Physicochemical Analysis of Water Extracts of Particulate Matter from Polluted Air in the Area of Kraków, Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Particulate Matter Sampling and Extraction
2.3. Preparation of Kraków PM Extracts
2.4. Analysis of the Carbon, Nitrogen, Hydrogen, Sulfur Elemental Content and Removal of Carbon Compounds
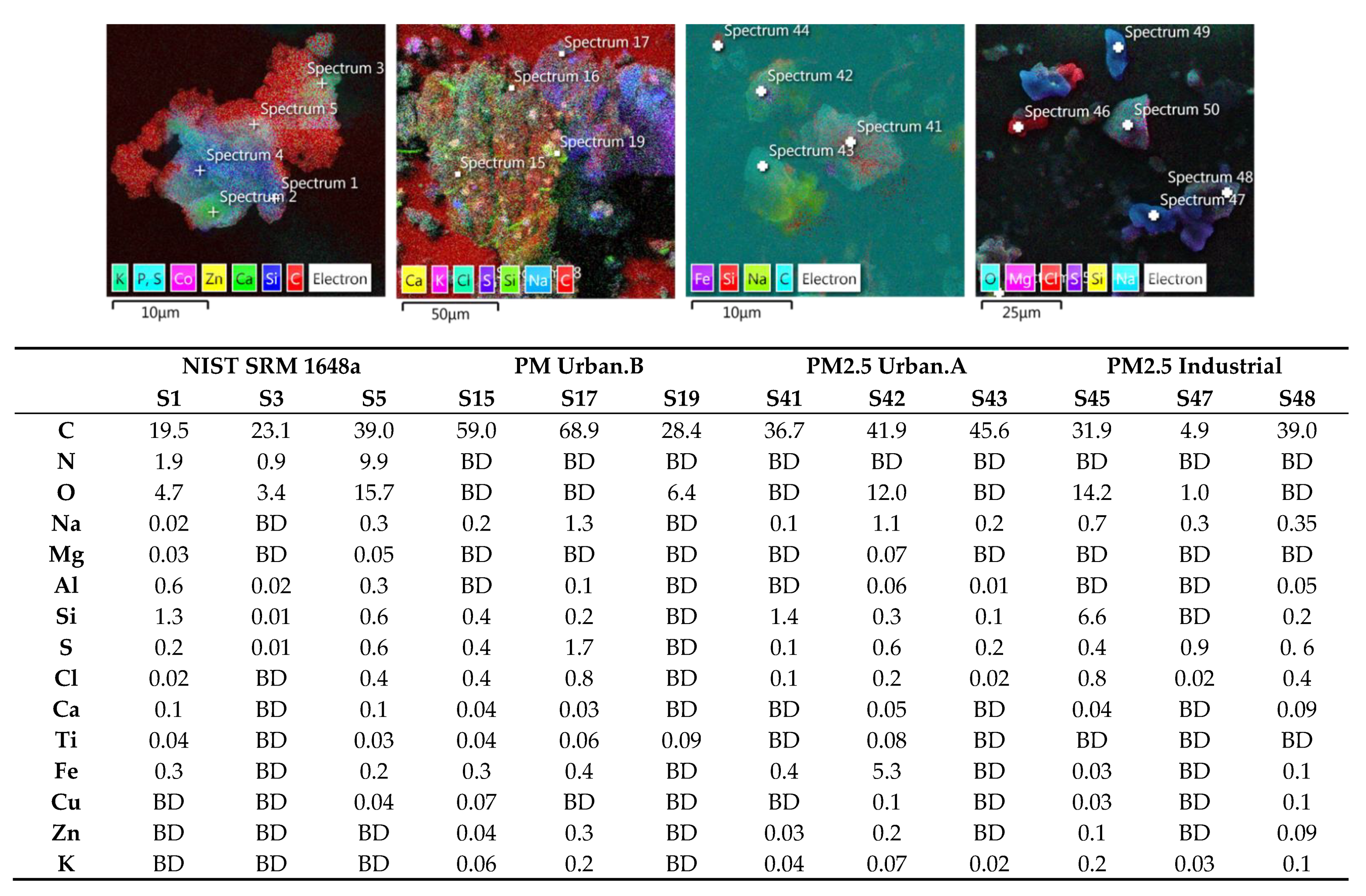
2.5. Morphology Characterization and Elemental Analysis of Kraków PM
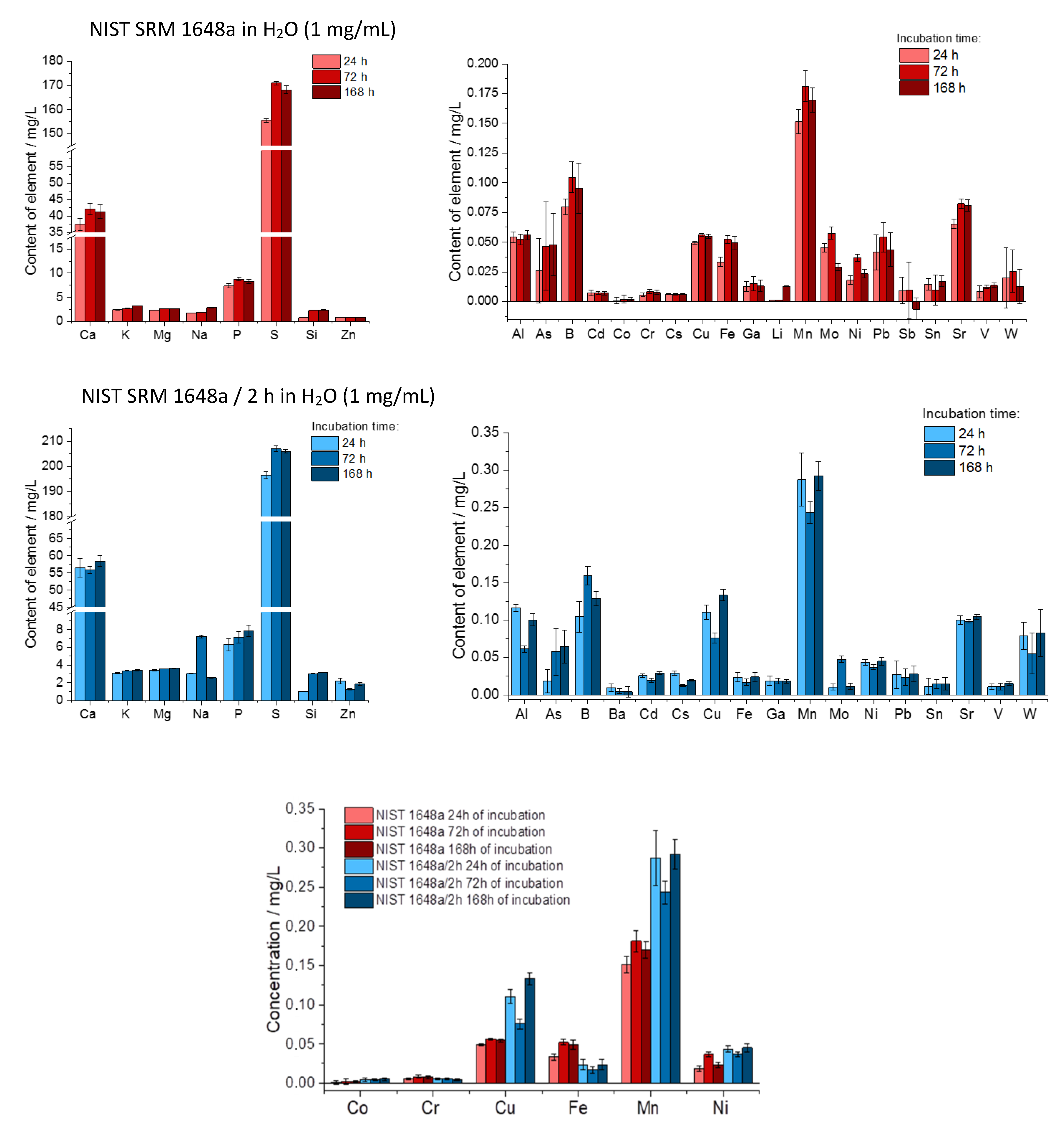
2.6. Determination of Elements Present in Water Extracts
2.7. Effect of Sonication Time, Filtration and Centrifugation on Particle Size Distribution (SRM/2 h and SRM 1648a)
3. Results
3.1. Analysis of the Carbon, Nitrogen, Hydrogen, Sulfur Elemental Content and Removal of Carbon Compounds
