The Role of Fossil Fuel Combustion Metals in PM2.5 Air Pollution Health Associations
Abstract
:1. Introduction
2. Sources and Concentrations of Trace Metals in Ambient Air PM
3. Source Apportionments of Particulate Matter
4. Review of Epidemiologic Studies in Human Populations
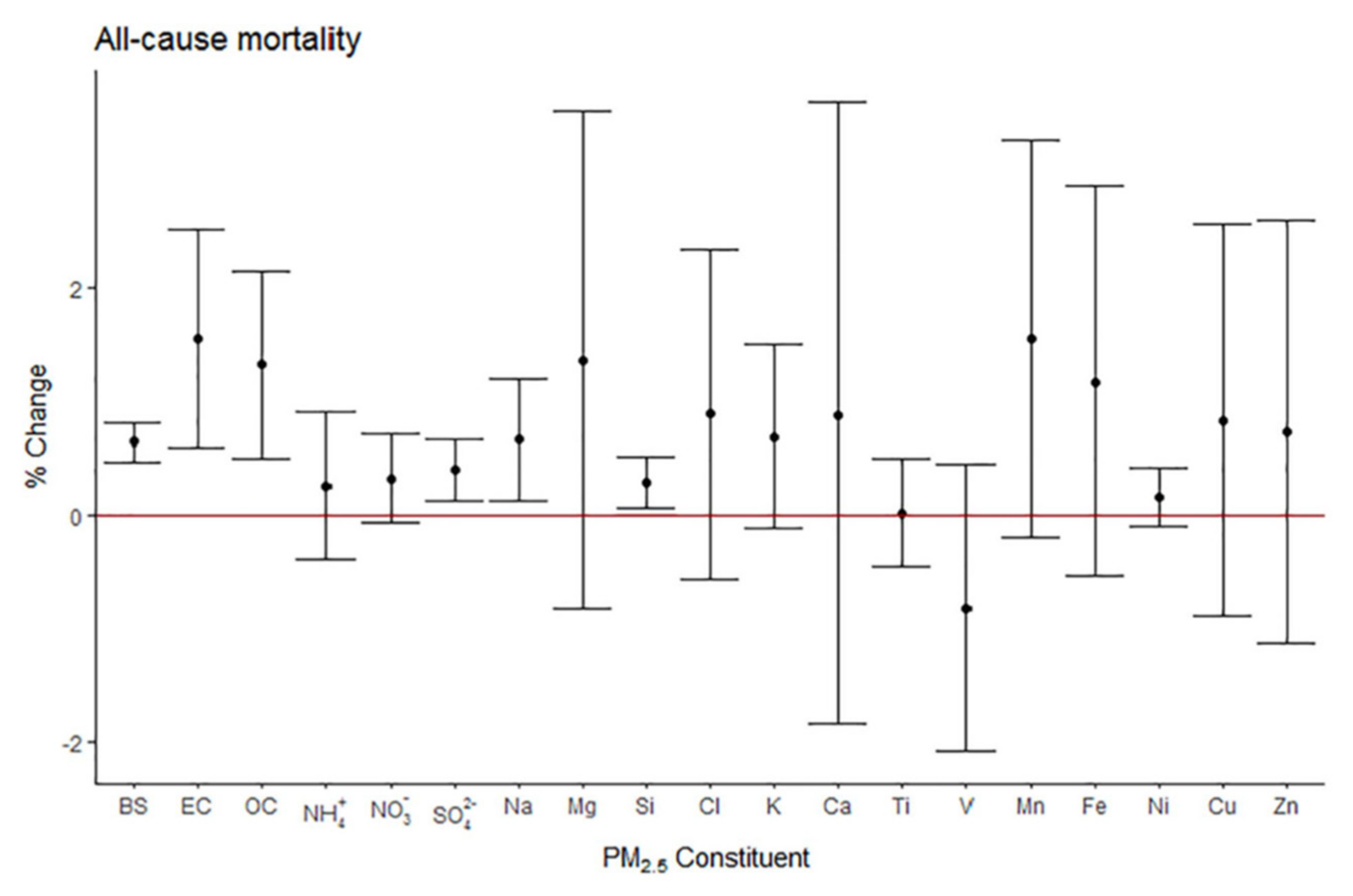
4.1. Health Effects of Short-Term Exposure to PM Metals or Their Source-Related Mass
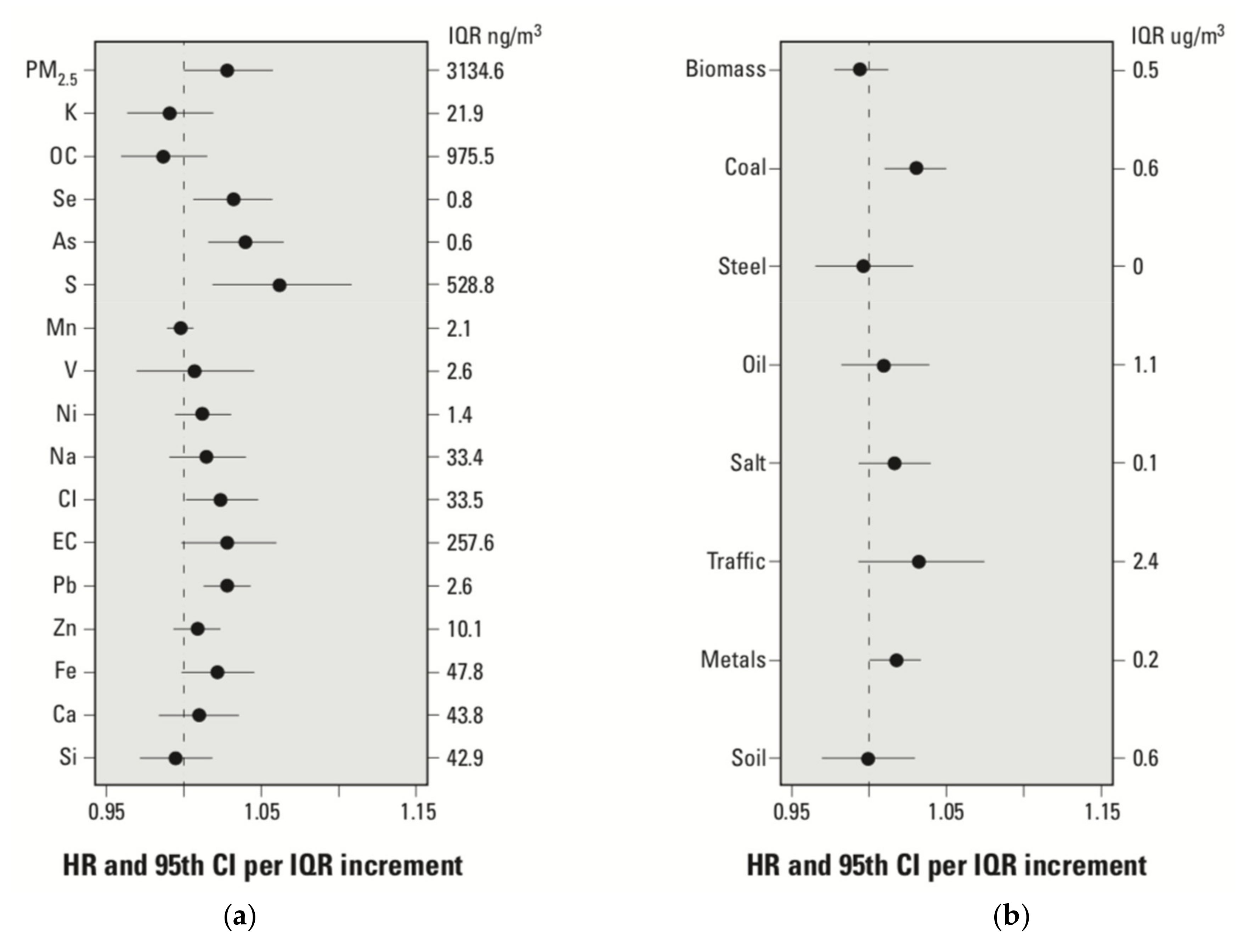
4.2. Adverse Health Effects of Long-Term Exposure to PM Constituent Metals or Source-Related PM Mass
5. Toxicology Studies of PM2.5 Exposure
5.1. Role of Metals in Oxidative Stress and Inflammation
5.2. Role of Metals in Cardiovascular Disease (CVD)
5.3. Translocation of Inhaled Metals from Lungs to Other Organs
5.4. Evidence from Concentrated Ambient Particles (CAPs) Inhalation Studies in Animals
5.5. Bioactivity of PM Associations with Elements and Sources
5.6. Effects of Metal Removal or Addition
6. Discussion
6.1. Where Do Metals Fit in the Larger Picture of PM-Associated Health Effects?
6.2. Are There Specific Metals That Can Account for Health Effects Associated with PM Mass?
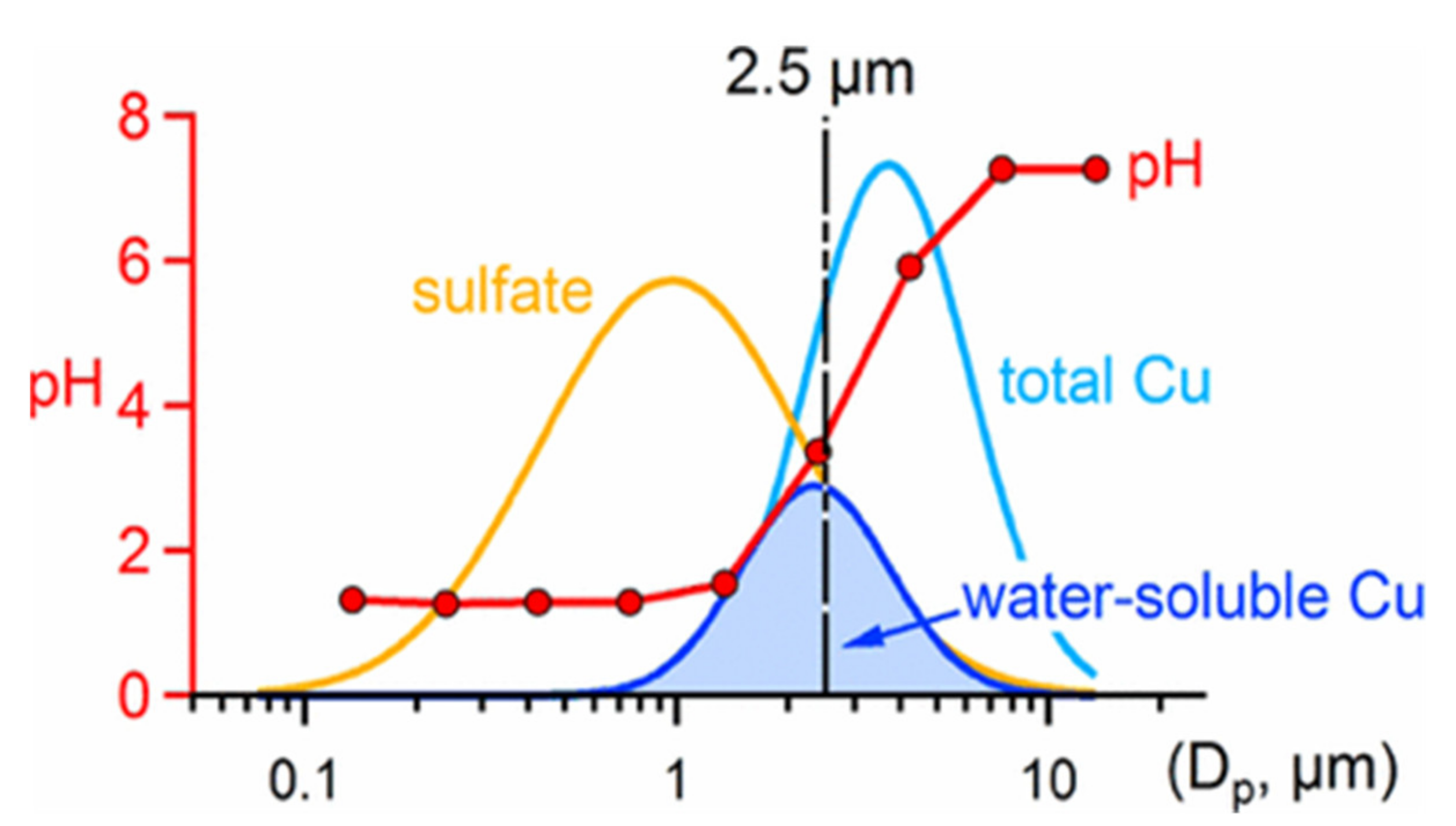
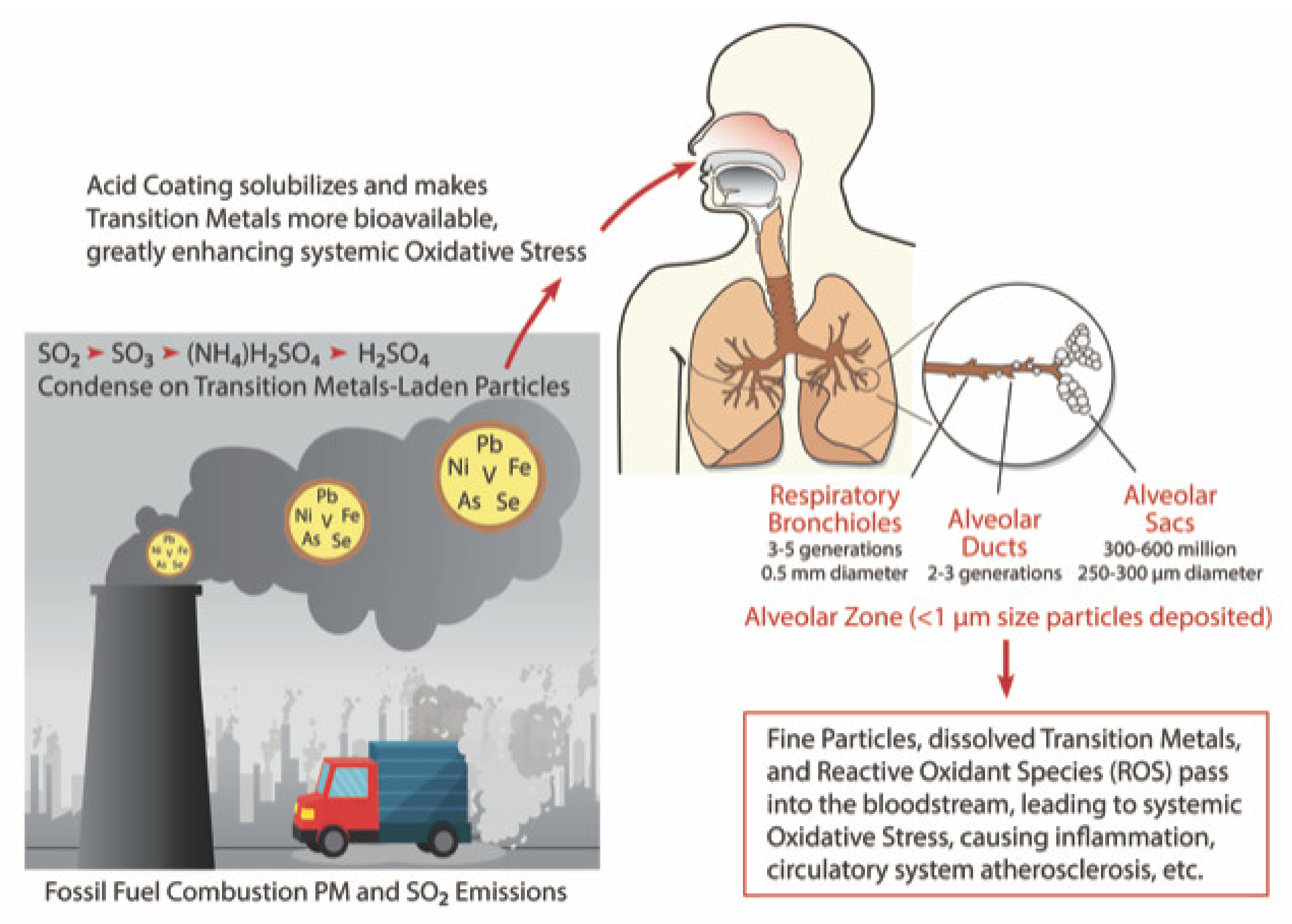
6.3. Acidic Sulfur as a Potentiator of Health Risks from Particulate Metals
- It often has correlated with adverse health effects as well or better than other widely used PM metrics in epidemiological studies for which comparisons were possible.
- It is stable and nonvolatile on sampling substrates.
- It can be analyzed easily and economically both continuously and in virtually all analytical laboratories.
- There is an existing body of historic sulfate epidemiology justifying its monitoring and regulation, along with PM2.5 mass.
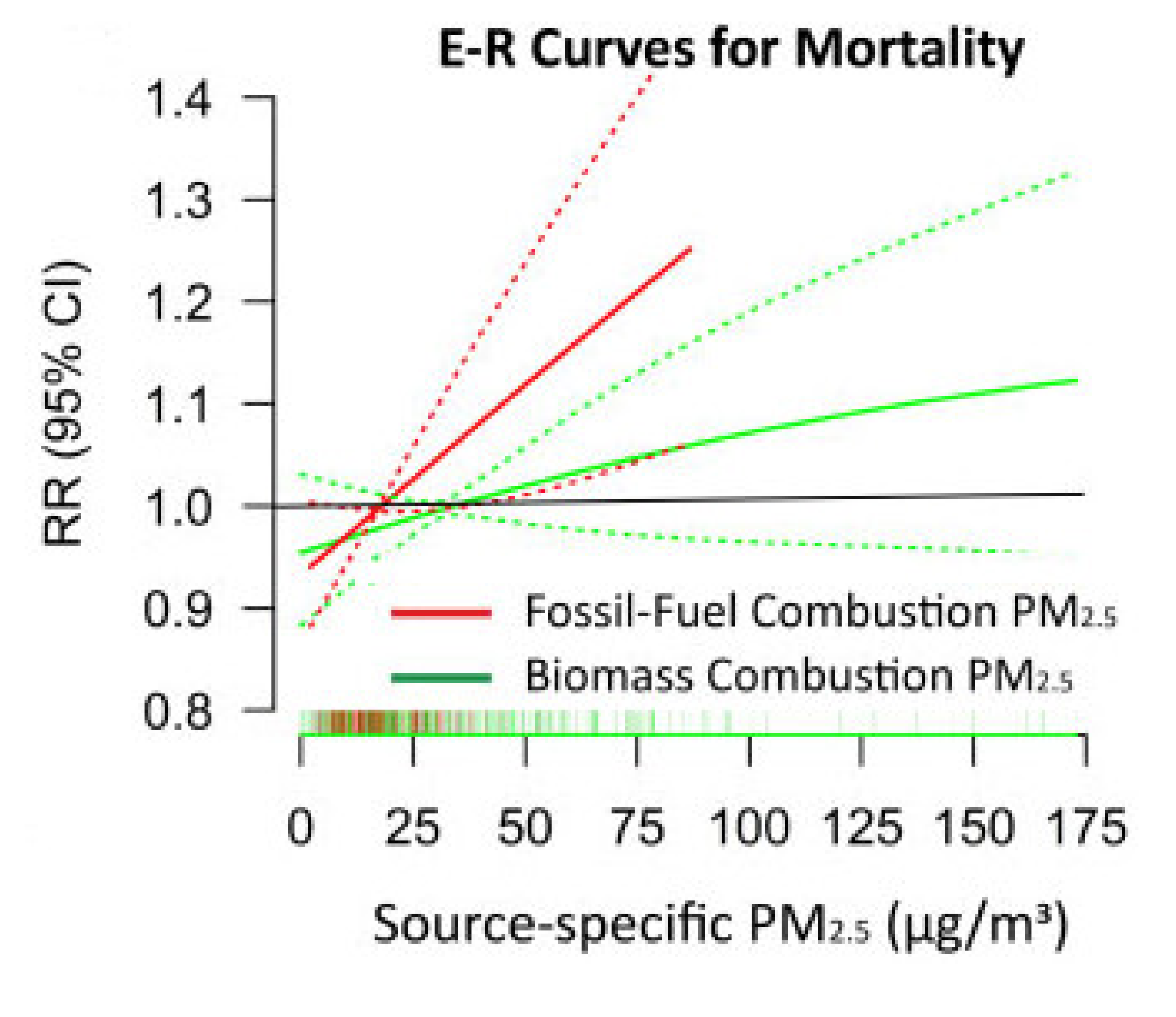
6.4. Role of Fossil Fuel Combustion Sources in Adverse Health Effects of PM
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Slim, S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Ozkaynak, H.; Thurston, G.D. Associations between 1980 U.S. Mortality Rates and Alternative Measures of Airborne Particle Concentration. Risk Anal. 1987, 7, 449–461. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A.; Burnett, R.T.; Thurston, G.D.; Thun, M.J.; Calle, E.E.; Krewski, D.; Godleski, J.J. Cardiovascular Mortality and Long-Term Exposure to Particulate Air Pollution. Circulation 2004, 109, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Pope, C.A. Mortality Effects of Longer Term Exposures to Fine Particulate Air Pollution: Review of Recent Epidemiological Evidence. Inhal. Toxicol. 2007, 19, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.D.; Burnett, R.T.; Turner, M.C.; Shi, Y.; Krewski, D.; Lall, R.; Ito, K.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; et al. Ischemic Heart Disease Mortality and Long-Term Exposure to Source-Related Components of U.S. Fine Particle Air Pollution. Environ. Health Perspect. 2016, 124, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.V. Health indices of the adverse effects of air pollution: The question of coherence. Environ. Res. 1992, 59, 336–349. [Google Scholar] [CrossRef]
- McConnell, R.; Islam, T.; Shankardass, K.; Jerrett, M.; Lurmann, F.; Gilliland, F.; Gauderman, J.; Avol, E.; Künzli, N.; Yao, L.; et al. Childhood incident asthma and traffic-related air pollution at home and school. Environ. Health Perspect. 2010, 118, 1021–1026. [Google Scholar] [CrossRef] [Green Version]
- Bråbäck, L.; Forsberg, B. Does traffic exhaust contribute to the development of asthma and allergic sensitization in children: Findings from recent cohort studies. Environ. Health A Glob. Access Sci. Source 2009, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.C.; Hwang, J.-S. Effects of Subchronic Exposures to Concentrated Ambient Particles (CAPs) in Mice: IV. Characterization of Acute and Chronic Effects of Ambient Air Fine Particulate Matter Exposures on Heart-Rate Variability. Inhal. Toxicol. 2005, 17, 209–216. [Google Scholar] [CrossRef]
