Emission Characteristics of Gaseous and Particulate Mercury from a Subcritical Power Plant Co-Firing Coal and Sludge
Abstract
1. Introduction
2. Experimental
2.1. Operating Conditions and Sample Characterization
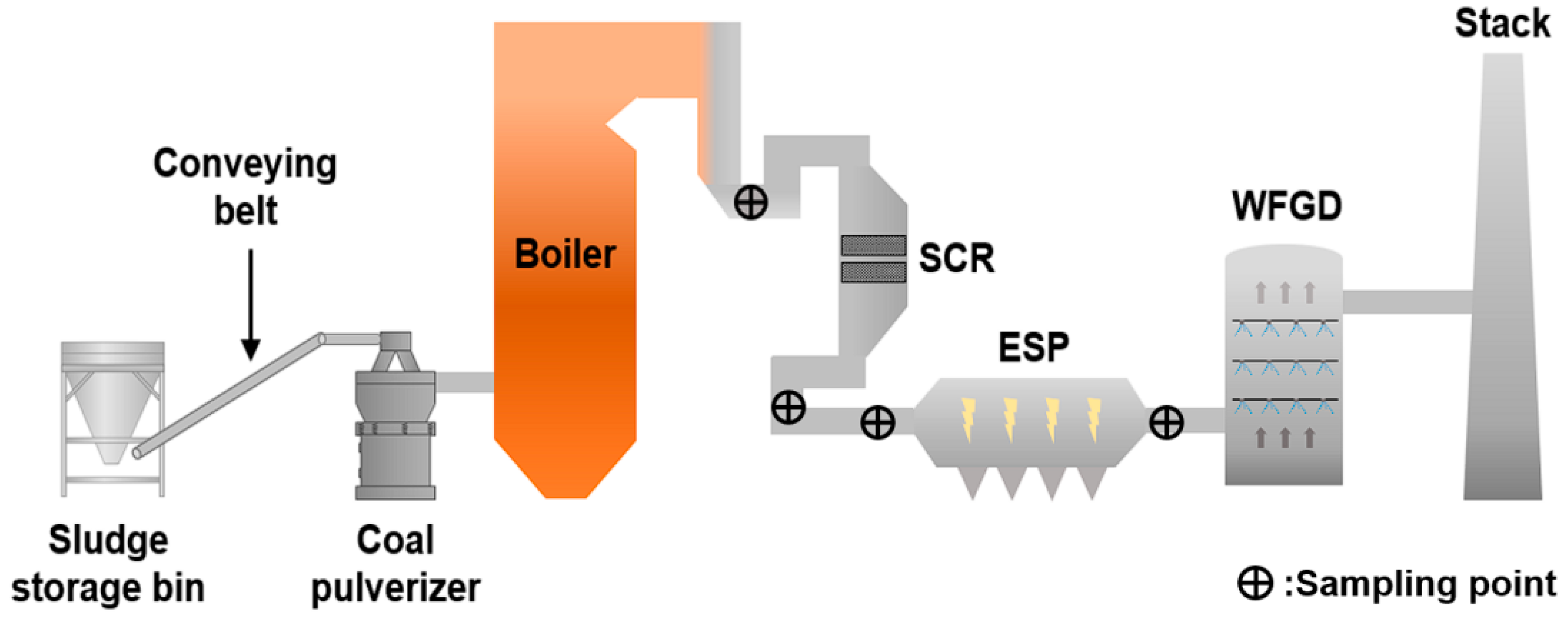
2.2. Co-Firing Procedures and Ultralow Emission of Boiler
2.3. Sampling Procedures and Analytical Method
