Occurrence of Volatile and Semi-Volatile Organic Pollutants in the Russian Arctic Atmosphere: The International Siberian Shelf Study Expedition (ISSS-2020)
Abstract
1. Introduction
2. Materials and Methods
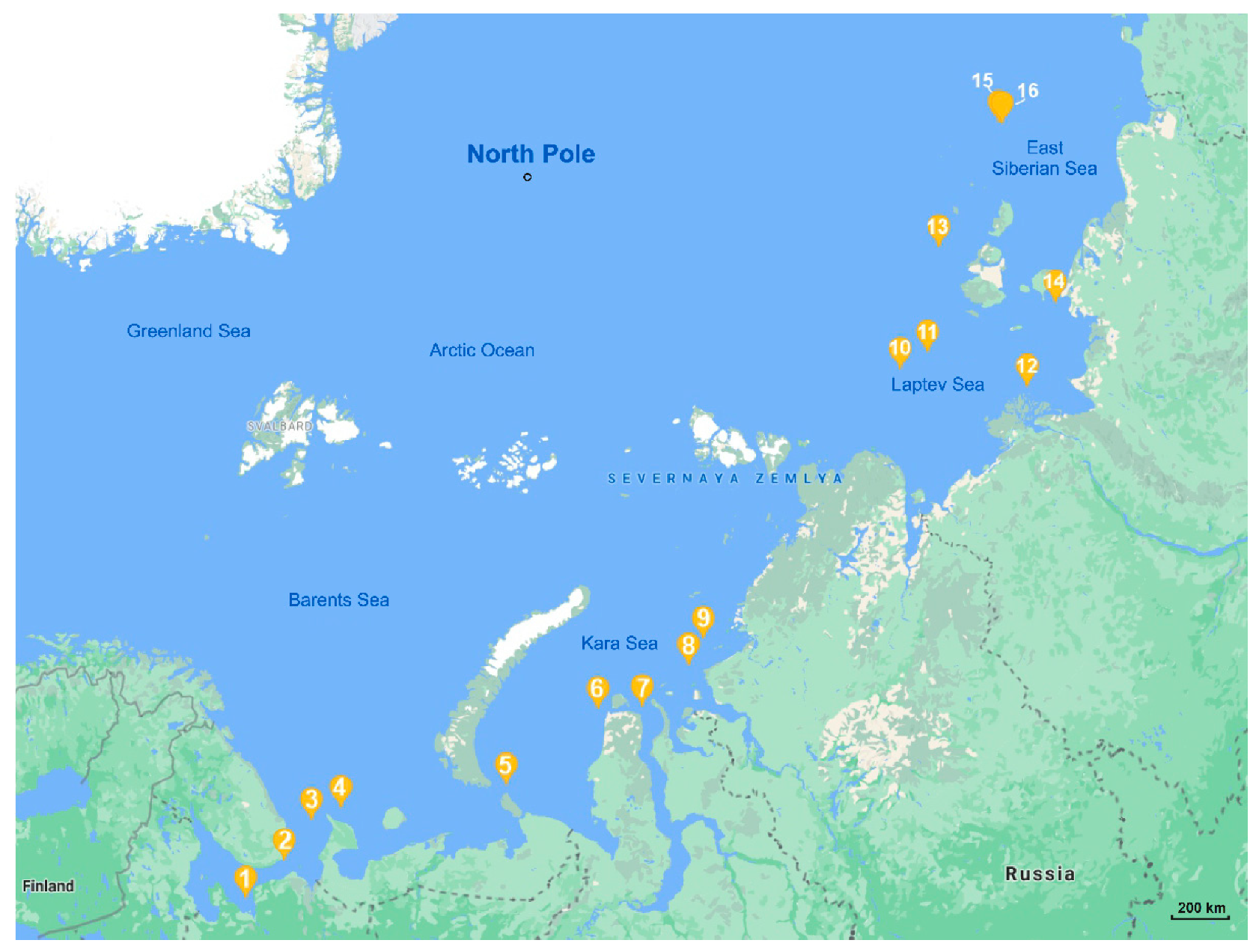
2.1. Air Samplng
2.2. TD-GC-HRMS Analysis
2.3. Analytes and Their Quantification
3. Results and Discussion
3.1. Method Sensitivity and General Description of the Samples
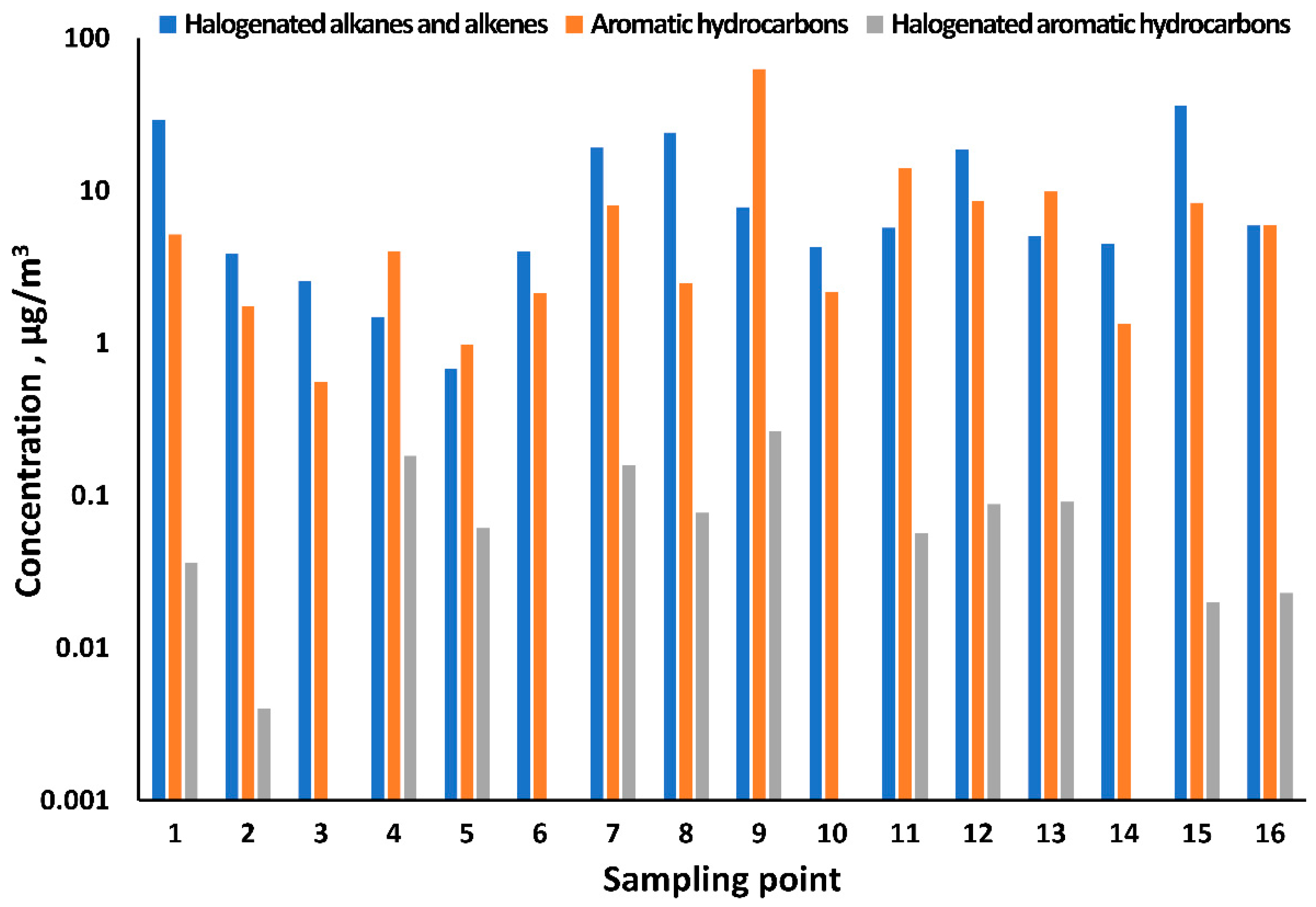
3.2. Volatile Organic Compounds
3.3. Semi-Volatile Organic Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cressey, D. Scientific challenges in the Arctic: Open water. Nature 2011, 478, 174–177. [Google Scholar] [CrossRef]
- Law, K.S.; Roiger, A.; Thomas, J.L.; Marelle, L.; Raut, J.C.; Dalsøren, S.; Fuglestvedt, J.; Tuccella, P.; Weinzierl, B.; Schlager, H. Local Arctic air pollution: Sources and impacts. Ambio 2017, 46, 453–463. [Google Scholar] [CrossRef]
- Law, K.S.; Stohl, A. Arctic Air Pollution: Origins and Impacts. Science 2007, 315, 1537–1540. [Google Scholar] [CrossRef]
- Kallenborn, R.; Hung, H.; Brorström-Lundén, E. Atmospheric Long-Range Transport of Persistent Organic Pollutants (POPs) into Polar Regions. Compr. Anal. Chem. 2015, 67, 411–432. [Google Scholar] [CrossRef]
- Ma, J.; Hung, H.; Macdonald, R.W. The influence of global climate change on the environmental fate of persistent organic pollutants: A review with emphasis on the Northern Hemisphere and the Arctic as a receptor. Glob. Planet. Change 2016, 146, 89–108. [Google Scholar] [CrossRef]
- Poliakova, O.V.; Lebedev, A.T.; Hanninen, O. Organic pollutants in snow of urban and rural Russia and Finland. Toxicol. Environ. Chem. 2000, 75, 181–194. [Google Scholar] [CrossRef]
- Lindwall, F.; Svendsen, S.S.; Nielsen, C.S.; Michelsena, A.; Rinnan, R. Warming increases isoprene emissions from an arctic fen. Sci. Total Environ. 2016, 553, 297–304. [Google Scholar] [CrossRef]
- Svendsen, S.S.; Lindwall, F.; Michelsena, A.; Rinnan, R. Biogenic volatile organic compound emissions along a high arctic soil moisture gradient. Sci. Total Environ. 2016, 573, 131–138. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, E.; Yoon, Y.J.; Park, J.Y.; Kim, T.-W.; Becagli, S.; Caiazzo, L.; Cappelletti, D.; Krejci, R.; Eleftheriadis, K.; et al. Influence of Biogenic Organics on the Chemical Composition of Arctic Aerosols. Glob. Biogeochem. Cycles. 2019, 33, 1238–1250. [Google Scholar] [CrossRef]
