Air Pollution Role as Risk Factor of Cardioinhibitory Carotid Hypersensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Protocol
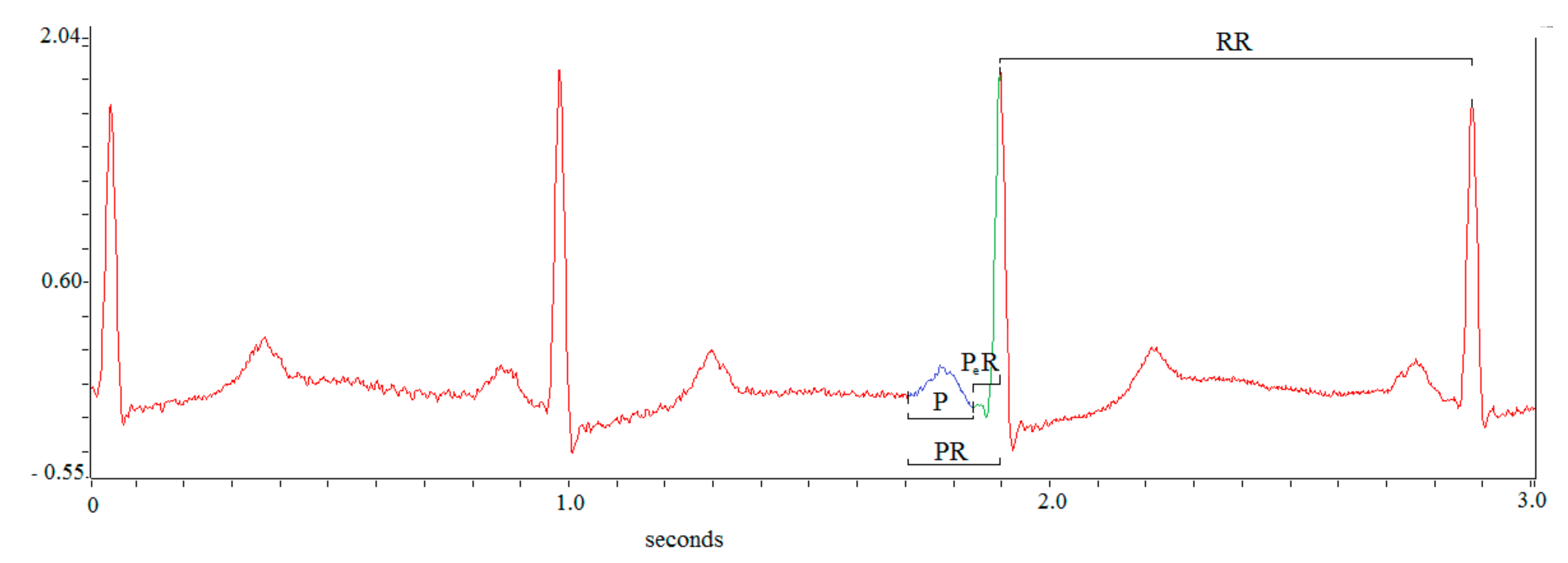
2.2. Offline Data Analysis
2.3. Statistical Analysis
3. Results
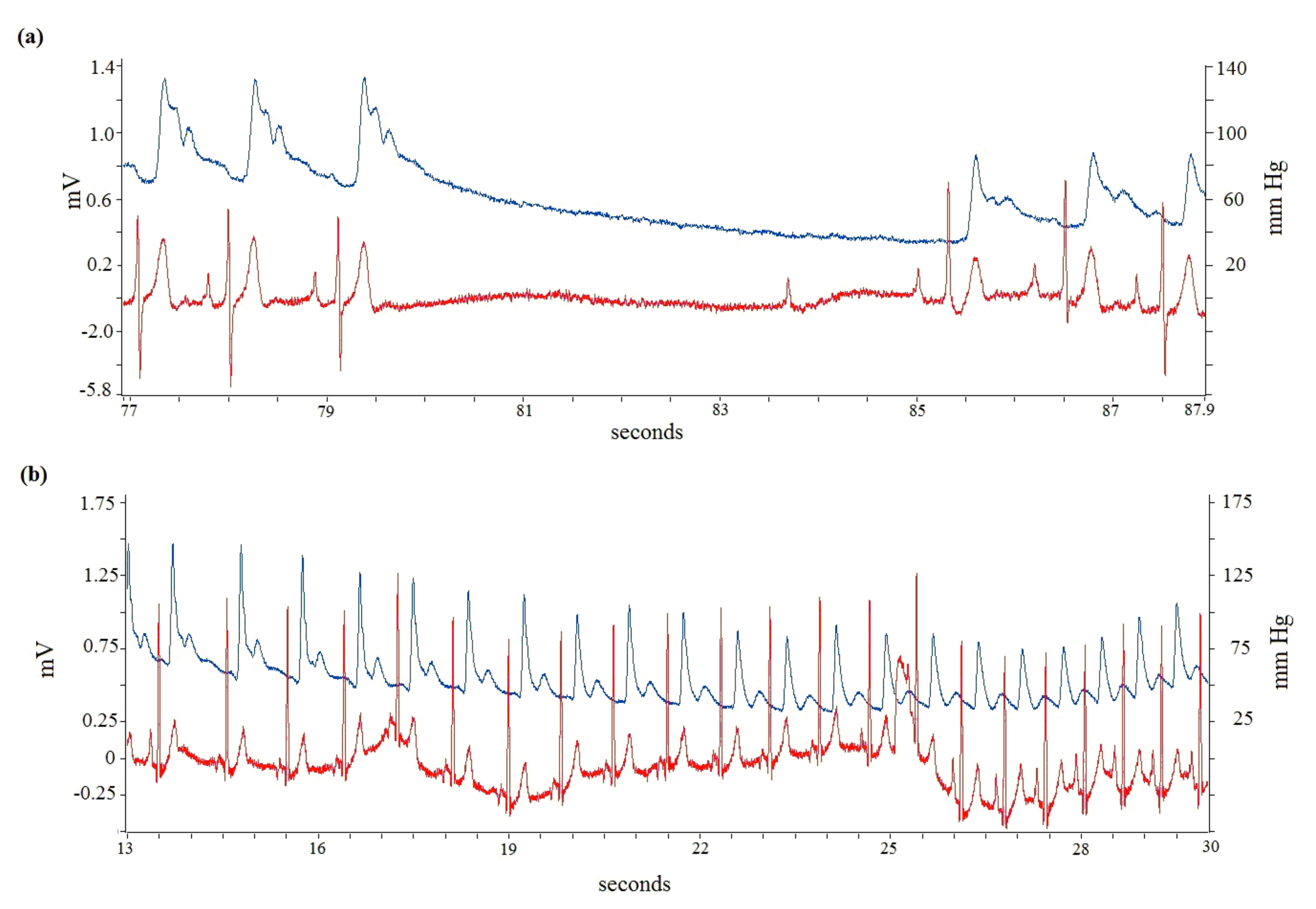
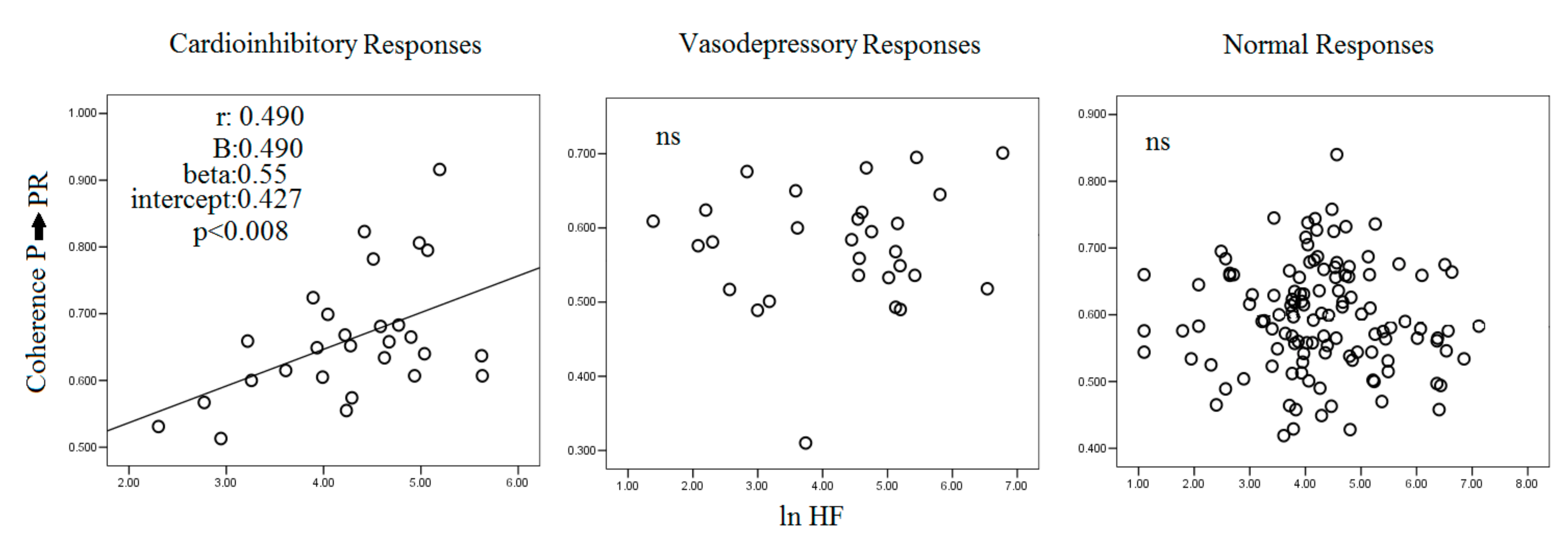
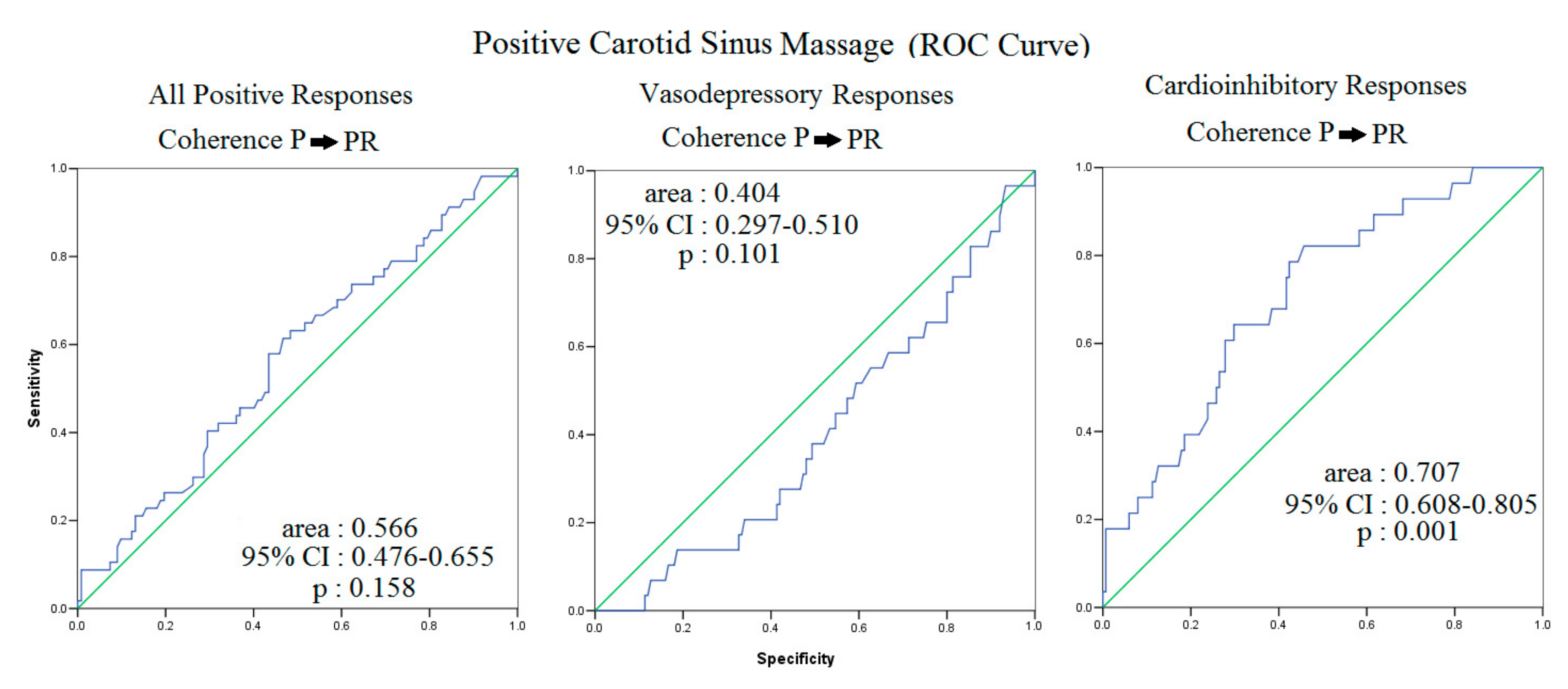
3.1. Carotid Sinus Hypersensitivity: The Cardioinhibitory Responses
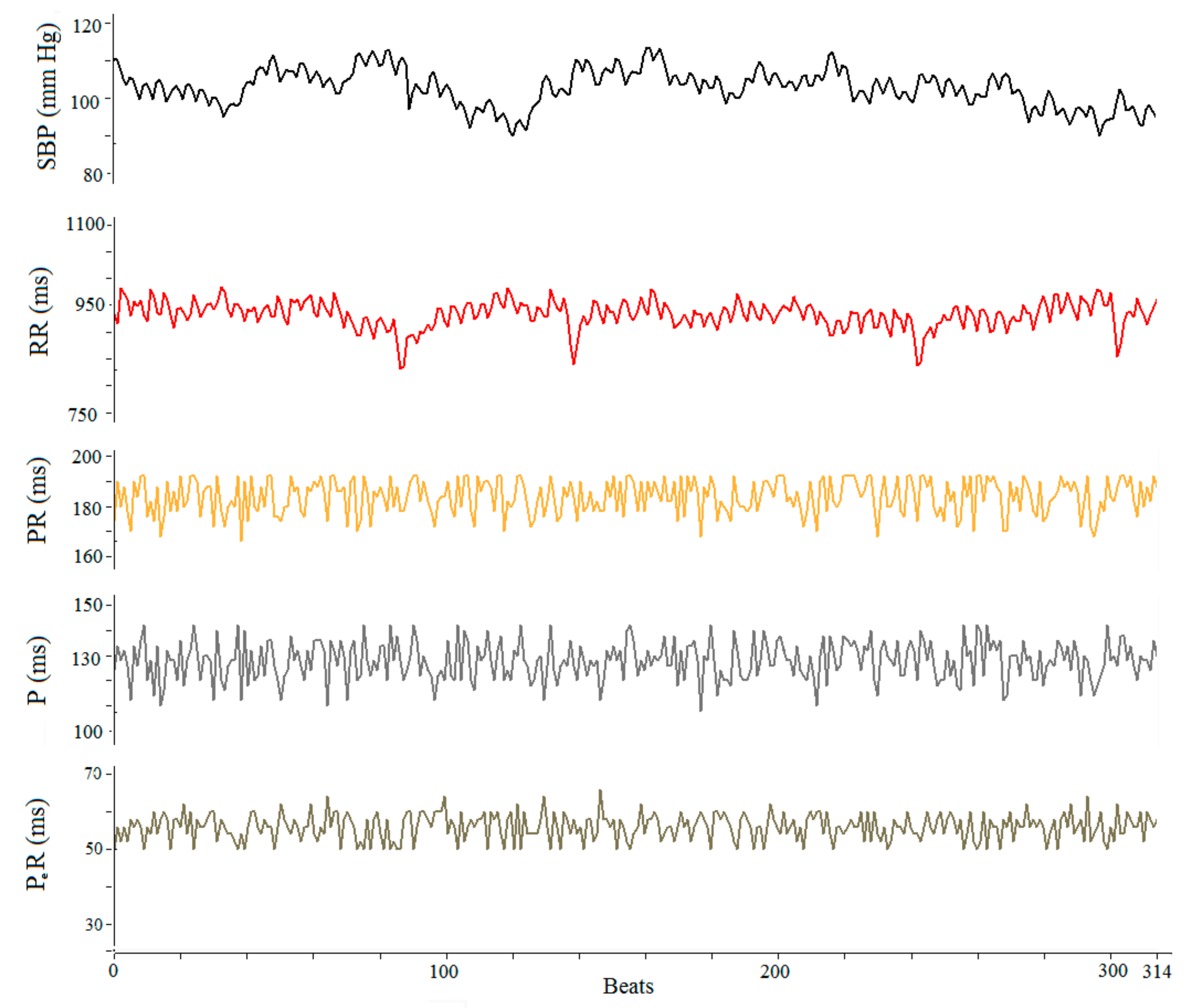
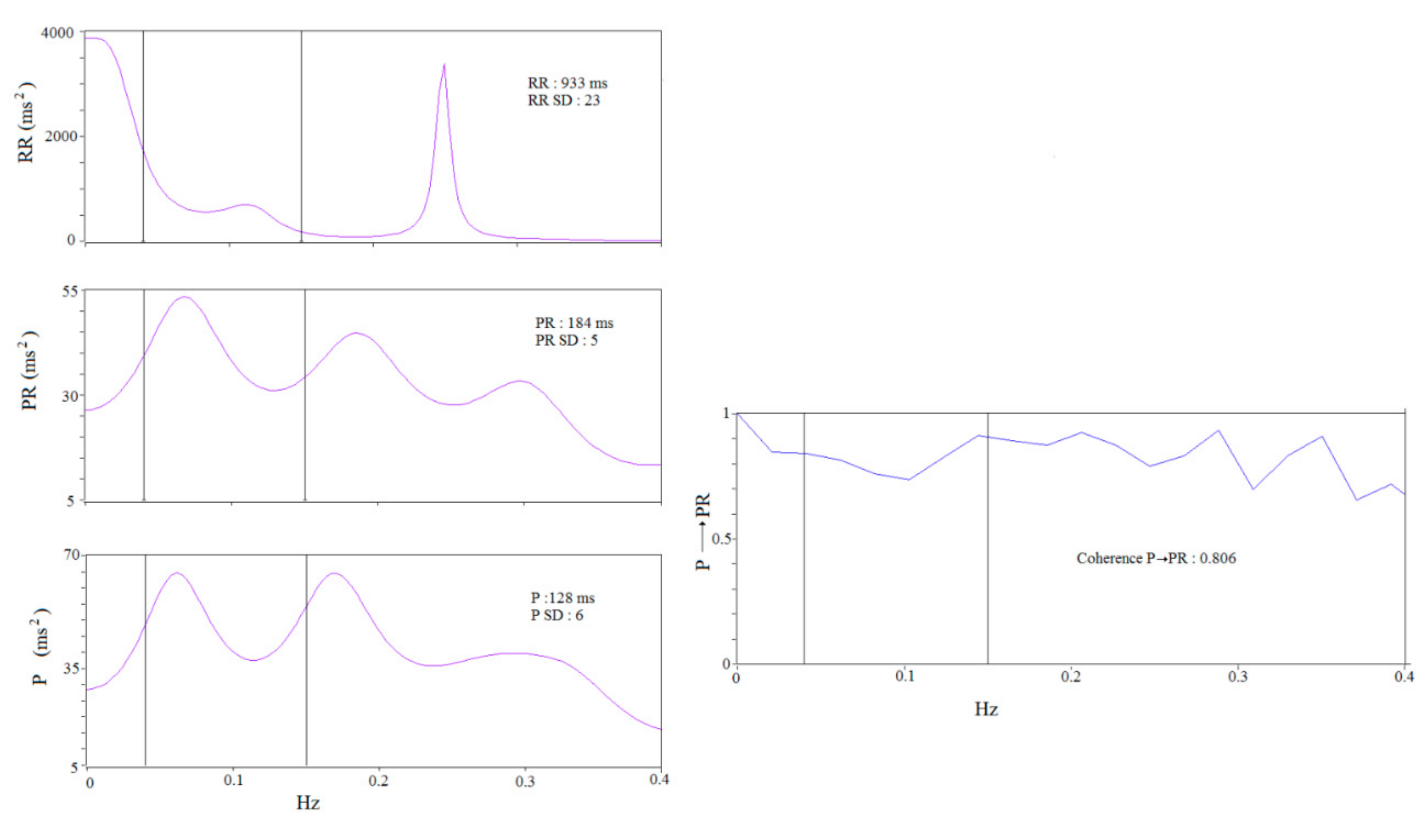
3.2. ECG Data
3.3. Pollution
4. Discussion
4.1. Ozone and RR, PR and P Wave Variability
4.2. RR, P Wave, PR Segment, SBP and Sinus Hypersensitivity
4.3. Possible Pathophysiologic Meaning of P→PR and P→PeR Coherence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mustafić, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Périer, M.-C.; Marijon, E.; Vernerey, D.; Empana, J.-P.; et al. Main Air Pollutants and Myocardial Infarction. JAMA J. Am. Med Assoc. 2012, 307, 713–721. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet 2013, 382, 1039–1048. [Google Scholar] [CrossRef]
- Fouillet, A.; Rey, G.; Wagner, V.; Laaidi, K.; Empereur-Bissonnet, P.; Le Tertre, A.; Frayssinet, P.; Bessemoulin, P.; Laurent, F.; De Crouy-Chanel, P.; et al. Has the impact of heat waves on mortality changed in France since the European heat wave of summer 2003? A study of the 2006 heat wave. Int. J. Epidemiol. 2008, 37, 309–317. [Google Scholar] [CrossRef]
- Newby, D.E.; Mannucci, P.M.; Tell, G.S.; Baccarelli, A.; Brook, R.D.; Donaldson, K.; Forastiere, F.; Franchini, M.; Franco, O.; Graham, I.; et al. Expert position paper on air pollution and cardiovascular disease. Eur. Heart J. 2014, 36, 83–93. [Google Scholar] [CrossRef]