3.2. Analysis of Organic and Inorganic Carbon Content
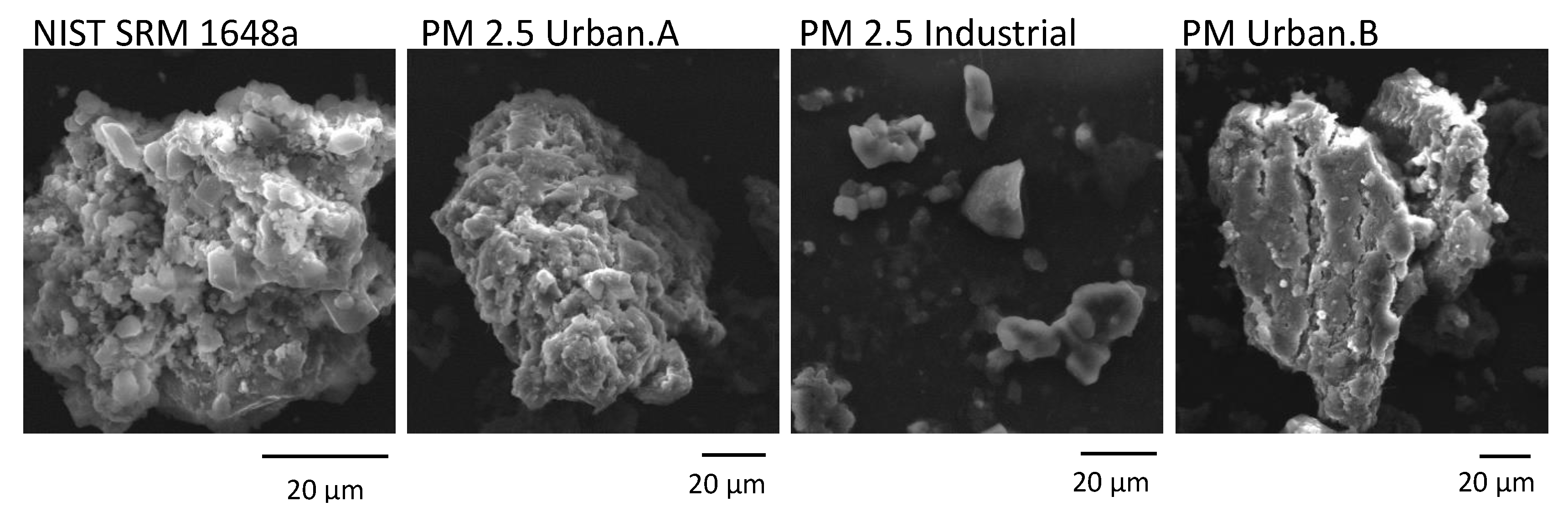
3.3. Analysis of Morphology of Particulate Matter from Different Kraków Locations
3.4. Determination of Elements Present in Water and PBS Extracts from Kraków and NIST SRM Particulate Matter
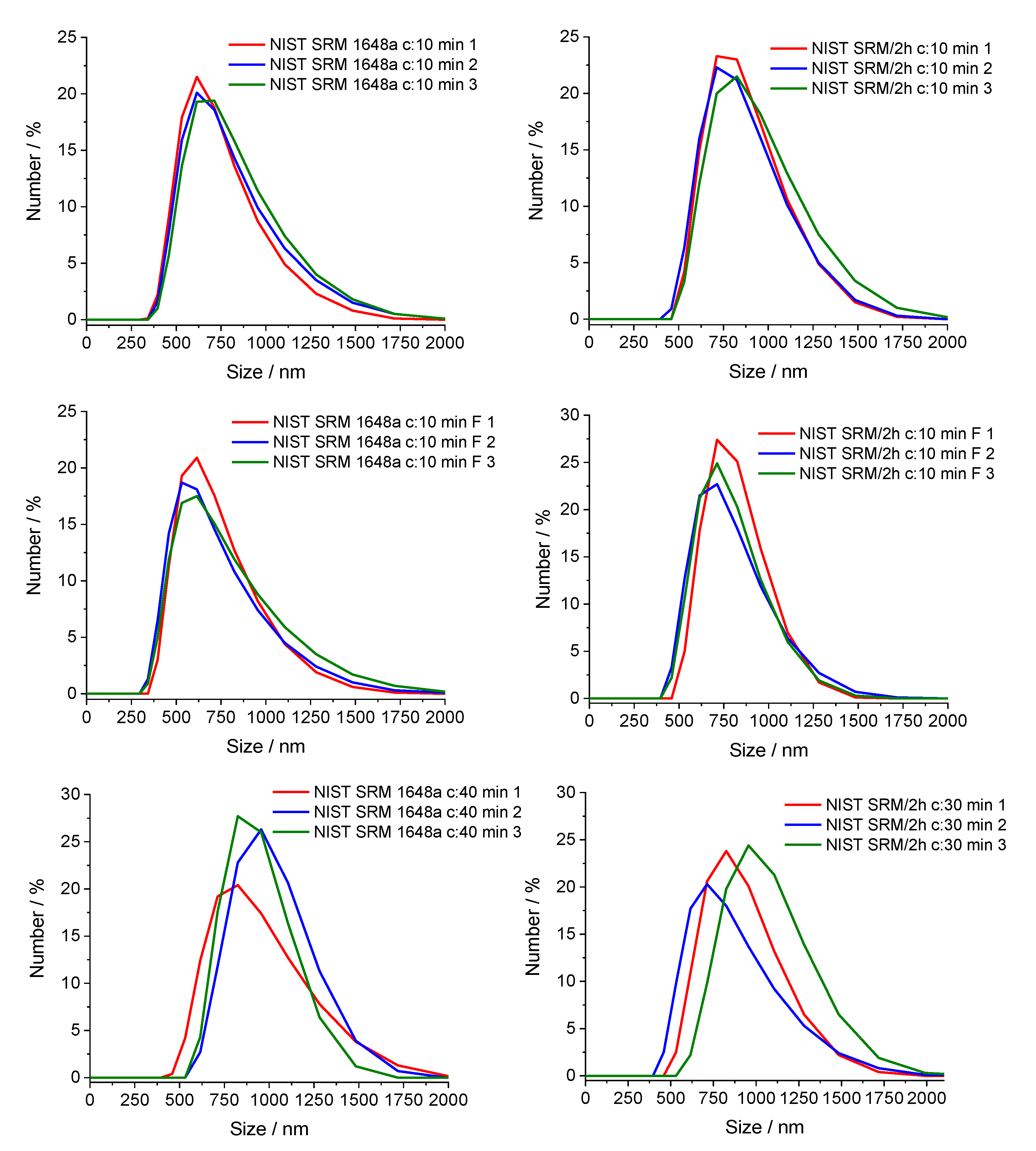
3.5. Effect of Sonication Time, Centrifugation and Filtration on Particle Size Distribution (NIST SRM/2 h and NIST SRM 1648a)
3.6. The Influence of the Extract Preparation Procedure on Particle Size Distribution (Kraków PM Samples)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Brito Beirao, C.A.; Ortiz, G.; De Leeuw, F.; Wiana, M. Air quality in Europe—2018 report. Eur. Environ. Agency 2018. [Google Scholar] [CrossRef]