- Chen, L.-C.; Hwang, J.-S.; Lall, R.; Thurston, G.; Lippmann, M. Alteration of cardiac function in ApoE−/−mice by subchronic urban and regional inhalation exposure to concentrated ambient PM2.5. Inhal. Toxicol. 2010, 22, 580–592. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Nadziejko, C.; Chen, L.C. Effects of Subchronic Exposures to Concentrated Ambient Particles (CAPs) in Mice: III. Acute and Chronic Effects of CAPs on Heart Rate, Heart-Rate Fluctuation, and Body Temperature. Inhal. Toxicol. 2005, 17, 199–207. [Google Scholar] [CrossRef]
- Quan, C.; Sun, Q.; Lippmann, M.; Chen, L.-C. Comparative effects of inhaled diesel exhaust and ambient fine particles on inflammation, atherosclerosis, and vascular dysfunction. Inhal. Toxicol. 2010, 22, 738–753. [Google Scholar] [CrossRef]
- Chen, L.C.; Nadziejko, C. Effects of Subchronic Exposures to Concentrated Ambient Particles (CAPs) in Mice: V. CAPs Exacerbate Aortic Plaque Development in Hyperlipidemic Mice. Inhal. Toxicol. 2005, 17, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q. Long-term Air Pollution Exposure and Acceleration of Atherosclerosis and Vascular Inflammation in an Animal Model. JAMA 2005, 294, 3003–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Yue, P.; Ying, Z.; Cardounel, A.J.; Brook, R.D.; Devlin, R.; Hwang, J.-S.; Zweier, J.L.; Chen, L.C.; Rajagopalan, S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1760–1766. [Google Scholar] [CrossRef] [Green Version]
- Geiger, A.; Cooper, J. Overview of Airborne Metals Regulations, Exposure Limits, Health Effects, and Contemporary Research; U.S. Environmental Protection Agency: Portland, OR, USA, 2010; pp. 1–64. [Google Scholar]
- USEPA. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2019); EPA/600/R-19/188; U.S. Environmental Protection Agency: Washington, DC, USA, 2019. [Google Scholar]
- Molina, C.; Toro, A.R.; Manzano, C.; Canepari, S.; Massimi, L.; Leiva-Guzmán, M. Airborne Aerosols and Human Health: Leapfrogging from Mass Concentration to Oxidative Potential. Atmosphere 2020, 11, 917. [Google Scholar] [CrossRef]
- Chen, L.-C.; Maciejczyk, P.; Thurston, G. Metals and air pollution. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G., Costa, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, In press. [Google Scholar]
- Lall, R.; Kendall, M.; Ito, K.; Thurston, G.D. Estimation of historical annual PM2.5 exposures for health effects assessment. Atmos. Environ. 2004, 38, 5217–5226. [Google Scholar] [CrossRef]
- Whitby, K.T.; Liu, B.Y.H.; Husar, R.B.; Barsic, N.J. The minnesota aerosol-analyzing system used in the Los Angeles smog project. J. Colloid Interface Sci. 1972, 39, 136–164. [Google Scholar] [CrossRef]
- Vu, T.V.; Delgado-Saborit, J.M.; Harrison, R.M. Review: Particle number size distributions from seven major sources and implications for source apportionment studies. Atmos. Environ. 2015, 122, 114–132. [Google Scholar] [CrossRef]
- EIA. U.S. Energy Information Administration. What is U.S. Electricity Generation by Energy Source? Available online: https://www.eia.gov/tools/faqs/faq.php?id=427&t=3 (accessed on 26 June 2021).
- Leikauf, G.D.; Kim, S.-H.; Jang, A.-S. Mechanisms of ultrafine particle-induced respiratory health effects. Exp. Mol. Med. 2020, 52, 329–337. [Google Scholar] [CrossRef]
- Chen, L.-C.; Maciejczyk, P. Size and Composition Matters: From Engineered Nanoparticles to Ambient Fine Particles. In A New Paradigm for Environmental Chemistry and Toxicology; Jiang, G., Li, X., Eds.; Springer: Singapore, 2020; pp. 241–260. [Google Scholar]