2.3.1. Mercury Sampling
2.3.2. PM10 Sampling
3. Results and Discussion
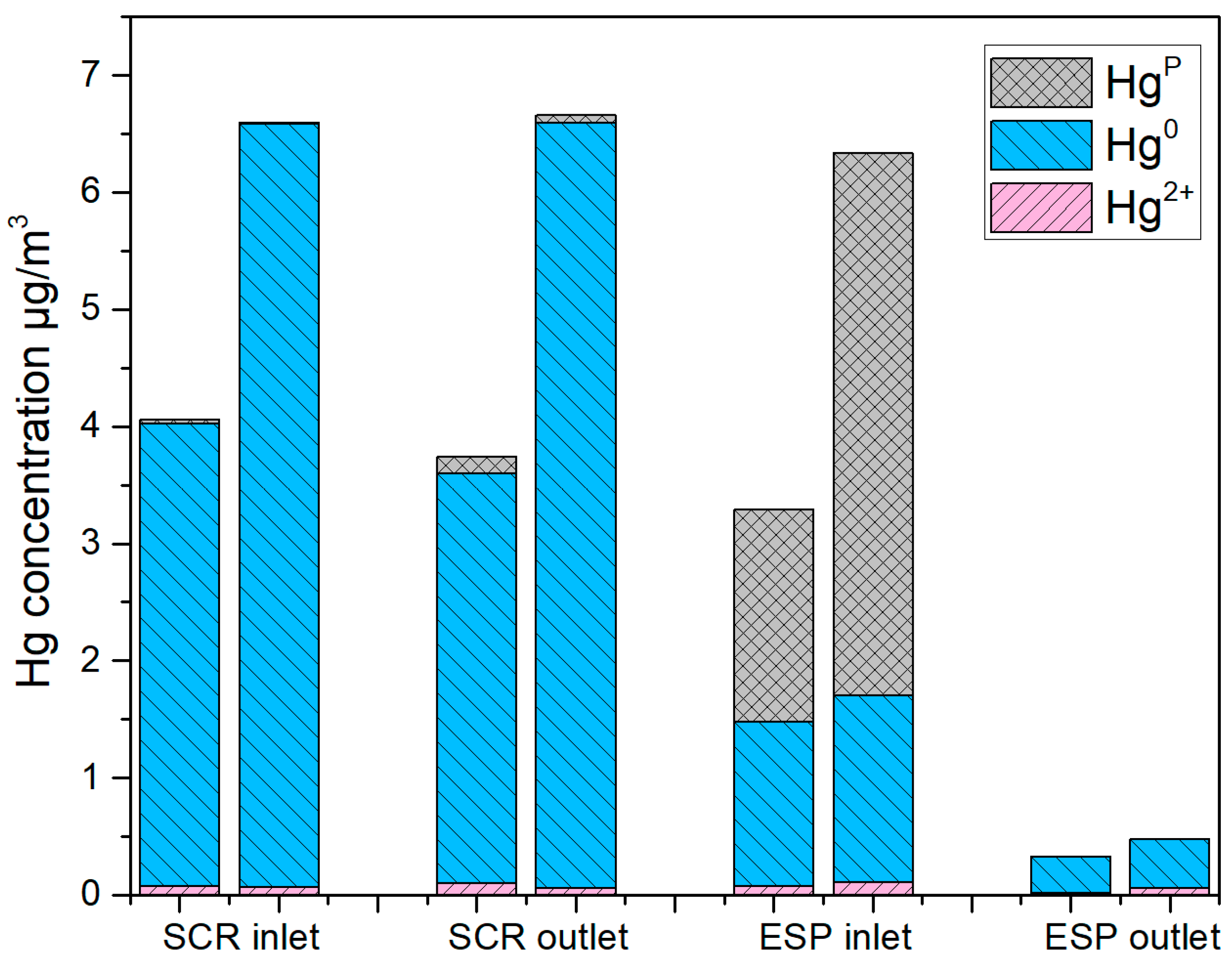
3.1. Mercury Concentration and Speciation at the Studied Sampling Sites
3.2. Mercury Mass Balance
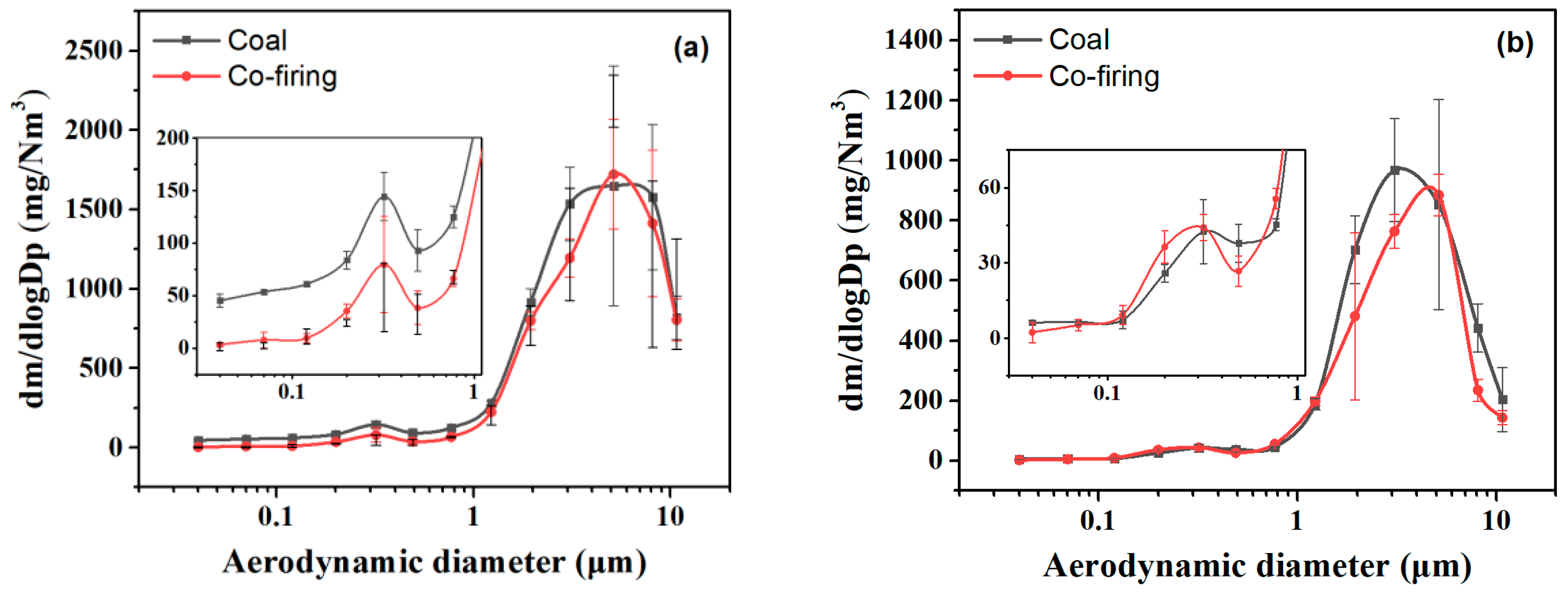
3.3. Emission Characteristics of PM10 in the SCR Outlet and the ESP Inlet
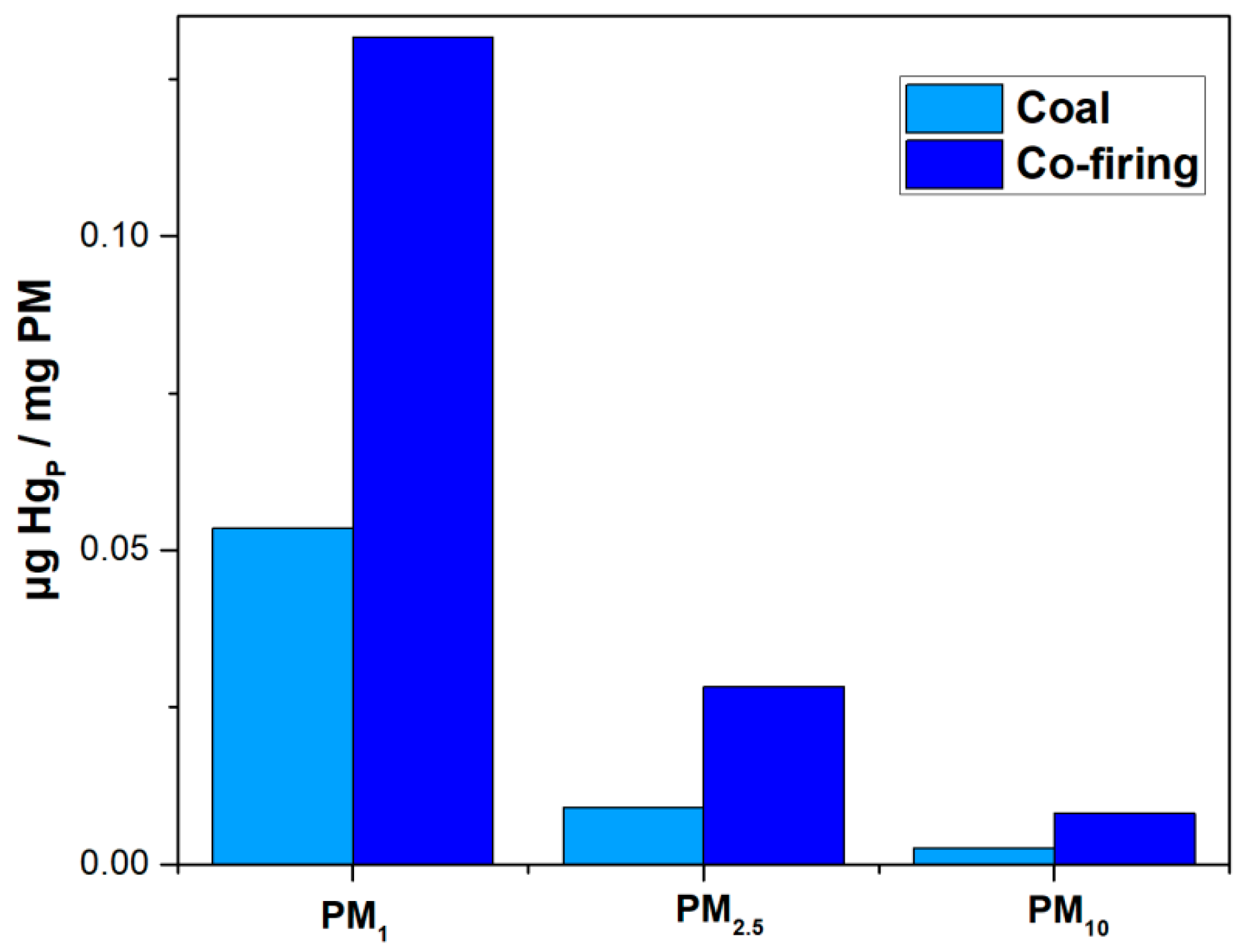