- Bokwa, A. The climate of the city and air pollution (In Polish). Aura 2016, 9, 8–13. [Google Scholar] [CrossRef]
- Stala-Szlugaj, K. Spalanie węgla kamiennego w sektorze komunalno-bytowym–wpływ na wielkość “niskiej emisji”. Rocz. Ochr. Sr. 2011, 113, 1877–1889. [Google Scholar]
- Zawada, M.; Starostka-Patyk, M. Energy efficiency in the context of low-stack emissions reduction on the example of the city of Czestochowa. Transp. Res. Procedia 2016, 16, 587–597. [Google Scholar] [CrossRef]
- Jędruszkiewicz, J.; Czarnecki, B.; Marosz, M. The variability of PM10 and PM2. 5 concentrations in selected Polish agglomerations: The role of meteorological conditions, 2006–2016. Int. J. Environ. Health Res. 2017, 27, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, L.; Dębska, B.; Gala, M. Report on the State of the Environment in the Lesser Poland Voivodship in 2016; Voivodship Inspectorate for Environmental Protection in Kraków: Kraków, Poland, 2017. (In Polish) [Google Scholar]
- Czarnecka, L.; Dębska, B.; Fiszer, P. Report on the State of the Environment in the LESSER Poland Voivodship in 2017; Voivodship Inspectorate for Environmental Protection in Kraków: Kraków, Poland, 2018. (In Polish) [Google Scholar]
- Cvetanoski, I. Skopje, Europe’s Most Polluted Capital. Osservatorio Balcani Caucaso Transeuropa. Available online: https://www.balcanicaucaso.org/eng/Areas/North-Macedonia/Skopje-Europe-s-most-polluted-capital-191702 (accessed on 16 April 2021).
