Methane Emissions from Surface of Mangrove River on Hainan Island, China
Abstract
:1. Introduction
2. Materials and Methods
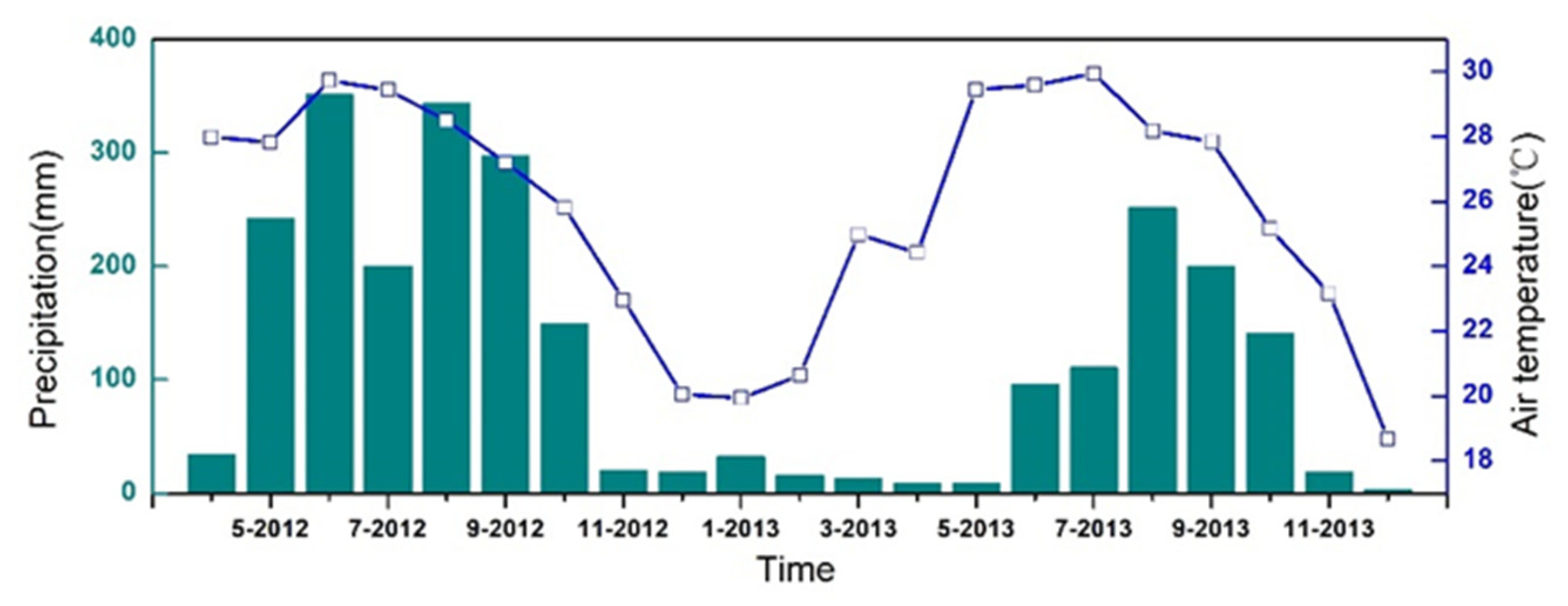
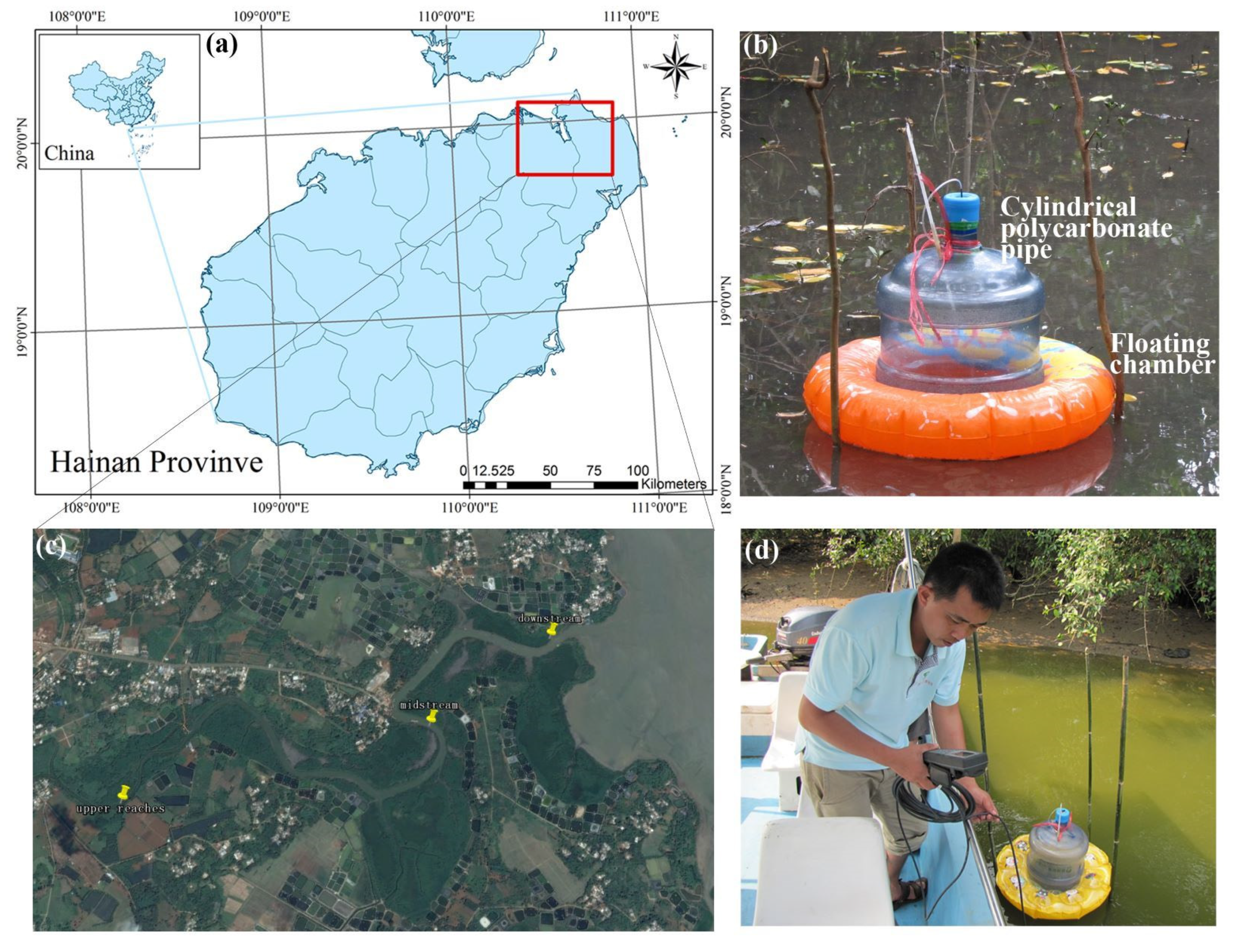
2.1. Study Site
2.2. Gas Sampling
2.3. Water Sampling and Analysis
2.4. Statistical Analysis
3. Results
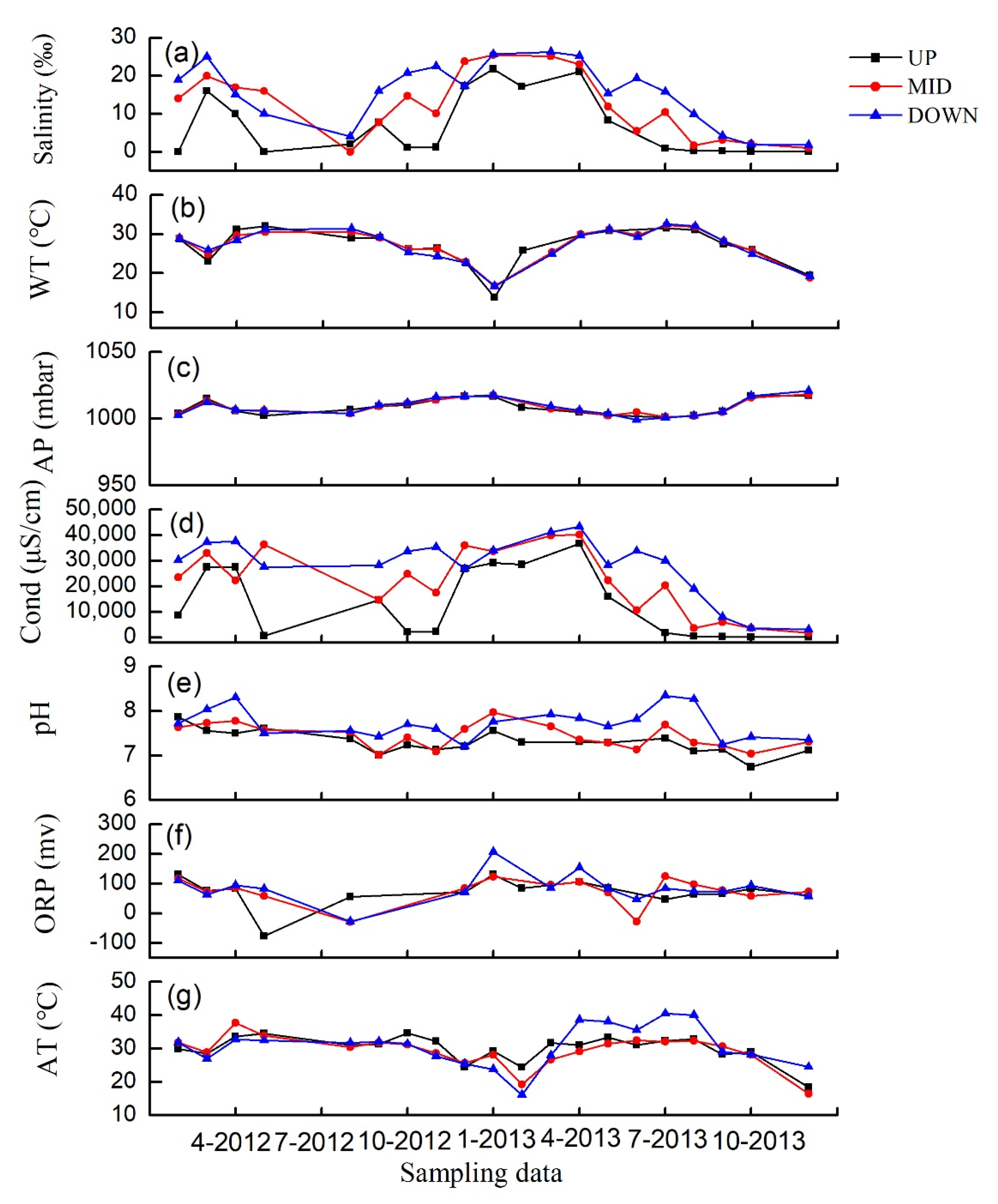
3.1. Water Characteristics and Environmental Factors
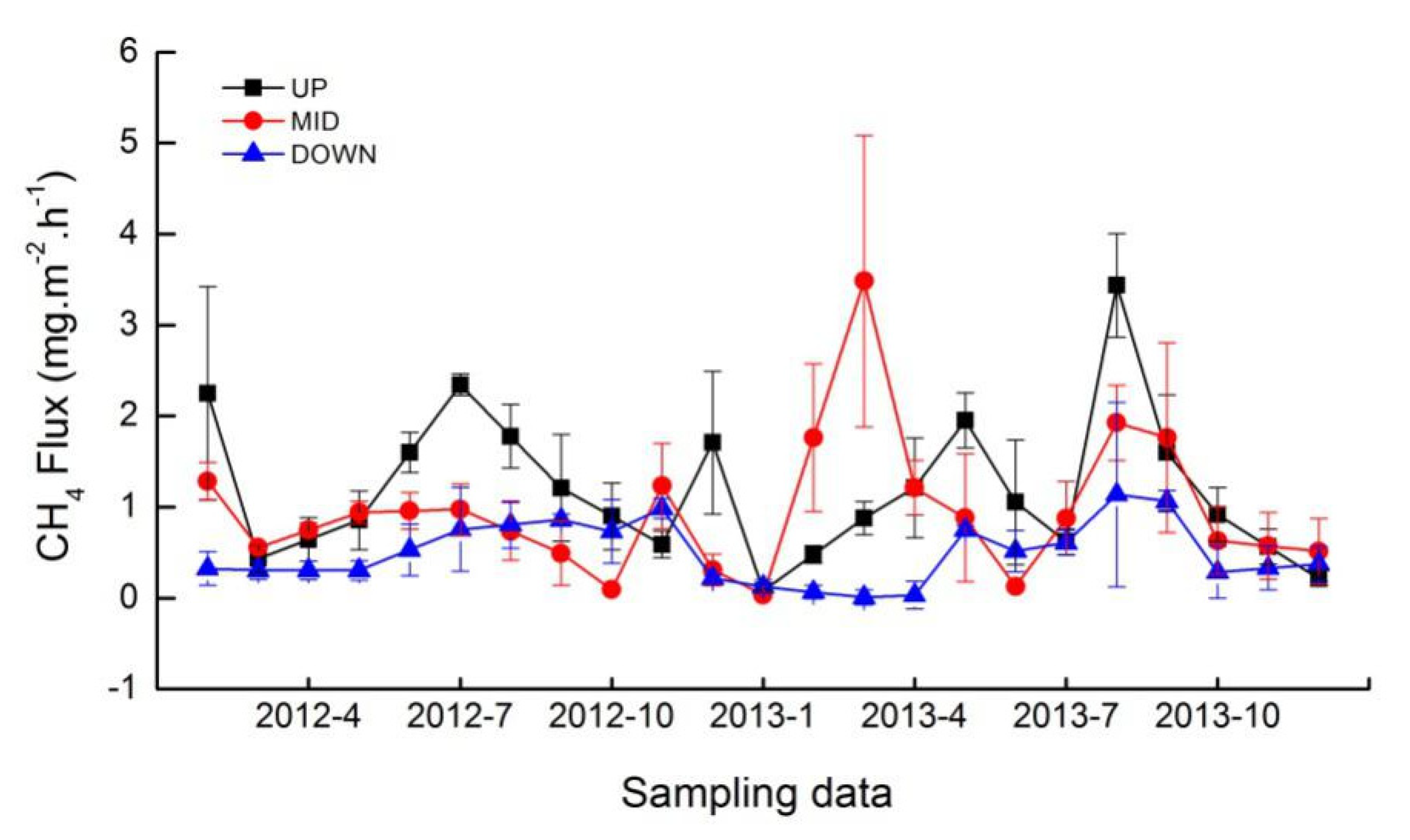
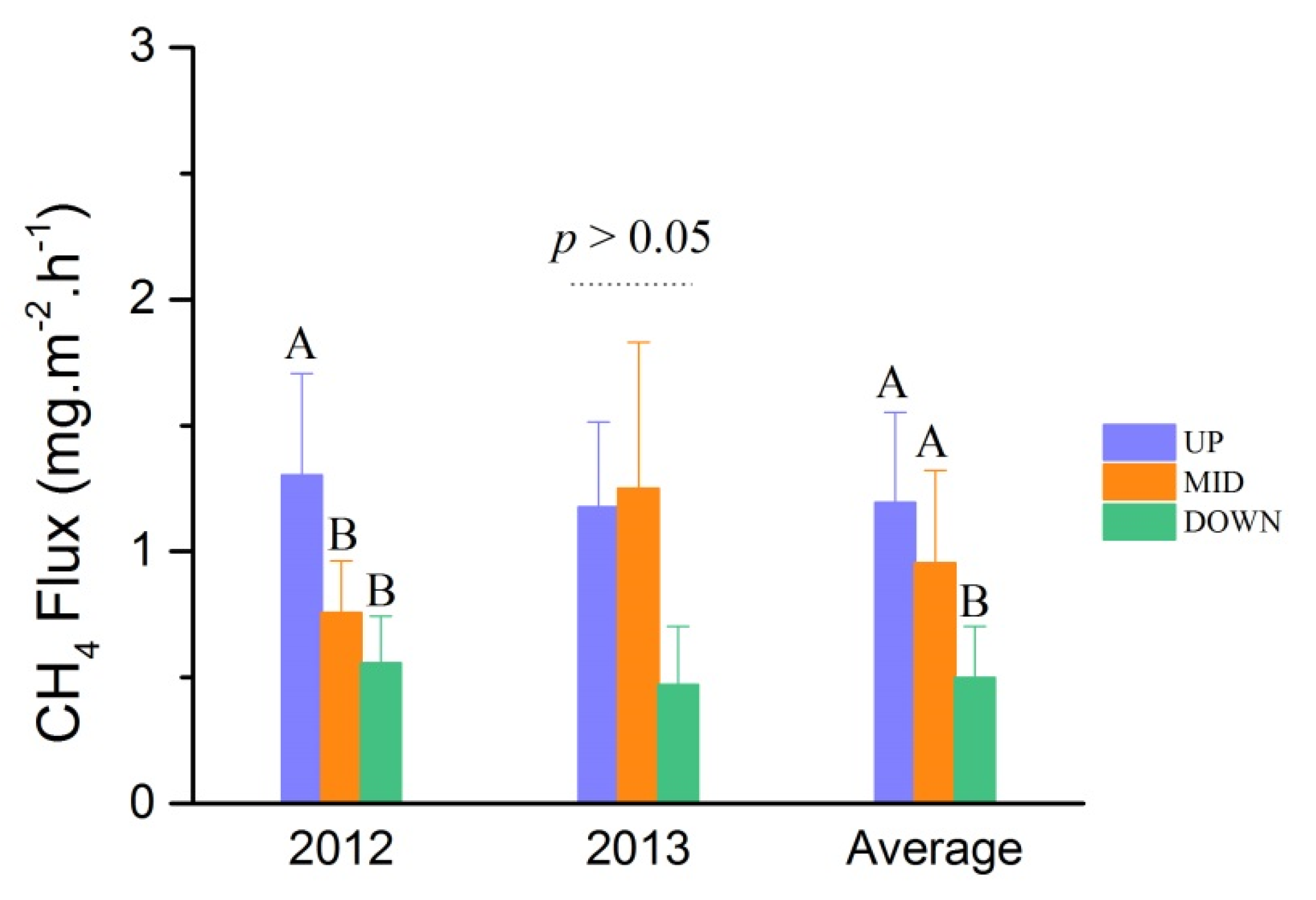
3.2. CH4 Fluxes
3.3. Relationship between Methane Fluxes and Factors
4. Discussion
4.1. Comparisons with Other Studies
4.2. Spatial and Seasonal Variations of CH4 Fluxes
4.3. Effect of Environmental Factors on CH4 Fluxes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Changes in Atmospheric Constituents and in Radiative Forcing; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Matousu, A.; Rulik, M.; Tuser, M.; Bednarik, A.; Simek, K.; Bussmann, I. Methane dynamics in a large river: A case study of the Elbe River. Aquat. Sci. 2019, 81, 12. [Google Scholar] [CrossRef]
- Selvam, B.P.; Natchimuthu, S.; Arunachalam, L.; Bastviken, D. Methane and carbon dioxide emissions from inland waters in India–implications for large scale greenhouse gas balances. Glob. Chang. Biol. 2014, 20, 3397–3407. [Google Scholar] [CrossRef] [Green Version]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.M.; Enrich-Prast, A. Freshwater Methane Emissions Offset the Continental Carbon Sink. Science 2011, 331, 50. [Google Scholar] [CrossRef] [Green Version]