- Blake, D.; Hinwood, A.L.; Horwitz, P. Peat fires and air quality: Volatile organic compounds and particulates. Chemosphere 2009, 76, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Kosyakov, D.S.; Ul’yanovskii, N.V.; Latkin, T.B.; Pokryshkin, S.A.; Berzhonskis, V.R.; Polyakova, O.V.; Lebedev, A.T. Peat burning—An important source of pyridines in the earth atmosphere. Environ. Pollut. 2020, 266, 115109. [Google Scholar] [CrossRef]
- Feltracco, M.; Barbaro, E.; Tedeschi, S.; Spolaor, A.; Turetta, C.; Vecchiato, M.; Morabito, E.; Zangrando, R.; Barbante, C.; Gambaro, A. Interannual variability of sugars in Arctic aerosol: Biomass burning and biogenic inputs. Sci. Total Environ. 2020, 706, 136089. [Google Scholar] [CrossRef]
- Newland, M.J.; Martinerie, P.; Witrant, E.; Helmig, D.; Worton, D.R.; Hogan, C.; Sturges, W.T.; Reeves, C.E. Changes to the chemical state of the Northern Hemisphere atmosphere during the second half of the twentieth century. Atmos. Chem. Phys. 2017, 17, 8269–8283. [Google Scholar] [CrossRef]
- Arnold, S.R.; Law, K.S.; Brock, C.A.; Thomas, J.L.; Starkweather, S.M.; Von Salzen, K.; Stohl, A.; Sharma, S.; Lund, M.T.; Flanner, M.G.; et al. Arctic air pollution: Challenges and opportunities for the next decade. Elem. Sci. Anthr. 2016, 4, 000104. [Google Scholar] [CrossRef]
- AMAP. Assessment 2015: Temporal Trends in Persistent Organic Pollutants in the Arctic. In Arctic Monitoring and Assessment Programme; AMAP: Oslo, Norway, 2016; 71p. [Google Scholar]
- Wong, F.; Hung, H.; Dryfhout-Clark, H.; Aas, W.; Bohlin-Nizzetto, P.; Breivik, K.; Mastromonaco, M.N.; Lundén, E.B.; Ólafsdóttir, K.; Sigurðsson, Á.; et al. Time trends of persistent organic pollutants (POPs) and Chemicals of Emerging Arctic Concern (CEAC) in Arctic air from 25 years of monitoring. Sci. Total Environ. 2021, 775, 145109. [Google Scholar] [CrossRef] [PubMed]
- Butt, C.M.; Berger, U.; Bossi, R.; Tomy, G.T. Levels and trends of poly- and perfluorinated compounds in the arctic environment. Sci. Total Environ. 2010, 408, 2936–2965. [Google Scholar] [CrossRef]
- Yu, Y.; Katsoyiannis, A.; Bohlin-Nizzetto, P.; Brorström-Lundén, E.; Ma, J.; Zhao, Y.; Wu, Z.; Tych, W.; Mindham, D.; Sverko, E.; et al. Polycyclic Aromatic Hydrocarbons Not Declining in Arctic Air Despite Global Emission Reduction. Environ. Sci. Technol. 2019, 53, 2375–2382. [Google Scholar] [CrossRef]
- Pernov, J.B.; Bossi, R.; Lebourgeois, T.; Nøjgaard, J.K.; Holzinger, R.; Hjorth, J.L.; Skov, H. Atmospheric VOC measurements at a High Arctic site: Characteristics and source apportionment. Atmos. Chem. Phys. 2021, 21, 2895–2916. [Google Scholar] [CrossRef]