- Perez, C.M.; Hazari, M.S.; Farraj, A.K. Role of Autonomic Reflex Arcs in Cardiovascular Responses to Air Pollution Exposure. Cardiovasc. Toxicol. 2015, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-S.; Eum, K.-D.; Fang, S.C.; Rodrigues, E.G.; Modest, G.A.; Christiani, D.C. Oxidative stress and systemic inflammation as modifiers of cardiac autonomic responses to particulate air pollution. Int. J. Cardiol. 2014, 176, 166–170. [Google Scholar] [CrossRef]
- Meo, S.A.; Suraya, F. Effect of environmental air pollution on cardiovascular diseases. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4890–4897. [Google Scholar] [PubMed]
- Arjomandi, M.; Wong, H.; Donde, A.; Frelinger, J.; Dalton, S.; Ching, W.; Power, K.; Balmes, J.R. Exposure to medium and high ambient levels of ozone causes adverse systemic inflammatory and cardiac autonomic effects. Am. J. Physiol. Circ. Physiol. 2015, 308, H1499–H1509. [Google Scholar] [CrossRef] [PubMed]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Hear. Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef]
- Zhao, A.; Chen, R.; Kuang, X.; Kan, H. Ambient Air Pollution and Daily Outpatient Visits for Cardiac Arrhythmia in Shanghai, China. J. Epidemiol. 2014, 24, 321–326. [Google Scholar] [CrossRef]
- Raza, A.; Bellander, T.; Bero-Bedada, G.; Dahlquist, M.; Hollenberg, J.; Jonsson, M.; Lind, T.; Rosenqvist, M.; Svensson, L.; Ljungman, P.L. Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur. Heart J. 2014, 35, 861–868. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Magrì, D. Syncope: Physiopathology, diagnosis and therapy. La Clin. Ter. 2015, 166, e216–e233. [Google Scholar]
- Pasquier, M.; Clair, M.; Pruvot, E.; Hugli, O.; Carron, P.-N. Carotid Sinus Massage. N. Engl. J. Med. 2017, 377, e21. [Google Scholar] [CrossRef] [PubMed]
- Galli, A.; Barbic, F.; Borella, M.; Costantino, G.; Perego, F.; Dipaola, F.; Casella, F.; Duca, P.G.; Diedrich, A.; Raj, S.; et al. Influence of Climate on Emergency Department Visits for Syncope: Role of Air Temperature Variability. PLoS ONE 2011, 6, e22719. [Google Scholar] [CrossRef]