- Council, N.R. Airborne Particles/Subcommittee on Airborne Particles, Committee on Medical and Biologic Effects of Environmental Pollutants, Division of Medical Sciences, Assembly of Life Sciences, National Research Council; University Park Press: Baltimore, MD, USA, 1979. [Google Scholar]
- USEPA. Compilation of Air Pollutant Emission Factors, Volume 1: Stationary Point and Area Sources. AP-42, Tables 1.3-1,1.3-2, 1.3-11, 1.4-2, 1.4-4; US Environmental Protection Agency, Office of Air Quality Planning and Standards: Research Triangle Park, NC, USA, 1995. Available online: http://www.epa.gov/ttn/chief/ap42/ch13/index.html (accessed on 26 June 2021).
- EEA. Key Air Quality Statistics for the Main Air PollutantsProd-ID: DAS-20-en. Published 28 August 2018. European Environmental Agency. Available online: https://www.eea.europa.eu/data-and-maps/dashboards/air-quality-statistics (accessed on 26 June 2021).
- Faroon, O.; Ashizawa, A.; Wright, S.; Tucker, P.; Jenkins, K.; Ingerman, L.; Rudisill, C. Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. In Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2012. [Google Scholar]
- Farahani, V.J.; Soleimanian, E.; Pirhadi, M.; Sioutas, C. Long-term trends in concentrations and sources of PM2.5–bound metals and elements in central Los Angeles. Atmos. Environ. 2021, 253, 118361. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Zhang, J.; Mao, Y.; Huang, X.; Qian, G. Evaluation for the heavy metal risk in fine particulate matter from the perspective of urban energy and industrial structure in China: A meta-analysis. J. Clean. Prod. 2020, 244, 118597. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Hsu, S.-C.; Chou, C.C.K.; Zhang, R.; Wu, Y.; Kao, S.-J.; Luo, L.; Huang, C.-H.; Lin, S.-H.; Huang, Y.-T. Wintertime haze deterioration in Beijing by industrial pollution deduced from trace metal fingerprints and enhanced health risk by heavy metals. Environ. Pollut. 2016, 208, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.F.S.; Hashizume, M.; Obase, Y.; Doi, M.; Tamura, K.; Tomari, S.; Kawano, T.; Fukushima, C.; Matsuse, H.; Chung, Y.; et al. Associations of chemical composition and sources of PM2.5 with lung function of severe asthmatic adults in a low air pollution environment of urban Nagasaki, Japan. Environ. Pollut. 2019, 252, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.M.; Teinilä, K.; Custódio, D.; Gomes Santos, A.; Xian, H.; Hillamo, R.; Alves, C.A.; Bittencourt de Andrade, J.; Olímpio da Rocha, G.; Kumar, P.; et al. Particulate pollutants in the Brazilian city of São Paulo: 1-year investigation for the chemical composition and source apportionment. Atmos. Chem. Phys. 2017, 17, 11943–11969. [Google Scholar] [CrossRef] [Green Version]
- Hernández, M.A.; Ramírez, O.; Benavides, J.A.; Franco, J.F. Urban cycling and air quality: Characterizing cyclist exposure to particulate-related pollution. Urban Clim. 2021, 36, 100767. [Google Scholar] [CrossRef]
- Garza-Galindo, R.; Morton-Bermea, O.; Hernández-Álvarez, E.; Ordoñez-Godínez, S.L.; Amador-Muñoz, O.; Beramendi-Orosco, L.E.; Retama, A.; Miranda, J.; Rosas-Pérez, I. Spatial and temporal distribution of metals in PM2.5 during 2013: Assessment of wind patterns to the impacts of geogenic and anthropogenic sources. Environ. Monit. Assess. 2019, 191, 165. [Google Scholar] [CrossRef]
- Huamán De La Cruz, A.; Bendezu Roca, Y.; Suarez-Salas, L.; Pomalaya, J.; Alvarez Tolentino, D.; Gioda, A. Chemical Characterization of PM2.5 at Rural and Urban Sites around the Metropolitan Area of Huancayo (Central Andes of Peru). Atmosphere 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Kuvarega, A.T.; Taru, P. Ambiental dust speciation and metal content variation in TSP, PM10 and PM2.5 in urban atmospheric air of Harare (Zimbabwe). Environ. Monit. Assess. 2008, 144, 1–14. [Google Scholar] [CrossRef]
- USEPA. National Lead Trends. Available online: https://www.epa.gov/air-trends/lead-trends (accessed on 26 June 2021).