- Röhler, L.; Schlabach, M.; Haglund, P.; Breivik, K.; Kallenborn, R.; Bohlin-Nizzetto, P. Non-target and suspect characterisation of organic contaminants in Arctic air—Part 2: Application of a new tool for identification and prioritisation of chemicals of emerging Arctic concern in air. Atmos. Chem. Phys. 2020, 20, 9031–9049. [Google Scholar] [CrossRef]
- Kos, G.; Kanthasami, V.; Adechina, N.; Ariya, P.A. Volatile organic compounds in Arctic snow: Concentrations and implications for atmospheric processes. Environ. Sci. Process. Impacts 2014, 16, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.T.; Mazur, D.M.; Polyakova, O.V.; Kosyakov, D.S.; Kozhevnikov, A.Y.; Latkin, T.B.; Andreeva, Y.I.; Artaev, V.B. Semi volatile organic compounds in the snow of Russian Arctic islands: Archipelago Novaya Zemlya. Environ. Pollut. 2018, 239, 416–427. [Google Scholar] [CrossRef]
- Mazur, D.M.; Latkin, T.B.; Kosyakov, D.S.; Kozhevnikov, A.Y.; Ul’yanovskii, N.V.; Kirilov, A.G.; Lebedev, A.T. Arctic snow pollution: A GC-HRMS case study of Franz Joseph Land archipelago. Environ. Pollut. 2020, 265, 114885. [Google Scholar] [CrossRef] [PubMed]
- Method 8260D Volatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); Revision 4; US Environmental Protection Agency: Washington, DC, USA, 2018.
- Method 8270E Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS); Revision 6; US Environmental Protection Agency: Washington, DC, USA, 2018.
- Zaikin, V.; Halket, J. A Handbook of Derivatives for Mass Spectrometry; IM Publications LLP: Chichester, UK, 2009. [Google Scholar]
- Hygienic Standards GN 2.1.6.695-98; Maximal Allowable Concentrations (MAC) of the Pollutants in the Atmospheric air. RF Ministry for Health: Moscow, Russia, 1998.
- Adcock, K.E.; Reeves, C.E.; Gooch, L.J.; Leedham Elvidge, E.C.; Ashfold, M.J.; Brenninkmeijer, C.A.; Chou, C.; Fraser, P.J.; Langenfelds, R.L.; Mohd Hanif, N.; et al. Continued increase of CFC-113a (CCl3CF3) mixing ratios in the global atmosphere: Emissions, occurrence and potential sources. Atmos. Chem. Phys. 2018, 18, 4737–4751. [Google Scholar] [CrossRef]
- Prinn, R.G.; Weiss, R.F.; Arduini, J.; Arnold, T.; Fraser, P.J.; Ganesan, A.L.; Gasore, J.; Harth, C.M.; Hermansen, O.; Kim, J.; et al. The ALE/GAGE/AGAGE Data Base. Available online: http://agage.mit.edu/data and https://agage2.eas.gatech.edu/data_archive/data_figures/global/pdf/CFC-113_glb.pdf (accessed on 27 April 2021).