- Martino, F. Sofia, Capital of Pollution. Osservatorio Balcani e Caucaso Transeuropa. Available online: https://www.balcanicaucaso.org/eng/Areas/Bulgaria/Sofia-capital-of-pollution-192015 (accessed on 16 April 2021).
- Samek, L.; Furman, L.; Mikrut, M.; Regiel-Futyra, A.; Macyk, W.; Stochel, G.; Van Eldik, R. Chemical composition of submicron and fine particulate matter collected in Krakow, Poland. Consequences for the APARIC project. Chemosphere 2017, 187, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.; Watters, R. Standard Reference Material 1648a, Certificate of Analysis; National Institute of Standards & Technology: Gaithersburg, MD, USA, 2008. [Google Scholar]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Mikrut, M.; Regiel-Futyra, A.; Macyk, W.; Stochel, G.; Van Eldik, R. Generation of hydroxyl radicals and singlet oxygen by particulate matter and its inorganic components. Environ. Pollut. 2018, 238, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Pio, C.; Alves, C.; Duarte, A. Identification, abundance and origin of atmospheric organic particulate matter in a Portuguese rural area. Atmos. Environ. 2001, 35, 1365–1375. [Google Scholar] [CrossRef]
- Styszko, K.; Szramowiat, K.; Kistler, M.; Giebl, A.K.; Socha, S.; Rosenberg, E.E.; Golas, J. Polycyclic aromatic hydrocarbons and their nitrated derivatives associated with PM10 from Kraków city during heating season, E3S Web of Conferences. EDP Sci. 2016. [Google Scholar] [CrossRef]
- Ravindra, K.; Bencs, L.; Wauters, E.; De Hoog, J.; Deutsch, F.; Roekens, E.; Bleux, N.; Berghmans, P.; Van Grieken, R. Seasonal and site-specific variation in vapour and aerosol phase PAHs over Flanders (Belgium) and their relation with anthropogenic activities. Atmos. Environ. 2006, 40, 771–785. [Google Scholar] [CrossRef]
- Wilczynska-Michalik, W.; Michalik, M. Composition and origin of dust particles in atmosphere in Kraków. Aura 2015, 3. (In Polish) [Google Scholar] [CrossRef]
- Espinosa, A.J.F.; Rodríguez, M.T.; de la Rosa, F.J.B.; Sánchez, J.C.J. A chemical speciation of trace metals for fine urban particles. Atmos. Environ. 2002, 36, 773–780. [Google Scholar] [CrossRef]
- Canepari, S.; Perrino, C.; Olivieri, F.; Astolfi, M.L. Characterisation of the traffic sources of PM through size-segregated sampling, sequential leaching and ICP analysis. Atmos. Environ. 2008, 42, 8161–8175. [Google Scholar] [CrossRef]
- Manousakas, M.; Papaefthymiou, H.; Eleftheriadis, K.; Katsanou, K. Determination of water-soluble and insoluble elements in PM2. 5 by ICP-MS. Sci. Total Environ. 2014, 493, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Pekney, N.J.; Davidson, C.I. Determination of trace elements in ambient aerosol samples. Anal. Chim. Acta 2005, 540, 269–277. [Google Scholar] [CrossRef]
