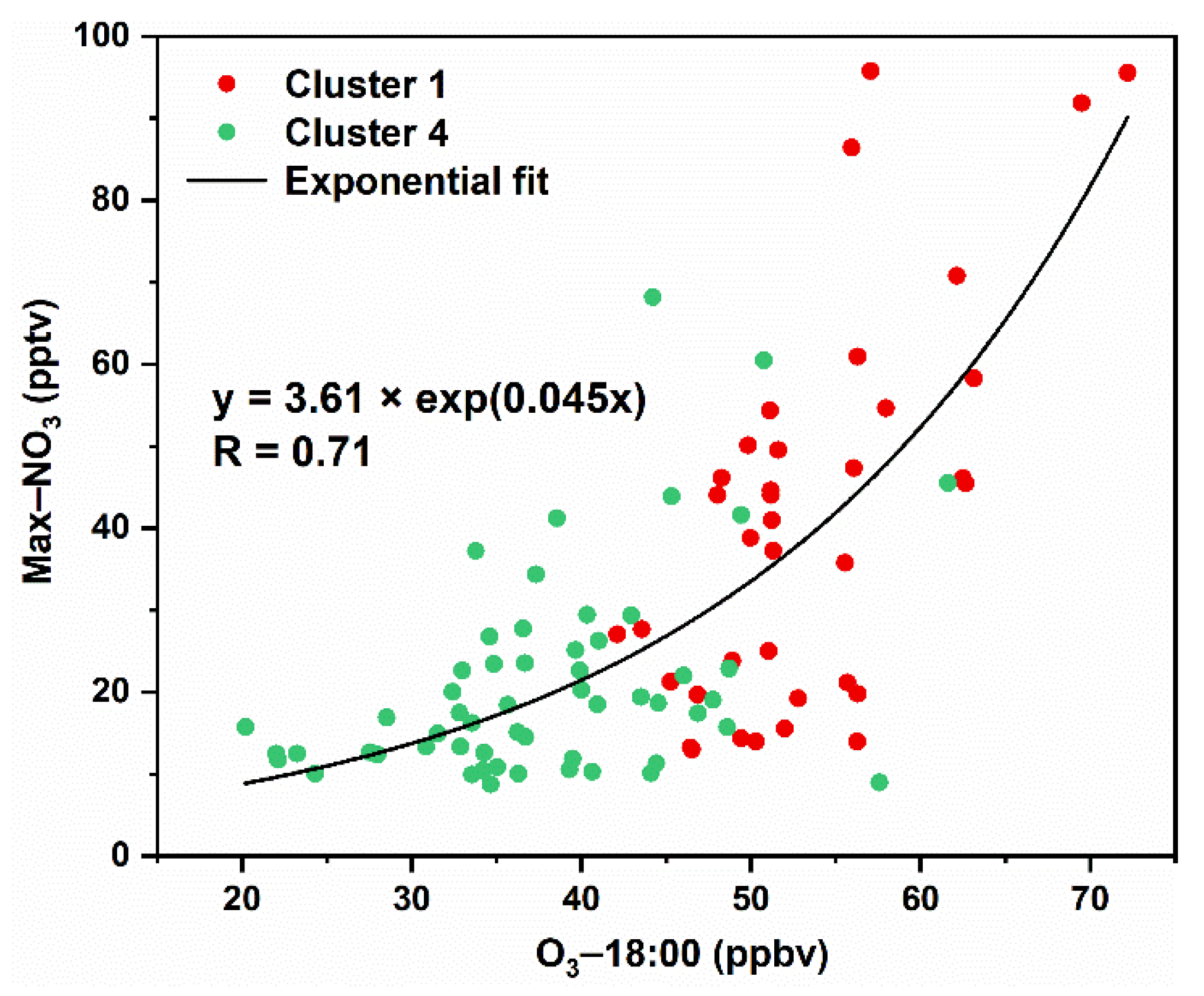
3.3. Interactions of Day- and Nighttime Atmospheric Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiu, W.Y.; Li, S.L.; Liu, Y.H.; Lu, K.D. Petrochemical and industrial sources of volatile organic compounds analyzed via regional wind-driven network in Shanghai. Atmosphere 2019, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, D.; Bian, J.; Bai, Z. Deep stratospheric intrusion and Russian wildfire induce enhanced tropospheric ozone pollution over the northern Tibetan Plateau. Atmos. Res. 2021, 259, 105662. [Google Scholar] [CrossRef]
- Alejandro, S.; Valdes, H.; Zaror, C.A. Natural zeolite reactivity towards ozone: The role of acid surface sites. Adv. Oxid. Technol. J. 2011, 14, 182–189. [Google Scholar] [CrossRef]
- Schnell, J.L.; Prather, M.J.; Josse, B.; Naik, V.; Horowitz, L.W.; Zeng, G.; Shindell, D.T.; Faluvegi, G. Effect of climate change on surface ozone over North America, Europe, and East Asia. Geophys. Res. Lett. 2016, 43, 3509–3518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, N.; Zheng, Y.Q.; Yue, X.; Harper, K.L. Mitigation of ozone damage to the world’s land ecosystems by source sector. Nat. Clim. Chang. 2020, 10, 134–137. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Xue, L.; Brimblecombe, P.; Lam, Y.F.; Li, L.; Zhang, L. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects. Sci. Total Environ. 2017, 575, 1582–1596. [Google Scholar] [CrossRef]
- Liu, P.F.; Song, H.Q.; Wang, T.H.; Wang, F.; Li, X.Y.; Miao, C.H.; Zhao, H.P. Effects of meteorological conditions and anthropogenic precursors on ground-level ozone concentrations in Chinese cities. Environ. Pollut. 2020, 262, 8. [Google Scholar] [CrossRef]
- Wang, N.; Lyu, X.P.; Deng, X.J.; Huang, X.; Jiang, F.; Ding, A.J. Aggravating O3 pollution due to NOx emission control in eastern China. Sci. Total Environ. 2019, 677, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Li, R.Y.; Chen, D.L.; Zhuang, Y.; Gao, B.B.; Yang, L.; Li, M.C. Understanding the causal influence of major meteorological factors on ground ozone concentrations across China. J. Clean. Prod. 2020, 242, 13. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhuang, Y.; Xie, X.M.; Chen, D.L.; Cheng, N.L.; Yang, L.; Li, R.Y. Understanding long-term variations of meteorological influences on ground ozone concentrations in Beijing During 2006–2016. Environ. Pollut. 2019, 245, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.P.; Yin, Y.Q.; Zhang, J.D.; Ji, X.; Deng, X.Y. Surface ozone and meteorological condition in a single year at an urban site in central-eastern China. Environ. Monit. Assess. 