- Yu, Z.; Li, Y. Marine volatile organic compounds and their impacts on marine aerosol—A review. Sci. Total Environ. 2021, 768, 145054. [Google Scholar] [CrossRef] [PubMed]
- Ballschmiter, K. Pattern and sources of naturally produced organohalogens in the marine environment: Biogenic formation of organohalogens. Chemosphere 2003, 52, 313–324. [Google Scholar] [CrossRef]
- Kurihara, M.; Iseda, M.; Ioriya, T.; Horimoto, N.; Kanda, J.; Ishimaru, T.; Yamaguchi, Y.; Hashimoto, S. Brominated methane compounds and isoprene in surface seawater of Sagami Bay: Concentrations, fluxes, and relationships with phytoplankton assemblages. Mar. Chem. 2012, 134–135, 71–79. [Google Scholar] [CrossRef]
- Shibazaki, A.; Ambiru, K.; Kurihara, M.; Tamegai, H.; Hashimoto, S. Phytoplankton as a temperate marine source of brominated methanes. Mar. Chem. 2016, 181, 44–50. [Google Scholar] [CrossRef]
- Chemicals of Emerging Arctic Concern. Summary for Policy-Makers; Arctic Monitoring and Assessment Programme (AMAP): Oslo, Norway, 2017; 16p. [Google Scholar]
- Kosyakov, D.S.; Ul’yanovskii, N.V.; Mazur, D.M.; Lebedev, A.T. Mass spectrometry in the study of air pollution in the Arctic. Lab. Pro. 2020, 13, 56–68. (In Russian) [Google Scholar] [CrossRef]
- Hansen, A.M.K.; Kristensen, K.; Nguyen, Q.T.; Zare, A.; Cozzi, F.; Nøjgaard, J.K.; Skov, H.; Brandt, J.; Christensen, J.H.; Ström, J.; et al. Organosulfates and organic acids in Arctic aerosols: Speciation, annual variation and concentration levels. Atmos. Chem. Phys. 2014, 14, 7807–7823. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. Biomass burning–a review of organic tracers for smoke from incomplete combustion. Appl. Geochem. 2002, 17, 129–162. [Google Scholar] [CrossRef]
- Polyakova, O.V.; Artaev, V.B.; Lebedev, A.T. Priority and emerging pollutants in the Moscow rain. Sci. Total Environ. 2018, 645, 1126–1134. [Google Scholar] [CrossRef]
- Polyakova, O.V.; Mazur, D.M.; Seregina, I.F.; Bolshov, M.A.; Lebedev, A.T. Estimation of contamination of atmosphere of Moscow in winter. J. Anal. Chem. 2012, 67, 1039–1049. [Google Scholar] [CrossRef]
- Lebedev, A.T.; Polyakova, O.V.; Mazur, D.M.; Artaev, V.B.; Canet, I.; Lallement, A.; Vaïtilingom, M.; Deguillaume, L.; Delort, A.-M. Detection of semi-volatile compounds in cloud waters by GC-GC-TOFMS. Evidence of phenols and phthalates as priority pollutants. Environ. Pollut. 2018, 241, 616–625. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency: Assessing and Managing Chemicals under TSCA. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/phthalates (accessed on 20 April 2021).
- Salapasidou, M.; Samara, C.; Voutsa, D. Endocrine disrupting compounds in the atmosphere of the urban area of Thessaloniki, Greece. Atmos. Environ. 2011, 45, 3720–3729. [Google Scholar] [CrossRef]
- Mazur, D.M.; Detenchuk, E.A.; Sosnova, A.A.; Artaev, V.B.; Lebedev, A.T. GC-HRMS with Complementary Ionization Techniques for Target and Non-target Screening for Chemical Exposure: Expanding the Insights of the Air Pollution Markers in Moscow Snow. Sci. Total Environ. 2021, 761, 144506. [Google Scholar] [CrossRef] [PubMed]
- Khaustov, A.; Redina, M. Polycyclic aromatic hydrocarbons in the snow cover of Moscow (case study of the RUDN University campus). Polycycl. Aromat. Compd. 2019. [Google Scholar] [CrossRef]
- He, J.; Balasubramanian, R. Semi-volatile organic compounds (SVOCs) in ambient air and rainwater in a tropical environment: Concentrations and temporal and seasonal trends. Chemosphere 2010, 78, 742–751. [Google Scholar] [CrossRef]
- Pokryshkin, S.A.; Kosyakov, D.S.; Kozhevnikov, A.Y.; Lakhmanov, D.E.; Ul’yanovskii, N.V. Highly Sensitive Determination of Chlorophenols in Sea Water by Gas Chromatography−Tandem Mass Spectrometry. J. Anal. Chem. 2018, 73, 991–998. [Google Scholar] [CrossRef]


| Number | Compounds | Tr, min | Concentration, μg/m3 at Sampling Point No. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |||
| Cl-alkanes, Cl-alkenes | ||||||||||||||||||
| 1 | Allyl chloride | 1.53 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2 | Methylene chloride | 1.64 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 3 | 1,1-Dichloroethene | 1.75 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 4 | 1,1-Dichloroethane | 1.81 | - | <0.017 | <0.017 | - | - | <0.017 | - | - | <0.017 | <0.017 | 0.019 | - | <0.017 | <0.017 | - | - |