- Sadeghi, B.; Choi, Y.; Yoon, S.; Flynn, J.; Kotsakis, A.; Lee, S. The characterization of fine particulate matter downwind of Houston: Using integrated factor analysis to identify anthropogenic and natural sources. Environ. Pollut. 2020, 262, 114345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, Q.; Li, L.; Han, Y.; Ye, Z.; Pongpiachan, S.; Zhang, Y.; Liu, S.; Tian, R.; Cao, J. Characteristics of PM2.5 at a High-Altitude Remote Site in the Southeastern Margin of the Tibetan Plateau in Premonsoon Season. Atmosphere 2019, 10, 645. [Google Scholar] [CrossRef] [Green Version]
- Parthasarathy, K.; Sahu, S.K.; Pandit, G.G. Comparison of Two Receptor Model Techniques for the Size Fractionated Particulate Matter Source Apportionment. Aerosol Air Qual. Res. 2016, 16, 1497–1508. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Dionisio, K.L.; Verissimo, T.G.; Kerr, A.S.; Coull, B.; Arku, R.E.; Koutrakis, P.; Spengler, J.D.; Hughes, A.F.; Vallarino, J.; et al. Chemical composition and sources of particle pollution in affluent and poor neighborhoods of Accra, Ghana. Environ. Res. Lett. 2013, 8, 044025. [Google Scholar] [CrossRef] [Green Version]
- Gaita, S.M.; Boman, J.; Gatari, M.J.; Pettersson, J.B.C.; Janhäll, S. Source apportionment and seasonal variation of PM2.5 in a Sub-Saharan African city: Nairobi, Kenya. Atmos. Chem. Phys. 2014, 14, 9977–9991. [Google Scholar] [CrossRef] [Green Version]
- Luckson, M.; Roelof, B.; Stuart, J.P.; Brigitte, L.; Johan, P.B.; Pieter, G.v.Z. Source apportionment of ambient PM10−2.5 and PM2.5 for the Vaal Triangle, South Africa. S. Afr. J. Sci. 2021, 117, 1–11. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2007. [Google Scholar]
- Jeong, C.-H.; Traub, A.; Huang, A.; Hilker, N.; Wang, J.M.; Herod, D.; Dabek-Zlotorzynska, E.; Celo, V.; Evans, G.J. Long-term analysis of PM2.5 from 2004 to 2017 in Toronto: Composition, sources, and oxidative potential. Environ. Pollut. 2020, 263, 114652. [Google Scholar] [CrossRef]
- Peltier, R.E.; Lippmann, M. Spatial and seasonal distribution of aerosol chemical components in New York City: (1) Incineration, coal combustion, and biomass burning. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 473–483. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Hoek, G.; Eeftens, M.; de Hoogh, K.; Beelen, R.; Beregszászi, T.; Cesaroni, G.; Cirach, M.; Cyrys, J.; De Nazelle, A.; et al. Spatial variation of PM elemental composition between and within 20 European study areas—Results of the ESCAPE project. Environ. Int. 2015, 84, 181–192. [Google Scholar] [CrossRef]
- Maciejczyk, P.; Chen, L.C. Effects of Subchronic Exposures to Concentrated Ambient Particles (CAPs) in Mice: VIII. Source-Related Daily Variations in In Vitro Responses to CAPs. Inhal. Toxicol. 2005, 17, 243–253. [Google Scholar] [CrossRef]
- Hernández-López, A.; Campo, J.; Mugica-Álvarez, V.; Hernández-Valle, B.L.; Mejía-Ponce, L.V.; Pineda-Santamaría, J.C.; Reynoso-Cruces, S.; Mendoza-Flores, J.; Rozanes-Valenzuela, D. A study of PM2.5 elemental composition in southwest Mexico City and development of receptor models with positive matrix factorization. Rev. Int. de Contam. Ambient. 2021, 37, 67–88. [Google Scholar] [CrossRef]
- Poulakis, E.; Theodosi, C.; Bressi, M.; Sciare, J.; Ghersi, V.; Mihalopoulos, N. Airborne mineral components and trace metals in Paris region: Spatial and temporal variability. Environ. Sci. Pollut. Res. 2015, 22, 14663–14672. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile for Vanadium; Agency for Toxic Substances and Disease Registry; Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012. [Google Scholar]
- Zou, Z.; Zhao, J.; Zhang, C.; Zhang, Y.; Yang, X.; Chen, J.; Xu, J.; Xue, R.; Zhou, B. Effects of cleaner ship fuels on air quality and implications for future policy: A case study of Chongming Ecological Island in China. J. Clean. Prod. 2020, 267, 122088. [Google Scholar] [CrossRef]
- Hopke, P.K.; Dai, Q.; Li, L.; Feng, Y. Global review of recent source apportionments for airborne particulate matter. Sci. Total Environ. 2020, 740, 140091. [Google Scholar] [CrossRef]
- Kim, E.; Hopke, P.K. Source Identifications of Airborne Fine Particles Using Positive Matrix Factorization and U.S. Environmental Protection Agency Positive Matrix Factorization. J. Air Waste Manag. Assoc. 2007, 57, 811–819. [Google Scholar] [CrossRef]
- Thurston, G.D.; Spengler, J.D. A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos. Environ. 1985, 19, 9–25. [Google Scholar] [CrossRef]
- Cooper, J.A.; Watson, J.G. Receptor Oriented Methods of Air Particulate Source Apportionment. J. Air Pollut. Control. Assoc. 1980, 30, 1116–1125. [Google Scholar] [CrossRef]
- Gordon, G.E. Receptor models. Environ. Sci. Technol. 1980, 14, 792–800. [Google Scholar] [CrossRef]
- Ondov, J.M.; Wexler, A.S. Where Do Particulate Toxins Reside? An Improved Paradigm for the Structure and Dynamics of the Urban Mid-Atlantic Aerosol. Environ. Sci. Technol. 1998, 32, 2547–2555. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Pacyna, J. Source inventories for atmospheric trace metals. In Atmospheric Particles; Harrison, R., Van Grieken, R., Eds.; Series on Analytical and Physical Chemistry of Environmental Systems; Wiley: Hoboken, NJ, USA, 1998; pp. 385–423. [Google Scholar]
- Galvão, E.S.; de Cassia Feroni, R.; D’Azeredo Orlando, M.T. A review of the main strategies used in the interpretation of similar chemical profiles yielded by receptor models in the source apportionment of particulate matter. Chemosphere 2021, 269, 128746. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Dai, Q.; Wu, J.; Zhang, Q.; Zhang, W.; Luo, R.; Cheng, Y.; Zhang, J.; Wang, L.; Yu, Z.; et al. Characteristics of the main primary source profiles of particulate matter across China from 1987 to 2017. Atmos. Chem. Phys. 2019, 19, 3223–3243. [Google Scholar] [CrossRef] [Green Version]
- Thurston, G.; Ito, K.; Lall, R.; Burnett, R.; Turner, M.; Krewski, D.; Shi, Y.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; et al. NPACT Study 4: Mortality and long-term exposure to PM2.5 and its components in the American Cancer Society’s CPS-II Cohort. In National Particle Component Toxicity (NPACT) Initiative Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components; Lippmann, M., Chen, L., Gordon, T., Ito, K., Thurston, G., Eds.; Health Effects Institute: Boston, MA, USA, 2013; pp. 127–166. [Google Scholar]
- Chow, J.C.; Watson, J.G. Review of PM2.5 and PM10 Apportionment for Fossil Fuel Combustion and Other Sources by the Chemical Mass Balance Receptor Model. Energy Fuels 2002, 16, 222–260. [Google Scholar] [CrossRef]
- Grieshop, A.P.; Lipsky, E.M.; Pekney, N.J.; Takahama, S.; Robinson, A.L. Fine particle emission factors from vehicles in a highway tunnel: Effects of fleet composition and season. Atmos. Environ. 2006, 40, 287–298. [Google Scholar] [CrossRef]
- Schauer, J.J.; Lough, G.C.; Shafer, M.M.; Christensen, W.F.; Arndt, M.F.; DeMinter, J.T.; Park, J.-S. Characterization of metals emitted from motor vehicles. Res. Rep. 2006, 133, 1–76, discussion 77–88. [Google Scholar]
- Geller, M.D.; Sardar, S.B.; Phuleria, H.; Fine, P.M.; Sioutas, C. Measurements of Particle Number and Mass Concentrations and Size Distributions in a Tunnel Environment. Environ. Sci. Technol. 2005, 39, 8653–8663. [Google Scholar] [CrossRef]
- Lough, G.C.; Schauer, J.J.; Park, J.-S.; Shafer, M.M.; DeMinter, J.T.; Weinstein, J.P. Emissions of Metals Associated with Motor Vehicle Roadways. Environ. Sci. Technol. 2005, 39, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.D.; Soares, D.; Serrano, L.M.V.; Walsh, R.P.D.; Dias-Ferreira, C.; Ferreira, C.S.S. Roads as sources of heavy metals in urban areas. The Covões catchment experiment, Coimbra, Portugal. J. Soils Sediments 2016, 16, 2622–2639. [Google Scholar] [CrossRef]
- Olmez, I.; Sheffield, A.E.; Gordon, G.E.; Houck, J.E.; Pritchett, L.C.; Cooper, J.A.; Dzubay, T.G.; Bennett, R.L. Compositions of Particles from Selected Sources in Philadelphia for Receptor Modeling Applications. JAPCA 1988, 38, 1392–1402. [Google Scholar] [CrossRef]
- Seidel, D.J.; Birnbaum, A.N. Effects of Independence Day fireworks on atmospheric concentrations of fine particulate matter in the United States. Atmos. Environ. 2015, 115, 192–198. [Google Scholar] [CrossRef]
- Hickey, C.; Gordon, C.; Galdanes, K.; Blaustein, M.; Horton, L.; Chillrud, S.; Ross, J.; Yinon, L.; Chen, L.C.; Gordon, T. Toxicity of particles emitted by fireworks. Part. Fibre Toxicol. 2020, 17, 28. [Google Scholar] [CrossRef]
- Thurston, G.D.; Ito, K.; Lall, R. A Source Apportionment of U.S. Fine Particulate Matter Air Pollution. Atmos. Environ. 2011, 45, 3924–3936. [Google Scholar] [CrossRef] [Green Version]
