Single-Particle Analysis of Atmospheric Aerosols: Applications of Raman Spectroscopy
Abstract
1. Introduction
2. Background
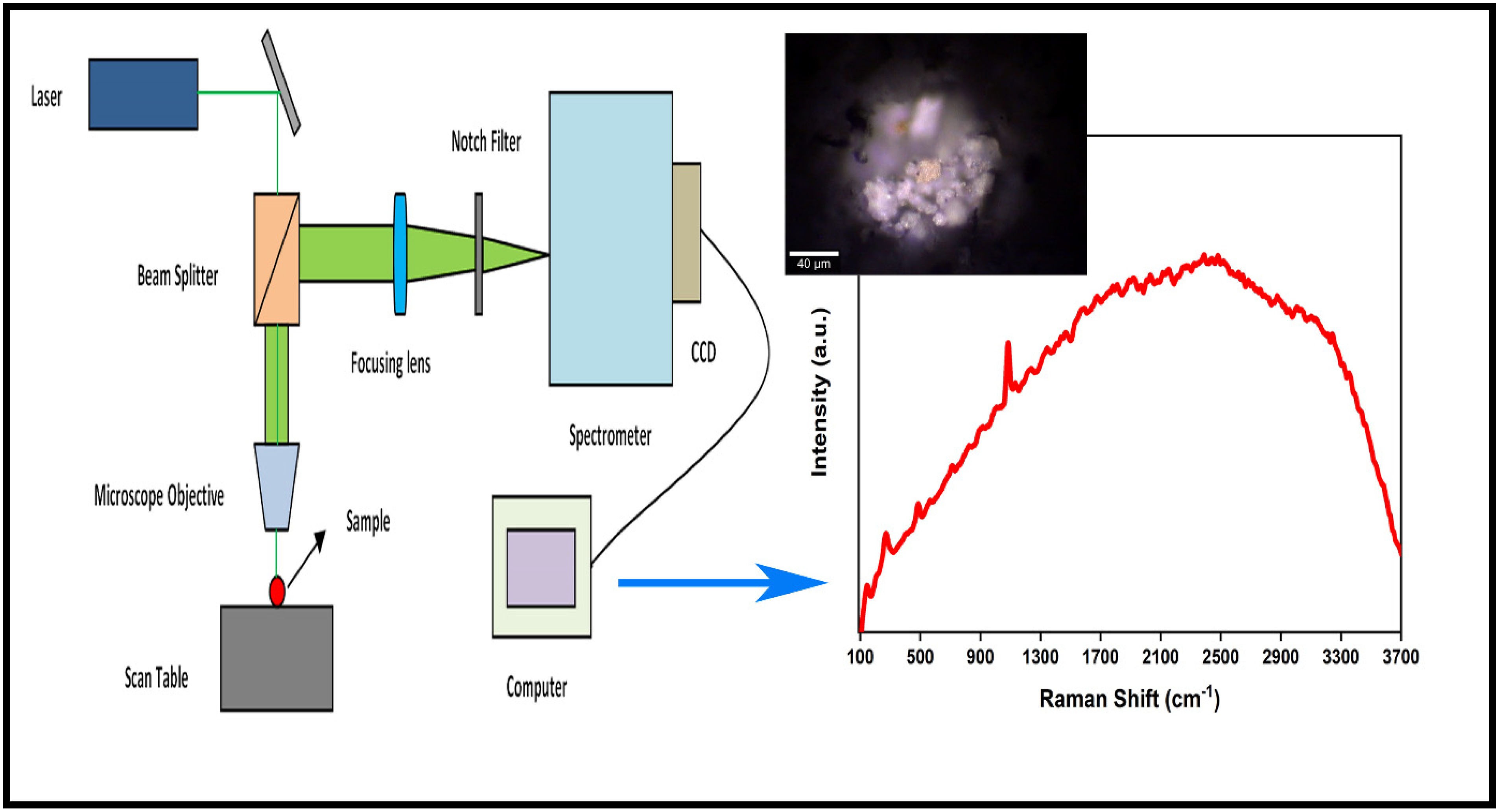
Raman Spectroscopy
3. Laboratory Studies
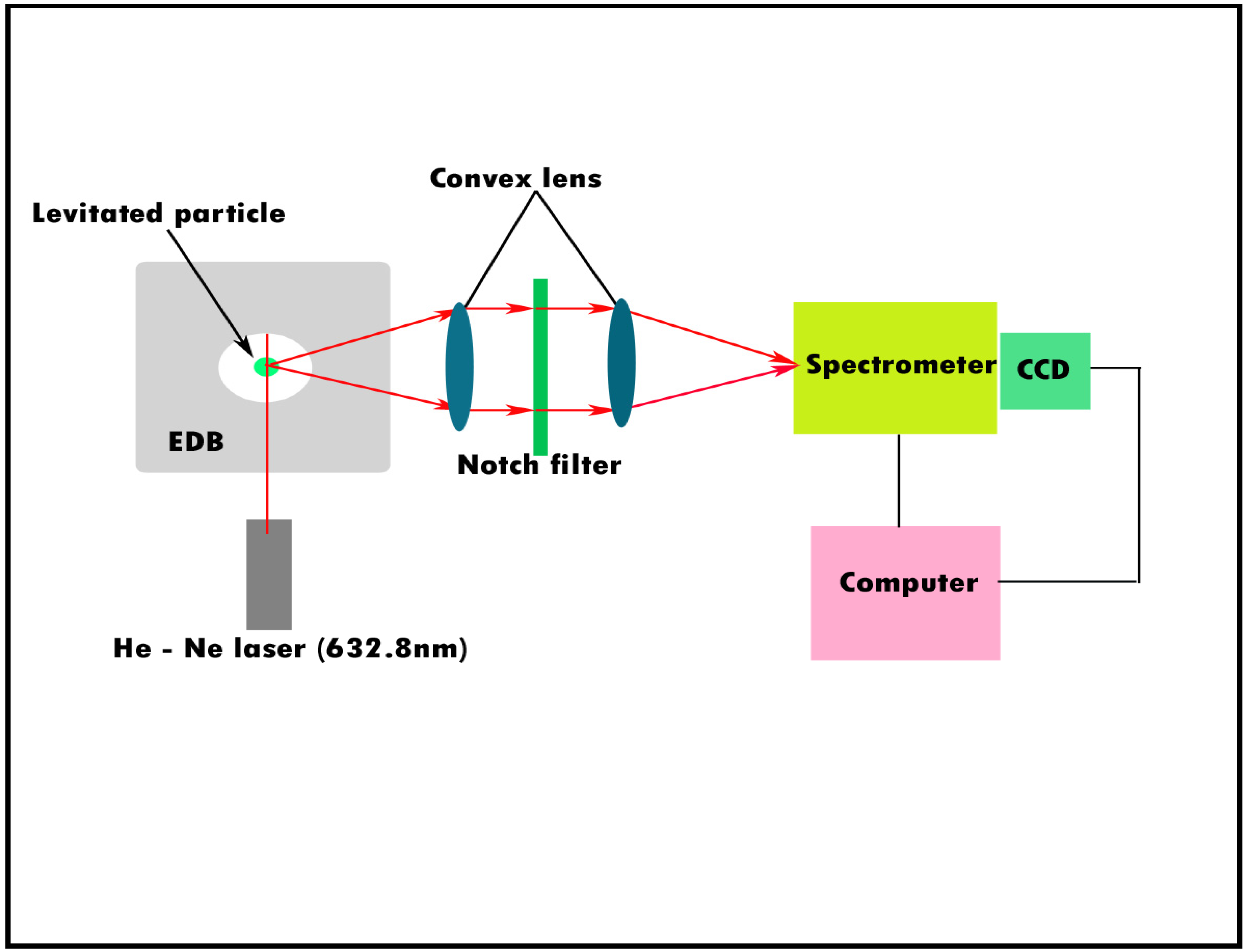
3.1. Hygroscopicity and Ice Nucleation Activity
3.2. Organic Aerosols
3.3. Mixed Salts/Mineral Dust
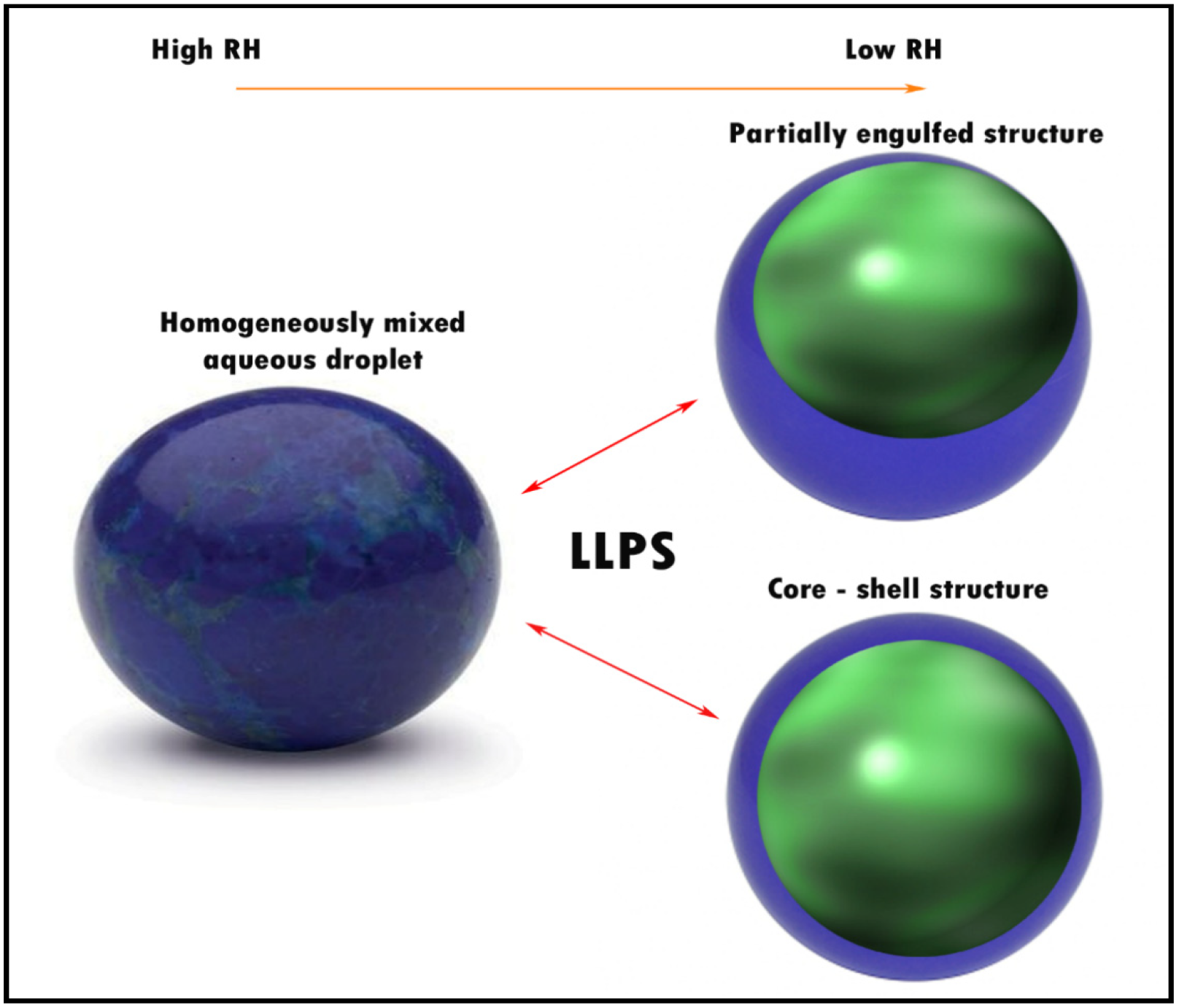
3.4. Liquid–Liquid Phase Separation (LLPS)
4. Ambient Aerosol Studies
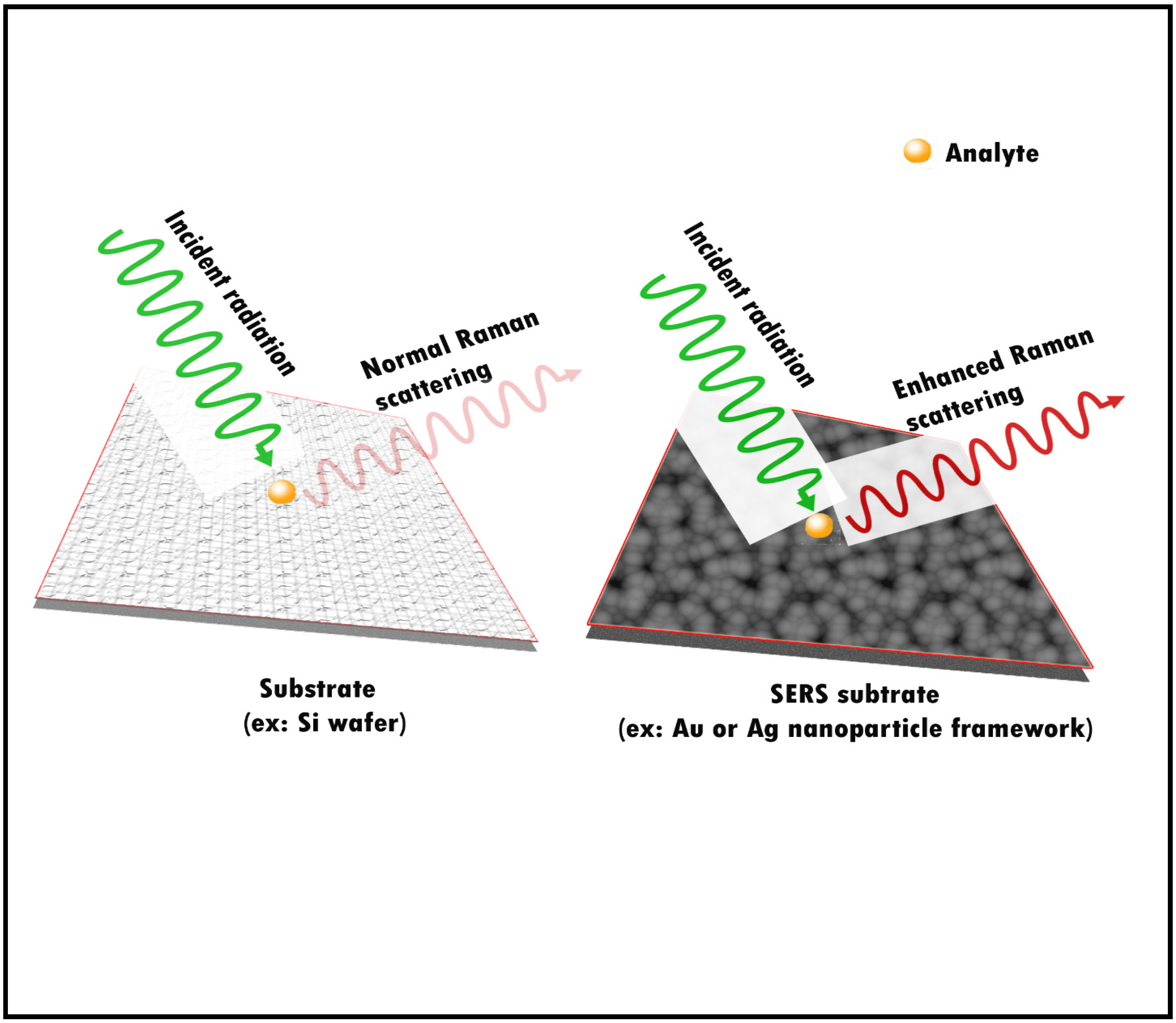
5. Surface-Enhanced Raman Spectroscopy (SERS)
6. Stimulated Raman Scattering (SRS) Microscopy
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMS | Aerosol mass spectrometry |
| ATOFMS | Aerosol time-of-flight mass spectrometry |
| ATR–FTIR | Attenuated total reflectance Fourier transform infrared |
| BC | Black carbon |
| BVOC | Biogenic volatile organic carbon |
| CCDs | Charge-coupled devices |
| DEPh | Diethyl phthalate |
| EDB | Electrodynamic balance |
| EELS | Electron energy loss spectrometry |
| fwhh | Full width at half height |
| TEM | Transmission electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| INPs | Ice-nucleating particles |
| LIBS | Laser-induced breakdown spectroscopy |
| LMMS | Laser microprobe mass spectrometry |
| LMWCA | Lower-molecular-weight carboxylic acids |
| LSPRs | Localized surface plasmon resonances |
| MRS | Micro-Raman spectroscopy |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PIXE | Proton-induced X-ray emission |
| PM | Particulate matter |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| RH | Relative humidity |
| SEM-EDX | Scanning electron microscopy–energy-dispersive X-ray |
| SERS | Surface-enhanced Raman spectroscopy |
| SNA | Sulfate–nitrate–ammonium |
| SOAs | Secondary organic aerosols |
| SIMS | Secondary ion mass spectrometry |
| SP-ICPMS | Single-particle inductively coupled mass spectrometry |
| TOF-SIMS | Time-of-flight–secondary ion mass spectrometry |
| VOCs | Volatile organic compounds |
| XAFS | X-ray absorption fine structure |
| XANES | X-ray absorption near-edge structure |
| XPS | X-ray photoelectron spectroscopy |
References
- Pöschl, U. Atmospheric Aerosols: Composition, Transformation, Climate and Health Effects. Angew. Chem. Int. Ed. 2005, 44, 7520–7540. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; de Reus, M.; Krejci, R.; Fischer, H.; Ström, J. Application of the variability-size relationship to atmospheric aerosol studies: Estimating aerosol lifetimes and ages. Atmos. Chem. Phys. 2002, 2, 133–145. [Google Scholar] [CrossRef]
- McMurry, P.H. A review of atmospheric aerosol measurements. Atmos. Environ. 2000, 34, 1959–1999. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.; Pitts, J. Chemistry of Upper and Lower Atmosphere; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Pratt, K.A.; Prather, K.A. Mass spectrometry of atmospheric aerosols—Recent developments and applications. Part II: On-line mass spectrometry techniques. Mass Spectrom. Rev. 2012, 31, 17–48. [Google Scholar] [CrossRef]
- Elmes, M.; Gasparon, M. Sampling and single particle analysis for the chemical characterisation of fine atmospheric particulates: A review. J. Environ. Manage. 2017, 202, 137–150. [Google Scholar] [CrossRef]
- Laskin, A.; Cowin, J.P. Automated Single-Particle SEM/EDX Analysis of Submicrometer Particles down to 0.1 μm. Anal. Chem. 2001, 73, 1023–1029. [Google Scholar] [CrossRef]