2009, 151, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Wang, G.L.; Yang, P.C. Study on the Sensitivity of Summer Ozone Density to the Enhanced Aerosol Loading over the Tibetan Plateau. Atmosphere 2020, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Tan, Z.; Lu, K.; Ma, X.; Li, X.; Chen, S.; Zhu, B.; Lin, L.; Li, Y.; Qiu, P.; et al. An explicit study of local ozone budget and NOx-VOCs sensitivity in Shenzhen China. Atmos. Environ. 2020, 224, 13. [Google Scholar] [CrossRef]
- Sun, L.Q.; Cheng, K.X.; Yang, H.D.; He, Q.S. An on-line monitoring technique for Trace Gases in Atmosphere Based on Differential optical absorption spectroscopy. In Proceedings of the 2nd Conference of the Optics-and-Photonics-Society-of-Singapore/International Conference on Optics in Precision Engineering and Nanotechnology (icOPEN), Singapore, 9–11 April 2013; Volume 8769. [Google Scholar]
- Allegrini, I.; Febo, A.; Giliberti, C.; Perrino, C. Intercomparison of DOAS and conventional analysers in the measurement of atmospheric pollutants in an urban background monitoring site of Rome. In Proceedings of the Conference on Remote Sensing of Vegetation and Water, and Standardization of Remote Sensing Methods, Munich, Germany, 18–19 June 1997; Volume 3107, pp. 64–73. [Google Scholar]
- Hoffman, S.; Sulkowski, W.; Krzyzanowski, K. The urban ozone monitoring by the DOAS technique application. J. Mol. Struct. 1995, 348, 187–189. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.S.; Wang, H.L.; Jing, S.G.; Lou, S.R.; Saiz-Lopez, A.; Zhou, B. Observationally constrained modeling of atmospheric oxidation capacity and photochemical reactivity in Shanghai, China. Atmos. Chem. Phys. 2020, 20, 1217–1232. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.H.; Wang, S.S.; Zhu, J.; Guo, Y.L.; Tang, G.Q.; Liu, B.X.; An, X.X.; Wang, Y.S.; Zhou, B. Vertically increased NO3 radical in the nocturnal boundary layer. Sci. Total Environ. 2021, 763, 12. [Google Scholar] [CrossRef]
- Voigt, S.; Orphal, J.; Burrows, J.P. The temperature and pressure dependence of the absorption cross-sections of NO2 in the 250–800 nm region measured by Fourier-transform spectroscopy. J. Photochem. Photobiol. A-Chem. 2002, 149, 1–7. [Google Scholar] [CrossRef]
- Meller, R.; Moortgat, G.K. Temperature dependence of the absorption cross sections of formaldehyde between 223 and 323 K in the wavelength range 225–375 nm. J. Geophys. Res.-Atmos. 2000, 105, 7089–7101. [Google Scholar] [CrossRef]
- Hermans, C.; Vandaele, A.C.; Fally, S. Fourier transform measurements of SO2 absorption cross sections: I. Temperature dependence in the 24,000–29,000 cm−1 (345–420 nm) region. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 756–765. [Google Scholar] [CrossRef]