- Aufdenkampe, A.K.; Mayorga, E.; Raymond, P.A.; Melack, J.M.; Doney, S.C.; Alin, S.R.; Aalto, R.E.; Yoo, K. Riverine coupling of biogeochemical cycles between land, oceans, and atmosphere. Front. Ecol. Environ. 2011, 9, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Butman, D.; Raymond, P.A. Significant efflux of carbon dioxide from streams and rivers in the United States. Nat. Geosci. 2011, 4, 839–842. [Google Scholar] [CrossRef]
- Cao, M.K.; Gregson, K.; Marshall, S. Global methane emission from wetlands and its sensitivity to climate change. Atmos. Environ. 1998, 32, 3293–3299. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Sarria-Guzman, Y.; Dendooven, L.; Luna-Guido, M. Methanogenesis and Methanotrophy in Soil: A Review. Pedosphere 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Zhe, L.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, 727–732. [Google Scholar]
- Dutta, M.K.; Bianchi, T.S.; Mukhopadhyay, S.K. Mangrove Methane Biogeochemistry in the Indian Sundarbans: A Proposed Budget. Front. Mar. Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sawakuchi, H.O.; Bastviken, D.; Sawakuchi, A.O.; Krusche, A.V.; Ballester, M.V.R.; Richey, J.E. Methane emissions from Amazonian Rivers and their contribution to the global methane budget. Glob. Chang. Biol. 2014, 20, 2829–2840. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Wang, F.; Guo, X.; Liu, F.; Dong, S. Carbon dioxide and methane fluxes across the sediment-water interface in different grass carp Ctenopharyngodon idella polyculture models. Aquac. Environ. Interact. 2017, 9, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Purvaja, R.; Ramesh, R.; Frenzel, P. Plant-mediated methane emission from an Indian mangrove. Glob. Chang. Biol. 2004, 10, 1825–1834. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Maher, D.T.; Ho, D.T.; Call, M.; Barr, J.G.; Eyre, B.D. Spatial and temporal variability of CO2 and CH4 gas transfer velocities and quantification of the CH4 microbubble flux in mangrove dominated estuaries. Limnol. Oceanogr. 2017, 62, 561–578. [Google Scholar] [CrossRef]
