Immediate and Delayed Meteorological Effects on COVID-19 Time-Varying Infectiousness in Tropical Cities
Abstract
1. Introduction
2. Materials and Methods
2.1. COVID-19-Related Data
2.2. Meteorological Data
2.3. Time-Varying Rt Estimation
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. Temperature, Relative Humidity, and Other Meteorological Variables and COVID-19 Infectiousnes
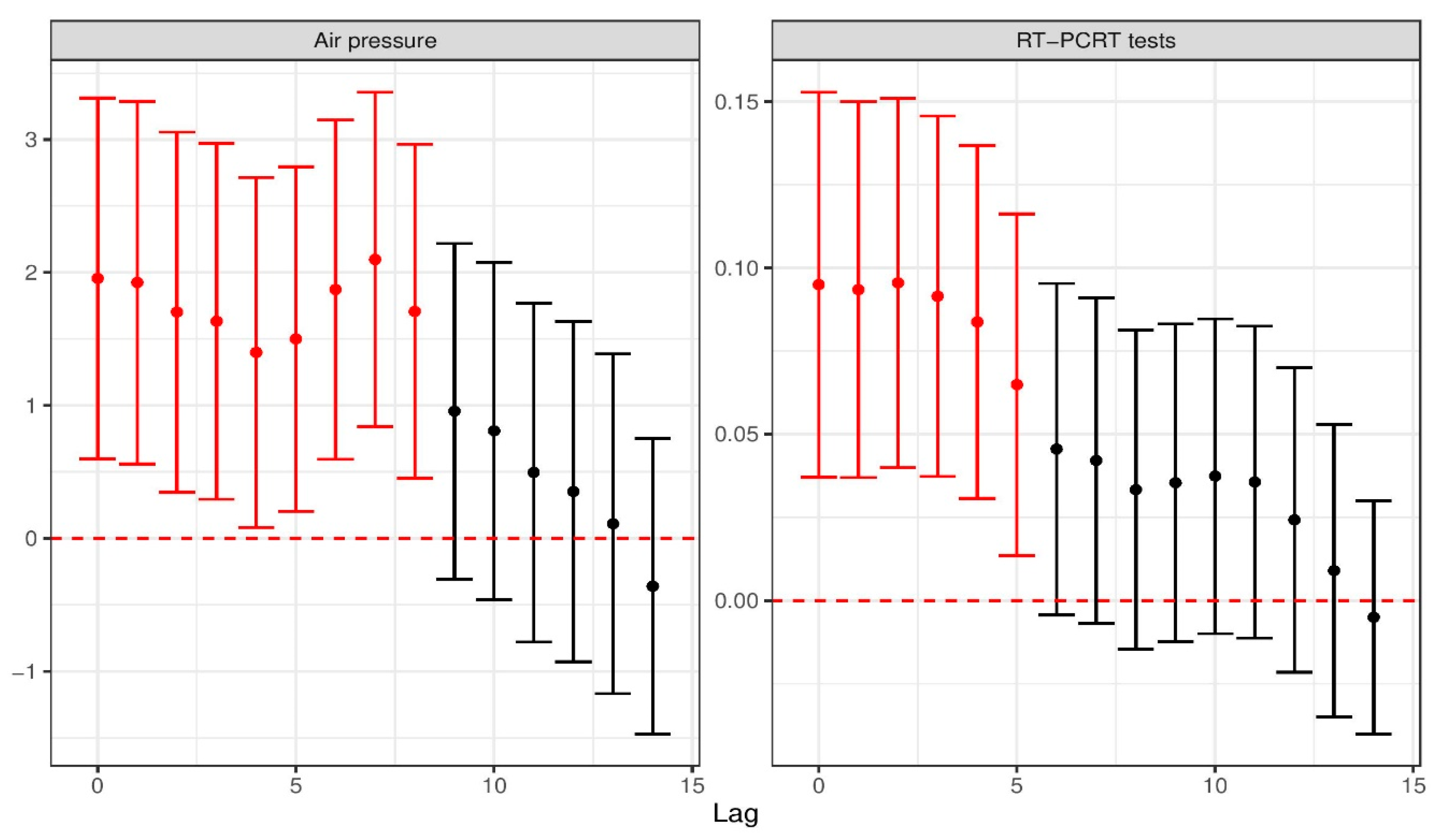
4.2. Air Pressure and COVID-19 Infectiousness
4.3. Short-Term Impact of Testing on COVID-19 Infectiousness
4.4. Community Measures and Its Effect on COVID-19 Infectiousnes
4.5. Limitations
4.6. Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Opening Remarks at the Mission Briefing on COVID-19. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-mission-briefing-on-covid-19 (accessed on 12 March 2020).