- Saraga, D.; Maggos, T.; Degrendele, C.; Klánová, J.; Horvat, M.; Kocman, D.; Kanduč, T.; Garcia Dos Santos, S.; Franco, R.; Gómez, P.M.; et al. Multi-city comparative PM2.5 source apportionment for fifteen sites in Europe: The ICARUS project. Sci. Total Environ. 2021, 751, 141855. [Google Scholar] [CrossRef]
- Deng, L.; Bi, C.; Jia, J.; Zeng, Y.; Chen, Z. Effects of heating activities in winter on characteristics of PM2.5-bound Pb, Cd and lead isotopes in cities of China. J. Clean. Prod. 2020, 265, 121826. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Achilleos, S.; Kioumourtzoglou, M.-A.; Wu, C.-D.; Schwartz, J.D.; Koutrakis, P.; Papatheodorou, S.I. Acute effects of fine particulate matter constituents on mortality: A systematic review and meta-regression analysis. Environ. Int. 2017, 109, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Brook, R.D.; Rajagopalan, S. Particulate Matter Air Pollution and Atherosclerosis. Curr. Atheroscler. Rep. 2010, 12, 291–300. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Integrated Science Assessment for Particulate Matter (Final Report); EPA/600/r-08/139f; U.S. Environmental Protection Agency: Washington, DC, USA, 2009. [Google Scholar]
- Yang, Y.; Ruan, Z.; Wang, X.; Yang, Y.; Mason, T.G.; Lin, H.; Tian, L. Short-term and long-term exposures to fine particulate matter constituents and health: A systematic review and meta-analysis. Environ. Pollut. 2019, 247, 874–882. [Google Scholar] [CrossRef] [PubMed]
- NRC. National Research Council: Research Priorities for Airborne Particulate Matter: III. Early Research Progress; National Research Council: Washington, DC, USA, 2001. [Google Scholar]
- Thurston, G.D.; Ito, K.; Mar, T.; Christensen, W.F.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Larson, T.V.; et al. Workgroup report: Workshop on source apportionment of particulate matter health effects—intercomparison of results and implications. Environ. Health Perspect. 2005, 113, 1768–1774. [Google Scholar] [CrossRef] [Green Version]
- Hopke, P.K.; Ito, K.; Mar, T.; Christensen, W.F.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Larson, T.V.; et al. PM source apportionment and health effects: 1. Intercomparison of source apportionment results. J. Expo. Sci. Environ. Epidemiol. 2005, 16, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Christensen, W.F.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Larson, T.V.; Neas, L.; Hopke, P.K.; et al. PM source apportionment and health effects: 2. An investigation of intermethod variability in associations between source-apportioned fine particle mass and daily mortality in Washington, DC. J. Expo. Sci. Environ. Epidemiol. 2005, 16, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Mar, T.F.; Ito, K.; Koenig, J.Q.; Larson, T.V.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Neas, L.; et al. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J. Expo. Sci. Environ. Epidemiol. 2005, 16, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Hedley, A.J.; Chau, P.Y.K.; Wong, C. The change in sub-species of particulate matter [PM10] before and after an intervention to restrict sulphur content of fuel in Hong Kong. In Proceedings of the Better Air Quality/Asian Development Bank, Agra, India, 6–8 December 2004. [Google Scholar]
- Hedley, A.J.; Wong, C.-M.; Thach, T.Q.; Ma, S.; Lam, T.-H.; Anderson, H.R. Cardiorespiratory and all-cause mortality after restrictions on sulphur content of fuel in Hong Kong: An intervention study. Lancet 2002, 360, 1646–1652. [Google Scholar] [CrossRef]
- Lippmann, M.; Ito, K.; Hwang, J.-S.; Maciejczyk, P.; Chen, L.-C. Cardiovascular effects of nickel in ambient air. Environ. Health Perspect. 2006, 114, 1662–1669. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Ebisu, K.; Zeger, S.L.; Samet, J.M.; Bell, M.L. Does the effect of PM10 on mortality depend on PM nickel and vanadium content? A reanalysis of the NMMAPS data. Environ. Health Perspect. 2007, 115, 1701–1703. [Google Scholar] [CrossRef] [Green Version]
- Laden, F.; Neas, L.M.; Dockery, D.W.; Schwartz, J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ. Health Perspect. 2000, 108, 941–947. [Google Scholar] [CrossRef]
- Burnett, R.T.; Brook, J.; Dann, T.; Delocla, C.; Philips, O.; Cakmak, S.; Vincent, R.; Goldberg, M.S.; Krewski, D. Association between Particulate- and Gas-Phase Components of Urban Air Pollution and Daily Mortality in Eight Canadian cities. Inhal. Toxicol. 2000, 12, 15–39. [Google Scholar] [CrossRef]
- Burnett, R.T.; Stieb, D.; Brook, J.R.; Cakmak, S.; Dales, R.; Raizenne, M.; Vincent, R.; Dann, T. Associations between Short-Term Changes in Nitrogen Dioxide and Mortality in Canadian Cities. Arch. Environ. Health Int. J. 2004, 59, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Malig, B.; Hasheminassab, S.; Berger, K.; Chang, E.; Sioutas, C. Associations of Source-Specific Fine Particulate Matter With Emergency Department Visits in California. Am. J. Epidemiol. 2016, 184, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Samet, J.M.; Dominici, F. Hospital admissions and chemical composition of fine particle air pollution. Am. J. Respir. Crit. Care Med. 2009, 179, 1115–1120. [Google Scholar] [CrossRef]
- Rahman, M.M.; Begum, B.A.; Hopke, P.K.; Nahar, K.; Newman, J.; Thurston, G.D. Cardiovascular morbidity and mortality associations with biomass and fossil-fuel combustion fine particulate-matter exposures in Dhaka, Bangladesh. Int. J. Epidemiol 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Thurston, G.; Ozkaynak, H.; Schatz, A. A chemical characterization and source apportionment of the IP network fine particle data. In Proceedings of the 77th Annual Meeting of the Air Pollution Control Association, San Francisco, CA, USA, 24–29 June 1984. [Google Scholar]
- Watson, J.; Chow, J.; Shah, J. Analysis of Inhalable and Fine Particulate Matter Measurements; Environmental Protection Agency: Washington, DC, USA, 1981. [Google Scholar]
- Thurston, G. Using source apportionment to determine the sources associated with PM health effects. In Proceedings of the 11th Annual Meeting of the International Society of Exposure Analysis, Charleston, NC, USA, 4–8 November 2001. [Google Scholar]
- Lipfert, F.W.; Baty, J.D.; Miller, J.P.; Wyzga, R.E. PM2.5 Constituents and Related Air Quality Variables As Predictors of Survival in a Cohort of U.S. Military Veterans. Inhal. Toxicol. 2006, 18, 645–657. [Google Scholar] [CrossRef]
- Chung, Y.; Dominici, F.; Wang, Y.; Coull, B.A.; Bell, M.L. Associations between long-term exposure to chemical constituents of fine particulate matter (PM2.5) and mortality in Medicare enrollees in the eastern United States. Environ. Health Perspect. 2015, 123, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Rodopoulou, S.; de Hoogh, K.; Strak, M.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Cesaroni, G.; et al. Long-Term Exposure to Fine Particle Elemental Components and Natural and Cause-Specific Mortality-a Pooled Analysis of Eight European Cohorts within the ELAPSE Project. Environ. Health Perspect. 2021, 129, 47009. [Google Scholar] [CrossRef]
- Zhang, Z.; Weichenthal, S.; Kwong, J.C.; Burnett, R.T.; Hatzopoulou, M.; Jerrett, M.; Van Donkelaar, A.; Bai, L.; Martin, R.V.; Copes, R.; et al. Long-term exposure to iron and copper in fine particulate air pollution and their combined impact on reactive oxygen species concentration in lung fluid: A population-based cohort study of cardiovascular disease incidence and mortality in Toronto, Canada. In Proceedings of the 32nd Annual Conference of the International Society for Environmental Epidemiology, Online, 14–17 August 2021. [Google Scholar] [CrossRef]
- Lim, C.C.; Hayes, R.B.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Thurston, G.D. Mediterranean Diet and the Association Between Air Pollution and Cardiovascular Disease Mortality Risk. Circulation 2019, 139, 1766–1775. [Google Scholar] [CrossRef]