- Huertas, I.E.; de la Paz, M.; Perez, F.F.; Navarro, G.; Flecha, S. Methane Emissions From the Salt Marshes of Donana Wetlands: Spatio-Temporal Variability and Controlling Factors. Front. Ecol. Evol. 2019, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kreuzwieser, J.; Buchholz, J.; Rennenberg, H. Emission of methane and nitrous oxide by Australian mangrove ecosystems. Plant Biol. 2003, 5, 423–431. [Google Scholar] [CrossRef]
- Allen, D.; Dalal, R.C.; Rennenberg, H.; Schmidt, S. Seasonal variation in nitrous oxide and methane emissions from subtropical estuary and coastal mangrove sediments, Australia. Plant Biol. 2011, 13, 126–133. [Google Scholar] [CrossRef]
- Sotomayor, D.; Corredor, J.E.; Morell, J.M. Methane flux from mangrove sediments along the southwestern coast of puerto rico. Estuaries 1994, 17, 140–147. [Google Scholar] [CrossRef]
- Truong, A.H.; Kim, M.T.; Thu, N.T.; Tung, N.N.; Trung, N.Q. Methane, Nitrous Oxide and Ammonia Emissions from Livestock Farming in the Red River Delta, Vietnam: An Inventory and Projection for 2000-2030. Sustainability 2018, 10, 3826. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.V.; Abril, G.; Bouillon, S. Carbon dynamics and CO2 and CH4 outgassing in the Mekong delta. Biogeosciences 2018, 15, 1093–1114. [Google Scholar] [CrossRef] [Green Version]
- Natchimuthu, S.; Wallin, M.B.; Klemedtsson, L.; Bastviken, D. Spatio-temporal patterns of stream methane and carbon dioxide emissions in a hemiboreal catchment in Southwest Swedend. Sci. Rep. 2017, 7, 39729. [Google Scholar] [CrossRef]