| 5 | Chloroprene | 1.85 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 6,7 | cis and trans -1,2-Dichloroethene | 1.93 | - | - | - | - | - | - | - | - | - | - | <0.033 | - | - | - | - | - |
| 8 | 2,2-Dichloropropane | 1.98 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | Chloroform | 2.02 | - | 0.36 | 0.17 | 0.12 | 0.014 | 0.22 | - | - | 1.9 | 0.29 | 0.42 | - | 0.40 | 0.26 | - | - |
| 10 | 1,1,1-Trichloroethane | 2.19 | 0.030 | 0.019 | 0.011 | 0.017 | <0.007 | 0.017 | - | <0.007 | 0.029 | 0.021 | 0.028 | 0.010 | 0.026 | 0.021 | 0.033 | 0.010 |
| 11 | 1,2-Dichloroethane | 2.25 | - | 0.24 | 0.14 | 0.17 | 0.018 | 0.16 | - | - | 0.34 | 0.17 | 0.27 | - | 0.25 | 0.16 | - | - |
| 12 | 1,1-Dichloropropene | 2.26 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 13 | Carbon tetrachloride | 2.33 | 1.1 | 1.2 | 0.89 | 0.72 | 0.25 | 1.2 | - | 0.1 | 1.7 | 1.4 | 1.8 | 0.26 | 1.5 | 1.4 | 0.62 | 0.65 |
| 14 | Trichloroethene | 2.73 | 0.005 | - | - | - | - | - | - | <0.005 | 0.009 | - | 0.023 | <0.005 | <0.005 | <0.005 | 0.007 | 0.007 |
| 15 | 1,2-Dichloropropane | 2.75 | 0.044 | 0.053 | 0.034 | 0.053 | 0.017 | 0.039 | - | 0.020 | 0.074 | 0.029 | 0.060 | 0.040 | 0.045 | 0.029 | 0.031 | 0.026 |
| 16 | cis-1,3-Dichloropropene | 3.36 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 17 | trans-1,3-Dichloropropene | 3.95 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 18 | 1,1,2-Trichloroethane | 4.19 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | - | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 |
| 19 | 1,3-Dichloropropane | 4.40 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 20 | Tetrachloroethene | 4.99 | 0.048 | 0.054 | 0.039 | 0.090 | 0.037 | 0.038 | 0.058 | 0.042 | 0.087 | 0.045 | 0.18 | 0.020 | 0.20 | 0.040 | 0.070 | 0.053 |
| 21 | 1,1,1,2-Tetrachloroethane | 6.61 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 22 | cis-1,4-Dichloro-2-butene | 8.42 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 23 | 1,1,2,2-Tetrachloroethane | 8.93 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 24 | 1,2,3-Trichloropropane | 6.61 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 |
| 25 | trans-1,4-Dichloro-2-butene | 9.17 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 26 | Pentachloroethane | 10.80 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 27 | Hexachloro-1,3-butadiene | 8.93 | 0.003 | 0.004 | 0.004 | 0.017 | 0.003 | 0.004 | 0.017 | 0.006 | 0.010 | 0.005 | 0.005 | 0.003 | 0.006 | 0.005 | 0.004 | 0.004 |
| 28 | Iodomethane | 1.61 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 29 | 1,1,2-Trichlorotrifluoroethane (CFC-113) | 1.61 | 28 | 1.8 | 1.2 | <0.075 | 0.1 | 2.2 | 19 | 24 | 3.4 | 2.2 | 2.8 | 18 | 2.5 | 2.4 | 35 | 5.1 |
| 30 | Bromochloromethane | 2.01 | - | <0.027 | <0.027 | - | - | <0.027 | - | - | - | <0.027 | <0.027 | - | <0.027 | <0.027 | - | - |
| 31 | Dibromomethane | 2.78 | - | 0.037 | 0.033 | 0.07 | 0.015 | 0.027 | - | <0.008 | 0.055 | 0.027 | 0.034 | <0.008 | 0.04 | 0.033 | 0.014 | 0.009 |
| 32 | Bromodichloromethane | 2.89 | - | 0.008 | <0.008 | 0.013 | <0.008 | <0.008 | - | <0.008 | 0.009 | <0.008 | 0.009 | <0.008 | 0.008 | <0.008 | <0.008 | <0.008 |
| 33 | Dibromochloromethane | 4.84 | <0.010 | <0.010 | <0.010 | 0.012 | 0.012 | <0.010 | - | <0.010 | 0.015 | <0.010 | <0.010 | <0.010 | <0.010 | <0.010 | <0.010 | <0.010 |
| 34 | 1,2-Dibromoethane (EDB) | 5.13 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 35 | Bromoform | 7.88 | 0.024 | 0.095 | 0.090 | 0.19 | 0.23 | 0.047 | 0.012 | 0.017 | 0.1 | 0.049 | 0.058 | 0.013 | 0.068 | 0.058 | 0.018 | 0.019 |
| N-compounds | ||||||||||||||||||
| 36 | Acrylonitrile | 1.61 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 37 | Propionitrile | 1.78 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 38 | Methacrylonitrile | 1.93 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 39 | Nitrobenzene | 13.92 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| S-compounds, O-compounds | ||||||||||||||||||
| 40 | Carbon disulfide | 1.66 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 41 | Methyl acrylate | 1.95 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 42 | Isobutyl alcohol | 2.04 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 43 | Tetrahydrofuran | 2.05 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 44 | 1,4-Dioxane | 2.82 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 45 | Methyl methacrylate | 2.86 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 46 | Ethyl methacrylate | 4.41 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Aromatic hydrocarbons | ||||||||||||||||||