- Stutz, J.; Kim, E.S.; Platt, U.; Bruno, P.; Perrino, C.; Febo, A. UV-visible absorption cross sections of nitrous acid. J. Geophys. Res.-Atmos. 2000, 105, 14585–14592. [Google Scholar] [CrossRef]
- Voigt, S.; Orphal, J.; Bogumil, K.; Burrows, J.P. The temperature dependence (203–293 K) of the absorption cross sections of O3 in the 230–850 nm region measured by Fourier-transform spectroscopy. J. Photochem. Photobiol. A-Chem. 2001, 143, 1–9. [Google Scholar] [CrossRef]
- Wu, J.; Xiao, B. Study on near-surface ozone pollution in Shanghai urban area. Guangzhou Chem. Ind. 2020, 48, 112–116, 156. [Google Scholar]
- Xu, J.M.; Tie, X.X.; Gao, W.; Lin, Y.F.; Fu, Q.Y. Measurement and model analyses of the ozone variation during 2006 to 2015 and its response to emission change in megacity Shanghai, China. Atmos. Chem. Phys. 2019, 19, 9017–9035. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Liu, Y.; Zhang, J.; Liu, B.; Li, Q.; Sun, J.; Wang, Y.; Xuan, Y.; Li, Y.; Pan, J.; et al. Bypassing the NOx titration trap in ozone pollution control in Beijing. Atmos. Res. 2021, 249, 105333. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Bian, J.; Xuan, Y.; Chen, H.; Bai, Z.; Wan, X.; Zheng, X.; Xia, X.; Lu, D. Long-term ozone variability in the vertical structure and integrated column over the North China Plain: Results based on ozonesonde and Dobson measurements during 2001–2019. Environ. Res. Lett. 2021, 16, 074053. [Google Scholar] [CrossRef]
- Guo, Y.L.; Wang, S.S.; Zhu, J.; Zhang, R.F.; Gao, S.; Saiz-Lopez, A.; Zhou, B. Atmospheric formaldehyde, glyoxal and their relations to ozone pollution under low- and high-NOx regimes in summertime Shanghai, China. Atmos. Res. 2021, 258, 10. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Bai, Y.; Liu, H.T.; Alatalo, J.M.; Jiang, B. Temporal variations in ambient air quality indicators in Shanghai municipality, China. Sci. Rep. 2020, 10, 11. [Google Scholar] [CrossRef]
- Li, D.D.; Xue, L.K.; Wen, L.; Wang, X.F.; Chen, T.S.; Mellouki, A.; Chen, J.M.; Wang, W.X. Characteristics and sources of nitrous acid in an urban atmosphere of northern China: Results from 1-yr continuous observations. Atmos. Environ. 2018, 182, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Tanvir, A.; Javed, Z.; Jian, Z.; Zhang, S.B.; Bilal, M.; Xue, R.B.; Wang, S.S.; Bin, Z. Ground-Based MAX-DOAS Observations of Tropospheric NO2 and HCHO During COVID-19 Lockdown and Spring Festival Over Shanghai, China. Remote Sens. 2021, 13, 488. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dai, C.H.; Wang, Z.W.; Guo, J.; Qin, P.F.; Zhang, X.S. Sensitivity analysis of atmospheric ozone formation to its precursors in summer of Changsha. Environ. Chem. 2019, 38, 531–538. [Google Scholar]