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Liu, J.; Liao, X.; Qian, S.; Yuan, J.; Wang, F.; Liu, Y.; Wang, Z.; Wang, F.-S.; Liu, L.; Zhang, Z. Community Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020, 26. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Heesterbeek, H.; Klinkenberg, D.; Hollingsworth, T.D. How will Country-Based Mitigation Measures Influence the Course of the COVID-19 Epidemic? Lancet 2020, 395, 931–934. [Google Scholar] [CrossRef]
- CSIS Southeast Asia Covid-19 Tracker. Available online: https://www.csis.org/programs/southeast-asia-program/southeast-asia-covid-19-tracker-0 (accessed on 17 April 2021).
- DOH. COVID-19 tracker: Philippines. Available online: https://ncovtracker.doh.gov.ph/ (accessed on 10 October 2020).
- Proclamation no. 922: Declaring a State of Public Health Emergency throughout the Philippines. Available online: https://www.officialgazette.gov.ph/downloads/2020/03mar/20200308-PROC-922-RRD.pdf (accessed on 10 October 2020).
- IATF. Recommendations for the Management of the Coronavirus Disease 2019 (COVID-19) Situation. Available online: https://doh.gov.ph/COVID-19/IATF-Resolutions (accessed on 10 October 2020).
- IATF. Omnibus Guidelines on the Implementation of Community Quarantine in the Philippines. Available online: https://doh.gov.ph/COVID-19/IATF-Resolutions (accessed on 10 October 2020).
- IATF. COVID-19 Inter-Agency Task Force for the Management of Emerging Infectious Diseases Resolutions. Available online: https://doh.gov.ph/COVID-19/IATF-Resolutions (accessed on 20 October 2020).
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, L.-M.; Wan, L.; Xiang, T.-X.; Le, A.; Liu, J.-M.; Peiris, M.; Poon, L.L.M.; Zhang, W. Viral Dynamics in Mild and Severe Cases of COVID-19. Lancet Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.-H.; Lou, L.-L.; Zhang, G.-J. The Clinical Dynamics of 18 Cases of COVID-19 Outside of Wuhan, China. Eur. Respir. J. 2020, 55, 2000398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Litvinova, M.; Wang, W.; Wang, Y.; Deng, X.; Chen, X.; Li, M.; Zheng, W.; Yi, L.; Chen, X.; et al. Evolving Epidemiology and Transmission Dynamics of Coronavirus Disease 2019 Outside Hubei Province, China: A Descriptive and Modelling Study. Lancet Infect. Dis. 2020, 20, 793–802. [Google Scholar] [CrossRef]
- Qi, H.; Xiao, S.; Shi, R.; Ward, M.P.; Chen, Y.; Tu, W.; Su, Q.; Wang, W.; Wang, X.; Zhang, Z. COVID-19 Transmission in Mainland China is Associated with Temperature and Humidity: A Time-Series Analysis. Sci. Total Environ. 2020, 728, 138778. [Google Scholar] [CrossRef]
- Şahin, M. Impact of Weather on COVID-19 Pandemic in TURKEY. Sci. Total Environ. 2020, 728, 138810. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, D.; Regoli, F. Role of the Chronic Air Pollution Levels in the Covid-19 Outbreak Risk in Italy. Environ. Pollut. 2020, 264, 114732. [Google Scholar] [CrossRef]
- Bashir, M.F.; Ma, B.; Bilal; Komal, B.; Bashir, M.A.; Tan, D.; Bashir, M. Correlation between Climate Indicators and COVID-19 Pandemic in New York, USA. Sci. Total Environ. 2020, 728, 138835. [Google Scholar] [CrossRef] [PubMed]
- Tosepu, R.; Gunawan, J.; Effendy, D.S.; Ahmad, L.O.A.I.; Lestari, H.; Bahar, H.; Asfian, P. Correlation between Weather and Covid-19 Pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020, 725, 138436. [Google Scholar] [CrossRef]
- McClymont, H.; Hu, W. Weather Variability and COVID-19 Transmission: A Review of Recent Research. Int. J. Environ. Res. Public Health 2021, 18, 396. [Google Scholar] [CrossRef]
- Bukhari, Q.; Massaro, J.M.; D’Agostino, S.R.B.; Khan, S. Effects of Weather on Coronavirus Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 5399. [Google Scholar] [CrossRef] [PubMed]