- Mäkelä, J.M.; Hoffmann, T.; Holzke, C.; Väkevä, M.; Suni, T.; Mattila, T.; Aalto, P.P.; Tapper, U.; Kauppinen, E.I.; O’Dowd, C.D. Biogenic iodine emissions and identification of end-products in coastal ultrafine particles during nucleation bursts. J. Geophys. Res. Atmos. 2002, 107, PAR 14-11–PAR 14-14. [Google Scholar] [CrossRef]
- Ma, C.-J.; Kasahara, M.; Höller, R.; Kamiya, T. Characteristics of single particles sampled in Japan during the Asian dust–storm period. Atmos. Environ. 2001, 35, 2707–2714. [Google Scholar] [CrossRef]
- Angyal, A.; Kertész, Z.; Szikszai, Z.; Szoboszlai, Z. Study of Cl-containing urban aerosol particles by ion beam analytical methods. Nucl. Instrum. Methods Phys. Res. B 2010, 268, 2211–2215. [Google Scholar] [CrossRef]
- Cheng, W.; Weng, L.-T.; Li, Y.; Lau, A.; Chan, C.K.; Chan, C.-M. Surface Chemical Composition of Size-Fractionated Urban Walkway Aerosols Determined by X-Ray Photoelectron Spectroscopy. Aerosol Sci. Technol. 2013, 47, 1118–1124. [Google Scholar] [CrossRef]
- Davidson, R.A.; Anderson, D.S.; Van Winkle, L.S.; Pinkerton, K.E.; Guo, T. Evolution of Silver Nanoparticles in the Rat Lung Investigated by X-ray Absorption Spectroscopy. J. Phys. Chem. A 2015, 119, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Sobanska, S.; Falgayrac, G.; Rimetz-Planchon, J.; Perdrix, E.; Brémard, C.; Barbillat, J. Resolving the internal structure of individual atmospheric aerosol particle by the combination of Atomic Force Microscopy, ESEM–EDX, Raman and ToF–SIMS imaging. Microchem. J. 2014, 114, 89–98. [Google Scholar] [CrossRef]
- Riemer, N.; West, M. Quantifying aerosol mixing state with entropy and diversity measures. Atmos. Chem. Phys. 2013, 13, 11423–11439. [Google Scholar] [CrossRef]
- Lee, S.-H.; Allen, H.C. Analytical Measurements of Atmospheric Urban Aerosol. Anal. Chem. 2012, 84, 1196–1201. [Google Scholar] [CrossRef]
- Zangmeister, C.D.; Pemberton, J.E. Raman Spectroscopy and Atomic Force Microscopy of the Reaction of Sulfuric Acid with Sodium Chloride. J. Am. Chem. Soc. 2000, 122, 12289–12296. [Google Scholar] [CrossRef]
- Craig, R.L.; Bondy, A.L.; Ault, A.P. Computer-controlled Raman microspectroscopy (CC-Raman): A method for the rapid characterization of individual atmospheric aerosol particles. Aerosol Sci. Technol. 2017, 51, 1099–1112. [Google Scholar] [CrossRef]
- Craig, R.; Bondy, A.; Ault, A. Surface Enhanced Raman Spectroscopy Enables Observations of Previously Undetectable Secondary Organic Aerosol Components at the Individual Particle Level. Anal. Chem. 2015, 87, 7510–7514. [Google Scholar] [CrossRef] [PubMed]
- Rindelaub, J.D.; Craig, R.L.; Nandy, L.; Bondy, A.L.; Dutcher, C.S.; Shepson, P.B.; Ault, A.P. Direct Measurement of pH in Individual Particles via Raman Microspectroscopy and Variation in Acidity with Relative Humidity. J. Phys. Chem. A 2016, 120, 911–917. [Google Scholar] [CrossRef]
- Tirella, P.N.; Craig, R.L.; Tubbs, D.B.; Olson, N.E.; Lei, Z.; Ault, A.P. Extending surface enhanced Raman spectroscopy (SERS) of atmospheric aerosol particles to the accumulation mode (150–800 nm). Environ. Sci. Process Impacts 2018, 20, 1570–1580. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Gardiner, D.J. Introduction to Raman scattering. In Practical Raman Spectroscopy; Springer: Berlin/Heidelberg, Germany, 1989; pp. 1–12. [Google Scholar]