- Alexander, P. Association of monthly frequencies of diverse diseases in the calls to the public emergency service of the city of Buenos Aires during 1999–2004 with meteorological variables and seasons. Int. J. Biometeorol. 2012, 57, 83–90. [Google Scholar] [CrossRef]
- Piccirillo, G.; Moscucci, F.; Pascucci, M.; Di Barba, D.; Montesanti, D.; Magrí, D. Effects of weather on neurally mediated syncope tests. Int. J. Cardiol. 2014, 176, 1411–1413. [Google Scholar] [CrossRef]
- Kowalska, M.; Kocot, K. Short-term exposure to ambient fine particulate matter (PM2.5 and PM10) and the risk of heart rhythm abnormalities and stroke. Postepy Hig. Med. Dosw. (Online) 2016, 70, 1017–1025. [Google Scholar] [CrossRef]
- Shutt, R.H.; Kauri, L.M.; Weichenthal, S.; Kumarathasan, P.; Vincent, R.; Thomson, E.M.; Liu, L.; Mahmud, M.; Cakmak, S.; Dales, R. Exposure to air pollution near a steel plant is associated with reduced heart rate variability: A randomised crossover study. Environ. Health 2017, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jiang, R.; Zhao, Z.; Song, W. Effects of ozone and fine particulate matter (PM2.5) on rat system inflammation and cardiac function. Toxicol. Lett. 2013, 217, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-H.; Bae, H.-J.; Yi, S.-M.; Park, E.; Lee, B.-E.; Hong, Y.-C. Vascular and cardiac autonomic function and PM2.5 constituents among the elderly: A longitudinal study. Sci. Total Environ. 2017, 607–608, 847–854. [Google Scholar] [CrossRef]
- Jia, X.; Song, X.; Shima, M.; Tamura, K.; Deng, F.; Guo, X. Acute effect of ambient ozone on heart rate variability in healthy elderly subjects. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, G.; Moscucci, F.; Fiorucci, C.; Di Iorio, C.; Mastropietri, F.; Magrì, D. Time- and frequency domain analysis of beat to beat P-wave duration, PR interval and RR interval can predict asystole as form of syncope during head-up tilt. Physiol. Meas. 2016, 37, 1910–1924. [Google Scholar] [CrossRef]