- Kodera, S.; Rashed, E.A.; Hirata, A. Correlation between COVID-19 Morbidity and Mortality Rates in Japan and Local Population Density, Temperature, and Absolute Humidity. Int. J. Environ. Res. Public Health 2020, 17, 5477. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-E.; Son, W.-S.; Ryu, Y.; Choi, S.B.; Kwon, O.; Ahn, I. Effects of Temperature, Humidity, and Diurnal Temperature Range on Influenza Incidence in a Temperate Region. Influ. Respir. Viruses 2020, 14, 11–18. [Google Scholar] [CrossRef]
- Li, J.; Rao, Y.; Sun, Q.; Wu, X.; Jin, J.; Bi, Y.; Chen, J.; Lei, F.; Liu, Q.; Duan, Z.; et al. Identification of Climate Factors Related to Human Infection with Avian Influenza A H7N9 and H5N1 Viruses in China. Sci. Rep. 2015, 5, 18094. [Google Scholar] [CrossRef]
- Gardner, E.G.; Kelton, D.; Poljak, Z.; Van Kerkhove, M.; Von Dobschuetz, S.; Greer, A.L. A Case-Crossover Analysis of the Impact of Weather on Primary Cases of Middle East Respiratory Syndrome. BMC Infect. Dis. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cori, A.; Ferguson, N.M.; Fraser, C.; Cauchemez, S. A New Framework and Software to Estimate Time-Varying Reproduction Numbers During Epidemics. Am. J. Epidemiol. 2013, 178, 1505–1512. [Google Scholar] [CrossRef]
- Al-Rousan, N.; Al-Najjar, H. The Correlation between the Spread of COVID-19 Infections and Weather Variables in 30 Chinese Provinces and the Impact of Chinese Government Mitigation Plans. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4565–4571. [Google Scholar] [PubMed]
- Chen, Y. COVID-19 Pandemic Imperils Weather Forecast. Geophys. Res. Lett. 2020, 47, e2020GL088613. [Google Scholar] [CrossRef]
- Gupta, S.; Raghuwanshi, G.S.; Chanda, A. Effect of Weather on COVID-19 Spread in the US: A Prediction Model for India in 2020. Sci. Total Environ. 2020, 728, 138860. [Google Scholar] [CrossRef] [PubMed]
- Ogaugwu, C.; Mogaji, H.; Ogaugwu, E.; Nebo, U.; Okoh, H.; Agbo, S.; Agbon, A. Effect of Weather on COVID-19 Transmission and Mortality in Lagos, Nigeria. Scientifica Cairo 2020, 2020, 1–6. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Mutz, Y.S.; Bernardes, P.C.; Conte-Junior, C.A. Relationship between COVID-19 and Weather: Case Study in a Tropical Country. Int. J. Hyg. Environ. Health 2020, 229, 113587. [Google Scholar] [CrossRef]
- Viglione, G. How COVID-19 Could Ruin Weather Forecasts and Climate Records. Nat. Cell Biol. 2020, 580, 440–441. [Google Scholar] [CrossRef]
- Seposo, X.T.; Dang, T.N.; Honda, Y. Effect Modification in the Temperature Extremes by Mortality Subgroups among the Tropical Cities of the Philippines. Glob. Health Action 2016, 9, 31500. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, M.Q.; Macadam, I.; Daron, J.; Katzfey, J.; Cinco, T.A.; Ares, E.D.; Jones, R.G. Projected Changes in Rainfall and Temperature over the Philippines from Multiple Dynamical Downscaling Models. Int. J. Clim. 2020, 40, 1784–1804. [Google Scholar] [CrossRef]
- Katul, G.G.; Mrad, A.; Bonetti, S.; Manoli, G.; Parolari, A.J. Global Convergence of COVID-19 Basic Reproduction Number and Estimation from Early-Time SIR Dynamics. PLoS ONE 2020, 15, e0239800. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Liu, L.; Wang, C.; Guo, H.; Hao, X.; Wang, Q.; Huang, J.; He, N.; Yu, H.; Lin, X.; et al. Association of Public Health Interventions With the Epidemiology of the COVID-19 Outbreak in Wuhan, China. JAMA 2020, 323, 1915. [Google Scholar] [CrossRef]
- NOAA-NCDC. Global Climate Station Summaries. Available online: https://www7.ncdc.noaa.gov/CDO/cdoselect.cmd?datasetabbv=SUMMARIES (accessed on 10 October 2020).