- Upstill-Goddard, R.C.; Salter, M.E.; Mann, P.J.; Barnes, J.; Poulsen, J.; Dinga, B.; Fiske, G.J.; Holmes, R.M. The riverine source of CH4 and N2O from the Republic of Congo, western Congo Basin. Biogeosciences 2017, 14, 2267–2281. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.G.; Jiang, H.H.; Wang, L.L.; Mou, X.J.; Sun, W.L. Seasonal and spatial variations of methane emissions from coastal marshes in the northern Yellow River estuary, China. Plant Soil 2013, 369, 317–333. [Google Scholar] [CrossRef] [Green Version]
- Qu, B.; Aho, K.S.; Li, C.; Kang, S.; Sillanpaa, M.; Yan, F.; Raymond, P.A. Greenhouse gases emissions in rivers of the Tibetan Plateau. Sci. Rep. 2017, 7, 16573. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xia, X.; Liu, S.; Zhang, S.; Li, S.; Wang, J.; Wang, G.; Gao, H.; Zhang, Z.; Wang, Q.; et al. Significant methane ebullition from alpine permafrost rivers on the East Qinghai–Tibet Plateau. Nat. Geosci. 2020, 13, 349–354. [Google Scholar] [CrossRef]
- Zhang, T.P.; Huang, X.Y.; Yang, Y.; Li, Y.L.; Dahlgren, R.A. Spatial and temporal variability in nitrous oxide and methane emissions in urban riparian zones of the Pearl River Delta. Environ. Sci. Pollut. Res. 2016, 23, 1552–1564. [Google Scholar] [CrossRef] [Green Version]
- Campeau, A.; Del Giorgio, P.A. Patterns in CH4 and CO2 concentrations across boreal rivers: Major drivers and implications for fluvial greenhouse emissions under climate change scenarios. Glob. Chang. Biol. 2014, 20, 1075–1088. [Google Scholar] [CrossRef]