- Epstein, A.E.; DiMarco, J.P.; Ellenbogen, K.A.; Estes, N.M.; Freedman, R.A.; Gettes, L.S.; Gillinov, A.M.; Gregoratos, G.; Hammill, S.C.; Hayes, D.L.; et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. Circulation 2013, 127, e283–e352. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Gonçalves, A.; Campos, J.; Frutuoso, C.; Silva, A.; Touguinha, C.; Freitas, J.; Maciel, M.J. The role of pacemaker in hypersensitive carotid sinus syndrome. Europace 2010, 13, 572–575. [Google Scholar] [CrossRef]
- Piccirillo, G.; Ogawa, M.; Song, J.; Chong, V.J.; Joung, B.; Han, S.; Magrì, D.; Chen, L.S.; Lin, S.-F.; Chen, P.-S. Power spectral analysis of heart rate variability and autonomic nervous system activity measured directly in healthy dogs and dogs with tachycardia-induced heart failure. Heart Rhythm. 2009, 6, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Magrì, D.; Sciomer, S.; Fedele, F.; Gualdi, G.; Casciani, E.; Pugliese, P.; Losardo, A.; Ferrazza, G.; Pasquazzi, E.; Schifano, E.; et al. Increased QT variability in young asymptomatic patients with β-thalassemia major. Eur. J. Haematol. 2007, 79, 322–329. [Google Scholar] [CrossRef]
- Piccirillo, G.; Cacciafesta, M.; Viola, E.; Santagada, E.; Nocco, M.; Lionetti, M.; Bucca, C.; Moisè, A.; Tarantini, S.; Marigliano, V. Influence of aging on cardiac baroreflex sensitivity determined non-invasively by power spectral analysis. Clin. Sci. 2001, 100, 267–274. [Google Scholar] [CrossRef]
- Piccirillo, G.; Di Giuseppe, V.; Nocco, M.; Lionetti, M.; Moisè, A.; Naso, C.; Tallarico, D.; Marigliano, V.; Cacciafesta, M.; Moisè, A. Influence of aging and other cardiovascular risk factors on baroreflex sensitivity. J. Am. Geriatr. Soc. 2001, 49, 1059–1065. [Google Scholar] [CrossRef]
- Sutton, R. Carotid sinus syndrome: Progress in understanding and management. Glob. Cardiol. Sci. Pract. 2014, 2014, 18. [Google Scholar] [CrossRef]