- Alula, M.T.; Mengesha, Z.T.; Mwenesongole, E. Advances in surface-enhanced Raman spectroscopy for analysis of pharmaceuticals: A review. Vib. Spectrosc. 2018, 98, 50–63. [Google Scholar] [CrossRef]
- Smith, E.; Dent, G. Modern Raman spectroscopy: A practical approach. John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ao, J.; Feng, Y.; Wu, S.; Wang, T.; Ling, J.; Zhang, L.; Ji, M. Rapid, 3D Chemical Profiling of Individual Atmospheric Aerosols with Stimulated Raman Scattering Microscopy. Small Methods 2020, 4, 1900600. [Google Scholar] [CrossRef]
- Ivleva, N.P.; McKeon, U.; Niessner, R.; Pöschl, U. Raman Microspectroscopic Analysis of Size-Resolved Atmospheric Aerosol Particle Samples Collected with an ELPI: Soot, Humic-Like Substances, and Inorganic Compounds. Aerosol Sci. Technol. 2007, 41, 655–671. [Google Scholar] [CrossRef]
- Ye, X.; Ma, Z.; Hu, D.; Yang, X.; Chen, J. Size-resolved hygroscopicity of submicrometer urban aerosols in Shanghai during wintertime. Atmos. Res. 2011, 99, 353–364. [Google Scholar] [CrossRef]
- Pringle, K.; Tost, H.; Pozzer, A.; Pöschl, U.; Lelieveld, J. Global distribution of the effective aerosol hygroscopicity parameter for CCN activation. Atmos. Chem. Phys 2010, 10, 5241–5255. [Google Scholar] [CrossRef]
- Randles, C.; Russell, L.; Ramaswamy, V. Hygroscopic and optical properties of organic sea salt aerosol and consequences for climate forcing. Geophys. Res. Lett. 2004, 31, L16108. [Google Scholar] [CrossRef]
- Weingartner, E.; Baltensperger, U.; Burtscher, H. Growth and structural change of combustion aerosols at high relative humidity. Environ. Sci. Technol. 1995, 29, 2982–2986. [Google Scholar] [CrossRef]
- Montgomery, J.F.; Rogak, S.N.; Green, S.I.; You, Y.; Bertram, A.K. Structural change of aerosol particle aggregates with exposure to elevated relative humidity. Environ. Sci. Technol. 2015, 49, 12054–12061. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chan, C.K. Understanding the Hygroscopic Properties of Supersaturated Droplets of Metal and Ammonium Sulfate Solutions Using Raman Spectroscopy. J. Phys. Chem. A 2002, 106, 285–292. [Google Scholar] [CrossRef]
- Wu, Z.; Nowak, A.; Poulain, L.; Herrmann, H.; Wiedensohler, A. Hygroscopic behavior of atmospherically relevant water-soluble carboxylic salts and their influence on the water uptake of ammonium sulfate. Atmos. Chem. Phys. 2011, 11, 12617–12626. [Google Scholar] [CrossRef]
- Yeung, M.C.; Lee, A.K.Y.; Chan, C.K. Phase Transition and Hygroscopic Properties of Internally Mixed Ammonium Sulfate and Adipic Acid (AS-AA) Particles by Optical Microscopic Imaging and Raman Spectroscopy. Aerosol Sci. Technol. 2009, 43, 387–399. [Google Scholar] [CrossRef]
- Choi, M.Y.; Chan, C.K. The effects of organic species on the hygroscopic behaviors of inorganic aerosols. Environ. Sci. Technol. 2002, 36, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; He, H.; Liu, C. Hygroscopic properties of oxalic acid and atmospherically relevant oxalates. Atmos. Environ. 2013, 69, 281–288. [Google Scholar] [CrossRef]
- DeMott, P.J.; Hill, T.C.J.; McCluskey, C.S.; Prather, K.A.; Collins, D.B.; Sullivan, R.C.; Ruppel, M.J.; Mason, R.H.; Irish, V.E.; Lee, T.; et al. Sea spray aerosol as a unique source of ice nucleating particles. Proc. Natl. Acad. Sci. USA 2016, 113, 5797–5803. [Google Scholar] [CrossRef]