- Wang, C.; Tong, C.; Chambers, L.G.; Liu, X. Identifying the Salinity Thresholds that Impact Greenhouse Gas Production in Subtropical Tidal Freshwater Marsh Soils. Wetlands 2017, 37, 559–571. [Google Scholar] [CrossRef]
- Yang, W.B.; Yuan, C.S.; Huang, B.Q.; Tong, C.; Yang, L. Emission Characteristics of Greenhouse Gases and Their Correlation with Water Quality at an Estuarine Mangrove Ecosystem—The Application of an In-Situ On-site NDIR Monitoring Technique. Wetlands 2018, 38, 723–738. [Google Scholar] [CrossRef]
- Ming, L.; Wang, L.J.; Wen-Hui, M.A.; Hong, M.L. A preliminary study of water pollution in Dongzhaigang national nature reserve. J. Hainan Norm. Univ. (Nat. Sci.) 2004, 2004, 282–285. [Google Scholar]
- Wang, Y.; Zuo, P.; Huang, Z.Q.; Zou, X.Q. Study of the Change of Mangrove Wetland Ecosystem and Driving Forces in Dongzhaigang. Sichuan Environ. 2006, 3, 44–49. [Google Scholar]
- Huang, X.; Wang, X.; Li, X.; Xin, K.; Yan, Z.; Sun, Y.; Bellerby, R. Distribution Pattern and Influencing Factors for Soil Organic Carbon (SOC) in Mangrove Communities at Dongzhaigang, China. J. Coast. Res. 2017, 34, 434–442. [Google Scholar] [CrossRef]
- Li, J.; Liao, Q.; Li, M.; Zhang, J.; Fungyee, T.N.; Xu, R. Community Structure and Biodiversity of Soil Ciliates at Dongzhaigang Mangrove Forest in Hainan Island, China. Appl. Environ. Soil Sci. 2010, 2010, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Guan, W.; Chen, H.; Liao, B.; Hu, J.; Peng, C.; Rui, J.; Tian, J.; Zhu, D.; He, Y. Archaeal communities in the sediments of different mangrove stands at Dongzhaigang, China. J. Soil Sediments 2016, 16, 1995–2004. [Google Scholar] [CrossRef]