- Amin, V.; Pavri, B.B. Carotid Sinus Syndrome. Cardiol. Rev. 2015, 23, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Yost, B.L.; Gleich, G.J.; Jacoby, D.B.; Fryer, A.D. The changing role of eosinophils in long-term hyperreactivity following a single ozone exposure. Am. J. Physiol. Cell. Mol. Physiol. 2005, 289, L627–L635. [Google Scholar] [CrossRef][Green Version]
- Rich, D.Q.; Schwartz, J.; Mittleman, M.; Link, M.; Luttmann-Gibson, H.; Catalano, P.J.; Speizer, F.E.; Dockery, D.W. Association of Short-term Ambient Air Pollution Concentrations and Ventricular Arrhythmias. Am. J. Epidemiol. 2005, 161, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Lisabeth, L.D.; Escobar, J.D.; Dvonch, J.T.; Sánchez, B.N.; Majersik, J.J.; Brown, D.; Smith, M.A.; Morgenstern, L.B. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann. Neurol. 2008, 64, 53–59. [Google Scholar] [CrossRef]
- Uchiyama, I.; Simomura, Y.; Yokoyama, E. Effects of acute exposure to ozone on heart rate and blood pressure of the conscious rat. Environ. Res. 1986, 41, 529–537. [Google Scholar] [CrossRef]
- Vaughan, T.R., Jr.; Moorman, W.J.; Lewis, T.R. Cardiopulmonary effects of acute exposure to ozone in the dog. Toxicol. Appl. Pharmacol. 1971, 20, 404–411. [Google Scholar] [CrossRef]
- Watkinson, W.P.; Campen, M.J.; Nolan, J.P.; Costa, D.L. Cardiovascular and systemic responses to inhaled pollutants in rodents: Effects of ozone and particulate matter. Environ. Health Perspect. 2001, 109, 539–546. [Google Scholar] [CrossRef]
- Gordon, T.; Taylor, B.F.; Amdur, M.O. Ozone Inhibition of Tissue Cholinesterase in Guinea Pigs. Arch. Environ. Health Int. J. 1981, 36, 284–288. [Google Scholar] [CrossRef]