- Wolf, M.J.; Zhang, Y.; Zhou, J.; Surratt, J.D.; Turpin, B.J.; Cziczo, D.J. Enhanced Ice Nucleation of Simulated Sea Salt Particles with the Addition of Anthropogenic Per- and Polyfluoroalkyl Substances. ACS Earth Space Chem. 2021, 5, 2074–2085. [Google Scholar] [CrossRef]
- Burrows, S.M.; Easter, R.C.; Liu, X.; Ma, P.L.; Wang, H.; Elliott, S.M.; Singh, B.; Zhang, K.; Rasch, P.J. OCEANFILMS (Organic Compounds from Ecosystems to Aerosols: Natural Films and Interfaces via Langmuir Molecular Surfactants) sea spray organic aerosol emissions—implementation in a global climate model and impacts on clouds. Atmos. Chem. Phys. 2022, 22, 5223–5251. [Google Scholar] [CrossRef]
- Baustian, K.J.; Wise, M.E.; Tolbert, M.A. Depositional ice nucleation on solid ammonium sulfate and glutaric acid particles. Atmos. Chem. Phys. 2010, 10, 2307–2317. [Google Scholar] [CrossRef]
- Wise, M.E.; Baustian, K.J.; Koop, T.; Freedman, M.A.; Jensen, E.J.; Tolbert, M.A. Depositional ice nucleation onto crystalline hydrated NaCl particles: A new mechanism for ice formation in the troposphere. Atmos. Chem. Phys. 2012, 12, 1121–1134. [Google Scholar] [CrossRef]
- Lupi, L.; Hudait, A.; Peters, B.; Grünwald, M.; Mullen, R.G.; Nguyen, A.H.; Molinero, V. Role of stacking disorder in ice nucleation. Nature 2017, 551, 218–222. [Google Scholar] [CrossRef]
- Llombart, P.; Noya, E.G.; MacDowell, L.G. Surface phase transitions and crystal habits of ice in the atmosphere. Sci. Adv. 2020, 6, eaay9322. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta A 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Andò, S.; Garzanti, E. Raman spectroscopy in heavy-mineral studies. Geol. Soc. Lond. Spec. Publ. 2014, 386, 395–412. [Google Scholar] [CrossRef]
- Hausler, T.; Gebhardt, P.; Iglesias, D.; Rameshan, C.; Marchesan, S.; Eder, D.; Grothe, H. Ice Nucleation Activity of Graphene and Graphene Oxides. J. Phys. Chem. C 2018, 122, 8182–8190. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Knutti, R. Anthropogenic and natural warming inferred from changes in Earth’s energy balance. Nat. Geosci. 2012, 5, 31–36. [Google Scholar] [CrossRef]
- Pandis, S.N.; Harley, R.A.; Cass, G.R.; Seinfeld, J.H. Secondary organic aerosol formation and transport. Atmos. Environ. 1992, 26, 2269–2282. [Google Scholar] [CrossRef]
- Huang, C.; Wang, H.; Li, L.; Wang, Q.; Lu, Q.; De Gouw, J.; Zhou, M.; Jing, S.; Lu, J.; Chen, C. VOC species and emission inventory from vehicles and their SOA formation potentials estimation in Shanghai, China. Atmos. Chem. Phys. 2015, 15, 11081–11096. [Google Scholar] [CrossRef]
- Lim, H.-J.; Carlton, A.G.; Turpin, B.J. Isoprene forms secondary organic aerosol through cloud processing: Model simulations. Environ. Sci. Technol. 2005, 39, 4441–4446. [Google Scholar] [CrossRef]
- Carlton, A.; Wiedinmyer, C.; Kroll, J. A review of Secondary Organic Aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009, 9, 4987–5005. [Google Scholar] [CrossRef]
- Bondy, A.L.; Craig, R.L.; Zhang, Z.; Gold, A.; Surratt, J.D.; Ault, A.P. Isoprene-Derived Organosulfates: Vibrational Mode Analysis by Raman Spectroscopy, Acidity-Dependent Spectral Modes, and Observation in Individual Atmospheric Particles. J. Phys. Chem. A 2018, 122, 303–315. [Google Scholar] [CrossRef]
- King, M.D.; Thompson, K.C.; Ward, A.D.; Pfrang, C.; Hughes, B.R. Oxidation of biogenic and water-soluble compounds in aqueous and organic aerosol droplets by ozone: A kinetic and product analysis approach using laser Raman tweezers. Faraday Discuss. 2008, 137, 173–192. [Google Scholar] [CrossRef]