- Liao, Q.Y.; Li, J.; Zhang, J.H.; Li, M.; Lu, Y.; Xu, R.L. An ecological analysis of soil sarcodina at Dongzhaigang mangrove in Hainan Island, China. Eur. J. Soil Biol. 2009, 45, 214–219. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Z.; Shi, F.; Wang, W. Are vegetated areas of mangroves attractive to juvenile and small fish? The case of Dongzhaigang Bay, Hainan Island, China. Estuar. Coast. Shelf Sci. 2009, 85, 208–216. [Google Scholar] [CrossRef]
- Chen, H.; Wu, N.; Yao, S.P.; Gao, Y.H.; Zhu, D.; Wang, Y.F.; Xiong, W.; Yuan, X.Z. High methane emissions from a littoral zone on the Qinghai-Tibetan Plateau. Atmos. Environ. 2009, 43, 4995–5000. [Google Scholar] [CrossRef]
- Zhu, D.; Chen, H.; Zhu, Q.A.; Wu, Y.; Wu, N. High Carbon Dioxide Evasion from an Alpine Peatland Lake: The Central Role of Terrestrial Dissolved Organic Carbon Input. Water Air. Soil Poll. 2012, 223, 2563–2569. [Google Scholar] [CrossRef]
- Bosse, U.; Frenzel, P. CH4 emissions from a West Siberian mire. Elektra 2001, 52, 99–114. [Google Scholar]
- Marani, L.; Alvala, P.C. Methane emissions from lakes and floodplains in Pantanal, Brazil. Atmos Environ. 2007, 41, 1627–1633. [Google Scholar] [CrossRef]
- Rajkumar, A.N.; Barnes, J.; Ramesh, R.; Purvaja, R.; Upstill-Goddard, R.C. Methane and nitrous oxide fluxes in the polluted Adyar River and estuary, SE India. Mar. Pollut. Bull. 2008, 56, 2043–2051. [Google Scholar] [CrossRef]
- Singh, S.N.; Kulshreshtha, K.; Agnihotri, S. Seasonal dynamics of methane emission from wetlands. Chemosphere–Glob. Chang. Sci. 2000, 2, 39–46. [Google Scholar] [CrossRef]