3.4. Relation of Emission Characteristics of PM10 and HgP
4. Conclusions
- (1)
- Before the SCR, Hg in flue gas from both single coal combustion and co-firing mainly existed as Hg0, and the higher content of Hg in sludge than coal led to the much higher Hg0 concentration for co-firing.
- (2)
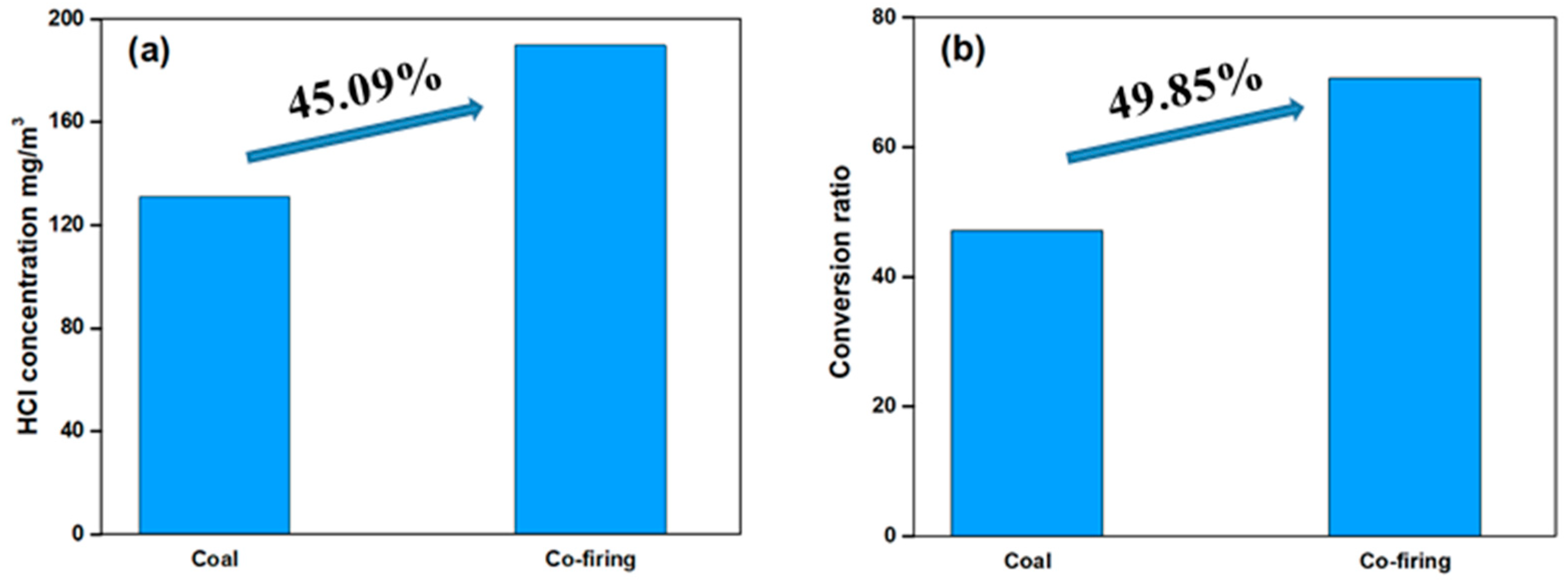
- Hgp concentration at the ESP inlet increased significantly, accompanied by a decrease in Hg0. The higher HCl concentration from co-firing derived from the much higher Cl content of sludge than coal, and the higher ash content of sludge containing more minerals capable of adsorbing Hg0, may lead to more transformation from Hg0 to Hg2+ and Hgp, when co-firing.