- Ganesan, K.; Raza, S.; Vijayaraghavan, R. Chemical warfare agents. J. Pharm. Bioallied Sci. 2010, 2, 166. [Google Scholar] [CrossRef] [PubMed]
- Kalume, A.; Beresnev, L.A.; Santarpia, J.; Pan, Y.L. Detection and characterization of chemical aerosol using laser-trapping single-particle Raman spectroscopy. Appl. Opt. 2017, 56, 6577–6582. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-C.; Tsai, Y.I.; Tsai, C.-H.; Hsieh, L.-Y. Carboxylic acids in PM2.5 over Pinus morrisonicola forest and related photoreaction mechanisms identified via Raman spectroscopy. Atmos. Environ. 2011, 45, 6741–6750. [Google Scholar] [CrossRef]
- Limbeck, A.; Puxbaum, H. Organic acids in continental background aerosols. Atmos. Environ. 1999, 33, 1847–1852. [Google Scholar] [CrossRef]
- Enders, G.; Dlugi, R.; Steinbrecher, R.; Clement, B.; Daiber, R.; Eijk, J.; Gäb, S.; Haziza, M.; Helas, G.; Herrmann, U. Biosphere/atmosphere interactions: Integrated research in a European coniferous forest ecosystem. Atmos. Environ. 1992, 26, 171–189. [Google Scholar] [CrossRef]
- Herckes, P.; Engling, G.; Kreidenweis, S.M.; Collett, J.L. Particle size distributions of organic aerosol constituents during the 2002 Yosemite aerosol characterization study. Environ. Sci. Technol. 2006, 40, 4554–4562. [Google Scholar] [CrossRef]
- Alves, C.A.; Pio, C.A.; Duarte, A.C. Particulate size distributed organic compounds in a forest atmosphere. Environ. Sci. Technol. 2000, 34, 4287–4293. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K.; Kanaya, Y.; Wang, Z. Contributions of biogenic volatile organic compounds to the formation of secondary organic aerosols over Mt. Tai, Central East China. Atmos. Environ. 2010, 44, 4817–4826. [Google Scholar] [CrossRef]
- Free, E.E. The Movement of Soil Material by the Wind; US Government Printing Office: Washington, DC, USA, 1911. [Google Scholar]
- Dixon, J.B.; Weed, S.B. Minerals in Soil Environments; Soil Science Society of America Inc. (SSSA): Madison, WI, USA, 1989. [Google Scholar]
- Hand, J.L.; Gill, T.; Schichtel, B. Spatial and seasonal variability in fine mineral dust and coarse aerosol mass at remote sites across the United States. J. Geophys. Res. Atmos. 2017, 122, 3080–3097. [Google Scholar] [CrossRef]
- Falkovich, A.H.; Schkolnik, G.; Ganor, E.; Rudich, Y. Adsorption of organic compounds pertinent to urban environments onto mineral dust particles. J. Geophys. Res. Atmos. 2004, 109, D02208. [Google Scholar] [CrossRef]
- Ponczek, M.; Hayeck, N.; Emmelin, C.; George, C. Heterogeneous photochemistry of dicarboxylic acids on mineral dust. Atmos. Environ. 2019, 212, 262–271. [Google Scholar] [CrossRef]
- Sullivan, R.; Guazzotti, S.; Sodeman, D.; Prather, K. Direct observations of the atmospheric processing of Asian mineral dust. Direct observations of the atmospheric processing of Asian mineral dust. Atmos. Chem. Phys. 2007, 7, 1213–1236. [Google Scholar] [CrossRef]
- Laskina, O.; Young, M.A.; Kleiber, P.D.; Grassian, V.H. Infrared extinction spectroscopy and micro-Raman spectroscopy of select components of mineral dust mixed with organic compounds. J. Geophys. Res. Atmos. 2013, 118, 6593–6606. [Google Scholar] [CrossRef]
- Tegen, I.; Schepanski, K. The global distribution of mineral dust. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Barcelona, Spain, 7–9 November 2007; p. 012001. [Google Scholar]