- Vodonos, A.; Awad, Y.A.; Schwartz, J. The concentration-response between long-term PM2.5 exposure and mortality; A meta-regression approach. Environ. Res. 2018, 166, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the air: A review of the effects of particulate matter air pollution on human health. J. Med Toxicol. Off. J. Am. Coll. Med Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassee, F.R.; Héroux, M.-E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Citron, J.; Willcocks, E.; Crowley, G.; Kwon, S.; Nolan, A. Genomics of Particulate Matter Exposure Associated Cardiopulmonary Disease: A Narrative Review. Int. J. Environ. Res. Public Health 2019, 16, 4335. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Ripley, S.; Weichenthal, S.; Godri Pollitt, K.J. Ambient particulate matter oxidative potential: Chemical determinants, associated health effects, and strategies for risk management. Free. Radic. Biol. Med. 2020, 151, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of Air Pollution Particles and Oxidative Stress in Cells, Tissues, and Living Systems. J. Toxicol. Environ. Health Part B 2012, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Danielsen, P.H.; Karottki, D.G.; Jantzen, K.; Roursgaard, M.; Klingberg, H.; Jensen, D.M.; Christophersen, D.V.; Hemmingsen, J.G.; Cao, Y.; et al. Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res./Rev. Mutat. Res. 2014, 762, 133–166. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, P.; Zhong, M.; Lippmann, M.; Chen, L.-C. Oxidant generation capacity of source-apportioned PM2.5. Inhal. Toxicol. 2010, 22, 29–36. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A. Environmental Stressors and Their Impact on Health and Disease with Focus on Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 735–740. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Fang, T.; Guo, H.; Zeng, L.; Verma, V.; Nenes, A.; Weber, R.J. Highly Acidic Ambient Particles, Soluble Metals, and Oxidative Potential: A Link between Sulfate and Aerosol Toxicity. Environ. Sci. Technol. 2017, 51, 2611–2620. [Google Scholar] [CrossRef]
- Verma, V.; Fang, T.; Xu, L.; Peltier, R.E.; Russell, A.G.; Ng, N.L.; Weber, R.J. Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM2.5. Environ. Sci. Technol. 2015, 49, 4646–4656. [Google Scholar] [CrossRef]
- Gao, D.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization of water-insoluble oxidative potential of PM2.5 using the dithiothreitol assay. Atmos. Environ. 2020, 224, 117327. [Google Scholar] [CrossRef]
- Shi, T.; Schins, R.P.F.; Knaapen, A.M.; Kuhlbusch, T.; Pitz, M.; Heinrich, J.; Borm, P.J.A. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J. Environ. Monit. 2003, 5, 550. [Google Scholar] [CrossRef]
- Park, J.; Park, E.H.; Schauer, J.J.; Yi, S.-M.; Heo, J. Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ. Int. 2018, 117, 276–283. [Google Scholar] [CrossRef]
- Forti, E.; Salovaara, S.; Cetin, Y.; Bulgheroni, A.; Tessadri, R.; Jennings, P.; Pfaller, W.; Prieto, P. In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicol. In Vitro 2011, 25, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fantel, A.G. Reactive oxygen species in developmental toxicity: Review and hypothesis. Teratology 1996, 53, 196–217. [Google Scholar] [CrossRef]
- Goldstein, S.; Meyerstein, D.; Czapski, G. The Fenton reagents. Free Radic. Biol. Med. 1993, 15, 435–445. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bertoli, I.; Manarini, F.; Russo, M. Ascorbate assay as a measure of oxidative potential for ambient particles: Evidence for the importance of cell-free surrogate lung fluid composition. Atmos. Environ. 2019, 211, 103–112. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Sedwick, P.N.; Church, T.M.; Baker, A.R.; Powell, C.F. Fractional solubility of aerosol iron: Synthesis of a global-scale data set. Geochim. Cosmochim. Acta 2012, 89, 173–189. [Google Scholar] [CrossRef]
- Costa, D.L.; Dreher, K.L. Bioavailable Transition Metals in Particulate Matter Mediate Cardiopulmonary Injury in Healthy and Compromised Animal Models. Environ. Health Perspect. 1997, 105, 1053–1060. [Google Scholar] [CrossRef]
- Gavett, S.H.; Madison, S.L.; Dreher, K.L.; Winsett, D.W.; McGee, J.K.; Costa, D.L. Metal and Sulfate Composition of Residual Oil Fly Ash Determines Airway Hyperreactivity and Lung Injury in Rats. Environ. Res. 1997, 72, 162–172. [Google Scholar] [CrossRef]
- Dreher, K.L.; Jaskot, R.H.; Lehmann, J.R.; Richards, J.H.; McGee, J.K.; Ghio, A.J.; Costa, D.L. Soluble transition metals mediate residual oil fly ash induced acute lung injury. J. Toxicol. Env. Health 1997, 50, 285–305. [Google Scholar]
- Schwartz, J.; Lepeule, J. Is Ambient PM 2.5 Sulfate Harmful? Schwartz and Lepeule Respond. Environ. Health Perspect. 2012, 120, a454–a455. [Google Scholar] [CrossRef]
- Oakes, M.; Ingall, E.D.; Lai, B.; Shafer, M.M.; Hays, M.D.; Liu, Z.G.; Russell, A.G.; Weber, R.J. Iron Solubility Related to Particle Sulfur Content in Source Emission and Ambient Fine Particles. Environ. Sci. Technol. 2012, 46, 6637–6644. [Google Scholar] [CrossRef]
- Shahpoury, P.; Zhang, Z.W.; Arangio, A.; Celo, V.; Dabek-Zlotorzynska, E.; Harner, T.; Nenes, A. The influence of chemical composition, aerosol acidity, and metal dissolution on the oxidative potential of fine particulate matter and redox potential of the lung lining fluid. Environ. Int. 2021, 148, 106343. [Google Scholar] [CrossRef]
- Brehmer, C.; Lai, A.; Clark, S.; Shan, M.; Ni, K.; Ezzati, M.; Yang, X.; Baumgartner, J.; Schauer, J.J.; Carter, E. The Oxidative Potential of Personal and Household PM2.5 in a Rural Setting in Southwestern China. Environ. Sci. Technol. 2019, 53, 2788–2798. [Google Scholar] [CrossRef]
- Lippmann, M.; Thurston, G.D. Sulfate concentrations as an indicator of ambient particulate matter air pollution for health risk evaluations. J. Expo. Anal. Environ. Epidemiol. 1996, 6, 123–146. [Google Scholar]
- Amdur, M.O.; Chen, L.C. Furnace-generated acid aerosols: Speciation and pulmonary effects. Environ. Health Perspect. 1989, 79, 147–150. [Google Scholar] [CrossRef]
- Lepeule, J.; Laden, F.; Dockery, D.; Schwartz, J. Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ. Health Perspect. 2012, 120, 965–970. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 9321–9333. [Google Scholar] [CrossRef] [Green Version]
- Shirai, T.; Yasueda, H.; Saito, A.; Taniguchi, M.; Akiyama, K.; Tsuchiya, T.; Suda, T.; Chida, K. Effect of Exposure and Sensitization to Indoor Allergens on Asthma Control Level. Allergol. Int. 2012, 61, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Wei, J.; Cheng, Y.; Subedi, K.; Verma, V. Synergistic and Antagonistic Interactions among the Particulate Matter Components in Generating Reactive Oxygen Species Based on the Dithiothreitol Assay. Environ. Sci. Technol. 2018, 52, 2261–2270. [Google Scholar] [CrossRef]
- Gao, D.; Godri Pollitt, K.J.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization and comparison of PM2.5 oxidative potential assessed by two acellular assays. Atmos. Chem. Phys. 2020, 20, 5197–5210. [Google Scholar] [CrossRef]
- Verma, V.; Fang, T.; Guo, H.; King, L.; Bates, J.T.; Peltier, R.E.; Edgerton, E.; Russell, A.G.; Weber, R.J. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: Spatiotemporal trends and source apportionment. Atmos. Chem. Phys. 2014, 14, 12915–12930. [Google Scholar] [CrossRef] [Green Version]
- Slawsky, E.; Ward-Caviness, C.K.; Neas, L.; Devlin, R.B.; Cascio, W.E.; Russell, A.G.; Huang, R.; Kraus, W.E.; Hauser, E.; Diaz-Sanchez, D.; et al. Evaluation of PM2.5 air pollution sources and cardiovascular health. Environ. Epidemiol. 2021, 5, e157. [Google Scholar] [CrossRef]
- Briedé, J.J.; de Kok, T.M.C.M.; Hogervorst, J.G.F.; Moonen, E.J.C.; op den Camp, C.L.B.; Kleinjans, J.C.S. Development and Application of an Electron Spin Resonance Spectrometry Method for the Determination of Oxygen Free Radical Formation by Particulate Matter. Environ. Sci. Technol. 2005, 39, 8420–8426. [Google Scholar] [CrossRef]
- Yang, A.; Janssen, N.A.H.; Brunekreef, B.; Cassee, F.R.; Hoek, G.; Gehring, U. Children’s respiratory health and oxidative potential of PM2.5: The PIAMA birth cohort study. Occup. Environ. Med. 2016, 73, 154–160. [Google Scholar] [CrossRef]
- Taghvaee, S.; Sowlat, M.H.; Diapouli, E.; Manousakas, M.I.; Vasilatou, V.; Eleftheriadis, K.; Sioutas, C. Source apportionment of the oxidative potential of fine ambient particulate matter (PM(2.5)) in Athens, Greece. Sci. Total Environ. 2019, 653, 1407–1416. [Google Scholar] [CrossRef]
- Guan, L.; Rui, W.; Bai, R.; Zhang, W.; Zhang, F.; Ding, W. Effects of Size-Fractionated Particulate Matter on Cellular Oxidant Radical Generation in Human Bronchial Epithelial BEAS-2B Cells. Int. J. Environ. Res. Public Health 2016, 13, 483. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Hong, Z.; Dong, W.; Deng, C.; Zhao, R.; Xu, J.; Zhuang, G.; Zhang, R. PM(2.5)-Induced Oxidative Stress and Mitochondrial Damage in the Nasal Mucosa of Rats. Int. J. Environ. Res. Public Health 2017, 14, 134. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Y.; Su, S.; Wang, B.; Zhu, X.; Wang, X.; Zeng, E.Y.; Xing, B.; Tao, S. Seasonal and spatial variations in the chemical components and the cellular effects of particulate matter collected in Northern China. Sci. Total Environ. 2018, 627, 1627–1637. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A.; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-C.; Lippmann, M. NPACT Study 1. Subchronic Inhalation Exposure of Mice to Concentrated Ambient PM 2.5 from Five Airsheds. In National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components; Lippmann, M., Chen, L.-C., Gordon, T., Ito, K., Thurston, G.D., Eds.; Health Effects Institute: Boston, MA, USA, 2013; pp. 15–53. [Google Scholar]