- (3)
- The removal efficiency of mercury after the ESP disposal was 92.12% under the coal firing condition and 92.83% under co-firing. The slightly higher efficiency after co-firing should be attributed to the complete removal of the higher concentration of Hgp in the ESP inlet.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, L.; Luo, J.; Chen, Y. Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 2015, 49, 4781–4782. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Hong, J.; Otaki, M.; Jolliet, O. Environmental and economic life cycle assessment for sewage sludge treatment processes in Japan. Waste Manag. 2009, 29, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Ma, L.; Xia, J.; Fang, Q.; Zhang, C.; Chen, G. Co-firing sludge in a pulverized coal-fired utility boiler: Combustion characteristics and economic impacts. Energy 2017, 119, 392–399. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Środa, K.; Kosowska-Golachowska, M.; Musiał, T.; Wolski, K. Combustion of pelleted sewage sludge with reference to coal and biomass. Fuel 2016, 170, 141–160. [Google Scholar] [CrossRef]
- Stelmach, S.; Wasielewski, R. Co-combustion of dried sewage sludge and coal in a pulverized coal boiler. J. Mater. Cycles Waste Manag. 2008, 10, 110–115. [Google Scholar] [CrossRef]
- You, C.A.; Xu, X.C. Coal combustion and its pollution control in China. Energy 2010, 35, 4467–4472. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Xue, Z.; Qu, Y.; Chai, F.; Hao, J. Atmospheric emissions estimation of Hg, As, and Se from coal-fired power plants in China, 2007. Sci. Total Environ. 2011, 409, 3078–3081. [Google Scholar] [CrossRef]
- Lee, S.J.; Seo, Y.C.; Jang, H.N.; Park, K.S.; Baek, J.I.; An, H.S.; Song, K.C. Speciation and mass distribution of mercury in a bituminous coal-fired power plant. Atmos. Environ. 2006, 40, 2215–2224. [Google Scholar] [CrossRef]
- Luo, G.; Yao, H.; Xu, M.; Gupta, R.; Xu, Z. Identifying modes of occurrence of mercury in coal by temperature programmed pyrolysis. Proc. Combust. Inst. 2011, 33, 2763–2769. [Google Scholar] [CrossRef]
- Xu, M.; Qiao, Y.; Zheng, C.; Li, L.; Liu, J. Modeling of homogeneous mercury speciation using detailed chemical kinetics. Combust. Flame 2003, 132, 208–218. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.; Liu, Y.; Huang, R.; Wang, Z.; Zhao, Y. A review on removal of mercury from flue gas utilizing existing air pollutant control devices (APCDs). J. Hazard. Mater. 2022, 427, 128132. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.; Yang, L.; Zhao, C.; Shen, X.; Zhang, M.; Chen, C. Experimental study on mercury transformation and removal in coal-fired boiler flue gases. Fuel Process. Technol. 2009, 90, 643–651. [Google Scholar] [CrossRef]
- Dajnak, D.; Clark, K.D.; Lockwood, F.C.; Reed, G. The prediction of mercury retention in ash from pulverised combustion of coal and sewage sludge. Fuel 2003, 82, 1901–1909. [Google Scholar] [CrossRef]
- Lopes, M.H.; Abelha, P.; Lapa, N.; Oliveira, J.S.; Cabrita, I.; Gulyurtlu, I. The behaviour of ashes and heavy metals during the co-combustion of sewage sludges in a fluidised bed. Waste Manag. 2003, 23, 859–870. [Google Scholar] [CrossRef]