- Wang, S.S.; Shi, C.Z.; Zhou, B.; Zhao, H.; Wang, Z.R.; Yang, S.N.; Chen, L.M. Observation of NO3 radicals over Shanghai, China. Atmos. Environ. 2013, 70, 401–409. [Google Scholar] [CrossRef]
- Govender, P.; Sivakumar, V. Application of k-means and hierarchical clustering techniques for analysis of air pollution: A review (1980–2019). Atmos. Pollut. Res. 2020, 11, 40–56. [Google Scholar] [CrossRef]
- Darby, L.S. Cluster analysis of surface winds in Houston, Texas, and the impact of wind patterns on ozone. J. Appl. Meteorol. 2005, 44, 1788–1806. [Google Scholar] [CrossRef]
- Awang, N.R.; Ramli, N.A.; Yahaya, A.S.; Elbayoumi, M. High Nighttime Ground-Level Ozone Concentrations in Kemaman: NO and NO2 Concentrations Attributions. Aerosol Air Qual. Res. 2015, 15, 1357–1366. [Google Scholar] [CrossRef]
- An, J.L.; Hang, Y.X.; Zhu, B.; Wang, D.D. Observational study of ozone concentrations in northern suburb of Nanjing. Ecol. Environ. Sci. 2010, 19, 1383–1386. [Google Scholar]
- Li, M.Y.; Yu, S.C.; Chen, X.; Li, Z.; Zhang, Y.B.; Wang, L.Q.; Liu, W.P.; Li, P.F.; Lichtfouse, E.; Rosenfeld, D.; et al. Large scale control of surface ozone by relative humidity observed during warm seasons in China. Environ. Chem. Lett. 2021, 19, 3981–3989. [Google Scholar] [CrossRef]
- Hong, Q.Q.; Liu, C.; Hu, Q.H.; Zhang, Y.L.; Xing, C.Z.; Su, W.J.; Ji, X.G.; Xiao, S.X. Evaluating the feasibility of formaldehyde derived from hyperspectral remote sensing as a proxy for volatile organic compounds. Atmos. Res. 2021, 264, 12. [Google Scholar] [CrossRef]
- Lin, H.T.; Wang, M.; Duan, Y.S.; Fu, Q.Y.; Ji, W.H.; Cui, H.X.; Jin, D.; Lin, Y.F.; Hu, K. O3 sensitivity and contributions of different NMHC sources in O3 formation at urban and suburban sites in Shanghai. Atmosphere 2020, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.F.; Yuan, Z.B.; Luo, H.H.; Wang, Y.R.; Xu, Y.Q.; Duan, Y.S.; Fu, Q.Y. Identification of long-term evolution of ozone sensitivity to precursors based on two-dimensional mutual verification. Sci. Total Environ. 2021, 760, 9. [Google Scholar] [CrossRef]
- Alicke, B.; Geyer, A.; Hofzumahaus, A.; Holland, F.; Konrad, S.; Patz, H.W.; Schafer, J.; Stutz, J.; Volz-Thomas, A.; Platt, U. OH formation by HONO photolysis during the BERLIOZ experiment. J. Geophys. Res.-Atmos. 2003, 108, 17. [Google Scholar] [CrossRef] [Green Version]