- Yu, Z.; Jang, M. Simulation of heterogeneous photooxidation of SO 2 and NO x in the presence of Gobi Desert dust particles under ambient sunlight. Atmos. Chem. Phys. 2018, 18, 14609–14622. [Google Scholar]
- Rkiouak, L.; Tang, M.J.; Camp, J.C.J.; McGregor, J.; Watson, I.M.; Cox, R.A.; Kalberer, M.; Ward, A.D.; Pope, F.D. Optical trapping and Raman spectroscopy of solid particles. Phys. Chem. Chem. Phys. 2014, 16, 11426–11434. [Google Scholar] [CrossRef]
- Tang, M.J.; Camp, J.C.; Rkiouak, L.; McGregor, J.; Watson, I.M.; Cox, R.A.; Kalberer, M.; Ward, A.D.; Pope, F.D. Heterogeneous interaction of SiO2 with N2O5: Aerosol flow tube and single particle optical levitation-Raman spectroscopy studies. J. Phys. Chem. A 2014, 118, 8817–8827. [Google Scholar] [CrossRef]
- Song, M.; Marcolli, C.; Krieger, U.; Zuend, A.; Peter, T. Liquid-liquid phase separation in aerosol particles: Dependence on O: C, organic functionalities, and compositional complexity. Geophys. Res. Lett. 2012, 39, L19801. [Google Scholar] [CrossRef]
- Marcolli, C.; Krieger, U.K. Relevance of Particle Morphology for Atmospheric Aerosol Processing. Trends Chem. 2020, 2, 1–3. [Google Scholar] [CrossRef]
- Wu, F.-M.; Wang, X.-W.; Jing, B.; Zhang, Y.-H.; Ge, M.-F. Liquid-liquid phase separation in internally mixed magnesium sulfate/glutaric acid particles. Atmos. Environ. 2018, 178, 286–292. [Google Scholar] [CrossRef]
- Brunamonti, S.; Krieger, U.K.; Marcolli, C.; Peter, T. Redistribution of black carbon in aerosol particles undergoing liquid-liquid phase separation. Geophys. Res. Lett. 2015, 42, 2532–2539. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Teng, X.; Liu, L.; Xu, Y.; Ren, L.; Shi, Z.; Zhang, Y.; Jiang, J.; Liu, D.; et al. Liquid-liquid phase separation reduces radiative absorption by aged black carbon aerosols. Commun. Earth Environ. 2022, 3, 128. [Google Scholar] [CrossRef]
- Sullivan, R.C.; Boyer-Chelmo, H.; Gorkowski, K.; Beydoun, H. Aerosol Optical Tweezers Elucidate the Chemistry, Acidity, Phase Separations, and Morphology of Atmospheric Microdroplets. Acc. Chem. Res. 2020, 53, 2498–2509. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.-K.; Meng, X.; Zhou, B.; Sun, R.; Wu, Z.; Hu, M.; Ye, A. Detecting the pH-dependent liquid-liquid phase separation of single levitated aerosol microdroplets via laser tweezers-Raman spectroscopy. Front. Phys. 2022, 10, 717. [Google Scholar] [CrossRef]
- Darimont, G.; Gilbert, B.; Cloots, R. Non-destructive evaluation of crystallinity and chemical composition by Raman spectroscopy in hydroxyapatite-coated implants. Mater. Lett. 2004, 58, 71–73. [Google Scholar] [CrossRef]
- Heidam, N.Z.; Christensen, J.; Wåhlin, P.; Skov, H. Arctic atmospheric contaminants in NE Greenland: Levels, variations, origins, transport, transformations and trends 1990–2001. Sci. Total Environ. 2004, 331, 5–28. [Google Scholar] [CrossRef]
- Batonneau, Y.; Sobanska, S.; Laureyns, J.; Bremard, C. Confocal Microprobe Raman Imaging of Urban Tropospheric Aerosol Particles. Environ. Sci. Technol. 2006, 40, 1300–1306. [Google Scholar] [CrossRef]
- Prospero, J. Mineral and sea salt aerosol concentrations in various ocean regions. J. Geophys. Res. Oceans 1979, 84, 725–731. [Google Scholar] [CrossRef]
- Deng, C.; Brooks, S.D.; Vidaurre, G.; Thornton, D.C.O. Using Raman Microspectroscopy to Determine Chemical Composition and Mixing State of Airborne Marine Aerosols over the Pacific Ocean. Aerosol Sci. Technol. 2013, 48, 193–206. [Google Scholar] [CrossRef]