- Sun, Q.; Yue, P.; Deiuliis, J.A.; Lumeng, C.N.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.C.; Lu, B.; Parthasarathy, S.; et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009, 119, 538–546. [Google Scholar] [CrossRef] [Green Version]
- Knuckles, T.L.; Jaskot, R.; Richards, J.H.; Miller, C.A.; Ledbetter, A.; McGee, J.; Linak, W.P.; Dreher, K.L. Biokinetically-Based In Vitro Cardiotoxicity of Residual Oil Fly Ash: Hazard Identification and Mechanisms of Injury. Cardiovasc. Toxicol. 2013, 13, 426–437. [Google Scholar] [CrossRef]
- Ku, T.; Zhang, Y.; Ji, X.; Li, G.; Sang, N. PM2.5-bound metal metabolic distribution and coupled lipid abnormality at different developmental windows. Environ. Pollut. 2017, 228, 354–362. [Google Scholar] [CrossRef]
- León-Mejía, G.; Machado, M.N.; Okuro, R.T.; Silva, L.F.O.; Telles, C.; Dias, J.; Niekraszewicz, L.; Da Silva, J.; Henriques, J.A.P.; Zin, W.A. Intratracheal instillation of coal and coal fly ash particles in mice induces DNA damage and translocation of metals to extrapulmonary tissues. Sci. Total Environ. 2018, 625, 589–599. [Google Scholar] [CrossRef]
- Potter, N.A.; Meltzer, G.Y.; Avenbuan, O.N.; Raja, A.; Zelikoff, J.T. Particulate Matter and Associated Metals: A Link with Neurotoxicity and Mental Health. Atmosphere 2021, 12, 425. [Google Scholar] [CrossRef]
- Cory-Slechta, D.; Sobolewski, M.; Oberdörster, G. Air Pollution-Related Brain Metal Dyshomeostasis as a Potential Risk Factor for Neurodevelopmental Disorders and Neurodegenerative Diseases. Atmosphere 2020, 11, 1098. [Google Scholar] [CrossRef]
- Klocke, C.; Sherina, V.; Graham, U.M.; Gunderson, J.; Allen, J.L.; Sobolewski, M.; Blum, J.L.; Zelikoff, J.T.; Cory-Slechta, D.A. Enhanced cerebellar myelination with concomitant iron elevation and ultrastructural irregularities following prenatal exposure to ambient particulate matter in the mouse. Inhal. Toxicol. 2018, 30, 381–396. [Google Scholar] [CrossRef]
- USEPA. Air Quality Criteria for Particulate Matter (Final Report, 2004); EPA 600/P-99/002aF-bF; Environmental Protection Agency: Washington, DC, USA, 2004. [Google Scholar]
- Gunnison, A.; Chen, L.C. Effects of Subchronic Exposures to Concentrated Ambient Particles in Mice: VI. Gene Expression in Heart and Lung Tissue. Inhal. Toxicol. 2005, 17, 225–233. [Google Scholar] [CrossRef]
- Lippmann, M.; Gordon, T.; Chen, L.C. Effects of Subchronic Exposures to Concentrated Ambient Particles (CAPs) in Mice: I. Introduction, Objectives, and Experimental Plan. Inhal. Toxicol. 2005, 17, 177–187. [Google Scholar] [CrossRef]
- Lippmann, M.; Gordon, T.; Chen, L.C. Effects of Subchronic Exposures to Concentrated Ambient Particles in Mice: IX. Integral Assessment and Human Health Implications of Subchronic Exposures of Mice to CAPs. Inhal. Toxicol. 2005, 17, 255–261. [Google Scholar] [CrossRef]
- Lippmann, M.; Hwang, J.-S.; Maciejczyk, P.; Chen, L.-C. PM source apportionment for short-term cardiac function changes in ApoE-/- mice. Environ. Health Perspect. 2005, 113, 1575–1579. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, B.; Makwana, O.; Pooler, M.; Chen, L.C. Effects of Subchronic Exposures to Concentrated Ambient Particles: VII. Degeneration of Dopaminergic Neurons in Apo E−/−Mice. Inhal. Toxicol. 2005, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, U.P.; Schladweiler, M.C.; Ledbetter, A.D.; McGee, J.K.; Walsh, L.; Gilmour, P.S.; Highfill, J.W.; Davies, D.; Pinkerton, K.E.; Richards, J.H.; et al. Consistent pulmonary and systemic responses from inhalation of fine concentrated ambient particles: Roles of rat strains used and physicochemical properties. Environ. Health Perspect. 2005, 113, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Xie, J.; Wong, C.K.C.; Chan, S.K.Y.; Abbaszade, G.; Schnelle-Kreis, J.; Zimmermann, R.; Li, J.; Zhang, G.; Fu, P.; et al. Contributions of City-Specific Fine Particulate Matter (PM2.5) to Differential In Vitro Oxidative Stress and Toxicity Implications between Beijing and Guangzhou of China. Environ. Sci. Technol. 2019, 53, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, X.; Qiu, X.; Jiang, X.; Fang, Y.; Wang, J.; Hu, D.; Zhu, T. Investigation of the chemical components of ambient fine particulate matter (PM2.5) associated with in vitro cellular responses to oxidative stress and inflammation. Environ. Int. 2020, 136, 105475. [Google Scholar] [CrossRef]
- Happo, M.S.; Salonen, R.O.; Hälinen, A.I.; Jalava, P.I.; Pennanen, A.S.; Dormans, J.A.M.A.; Gerlofs-Nijland, M.E.; Cassee, F.R.; Kosma, V.M.; Sillanpää, M.; et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhal. Toxicol. 2010, 22, 402–416. [Google Scholar] [CrossRef]
- Mirowsky, J.; Hickey, C.; Horton, L.; Blaustein, M.; Galdanes, K.; Peltier, R.E.; Chillrud, S.; Chen, L.C.; Ross, J.; Nadas, A.; et al. The effect of particle size, location and season on the toxicity of urban and rural particulate matter. Inhal. Toxicol. 2013, 25, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Mirowsky, J.E.; Jin, L.; Thurston, G.; Lighthall, D.; Tyner, T.; Horton, L.; Galdanes, K.; Chillrud, S.; Ross, J.; Pinkerton, K.E.; et al. In vitro and in vivo toxicity of urban and rural particulate matter from California. Atmos. Environ. 2015, 103, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Thomson, E.M.; Breznan, D.; Karthikeyan, S.; MacKinnon-Roy, C.; Charland, J.-P.; Dabek-Zlotorzynska, E.; Celo, V.; Kumarathasan, P.; Brook, J.R.; Vincent, R. Cytotoxic and inflammatory potential of size-fractionated particulate matter collected repeatedly within a small urban area. Part. Fibre Toxicol. 2015, 12, 24. [Google Scholar] [CrossRef] [Green Version]
- Thomson, E.M.; Breznan, D.; Karthikeyan, S.; MacKinnon-Roy, C.; Vuong, N.Q.; Dabek-Zlotorzynska, E.; Celo, V.; Charland, J.-P.; Kumarathasan, P.; Brook, J.R.; et al. Contrasting biological potency of particulate matter collected at sites impacted by distinct industrial sources. Part. Fibre Toxicol. 2016, 13, 65. [Google Scholar] [CrossRef] [Green Version]
- Van Den Heuvel, R.; Den Hond, E.; Govarts, E.; Colles, A.; Koppen, G.; Staelens, J.; Mampaey, M.; Janssen, N.; Schoeters, G. Identification of PM 10 characteristics involved in cellular responses in human bronchial epithelial cells (Beas-2B). Environ. Res. 2016, 149, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ouyang, W.; Shu, Y.; Tian, Y.; Feng, Y.; Zhang, T.; Chen, W. Incorporating bioaccessibility into health risk assessment of heavy metals in particulate matter originated from different sources of atmospheric pollution. Environ. Pollut. 2019, 254. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Ge, X.; Nie, D.; Wang, M.; Zhou, H.; Chen, M. In vitro toxicity evaluation of heavy metals in urban air particulate matter on human lung epithelial cells. Sci. Total Environ. 2019, 678, 301–308. [Google Scholar] [CrossRef]
- Cuevas, A.K.; Niu, J.; Zhong, M.; Liberda, E.N.; Ghio, A.; Qu, Q.; Chen, L.C. Metal rich particulate matter impairs acetylcholine-mediated vasorelaxation of microvessels in mice. Part. Fibre Toxicol. 2015, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Rao, X.; Wang, T.-Y.; Jiang, S.Y.; Ying, Z.; Liu, C.; Wang, A.; Zhong, M.; Deiuliis, J.A.; Maiseyeu, A.; et al. Effect of co-exposure to nickel and particulate matter on insulin resistance and mitochondrial dysfunction in a mouse model. Part. Fibre Toxicol. 2012, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ying, Z.; Xu, X.; Chen, M.; Liu, D.; Zhong, M.; Chen, L.-c.; Sun, Q.; Rajagopalan, S. A synergistic vascular effect of airborne particulate matter and nickel in a mouse model. Toxicol. Sci. Off. J. Soc. Toxicol. 2013, 135, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Pardo, M.; Porat, Z.; Rudich, A.; Schauer, J.J.; Rudich, Y. Repeated exposures to roadside particulate matter extracts suppresses pulmonary defense mechanisms, resulting in lipid and protein oxidative damage. Environ. Pollut. 2016, 210, 227–237. [Google Scholar] [CrossRef]
- Pardo, M.; Shafer, M.M.; Rudich, A.; Schauer, J.J.; Rudich, Y. Single Exposure to near Roadway Particulate Matter Leads to Confined Inflammatory and Defense Responses: Possible Role of Metals. Environ. Sci. Technol. 2015, 49, 8777–8785. [Google Scholar] [CrossRef]
- Li, H.; Wan, Y.; Chen, X.; Cheng, L.; Yang, X.; Xia, W.; Xu, S.; Zhang, H. A multiregional survey of nickel in outdoor air particulate matter in China: Implication for human exposure. Chemosphere 2018, 199, 702–708. [Google Scholar] [CrossRef]