| 47 | Benzene | 2.31 | 2.9 | 1.0 | 0.43 | 2.4 | 0.47 | 0.54 | 2.6 | 0.079 | 1.5 | 0.9 | 3.6 | 5.9 | 2.3 | 0.93 | 5.1 | 3.2 |
| 48 | Toluene | 3.90 | 1.1 | 0.37 | 0.074 | 0.62 | 0.31 | 0.11 | 3.1 | 1.6 | 1.6 | 0.29 | 1.8 | 1.3 | 1.1 | 0.24 | 1.9 | 1.8 |
| 49 | Ethylbenzene | 6.89 | 0.14 | 0.084 | 0.004 | 0.14 | 0.018 | 0.180 | 0.34 | 0.16 | 8.2 | 0.19 | 1.6 | 0.24 | 0.79 | 0.030 | 0.19 | 0.18 |
| 50,51 | M,p-Xylene | 7.20 | 0.30 | 0.090 | 0.008 | 0.22 | 0.028 | 0.43 | 0.8 | 0.24 | 18 | 0.25 | 2.3 | 0.48 | 1.4 | 0.025 | 0.28 | 0.38 |
| 52 | Styrene | 7.93 | 0.13 | 0.026 | 0.011 | 0.20 | 0.062 | 0.022 | 0.38 | 0.13 | 0.76 | 0.032 | 0.20 | 0.10 | 0.17 | 0.006 | 0.11 | 0.12 |
| 53 | o-Xylene | 7.96 | 0.49 | 0.060 | 0.017 | 0.19 | 0.040 | 0.77 | 0.42 | 0.092 | 30 | 0.26 | 1.9 | 0.22 | 1.2 | 0.038 | 0.20 | 0.17 |
| 54 | Isopropylbenzene | 9.01 | 0.023 | 0.005 | 0.002 | 0.011 | 0.003 | 0.021 | 0.037 | 0.011 | 1.2 | 0.019 | 0.18 | 0.011 | 0.12 | 0.004 | 0.030 | 0.014 |
| 55 | n-Propylbenzene | 10.00 | 0.036 | 0.011 | <0.002 | 0.025 | 0.008 | 0.004 | 0.15 | 0.038 | 0.11 | 0.027 | 0.28 | 0.078 | 0.22 | 0.005 | 0.10 | 0.060 |
| 56 | 1,3,5-Trimethylbenzene | 10.51 | 0.006 | 0.007 | <0.004 | 0.039 | 0.006 | 0.013 | 0.024 | 0.007 | 0.26 | 0.017 | 0.23 | 0.031 | 0.21 | - | 0.035 | 0.004 |
| 57 | tert-Butylbenzene | 11.18 | - | - | - | - | - | - | 0.006 | 0.004 | - | - | - | 0.004 | - | - | 0.029 | - |
| 58 | 1,2,4-Trimethylbenzene | 11.26 | 0.015 | 0.021 | <0.007 | 0.054 | 0.009 | 0.011 | 0.056 | 0.046 | 0.70 | 0.11 | 1.5 | 0.065 | 2.0 | <0.007 | 0.27 | 0.011 |
| 59 | sec-Butylbenzene | 11.73 | 0.002 | 0.001 | - | 0.005 | 0.001 | 0.001 | 0.011 | 0.004 | 0.064 | 0.010 | 0.11 | 0.008 | 0.12 | <0.004 | 0.016 | 0.002 |
| 60 | 4-Isopropyltoluene | 12.21 | 0.017 | 0.042 | 0.013 | 0.036 | 0.018 | 0.038 | 0.033 | 0.053 | 0.20 | 0.059 | 0.29 | 0.030 | 0.14 | 0.062 | 0.037 | 0.021 |
| 61 | n-Butylbenzene | 13.11 | 0.007 | 0.006 | <0.005 | 0.027 | 0.005 | <0.005 | 0.073 | 0.030 | 0.083 | 0.015 | 0.088 | 0.065 | 0.11 | <0.005 | 0.030 | 0.012 |
| Halogenated aromatic hydrocarbons | ||||||||||||||||||
| 62 | Chlorobenzene | 6.35 | 0.036 | 0.004 | <0.004 | 0.10 | 0.042 | <0.004 | 0.080 | 0.075 | 0.10 | <0.004 | 0.036 | 0.068 | 0.008 | - | 0.020 | 0.023 |
| 63 | Bromobenzene | 9.2 | - | - | - | <0.007 | <0.007 | - | <0.007 | <0.007 | <0.007 | - | <0.007 | <0.007 | <0.007 | <0.007 | <0.007 | <0.007 |
| 64 | 2-Chlorotoluene | 9.86 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 65 | 4-Chlorotoluene | 10.12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 66 | 1,3-Dichlorobenzene | 11.58 | - | - | - | 0.029 | 0.010 | - | 0.022 | <0.008 | 0.015 | - | <0.008 | 0.011 | - | - | - | <0.008 |
| 67 | 1,4-Dichlorobenzene | 11.87 | <0.008 | <0.008 | <0.008 | 0.021 | <0.008 | <0.008 | 0.025 | <0.008 | 0.027 | <0.008 | 0.009 | <0.008 | 0.083 | <0.008 | <0.008 | <0.008 |
| 68 | 1,2-Dichlorobenzene | 12.38 | - | <0.005 | <0.005 | 0.011 | 0.005 | <0.005 | 0.012 | <0.005 | 0.096 | <0.005 | 0.007 | <0.005 | <0.005 | <0.005 | - | - |
| 69 | 1,2-Dibromo-3-chloropropane | 13.98 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 70 | 1,2,4-Trichlorobenzene | 16.31 | - | <0.002 | <0.002 | 0.012 | 0.002 | <0.002 | 0.009 | - | 0.022 | <0.002 | 0.003 | 0.003 | <0.002 | <0.002 | - | - |
| 71 | 1,2,3-Trichlorobenzene | 17.11 | - | - | - | 0.009 | 0.002 | - | 0.010 | 0.002 | 0.004 | - | 0.002 | 0.006 | <0.002 | - | <0.002 | <0.002 |
| Number | Compounds | Tr, min | Concentration, μg/m3, at Sampling Point | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |||
| N-compounds | ||||||||||||||||||
| 72 | N-Nitrosodimethylamine | 3.35 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 73 | Pyridine | 3.43 | 0.092 | 0.035 | <0.017 | 0.21 | 0.094 | <0.017 | - | - | 0.22 | 0.034 | 0.084 | 0.14 | 0.064 | <0.017 | 0.062 | - |
| 74 | Aniline | 10.72 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 75 | N-Nitroso-di-n-propylamine | 13.50 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 76 | 2-Nitrophenol | 15.12 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 77 | 4-Chloroaniline | 16.95 | - | <0.033 | - | <0.033 | <0.033 | - | - | - | - | <0.033 | <0.033 | - | <0.033 | <0.033 | - | - |