- Sarti, E.; Pasti, L.; Rossi, M.; Ascanelli, M.; Pagnoni, A.; Trombini, M.; Remelli, M. The composition of PM1 and PM2. 5 samples, metals and their water soluble fractions in the Bologna area (Italy). Atmos. Pollut. Res. 2015, 6, 708–718. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Yang, F.; Chan, K.L.; Ning, Z. Water solubility of metals in coarse PM and PM2. 5 in typical urban environment in Hong Kong. Atmos. Pollut. Res. 2014, 5, 236–244. [Google Scholar] [CrossRef]
- Zajusz-Zubek, E.; Mainka, A.; Kaczmarek, K. Determination of water-soluble elements in PM2.5, PM10, and PM2.5-10 collected in the surroundings of power plants. In Proceedings of the E3S Web of Conferences, Zakopane, Poland, 18–21 October 2017; Volume 28, p. 01042. [Google Scholar] [CrossRef]




| Content (in %) | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Solvent | Sample Preparation: 3 Days of Incubation | Sample Preparation: 3 Days of Incubation, 10 Min Centrifugation | Sample Preparation: 3 Days of Incubation, Filtration, 10 Min Centrifugation | |||
| TOC | IC | TOC | IC | TOC | IC | ||
| NIST SRM 1648a | H2O | 21.6 ± 0.5 | 0.1 ± 0.1 | 4.9 ± 0.3 | - | 3.1 ± 0.1 | 0.1 ± 0.1 |
| PBS | 19 ± 3 | 1.5 ± 0.1 | 3.6 ± 0.1 | 0.5 ± 0.1 | 4.1 ± 0.3 | 0.4 ± 0.1 | |
| NIST SRM/2 h | H2O | 4.0 ± 0.2 | 0.2 ± 0.1 | 2.3 ± 0.3 | - | 3.9 ± 0.2 | - |
| PBS | 3.4 ± 0.4 | 0.5 ± 0.1 | 1.2 ± 0.2 | 0.4 ± 0.1 | 1.6 ± 0.1 | 0.3 ± 0.1 | |
| PM2.5 Urban.A | H2O | 31 ± 2 | 0.2 ± 0.1 | 14.4 ± 0.3 | 0.1 ± 0.1 | 11.0 ± 0.7 | 0.1 ± 0.1 |
| PBS | 45 ± 4 | 1.3 ± 0.1 | 16.9 ± 0.2 | 0.6 ± 0.1 | 16.5 ± 0.2 | 0.5 ± 0.1 | |
| PM2.5 Industrial | H2O | 47.4 ± 0.2 | 0.2 ± 0.1 | 7.4 ± 0.2 | 0.1 ± 0.1 | 11.9 ± 0.1 | - |
| PBS | 38 ± 3 | 1.2 ± 0.1 | 10.0 ± 0.4 | 0.8 ± 0.1 | 10.3 ± 0.5 | 0.5 ± 0.1 | |
| PM Urban.B | H2O | 48 ± 5 | 0.2 ± 0.1 | 10.4 ± 0.6 | 0.1 ± 0.1 | 9.5 ± 0.3 | 0.1 ± 0.1 |
| PBS | 46 ± 3 | 1.0 ± 0.1 | 14 ± 1 | 0.5 ± 0.1 | 11 ± 1 | 0.5 ± 0.1 | |
| PM Urban.B/10 h | H2O | 19 ± 2 | 0.1 ± 0.1 | 9.2 ± 0.1 | 0.3 ± 0.1 | 11.1 ± 0.4 | 0.1 ± 0.1 |
| PBS | 25 ± 3 | 0.7 ± 0.1 | 11.1 ± 0.4 | 0.7 ± 0.1 | 12.8 ± 0.2 | 0.8 ± 0.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikrut, M.; Macyk, W.; van Eldik, R.; Stochel, G. Physicochemical Analysis of Water Extracts of Particulate Matter from Polluted Air in the Area of Kraków, Poland. Atmosphere 2021, 12, 565. https://doi.org/10.3390/atmos12050565
Mikrut M, Macyk W, van Eldik R, Stochel G. Physicochemical Analysis of Water Extracts of Particulate Matter from Polluted Air in the Area of Kraków, Poland. Atmosphere. 2021; 12(5):565. https://doi.org/10.3390/atmos12050565
Chicago/Turabian StyleMikrut, Magdalena, Wojciech Macyk, Rudi van Eldik, and Grażyna Stochel. 2021. "Physicochemical Analysis of Water Extracts of Particulate Matter from Polluted Air in the Area of Kraków, Poland" Atmosphere 12, no. 5: 565. https://doi.org/10.3390/atmos12050565
APA StyleMikrut, M., Macyk, W., van Eldik, R., & Stochel, G. (2021). Physicochemical Analysis of Water Extracts of Particulate Matter from Polluted Air in the Area of Kraków, Poland. Atmosphere, 12(5), 565. https://doi.org/10.3390/atmos12050565