- Li, C.; Duan, Y.; Tang, H.; Zhu, C.; Zheng, Y.; Huang, T. Mercury emissions monitoring in a coal-fired power plant by using the EPA method 30B based on a calcium-based sorbent trap. Fuel 2018, 221, 171–178. [Google Scholar] [CrossRef]
- Liu, S.; Hao, H.; Jia, W.; Cao, Y.; Chen, C. Effects of ultralow-emission retrofitting on mercury emission from a coal-fired power plant. Energy Fuels 2020, 34, 7502–7508. [Google Scholar] [CrossRef]
- Wen, M.; Wu, Q.; Li, G.; Wang, S.; Li, Z.; Tang, Y.; Liu, T. Impact of ultra-low emission technology retrofit on the mercury emissions and cross-media transfer in coal-fired power plants. J. Hazard. Mater. 2020, 396, 122729. [Google Scholar] [CrossRef]
- Liu, T.; Wen, C.; Zhou, K.; Li, C.; Zhu, Y.; Wang, D.; Jing, Z. Influence of collaborative disposal of sewage sludge in the fly ash partitioning and PM10 emission from a subcritical coal-fired boiler. Fuel 2023, 331, 125871. [Google Scholar] [CrossRef]
- Meij, R.; Te Winkel, H. Mercury emissions from coal-fired power stations: The current state of the art in the Netherlands. Sci. Total Environ. 2006, 368, 393–396. [Google Scholar] [CrossRef]
- Hower, J.C.; Senior, C.L.; Suuberg, E.M.; Hurt, R.H.; Wilcox, J.L.; Olson, E.S. Mercury capture by native fly ash carbons in coal-fired power plants. Prog. Energy Combust. Sci. 2010, 36, 510–529. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). EPA Method 30B; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2008.
- Prestbo, E.M.; Bloom, N.S. Mercury speciation adsorption (MESA) method for combustion flue gas: Methodology, artifacts, intercomparison, and atmospheric implications. Water Air Soil Pollut. 1995, 80, 145–158. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.; Xu, J.; Chang, G.; Xu, M.; Zhou, Z.; Liu, X.; Liu, L. Catalytic oxidation of Hg0 over Mn-doped CeO2-ZrO2 solid solution and MnOx/CeO2-ZrO2 supported catalysts: Characterization, catalytic activity and SO2 resistance. Fuel 2022, 310, 122317. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhao, B.; Shao, H.; Xu, Y.; Xu, M. Elemental mercury oxidation over manganese-based perovskite-type catalyst at low temperature. Chem. Eng. J. 2016, 288, 701–710. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Liu, X.W.; Zhao, B.; Chen, Z.G.; Shao, H.Z.; Wang, L.L.; Xu, M.H. Effects of existing energy saving and air pollution control devices on mercury removal in coal-fired power plants. Fuel Process. Technol. 2015, 131, 99–108. [Google Scholar] [CrossRef]
- Wang, H.; Duan, Y.; Ying, Z.; Xue, Y. Studies on mercury adsorption species and desorption activation energy on activated carbon under oxy combustion. J. Fuel Chem. Technol. 2008, 36, 23–29. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhang, L.; Li, G.H.; Wu, Y.; Hao, J.M.; Pirrone, N.; Ancora, M.P. Mercury emission and speciation of coal-fired power plants in China. Atmos. Chem. Phys. 2010, 10, 1183–1192. [Google Scholar] [CrossRef]
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- Sheng, C.; Li, Y.; Liu, X.; Yao, H.; Xu, M. Ash particle formation during O2/CO2 combustion of pulverized coals. Fuel Process.Technol. 2007, 88, 1021–1028. [Google Scholar] [CrossRef]
- Xu, M.; Yu, D.; Yao, H.; Liu, X.; Qiao, Y. Coal combustion-generated aerosols: Formation and properties. Proc. Combust. Inst. 2011, 33, 1681–1697. [Google Scholar] [CrossRef]