| 78 | 2-Nitroaniline | 21.58 | - | <0.035 | <0.035 | 0.042 | <0.035 | <0.035 | <0.035 | - | <0.035 | <0.035 | <0.035 | - | <0.035 | <0.035 | - | - |
| 79 | 1,4-Dinitrobenzene | 22.17 | - | - | - | <0.074 | - | - | - | - | - | - | - | - | - | - | - | - |
| 80 | 1,3-Dinitrobenzene | 22.43 | - | - | - | <0.05 | <0.05 | - | - | - | <0.05 | - | - | - | - | - | - | - |
| 81 | 2,6-Dinitrotoluene | 22.62 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 82 | 1,2-Dinitrobenzene | 22.74 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 83 | 3-Nitroaniline | 23.20 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 84 | 2,4-Dinitrophenol | 23.62 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 85 | 2,4-Dinitrotoluene | 24.13 | - | - | - | 0.015 | - | - | - | - | - | - | - | - | - | - | - | - |
| 86 | 4-Nitroaniline | 25.55 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 87 | 4,6-Dinitro-2-methylphenol | 25.67 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 88 | Diphenylamine | 25.97 | - | - | - | 0.022 | - | - | - | - | - | - | - | - | - | - | - | - |
| 89 | Azobenzene | 26.05 | - | - | - | - | - | - | - | - | 0.023 | <0.005 | - | <0.005 | - | <0.005 | <0.005 | <0.005 |
| 90 | Carbazole | 29.88 | - | - | - | 0.017 | <0.017 | <0.017 | <0.017 | - | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | <0.017 | - | <0.017 |
| CHO-compounds | ||||||||||||||||||
| 91 | Phenol | 10.9 | - | 0.68 | 0.78 | 6.0 | 1.4 | 0.80 | - | - | 3.5 | 1.0 | 1.4 | 1.4 | 0.89 | 0.59 | 0.43 | - |
| 92 | Benzyl alcohol | 12.55 | - | 0.024 | <0.017 | 0.11 | 0.033 | 0.025 | - | - | 36 | 0.017 | 0.076 | 0.27 | 0.056 | 0.023 | - | - |
| 93 | 2-Methylphenol | 13.05 | - | - | - | 0.072 | - | - | - | - | 0.020 | - | 0.013 | 0.030 | 0.018 | - | 0.014 | - |
| 94,95 | 3- and 4-Methylphenol | 13.5 | - | 0.16 | 0.060 | 0.58 | 0.15 | 0.064 | - | - | 0.88 | 0.069 | 0.18 | 0.070 | 0.21 | 0.043 | 0.041 | - |
| 96 | 2,4-Dimethylphenol | 15.6 | - | <0.033 | - | 0.038 | - | - | - | - | 0.038 | - | <0.033 | - | <0.033 | - | - | - |
| 97 | Benzoic acid | 16.23 | 1.2 | - | 235 | 165 | 49 | 161 | 20 | 6.7 | 1222 | 17 | 191 | 24 | 0.53 | 50 | 7.0 | 3.3 |
| 98 | Isophorone | 14.87 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 | <0.002 |
| Halogenated SVOCs | ||||||||||||||||||
| 99 | 2-Chlorophenol | 11.07 | - | - | <0.008 | 0.086 | 0.020 | - | - | - | 0.008 | - | <0.008 | 0.022 | <0.008 | - | - | - |
| 100 | 2,4-Dichlorophenol | 16.07 | - | - | - | 0.054 | - | - | 0.059 | <0.033 | <0.033 | - | <0.033 | <0.033 | <0.033 | <0.033 | - | - |
| 101 | 4-Chloro-3-methylphenol | 19.03 | - | - | - | 0.036 | - | - | - | - | <0.025 | - | <0.025 | - | <0.025 | <0.025 | - | - |
| 102 | 2,4,6-Trichlorophenol | 20.43 | - | - | - | 0.059 | - | - | 0.040 | <0.010 | 0.021 | - | <0.010 | <0.010 | <0.010 | <0.010 | <0.010 | <0.010 |
| 103 | 2,4,5-Trichlorophenol | 20.53 | - | - | - | 0.057 | - | - | 0.059 | <0.013 | 0.023 | <0.013 | <0.013 | <0.013 | - | <0.013 | - | <0.013 |
| 104 | 2,3,5,6-Tetrachlorophenol | 24.39 | - | - | - | 0.044 | - | - | 0.062 | - | 0.037 | - | - | <0.033 | - | - | - | <0.033 |
| 105 | 2,3,4,6-Tetrachlorophenol | 24.55 | - | - | - | 0.047 | - | - | 0.066 | <0.025 | 0.033 | - | <0.025 | <0.025 | - | - | - | <0.025 |
| 106 | 4-Chlorophenyl phenyl ether | 25.49 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 107 | Pentachlorophenol | 28.19 | - | - | - | - | - | - | <0.21 | - | <0.21 | - | - | - | - | - | - | - |
| 108 | Bis(2-chloroethyl)ether | 11.07 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 109 | Hexachloroethane | 13.57 | - | 0.004 | 0.005 | 0.007 | 0.003 | 0.005 | - | - | 0.009 | 0.004 | 0.006 | - | 0.005 | 0.005 | - | - |
| 110 | Bis(2-chloroethoxy)methane | 15.92 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 111 | Hexachlorocyclopentadiene | 19.91 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 112 | 2-Chloronaphthalene | 21.02 | - | - | - | 0.016 | <0.002 | - | 0.005 | - | <0.002 | - | 0.003 | - | 0.002 | 0.002 | - | - |
| 113 | Hexachlorobenzene | 27.35 | - | - | - | 0.012 | - | - | 0.017 | - | <0.005 | <0.005 | <0.005 | - | <0.005 | 0.005 | - | - |
| 114 | 4-Bromophenyl phenyl ether | 27.33 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Polycyclic aromatic hydrocarbons | ||||||||||||||||||
| 115 | Naphthalene | 16.51 | 0.025 | 0.12 | 0.051 | 0.11 | 0.012 | 0.058 | 0.025 | 0.004 | 0.10 | 0.011 | 0.008 | 0.18 | 0.013 | 0.20 | 0.010 | 0.30 |