- Devol, A.H.; Richey, J.E.; Clark, W.A.; King, S.L.; Martinelli, L.A. Methane emissions to the troposphere from the Amazon floodplain. J. Geophys. Res. Atmos. 1988, 93, 1583–1592. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.K.; Biswas, H.; De, T.K.; Sen, B.K.; Sen, S.; Jana, T.K. Impact of Sundarban mangrove biosphere on the carbon dioxide and methane mixing ratios at the NE Coast of Bay of Bengal, India. Atmos. Environ. 2002, 36, 629–638. [Google Scholar] [CrossRef]
- Bartlett, K.B.; Crill, P.M.; Bonassi, J.A.; Richey, J.E.; Harriss, R.C. Methane flux from the Amazon River floodplain: Emissions during rising water. J. Geophys. Res. Atmos. 1990, 95, 16773–16788. [Google Scholar] [CrossRef]
- Poffenbarger, H.J.; Needelman, B.A.; Megonigal, J.P. Salinity Influence on Methane Emissions from Tidal Marshes. Wetlands 2011, 31, 831–842. [Google Scholar] [CrossRef]
| Environmental Factors | Upper Reaches | Midstream | Downstream |
|---|---|---|---|
| Air Temperature (°C) | 30.11 ± 3.95 | 29.3 ± 4.77 | 30.75 ± 6.09 |
| Salinity (‰) | 6.95 ± 6.16 b | 12.23 ± 8.65 ab | 15.52 ± 8.20 a |
| Water Temperature (°C) | 26.86 ± 4.74 | 27.27 ± 4.22 | 27.13 ± 4.35 |
| Atmospheric pressure (mbar) | 1008.94 ± 5.87 | 1008.72 ± 5.76 | 1008.88 ± 6.52 |
| Conductivity (μS/cm) | 1.31 ± 1.29 b | 2.16 ± 1.30 ab | 2.78 ± 1.20 a |
| pH | 7.30 ± 0.26 b | 7.44 ± 0.27 b | 7.72 ± 0.34 a |
| ORP (mv) | 71.39 ± 47.74 | 74.58 ± 45.36 | 84.84 ± 49.26 |
| DOC (mg/L) | 2.98 ± 2.60 | 3.63 ± 2.34 | 2.61 ± 2.38 |
| -N (mg/L) | 0.48 ± 0.44 | 0.74 ± 0.73 | 0.35 ± 0.32 |
| NO3−-N (mg/L) | 0.27 ± 0.26 | 0.24 ± 0.23 | 0.23 ± 0.22 |
| (mg/L) | 0.15 ± 0.14 b | 0.23 ± 0.16 b | 0.57 ± 0.56 a |
| Regions | Salinity | WT | AP | pH | Cond | ORP mV | AT | |
|---|---|---|---|---|---|---|---|---|
| UP | r | −0.29 | 0.490 * | −0.505 * | −0.06 | −0.22 | 0.06 | 0.26 |
| p | 0.24 | 0.04 | 0.03 | 0.81 | 0.40 | 0.85 | 0.27 | |