- Yu, D.; Xu, M.; Yao, H.; Sui, J.; Liu, X.; Yu, Y.; Cao, Q. Use of elemental size distributions in identifying particle formation modes. Proc. Combust. Inst. 2007, 31, 1921–1928. [Google Scholar] [CrossRef]
- Földi, C.; Andrée, C.A.; Mansfeldt, T. Sequential extraction of inorganic mercury in dumped blast furnace sludge. Environ. Sci. Pollut. Res. Int. 2015, 22, 15755–15762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, X.; Wu, F. Development and evaluation of magnetic iron-carbon sorbents for mercury removal in coal combustion flue gas. J. Energy Inst. 2020, 93, 1615–1623. [Google Scholar] [CrossRef]
- Dunham, G.E.; DeWall, R.A.; Senior, C.L. Fixed-bed studies of the interactions between mercury and coal combustion fly ash. Fuel Process. Technol. 2003, 82, 197–213. [Google Scholar] [CrossRef]
- Rompalski, P.; Smoliński, A.; Krztoń, H.; Gazdowicz, J.; Howaniec, N.; Rog, L. Determination of mercury content in hard coal and fly ash using X-ray diffraction and scanning electron microscopy coupled with chemical analysis. Arab. J. Chem. 2019, 12, 3927–3942. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Wang, G.; Wang, Q.; Zeng, B.; Xiao, Z.; Li, Z. Leachability of mercury in coal fly ash from coal-fired power plants in southwest China. Front. Environ. Sci. 2022, 10, 887837. [Google Scholar] [CrossRef]
- Yokoyama, T.; Asakura, K.; Matsuda, H.; Ito, S.; Noda, N. Mercury emissions from a coal-fired power plant in Japan. Sci. Total Environ. 2000, 259, 97–103. [Google Scholar] [CrossRef]
| Proximate analysis (wt%, db a) | VM | Ash | FC | Mad | |||
| coal | 30.31 | 16.98 | 52.71 | 1.09 | |||
| sludge | 40.75 | 56.12 | 3.13 | 0.90 | |||
| Ultimate analysis (wt%, ad b) | C | H | N | S | O c | Hg (μg/g) | Cl (μg/g) |
| coal | 66.69 | 4.25 | 0.76 | 0.32 | 10.23 | 0.07 | 133 |
| sludge | 21.80 | 3.67 | 3.41 | 0.72 | 14.10 | 0.96 | 450 |
| Boiler Load (MW) | NOX (mg/m3) | SO2 (mg/m3) | PM (mg/m3) | |
|---|---|---|---|---|
| coal | 373.2 | 27.6 | 14 | 1.17 |
| co-firing | 381.2 | 35.7 | 15.6 | 0.95 |
| Sample (μg/g) | Desulfurization Gypsum | Desulfurization Waste Water | Bottom Slag | Fly Ash | Coal | Sludge |
|---|---|---|---|---|---|---|
| Coal | 0.71 | 1.00 | 0.002 | 1.26 | 0.07 | / |
| Co-firing | 0.81 | 1.57 | 0.009 | 0.91 | 0.07 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wen, C.; Wang, D.; Zhao, C.; Li, R. Emission Characteristics of Gaseous and Particulate Mercury from a Subcritical Power Plant Co-Firing Coal and Sludge. Atmosphere 2022, 13, 1656. https://doi.org/10.3390/atmos13101656
Li C, Wen C, Wang D, Zhao C, Li R. Emission Characteristics of Gaseous and Particulate Mercury from a Subcritical Power Plant Co-Firing Coal and Sludge. Atmosphere. 2022; 13(10):1656. https://doi.org/10.3390/atmos13101656
Chicago/Turabian StyleLi, Changkang, Chang Wen, Dapeng Wang, Changxi Zhao, and Rui Li. 2022. "Emission Characteristics of Gaseous and Particulate Mercury from a Subcritical Power Plant Co-Firing Coal and Sludge" Atmosphere 13, no. 10: 1656. https://doi.org/10.3390/atmos13101656
APA StyleLi, C., Wen, C., Wang, D., Zhao, C., & Li, R. (2022). Emission Characteristics of Gaseous and Particulate Mercury from a Subcritical Power Plant Co-Firing Coal and Sludge. Atmosphere, 13(10), 1656. https://doi.org/10.3390/atmos13101656