| 116 | 2-Methylnaphthalene | 19.21 | 0.004 | 0.029 | 0.014 | 0.046 | 0.003 | 0.11 | 0.003 | - | 0.034 | <0.002 | - | 0.026 | 0.007 | 0.16 | <0.002 | 0.42 |
| 117 | 1-Methylnaphthalene | 19.56 | 0.004 | 0.025 | 0.012 | 0.039 | 0.003 | 0.070 | 0.003 | - | 0.031 | <0.002 | - | 0.016 | 0.005 | 0.11 | 0.002 | 0.28 |
| 118 | Acenaphthylene | 22.55 | - | 0.005 | - | - | - | - | <0.002 | - | 0.029 | 0.002 | - | 0.005 | <0.002 | 0.004 | 0.003 | 0.007 |
| 119 | Acenaphthene | 23.26 | - | 0.004 | - | - | - | 0.002 | <0.002 | - | 0.007 | <0.002 | - | 0.004 | <0.002 | 0.007 | 0.002 | 0.009 |
| 120 | Dibenzofuran | 23.94 | - | 0.015 | <0.002 | 0.008 | 0.002 | 0.002 | 0.004 | 0.003 | 0.092 | 0.009 | 0.004 | 0.056 | 0.008 | 0.048 | 0.011 | 0.040 |
| 121 | Fluorene | 25.25 | - | 0.005 | - | 0.005 | - | 0.004 | 0.003 | <0.002 | 0.009 | 0.003 | <0.002 | 0.010 | 0.002 | 0.017 | 0.003 | 0.018 |
| 122 | Phenanthrene | 28.86 | - | <0.022 | - | 0.022 | <0.022 | <0.022 | <0.022 | <0.022 | 0.10 | <0.022 | <0.022 | 0.068 | <0.022 | 0.078 | <0.022 | 0.10 |
| 123 | Anthracene | 29.06 | - | - | - | - | <0.007 | - | <0.007 | <0.007 | 0.039 | <0.007 | 0.031 | 0.009 | <0.007 | 0.010 | <0.007 | 0.013 |
| 124 | Fluoranthene | 33.45 | - | 0.010 | - | <0.004 | - | - | <0.004 | <0.004 | 0.078 | 0.008 | <0.004 | 0.018 | 0.003 | 0.012 | 0.009 | 0.014 |
| 125 | Pyrene | 34.26 | - | 0.013 | - | 0.003 | - | - | <0.004 | <0.004 | 0.069 | 0.010 | <0.004 | 0.017 | <0.004 | 0.014 | 0.009 | 0.022 |
| 126 | Benz(a)anthracene | 38.98 | - | - | - | - | - | - | <0.033 | <0.033 | 0.033 | <0.033 | <0.033 | <0.033 | <0.033 | <0.033 | <0.033 | <0.033 |
| 127 | Chrysene | 39.1 | - | - | - | - | - | - | <0.025 | <0.025 | 0.051 | <0.025 | <0.025 | <0.025 | <0.025 | <0.025 | <0.025 | <0.025 |
| 128 | Benzo[b]fluoranthene | 42.94 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 129 | Benzo[k]fluoranthene | 43.03 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 130 | Benzo[a]pyrene | 43.93 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 131 | Benzo[g,h,i]perylene | 48.27 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Phthalates | ||||||||||||||||||
| 132 | Dimethylphthalate | 22.51 | - | - | - | 0.030 | <0.004 | <0.004 | - | - | 0.015 | - | 0.009 | - | 0.008 | <0.004 | - | - |
| 133 | Diethylphthalate | 25.31 | - | - | 0.012 | 0.11 | 0.007 | 0.012 | 0.13 | 0.026 | 0.066 | - | 0.077 | 0.46 | 0.014 | 0.002 | 0.007 | - |
| 134 | Di-n-butyl phthalate | 31.76 | - | - | 0.008 | 0.22 | 0.024 | 0.031 | - | - | 2.0 | 0.010 | 0.82 | - | 0.026 | 0.008 | - | - |
| 135 | Benzyl butyl phthalate | 37.5 | - | - | - | 0.009 | <0.004 | <0.004 | <0.004 | - | 0.25 | - | 0.005 | - | <0.004 | <0.004 | - | - |
| 136 | Bis(2-ethylhexyl)adipate | 38.18 | - | - | - | <0.025 | <0.025 | <0.025 | - | - | 0.52 | <0.025 | <0.025 | <0.025 | <0.025 | <0.025 | - | - |
| 137 | Bis(2-ethylhexyl)phthalate | 40.01 | 0.011 | 0.016 | 0.018 | 0.6 | 3.1 | 0.80 | 0.093 | 0.016 | 28 | 0.57 | 1.5 | 0.036 | 0.57 | 0.17 | 0.42 | 0.031 |
| 138 | Di-n-octyl phthalate | 42.42 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosyakov, D.S.; Shavrina, I.S.; Ul’yanovskii, N.V.; Lakhmanov, D.E.; Lebedev, A.T. Occurrence of Volatile and Semi-Volatile Organic Pollutants in the Russian Arctic Atmosphere: The International Siberian Shelf Study Expedition (ISSS-2020). Atmosphere 2021, 12, 767. https://doi.org/10.3390/atmos12060767
Kosyakov DS, Shavrina IS, Ul’yanovskii NV, Lakhmanov DE, Lebedev AT. Occurrence of Volatile and Semi-Volatile Organic Pollutants in the Russian Arctic Atmosphere: The International Siberian Shelf Study Expedition (ISSS-2020). Atmosphere. 2021; 12(6):767. https://doi.org/10.3390/atmos12060767
Chicago/Turabian StyleKosyakov, Dmitry S., Irina S. Shavrina, Nikolay V. Ul’yanovskii, Dmitry E. Lakhmanov, and Albert T. Lebedev. 2021. "Occurrence of Volatile and Semi-Volatile Organic Pollutants in the Russian Arctic Atmosphere: The International Siberian Shelf Study Expedition (ISSS-2020)" Atmosphere 12, no. 6: 767. https://doi.org/10.3390/atmos12060767
APA StyleKosyakov, D. S., Shavrina, I. S., Ul’yanovskii, N. V., Lakhmanov, D. E., & Lebedev, A. T. (2021). Occurrence of Volatile and Semi-Volatile Organic Pollutants in the Russian Arctic Atmosphere: The International Siberian Shelf Study Expedition (ISSS-2020). Atmosphere, 12(6), 767. https://doi.org/10.3390/atmos12060767