- Towers, S.; Patterson-Lomba, O.; Castillo-Chavez, C. Temporal Variations in the Effective Reproduction Number of the 2014 West Africa Ebola Outbreak. PLoS Curr. 2014, 6, 6. [Google Scholar] [CrossRef]
- Cori, A. EpiEstim: Estimate Time Varying Reproduction Numbers from Epidemic Curves. Available online: https://rdrr.io/cran/EpiEstim/ (accessed on 10 October 2020).
- Team, R.C. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing (accessed on 10 October 2020).
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Qin, J.; You, C.; Lin, Q.; Hu, T.; Yu, S.; Zhou, X.-H. Estimation of Incubation Period Distribution of COVID-19 Using Disease Onset forward Time: A Novel Cross-Sectional and forward Follow-Up Study. Sci. Adv. 2020, 6, eabc1202. [Google Scholar] [CrossRef] [PubMed]
- Smith, G. Step Away from Stepwise. J. Big Data 2018, 5, 32. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.; Yao, J.; Zhang, X.; Li, L.; Xu, X.; He, X.; Wang, B.; Fu, S.; Niu, T.; et al. Impact of Meteorological Factors on the COVID-19 Transmission: A Multi-City Study in China. Sci. Total Environ. 2020, 726, 138513. [Google Scholar] [CrossRef] [PubMed]
- Tobías, A.; Molina, T. Is Temperature Reducing the Transmission of COVID-19? Environ. Res. 2020, 186, 109553. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Pan, J.; Liu, Z.; Meng, X.; Wang, W.; Kan, H.; Wang, W. No Association of COVID-19 Transmission with Temperature or UV Radiation in Chinese Cities. Eur. Respir. J. 2020, 55, 2000517. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, K.; Feng, K.; Lin, X.; Lv, W.; Chen, K.; Wang, F. Impact of Temperature and Relative Humidity on the Transmission of COVID-19: A Modelling Study in China and the United States. BMJ Open 2021, 11, e043863. [Google Scholar] [CrossRef]
- Haque, S.E.; Rahman, M. Association between Temperature, Humidity, and COVID-19 Outbreaks in Bangladesh. Environ. Sci. Policy 2020, 114, 253–255. [Google Scholar] [CrossRef]
- Sajadi, M.M.; Habibzadeh, P.; Vintzileos, A.; Shokouhi, S.; Miralles-Wilhelm, F.; Amoroso, A. Temperature, Humidity, and Latitude Analysis to Estimate Potential Spread and Seasonality of Coronavirus Disease 2019 (COVID-19). JAMA Netw. Open 2020, 3, e2011834. [Google Scholar] [CrossRef]
- Notari, A. Temperature Dependence of COVID-19 Transmission. Sci. Total Environ. 2021, 763, 144390. [Google Scholar] [CrossRef] [PubMed]
- Brauner, J.M.; Mindermann, S.; Sharma, M.; Johnston, D.; Salvatier, J.; Gavenčiak, T.; Stephenson, A.B.; Leech, G.; Altman, G.; Mikulik, V.; et al. Inferring the Effectiveness of Government Interventions against COVID-19. Science 2021, 371, eabd9338. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Dong, Z.; Zeng, W.; Ma, W.; Zhao, D.; Sun, X.; Gong, S.; Xiao, J.; Li, T.; Hu, W. The Effects of Meteorological Factors on Influenza among Children in Guangzhou, China. Influ. Respir. Viruses 2018, 13, 166–175. [Google Scholar] [CrossRef]