- Chen, Y.; Graziano, J.H.; Parvez, F.; Liu, M.; Slavkovich, V.; Kalra, T.; Argos, M.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 2011, 342, d2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope Iii, C.A. Lung Cancer, Cardiopulmonary Mortality, and Long-term Exposure to Fine Particulate Air Pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.C.; Lam, H.F.; Kim, E.J.; Guty, J.; Amdur, M.O. Pulmonary effects of ultrafine coal fly ash inhaled by guinea pigs. J. Toxicol. Environ. Health 1990, 29, 169–184. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, X.; Feng, J.; Fan, Q.; Zhang, Y.; Yang, X. Influence of Ship Emissions on Urban Air Quality: A Comprehensive Study Using Highly Time-Resolved Online Measurements and Numerical Simulation in Shanghai. Environ. Sci. Technol. 2016, 51, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Lin, Y.; Pan, J.; Zhang, Y.; Louie, P.K.K.; Li, M.; Fu, Q. Atmospheric pollution from ships and its impact on local air quality at a port site in Shanghai. Atmos. Chem. Phys. 2019, 19, 6315–6330. [Google Scholar] [CrossRef] [Green Version]
- Peltier, R.E.; Lippmann, M. Residual oil combustion: 2. Distributions of airborne nickel and vanadium within New York City. J. Expo. Sci. Environ. Epidemiol. 2009, 20, 342–350. [Google Scholar] [CrossRef]
- HEI. Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects; HEI Special Report 17; Health Effects Institute: Boston, MA, USA, 2010. [Google Scholar]
- McMurry, P.H. A review of atmospheric aerosol measurements. Atmos. Environ. 2000, 34, 1959–1999. [Google Scholar] [CrossRef]


| Pollutant | PM Emissions (lb/MMBtu) | PM Emissions as % of PM Mass | ||||
|---|---|---|---|---|---|---|
| Natural Gas | Distillate Oil | Residual Oil | Natural Gas | Distillate Oil | Residual Oil | |
| Antimony | 3.5 × 10−5 | 0.070 | ||||
| Arsenic | 2.0 × 10−7 | 4.0 × 10−6 | 8.8 × 10−6 | 0.003 | 0.017 | 0.018 |
| Barium | 4.3 × 10−6 | 1.7 × 10−5 | 0.058 | 0.034 | ||
| Beryllium | <1.2 × 10−8 | 3.0 × 10−6 | 1.9 × 10−7 | 0.013 | 0.001 | |
| Cadmium | 1.1 × 10−6 | 3.0 × 10−6 | 2.7 × 10−6 | 0.014 | 0.013 | 0.005 |
| Chloride | 2.3 × 10−3 | 4.641 | ||||
| Chromium | 1.4 × 10−6 | 3.0 × 10−6 | 5.6 × 10−6 | 0.018 | 0.013 | 0.011 |
| Cobalt | 8.2 × 10−8 | 4.0 × 10−5 | 0.001 | 0.081 | ||
| Copper | 8.3 × 10−7 | 6.0 × 10−6 | 1.2 × 10−5 | 0.011 | 0.025 | 0.024 |
| Fluoride | 2.5 × 10−4 | 0.499 | ||||
| Lead | 4.9 × 10−7 | 9.0 × 10−6 | 1.0 × 10−5 | 0.007 | 0.038 | 0.020 |
| Manganese | 3.7 × 10−7 | 6.0 × 10−6 | 2.0 × 10−5 | 0.005 | 0.025 | 0.040 |
| Mercury | 2.5 × 10−7 | 3.0 × 10−6 | 7.5 × 10−7 | 0.003 | 0.013 | 0.002 |
| Molybdenum | 1.1 × 10−6 | 5.2 × 10−6 | 0.014 | 0.011 | ||
| Nickel | 2.1 × 10−6 | 3.0 × 10−6 | 5.6 × 10−4 | 0.028 | 0.013 | 1.130 |
| Phosphorus | 6.3 × 10−5 | 0.127 | ||||
| Selenium | <2.4 × 10−8 | 15.0 × 10−6 | 4.6 × 10−6 | 0.063 | 0.009 | |
| Vanadium | 2.3 × 10−6 | 2.1 × 10−4 | 0.030 | 0.425 | ||
| Zinc | 2.8 × 10−5 | 4.0 × 10−6 | 1.9 × 10−4 | 0.382 | 0.017 | 0.389 |
| Total PM | 7.5 × 10−3 | 2.4 × 10−2 | 5.0 × 10−2 | |||
| Metal | European Union | North America | Asia | Central and South America | Africa |
|---|---|---|---|---|---|
| Cd | 0.05–2.9 rural [29] 0.1–14.1 urban [29] | Less than 0.2 US [30] 6 Los Angeles [31] | 2.3–55 China [32] 0.1–8.4 Beijing [33] 0.2 Nagasaki [34] | 0.1–15.1 Sao Paulo [35] 0.05 Bogota [36] 0.03–13 Mexico City [37] 1–8 Huancayo, Peru [38] | 5 Harare, Zimbabwe [39] |
| Pb | 1–115 rural [29] 1–330 urban [29] | 32 US [40] 5 Los Angeles [31] 0.8–17 Houston [41] | 100–1100 China [32] 6–715 Beijing [33] 7.8 Nagasaki [34] 1.3–12.6 SE Tibetan Plateau [42] 37 Mumbai [43] | 3–172 Sao Paulo [35] 1.6 Bogota [36] 1.1–125 Mexico City [37] 5–153 Huancayo, Peru [38] | 185 Harare, Zimbabwe [39] 6–31 Accra, Ghana [44] 22 Nairobi, Kenya [45] 30–180 Vaal Triangle, South Africa [46] |
| As | 0.06–3.3 rural [29] 0.1–30.2 urban [29] | 1–3 US rural [47] 20–100 US urban [47] 0.46 Toronto [48] 0.85 New York City [49] | Beijing 0.5–27 [33] 0.4–15.7 SE Tibetan Plateau [42] 1.4 Nagasaki [34] 13.7 Mumbai [43] | 0.06–7.1 Sao Paulo [35] 0.12 Bogota [36] 2–14 Huancayo, Peru [38] | |
| Fe | 67–283 [50]: 92 Northern 119 West/Centra l224 South | 26 rural New York [51] 4–488 NYC [49] 190 Los Angeles [31] 36–1270 Houston [41] 42 Toronto [48] | 81–4300 Beijing [33] 30–940 SE Tibetan Plateau [42] 90 Nagasaki [34] 316 Mumbai [43] | 140–2056 Sao Paulo [35] 124 Bogota [36] 350 Mexico City [52] 932–5064 Huancayo, Peru [38] | 346–539 Accra, Ghana [44] 530 Nairobi, Kenya [45] 90–22870 Vaal Triangle, South Africa [46] |
| Ni | 0.6–4.4 [50]: 1.1 Northern 0.9 West/Central 3.1 South | 3 NYC summer [49] 9.8 NYC winter [49] 3 Los Angeles [31] 0.3–5.7 Houston [41] 0.17 Toronto [48] | 6–127 China [32] 0.7–257 Beijing [33] 0.4–5.1 SE Tibetan Plateau [42] 1.3 Nagasaki [34] 6.7 Mumbai [43] | 2.3–16.1 Sao Paulo [35] 0.49 Bogota [36] 1.2–114 Mexico City [37] 4–22 Huancayo, Peru [38] | 180 Harare, Zimbabwe [39] 3–4 Accra, Ghana [44] 4 Nairobi, Kenya [45] 10–2470 Vaal Triangle, South Africa [46] |
| Cu | 1.8–14.2 [50]: 3.2 Northern 5.3 West/Central 10.1 South | 3 rural New York [51] 6.3 NYC [49] 10 Los Angeles [31] 0.5–36 Houston [41] 2.57 Toronto [48] | 31–231 China [32] 2.5–80 Beijing [33] 6.7–73.2 SE Tibetan Plateau [42] 2.3 Nagasaki [34] 6.7 Mumbai [43] | 7–390 Sao Paulo [35] 19.4 Bogota [36] 0.64–5181 Mexico City [37] 12–77 Huancayo, Peru [38] | 2–6 Accra, Ghana [44] 11 Nairobi, Kenya [45] 10–240 Vaal Triangle, South Africa [46] |
| Zn | 11.7–57.8 [50]: 13.4 Northern 26.5 West/Central 34.9 South | 6 rural New York [51] 33 NYC [49] 10 Los Angeles [31] 0.6–53 Houston [41] 9.1 Toronto [48] | 13–748 Beijing [33] 0.8–88.3 SE Tibetan Plateau [42] 26 Nagasaki [34] 61 Mumbai [43] | <DL-673 Sao Paulo [35] 13 Bogota [36] 58 Mexico City [52] 145–719 Huancayo, Peru [38] | 15–45 Accra, Ghana [44] 91 Nairobi, Kenya [45] 110–690 Vaal Triangle, South Africa [46] |
| Mn | 2.5–5.6 Paris [53] | 2 rural New York [51] 3.1–4.3 NYC [49] 12 Los Angeles [31] 0.8–21 Houston [41] 1.68 Toronto [48] | 3–223 Beijing [33] 1.9–21.5 SE Tibetan Plateau [42] 5.6 Nagasaki [34] 10.5 Mumbai [43] | <DL-64 Sao Paulo [35] 1.9 Bogota [36] 1.5–567 Mexico City [37] 16–182 Huancayo, Peru [38] | 7–9 Accra, Ghana [44] 41 Nairobi, Kenya [45] <DL-120 Vaal Triangle, South Africa [46] |
| V | 0.7–9.7 [50]: 1.9 Northern 1.3 West/Central 6.1 South | US mean 11 [54] 3 rural New York [51] 14.5 NYC [49] 1 Los Angeles [31] | 5.69 Shanghai [55] 0.4–19 Beijing [33] 2.6 Nagasaki [34] 13.1 Mumbai [43] | 0.2 Bogota [36] 0.09–761 Mexico City [37] 1–15 Huancayo, Peru [38] | 1 Accra, Ghana [44] 10–50 Vaal Triangle, South Africa [46] |
| Source Category | Types of Sources | Element Markers |
|---|---|---|
| Coal combustion | Coal-fired power plants, coal-fired industrial boilers, and residential combustion | Crustal materials (Na, Ca, Mg, Al, Fe, Cl), OC, EC, sulfate, and trace metals (As, Se, Cd, Cr, Cu, Ni, Pb, Hg, Zn) [64,65,66] |
| Oil combustion | Power plants, industrial boilers, maritime/shipping industry | Ni and V [58,67] |
| Industrial emissions | Industrial, mining, and manufacturing processes | Highly dependent on location; EC,OC, Ca, S, Mn, Fe, Cr, Zn, Pb [64] |
| Traffic | (1) Vehicle tailpipe | Ca, Mn, Fe, Cu, Ni, Zn, Cr, and Ba. Specifically: Ca, Cu, and Ni for gasoline-fueled vehicles [68,69], Ti and Ba for diesel-fueled vehicles [70,71] |
| (2) Vehicle non-tailpipe sources (i.e., tire abrasion, brake and engine wear) | Zn (brake and tire wear, motor oil), Cu (brake wear), Pb (brake, oil, additives, gasoline), Cd (tire wear), Ni (brake wear), Cr (brake and tire wear) [72] | |
| (3) Dust resuspension from the roadway | Cd, Pb (as legacy soil contamination from Pb-gasoline days), Cu, Ba, and Sb (from brake wear), as well as Ca and Zn from lubricating oil [64] | |
| Fugitive dust | Crustal/soil and road dust resuspension | Si, Al, Ca, Fe, Na, Mg, Ti |
| Biomass burning | Residential wood combustion other types of solid fuels combustion, wildfires, burning of agricultural residues | K, OC, EC, and organic markers |
| Waste incineration | Municipal waste incineration | Pb, Zn [73] |
| Fireworks | Fireworks | Pb, Ti, Sr, Ba, Cu [74,75] |
| Emission Source Class | Mean US PM2.5 (µg/m3) | Percent Mortality Effect Estimate (%) |
|---|---|---|
| Soil | 4.4 | 0.4 |
| Auto emissions | 2.9 | 0.6 |
| Oil combustion | 3.8 | 0.6 |
| Metals (iron/steel) | 1.1 | 1.2 a |
| Coal burning | 11.0 | 7.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciejczyk, P.; Chen, L.-C.; Thurston, G. The Role of Fossil Fuel Combustion Metals in PM2.5 Air Pollution Health Associations. Atmosphere 2021, 12, 1086. https://doi.org/10.3390/atmos12091086
Maciejczyk P, Chen L-C, Thurston G. The Role of Fossil Fuel Combustion Metals in PM2.5 Air Pollution Health Associations. Atmosphere. 2021; 12(9):1086. https://doi.org/10.3390/atmos12091086
Chicago/Turabian StyleMaciejczyk, Polina, Lung-Chi Chen, and George Thurston. 2021. "The Role of Fossil Fuel Combustion Metals in PM2.5 Air Pollution Health Associations" Atmosphere 12, no. 9: 1086. https://doi.org/10.3390/atmos12091086
APA StyleMaciejczyk, P., Chen, L.-C., & Thurston, G. (2021). The Role of Fossil Fuel Combustion Metals in PM2.5 Air Pollution Health Associations. Atmosphere, 12(9), 1086. https://doi.org/10.3390/atmos12091086