- Iordanidis, A.; Garcia-Guinea, J.; Garas, S.; Asvesta, A.; Triantafyllou, A.G. Application of μRaman Microscopy to the Identification of Individual Airborne Particles: Preliminary Results from Kozani’s Area, Northern Greece. Part. Sci. Technol. 2014, 32, 355–359. [Google Scholar] [CrossRef]
- Vineyard, M.F.; LaBrake, S.M.; Ali, S.F.; Nadareski, B.J.; Safiq, A.D.; Smith, J.W.; Yoskowitz, J.T. Characterization of atmospheric aerosols in the Adirondack Mountains using PIXE, SEM/EDX, and Micro-Raman spectroscopies. Nucl. Instrum. Methods Phys. Res. B 2015, 350, 77–80. [Google Scholar] [CrossRef]
- Gonzalez, L.T.; Longoria-Rodriguez, F.E.; Sanchez-Dominguez, M.; Leyva-Porras, C.; Acuna-Askar, K.; Kharissov, B.I.; Arizpe-Zapata, A.; Alfaro-Barbosa, J.M. Seasonal variation and chemical composition of particulate matter: A study by XPS, ICP-AES and sequential microanalysis using Raman with SEM/EDS. J. Environ. Sci. 2018, 74, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the environment: A review of analytical methods, distribution, and biological effects. Trends Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Verla, E.N.; Ibe, F.C.; Amaobi, C.E. Airborne microplastics: A review study on method for analysis, occurrence, movement and risks. Environ. Monit. Assess. 2019, 191, 668. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.; Ribeiro-Claro, P.J. Identification of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, D.; Goldbeck, C.; Humpf, H.-U.; Fürst, P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018, 129, 154–162. [Google Scholar] [CrossRef]
- Kniggendorf, A.-K.; Wetzel, C.; Roth, B. Microplastics detection in streaming tap water with Raman spectroscopy. Sensors 2019, 19, 1839. [Google Scholar] [CrossRef]
- Klein, M.; Fischer, E.K. Microplastic abundance in atmospheric deposition within the Metropolitan area of Hamburg, Germany. Sci. Total Environ. 2019, 685, 96–103. [Google Scholar] [CrossRef]
- Sobhani, Z.; Al Amin, M.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics by Raman mapping. Anal. Chim. Acta 2019, 1077, 191–199. [Google Scholar] [CrossRef]
- Ong, T.T.X.; Blanch, E.W.; Jones, O.A.H. Surface Enhanced Raman Spectroscopy in environmental analysis, monitoring and assessment. Sci. Total Environ. 2020, 720, 137601. [Google Scholar] [CrossRef]
- Fu, Y.; Kuppe, C.; Valev, V.K.; Fu, H.; Zhang, L.; Chen, J. Surface-Enhanced Raman Spectroscopy: A Facile and Rapid Method for the Chemical Component Study of Individual Atmospheric Aerosol. Environ. Sci. Technol. 2017, 51, 6260–6267. [Google Scholar] [CrossRef]
- Hu, J.; Duan, F.; He, K.; Ma, Y.; Dong, S.; Liu, X. Characteristics and mixing state of S-rich particles in haze episodes in Beijing. Front Environ. Sci. Eng. 2016, 10, 12. [Google Scholar] [CrossRef]
- Eom, H.-J.; Gupta, D.; Li, X.; Jung, H.-J.; Kim, H.; Ro, C.-U. Influence of collecting substrates on the characterization of hygroscopic properties of inorganic aerosol particles. Anal. Chem. 2014, 86, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Yang, F.; Ma, Y.; Zhang, Q.; Yao, X.; Chan, C.K.; Cadle, S.; Chan, T.; Mulawa, P. The characteristics of PM2.5 in Beijing, China. Atmos. Environ. 2001, 35, 4959–4970. [Google Scholar] [CrossRef]