- Chuang, K.-J.; Chan, C.-C.; Su, T.-C.; Lee, C.-T.; Tang, C.-S. The Effect of Urban Air Pollution on Inflammation, Oxidative Stress, Coagulation, and Autonomic Dysfunction in Young Adults. Am. J. Respir. Crit. Care Med. 2007, 176, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Tank, J.; Biller, H.; Heusser, K.; Holz, O.; Diedrich, A.; Framke, T.; Koch, A.; Grosshennig, A.; Koch, W.; Krug, N.; et al. Effect of Acute Ozone Induced Airway Inflammation on Human Sympathetic Nerve Traffic: A Randomized, Placebo Controlled, Crossover Study. PLoS ONE 2011, 6, e18737. [Google Scholar] [CrossRef]
- Krediet, C.T.P.; Parry, S.W.; Jardine, D.L.; Benditt, D.G.; Brignole, M.; Wieling, W. The history of diagnosing carotid sinus hypersensitivity: Why are the current criteria too sensitive? Europace 2011, 13, 14–22. [Google Scholar] [CrossRef] [PubMed]
| Positive Sinus Carotid Massage | ||||
|---|---|---|---|---|
| Variables | Subjects with Cardioinhibitory Response N = 28 | Subjects with Vasodepressory Response N = 29 | Subjects with Normal Response N = 122 | p Values |
| Age, yrs | 72 ± 10 | 71 ± 10 | 67 ± 10 | 0.052 |
| M/F, | 20/8 | 14/15 | 36/86 | 0.107 |
| BMI, kg/m2 | 26 ± 4 | 26 ± 4 | 26 ± 3 | 0.851 |
| HR, beats/min | 67 ± 11 | 69 ± 11 | 70 ± 9 | 0.338 |
| SBP, mm Hg | 127 ± 22 | 120 ± 18 | 125 ± 20 | 0.117 |
| DBP, mm Hg | 74 ± 9 | 70 ± 8 | 73 ± 9 | 0.431 |
| Positive Sinus Carotid Massage | ||||
|---|---|---|---|---|
| Variables | Subjects with Cardioinhibitory Response N = 28 | Subjects with Vasodepressory Response N = 29 | Subjects with Normal Response N = 122 | p Values |
| Carbon Monoxide, mg/m3 | 0.535 ± 0.270 | 0.610 ± 0.255 | 0.655 ± 0.309 | 0.062 |
| Ozone, μg/m3 | 106 ± 36 | 90 ± 40 | 86 ± 40 | 0.075 |
| Nitrogen Dioxide, μg/m3 | 86 ± 33 | 92 ± 20 | 89 ± 26 | 0.533 |
| Particulate Matter 10 μm, μg/m3 | 28 ± 14 | 28 ± 12 | 28 ± 13 | 0.905 |
| Particulate Matter 2.5 μm, μg/m3 * | 20 ± 8 | 19 ± 9 | 21 ± 8 | 0.589 |
| Benzene, mg/m3 | 1.10 ± 0.23 | 1.14 ± 0.25 | 1.17 ± 0.21 | 0.133 |
| Maximum Temperature, °C | 23 ± 7 | 21 ± 7 | 20 ± 8 | 0.129 |