| n | 18 | 18 | 18 | 18 | 170 | 15 | 20 | |
| MID | r | 0.08 | 0.25 | −0.39 | 0.01 | 0.10 | 0.28 | −0.11 |
| p | 0.73 | 0.30 | 0.09 | 0.97 | 0.68 | 0.30 | 0.63 | |
| n | 19 | 19 | 19 | 19 | 18 | 16 | 20 | |
| DOWN | r | −0.38 | 0.41 | −0.29 | −0.06 | −0.32 | −0.50 | 0.41 |
| p | 0.11 | 0.08 | 0.23 | 0.81 | 0.20 | 0.05 | 0.07 | |
| n | 19 | 19 | 19 | 19 | 18 | 16 | 20 |
| Name of River | Country | Type | CH4 Flux (mg m−2 h−1) | Reference | ||
|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | ||||
| Yennisei River | Russia | Mires | ND | ND | 1.04 | [39] |
| Miranda River | Brazil | Open water | 0.067 | 91.1 | 5.93 | [40] |
| Adyar River | India | Open water | 0.0013 | 76.1 | 0.53–15.3 * | [41] |
| Andhra Pradesh | India | Water bodies | 0.006 | 34.73 | 5.2 | [3] |
| Sundarbans | Bangladesh | Mangrove | −0.66 | 1.33 | 0.67 | [44] |
| Yanfeng river | China | Open water | 0.011 | 5.156 | 0.96 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Guan, W.; Chen, H. Methane Emissions from Surface of Mangrove River on Hainan Island, China. Atmosphere 2021, 12, 1126. https://doi.org/10.3390/atmos12091126
Hu J, Guan W, Chen H. Methane Emissions from Surface of Mangrove River on Hainan Island, China. Atmosphere. 2021; 12(9):1126. https://doi.org/10.3390/atmos12091126
Chicago/Turabian StyleHu, Ji, Wei Guan, and Huai Chen. 2021. "Methane Emissions from Surface of Mangrove River on Hainan Island, China" Atmosphere 12, no. 9: 1126. https://doi.org/10.3390/atmos12091126
APA StyleHu, J., Guan, W., & Chen, H. (2021). Methane Emissions from Surface of Mangrove River on Hainan Island, China. Atmosphere, 12(9), 1126. https://doi.org/10.3390/atmos12091126