- Sun, Z.; Duan, F.; He, K.; Du, J.; Yang, L.; Li, H.; Ma, T.; Yang, S. Physicochemical analysis of individual atmospheric fine particles based on effective surface-enhanced Raman spectroscopy. J. Environ. Sci. 2019, 75, 388–395. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Zhang, Y.; Wang, L.; Deng, J.; Gao, Y.; Yu, L.; Zhang, J.; Sun, H. Widespread distribution of PET and PC microplastics in dust in urban China and their estimated human exposure. Environ. Int. 2019, 128, 116–124. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Jia, P.; Feng, Y.; Fu, S.; Yang, J.; Xiong, L.; Su, F.; Wu, Y.; Huang, Y. Ag Nanoparticle-Decorated Mesoporous Silica as a Dual-Mode Raman Sensing Platform for Detection of Volatile Organic Compounds. ACS Appl. Nano Mater. 2021, 4, 1019–1028. [Google Scholar] [CrossRef]
- Freudiger, C.W.; Min, W.; Saar, B.G.; Lu, S.; Holtom, G.R.; He, C.; Tsai, J.C.; Kang, J.X.; Xie, X.S. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 2008, 322, 1857–1861. [Google Scholar] [CrossRef]
- Nie, S.; Emory Steven, R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Miao, Y.; Wild, J.; Min, W.; Yang, Y. Emerging applications of stimulated Raman scattering microscopy in materials science. Matter 2021, 4, 1460–1483. [Google Scholar] [CrossRef]
- Houle, M.-A.; Burruss, R.C.; Ridsdale, A.; Moffatt, D.J.; Légaré, F.; Stolow, A. Rapid 3D chemical-specific imaging of minerals using stimulated Raman scattering microscopy. J. Raman Spectrosc. 2017, 48, 726–735. [Google Scholar] [CrossRef]
- Zada, L.; Leslie, H.A.; Vethaak, A.D.; Tinnevelt, G.H.; Jansen, J.J.; de Boer, J.F.; Ariese, F. Fast microplastics identification with stimulated Raman scattering microscopy. J. Raman Spectrosc. 2018, 49, 1136–1144. [Google Scholar] [CrossRef]

| Offline Techniques | Online Techniques |
|---|---|
| Scanning electron microscopy–energy-dispersive X-ray (SEM-EDX) Micro-Raman spectroscopy (MRS) High-resolution transmission electron microscopy (HRTEM) X-ray photoelectron spectroscopy (XPS) Nano-scale secondary ion mass spectrometry (Nano SIMS) Time-of-flight SIMS (TOF-SIMS) X-ray absorption fine structure spectroscopy (XAFS) X-ray absorption near-edge structure (XANES) spectroscopy Electron energy loss spectrometry (EELS) Proton-induced X-ray emission (PIXE) Single-particle inductively coupled mass spectrometry (SP-ICPMS) Laser microprobe mass spectrometry (LMMS) | Aerosol time-of-flight mass spectrometer (ATOFMS) Laser-induced breakdown spectroscopy (LIBS) Aerosol mass spectrometry (AMS) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moorchilot, V.S.; Aravind, U.K.; Menacherry, S.P.M.; Aravindakumar, C.T. Single-Particle Analysis of Atmospheric Aerosols: Applications of Raman Spectroscopy. Atmosphere 2022, 13, 1779. https://doi.org/10.3390/atmos13111779
Moorchilot VS, Aravind UK, Menacherry SPM, Aravindakumar CT. Single-Particle Analysis of Atmospheric Aerosols: Applications of Raman Spectroscopy. Atmosphere. 2022; 13(11):1779. https://doi.org/10.3390/atmos13111779
Chicago/Turabian StyleMoorchilot, Vishnu S., Usha K. Aravind, Sunil Paul M. Menacherry, and Charuvila T. Aravindakumar. 2022. "Single-Particle Analysis of Atmospheric Aerosols: Applications of Raman Spectroscopy" Atmosphere 13, no. 11: 1779. https://doi.org/10.3390/atmos13111779
APA StyleMoorchilot, V. S., Aravind, U. K., Menacherry, S. P. M., & Aravindakumar, C. T. (2022). Single-Particle Analysis of Atmospheric Aerosols: Applications of Raman Spectroscopy. Atmosphere, 13(11), 1779. https://doi.org/10.3390/atmos13111779