| Minimum Temperature, °C | 12 ± 7 | 10 ± 7 | 10 ± 7 | 0.209 |
| Mean Temperature, °C | 18 ±7 | 15 ± 7 | 15 ± 7 | 0.162 |
| Relative Humidity, % | 71 ± 12 | 73 ± 12 | 73 ± 12 | 0.642 |
| ≥75th Percentile | ≤75th Percentile | |||
|---|---|---|---|---|
| Variables (75th Percentile) | Subjects with Positive Cardioinhibitory Response N (%) | Subjects with Positive Cardioinhibitory Response N (%) | χ2 | p Values |
| Carbon Monoxide, (0.8 mg/m3) | 7 (14%) | 21 (16%) | 0 | 0.0772 |
| Ozone, (117 μg/m3) | 15 (32%) | 13 (10%) | 7.4 | 0.0067 |
| Nitrogen Dioxide, (102 μg/m3) | 8 (18%) | 20 (15%) | 0 | 0.675 |
| Particulate 10 μm, (32 μg/m3) | 8 (17%) | 20 (15%) | 0 | 0.784 |
| Particulate 2.5 μm, (22 μg/m3) | 9 (15%) | 19 (16%) | 0 | 0.886 |
| Benzene, (1.4 mg/m3) | 8 (15%) | 20 (16%) | 0 | 0.896 |
| Maximum Temperature, (27 °C) | 10 (20%) | 18 (14%) | 0.8 | 0.384 |
| Minimum Temperature, (°C) | 9 (20%) | 19 (14%) | 0.5 | 0.413 |
| Mean Temperature, °C | 12 (26%) | 16 (12%) | 3.3 | 0.0601 |
| Relative Humidity, %, | 8 (17%) | 20 (15%) | 0.1 | 0.784 |
| Positive Sinus Carotid Massage | ||||
|---|---|---|---|---|
| Variables | Subjects with Cardioinhibitory Response N = 28 | Subjects with Vasodepressory Response N = 29 | Subjects with Normal Response N = 122 | p Values |
| RR mean, ms | 902 ± 85 | 901 ± 156 | 853 ± 120 | 0.053 |
| RR standard deviation | 25 ± 9 | 25 ± 10 | 27 ± 12 | 0.455 |
| PR mean, ms | 221 ± 28 | 209 ± 27 | 210 ± 26 | 0.143 |
| PR standard deviation | 6 ± 1 | 6 ± 1 | 6 ± 1 | 0.171 |
| P mean, ms | 121 ± 19 | 119 ± 21 | 119 ± 18 | 0.866 |
| P standard deviation | 8 ± 2 | 8 ± 2 | 8 ± 2 | 0.141 |
| PeR mean, ms | 95 ± 25 | 90 ± 18 | 90 ± 25 | 0.758 |
| PeR, standard deviation | 5 ± 2 * | 5 ± 1 | 6 ± 1 | 0.020 |
| P/PeR mean | 1.34 ± 0.40 | 1.37 ± 0.37 | 1.41 ± 0.46 | 0.766 |
| P/PeR standard deviation | 1.55 ± 0.72 *# | 1.48 ± 0.21 | 1.47 ± 0.20 | 0.003 |