- Harris, G.W.; Carter, W.P.L.; Winer, A.M.; Pitts, J.N.; Platt, U.; Perner, D. Observations of nitrous-acid in the Los-Angeles atmosphere and implications for predictions of ozone precursor relationships. Environ. Sci. Technol. 1982, 16, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, T.; Zhang, L.; Li, Q.Y.; Wang, Z.; Xia, M.; Yun, H.; Wang, W.H.; Yu, C.; Yue, D.L.; et al. The significant contribution of HONO to secondary pollutants during a severe winter pollution event in southern China. Atmos. Chem. Phys. 2019, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jia, C.H.; Tong, S.R.; Zhang, W.Q.; Zhang, X.R.; Li, W.R.; Wang, Z.; Wang, L.L.; Liu, Z.R.; Hu, B.; Zhao, P.S.; et al. Pollution characteristics and potential sources of nitrous acid (HONO) in early autumn 2018 of Beijing. Sci. Total Environ. 2020, 735, 11. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.S.; Stutz, J. Nighttime radical observations and chemistry. Chem. Soc. Rev. 2012, 41, 6405–6447. [Google Scholar] [CrossRef]
- Pye, H.O.T.; Chan, A.W.H.; Barkley, M.P.; Seinfeld, J.H. Global modeling of organic aerosol: The importance of reactive nitrogen (NOx and NO3). Atmos. Chem. Phys. 2010, 10, 11261–11276. [Google Scholar] [CrossRef] [Green Version]




| Period | Species | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 |
|---|---|---|---|---|---|
| After midnight | O3 level | higher | higher | lower | lower |
| NO titration | weak | weak | intense | intense | |
| HONO level | lower | // | // | higher | |
| Daytime | O3 increase | lower | // | // | higher |
| Photochemical level | intense | weak | weak | intense | |
| Meteorological factors for photochemical reaction (T, RH, WS, R) | favorable | adverse | adverse | favorable | |
| Before midnight | O3-18:00 | higher | // | // | lower |
| max-NO3 | higher | // | // | lower | |
| BLH | lower | higher | higher | lower | |
| O3 accumulation | yes | / | / | yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, S.; Zhang, S.; Gu, C.; Tanvir, A.; Zhang, R.; Zhou, B. Clustering Analysis on Drivers of O3 Diurnal Pattern and Interactions with Nighttime NO3 and HONO. Atmosphere 2022, 13, 351. https://doi.org/10.3390/atmos13020351
Wang X, Wang S, Zhang S, Gu C, Tanvir A, Zhang R, Zhou B. Clustering Analysis on Drivers of O3 Diurnal Pattern and Interactions with Nighttime NO3 and HONO. Atmosphere. 2022; 13(2):351. https://doi.org/10.3390/atmos13020351
Chicago/Turabian StyleWang, Xue, Shanshan Wang, Sanbao Zhang, Chuanqi Gu, Aimon Tanvir, Ruifeng Zhang, and Bin Zhou. 2022. "Clustering Analysis on Drivers of O3 Diurnal Pattern and Interactions with Nighttime NO3 and HONO" Atmosphere 13, no. 2: 351. https://doi.org/10.3390/atmos13020351
APA StyleWang, X., Wang, S., Zhang, S., Gu, C., Tanvir, A., Zhang, R., & Zhou, B. (2022). Clustering Analysis on Drivers of O3 Diurnal Pattern and Interactions with Nighttime NO3 and HONO. Atmosphere, 13(2), 351. https://doi.org/10.3390/atmos13020351