| P → PR, coherence | 0.662 ± 0.093 **## | 0.547 ± 0.080 | 0.596 ± 0.081 | <0.001 |
| P → PeR, coherence | 0.454 ± 0.107 *# | 0.547 ± 0.086 | 0.527 ± 0.089 | <0.001 |
| Positive Sinus Carotid Massage | ||||
|---|---|---|---|---|
| Variables | Subjects with Cardioinhibitory Response N = 28 | Subjects with Vasodepressory Response N = 29 | Subjects with Normal Response N = 122 | p Values |
| TPRR, ms2 | 515 (786) | 586 (668) | 643 (889) | 0.617 |
| VLFRR, ms2 | 381 (491) | 263 (415) | 331 (536) | 0.677 |
| LFRR, ms2 | 98 (130) | 90 (258) | 122 (140) | 0.861 |
| HFRR, ms2 | 83 (90) | 96 (158) | 70 (126) | 0.924 |
| LF/HF | 1.47 (1.42) | 1.38 (1.41) | 1.45 (1.77) | 0.837 |
| LFRR, nu | 51 ± 16 | 52 ± 16 | 53 ± 17 | 0.837 |
| HFRR, nu | 41 ± 18 | 39 ± 17 | 38 ± 17 | 0.762 |
| TPSBP, mm Hg2 | 25 (39) | 31 (27) | 40 (29) | 0.905 |
| VLFSBP, mm Hg2 | 17 (28) | 22 (31) | 20 (27) | 0.858 |
| LFSBP, mm Hg2 | 4 (5) | 4 (5) | 4 (5) | 0.529 |
| HFSBP, mm Hg2 | 2 (3) | 3 (2) | 2 (3) | 0.607 |
| αLF, ms/mm Hg | 6 (4) | 6 (7) | 6 (5) | 0.825 |
| αHF, ms/mm Hg | 7 (6) | 6 (5) | 7 (6) | 0.342 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccirillo, G.; Moscucci, F.; Magrì, D. Air Pollution Role as Risk Factor of Cardioinhibitory Carotid Hypersensitivity. Atmosphere 2022, 13, 123. https://doi.org/10.3390/atmos13010123
Piccirillo G, Moscucci F, Magrì D. Air Pollution Role as Risk Factor of Cardioinhibitory Carotid Hypersensitivity. Atmosphere. 2022; 13(1):123. https://doi.org/10.3390/atmos13010123
Chicago/Turabian StylePiccirillo, Gianfranco, Federica Moscucci, and Damiano Magrì. 2022. "Air Pollution Role as Risk Factor of Cardioinhibitory Carotid Hypersensitivity" Atmosphere 13, no. 1: 123. https://doi.org/10.3390/atmos13010123
APA StylePiccirillo, G., Moscucci, F., & Magrì, D. (2022). Air Pollution Role as Risk Factor of Cardioinhibitory Carotid Hypersensitivity. Atmosphere, 13(1), 123. https://doi.org/10.3390/atmos13010123