- Bhaganagar, K.; Bhimireddy, S. Local Atmospheric Factors that Enhance Air-Borne Dispersion of Coronavirus-High-Fidelity Numerical Simulation of COVID19 Case Study in Real-Time. Environ. Res. 2020, 191, 110170. [Google Scholar] [CrossRef]
- Riddell, S.; Goldie, S.; Hill, A.; Eagles, D.; Drew, T.W. The Effect of Temperature on Persistence of SARS-CoV-2 on Common Surfaces. Virol. J. 2020, 17, 145. [Google Scholar] [CrossRef]
- Marr, L.C.; Tang, J.W.; Van Mullekom, J.; Lakdawala, S.S. Mechanistic Insights into the Effect of Humidity on Airborne Influenza Virus Survival, Transmission and Incidence. J. R. Soc. Interface 2019, 16, 20180298. [Google Scholar] [CrossRef]
- Hellewell, J.; Abbott, S.; Gimma, A.; Bosse, N.I.; Jarvis, C.I.; Russell, T.W.; Munday, J.D.; Kucharski, A.J.; Edmunds, W.J.; Sun, F. Feasibility of Controlling COVID-19 Outbreaks by Isolation of Cases and Contacts. Lancet Glob. Health 2020, 8, e488–e496. [Google Scholar] [CrossRef]
- Grassly, N.C.; Pons-Salort, M.; Parker, E.P.K.; White, P.J.; Ferguson, N.M.; Ainslie, K.; Baguelin, M.; Bhatt, S.; Boonyasiri, A.; Brazeau, N.; et al. Comparison of Molecular Testing Strategies for COVID-19 Control: A Mathematical Modelling Study. Lancet Infect. Dis. 2020, 20, 1381–1389. [Google Scholar] [CrossRef]
- Liu, P.-Y.; He, S.; Rong, L.-B.; Tang, S.-Y. The Effect of Control Measures on COVID-19 Transmission in Italy: Comparison with Guangdong Province in China. Infect. Dis. Poverty 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Wu, D.; Lu, J.; Liu, Y.; Zhang, Z.; Luo, L. Positive Effects of COVID-19 Control Measures on Influenza Prevention. Int. J. Infect. Dis. 2020, 95, 345–346. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Yang, C.-H.; Gutierrez, B.; Wu, C.-H.; Klein, B.; Pigott, D.M.; Du Plessis, L.; Faria, N.R.; Li, R.; Hanage, W.P.; et al. The Effect of Human Mobility and Control Measures on the COVID-19 Epidemic in China. Science 2020, 368, 493–497. [Google Scholar] [CrossRef]
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the Effects of Non-Pharmaceutical Interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef]
- Lau, H.; Khosrawipour, V.; Kocbach, P.; Mikolajczyk, A.; Schubert, J.; Bania, J.; Khosrawipour, T. The Positive Impact of Lockdown in Wuhan on Containing the COVID-19 Outbreak in China. J. Travel Med. 2020, 27, 3. [Google Scholar] [CrossRef]
- Chinazzi, M.; Davis, J.T.; Ajelli, M.; Gioannini, C.; Litvinova, M.; Merler, S.; Piontti, A.P.Y.; Mu, K.; Rossi, L.; Sun, K.; et al. The Effect of Travel Restrictions on the Spread of the 2019 Novel Coronavirus (COVID-19) Outbreak. Science 2020, 368, 395–400. [Google Scholar] [CrossRef]
- Schlosser, F.; Maier, B.F.; Jack, O.; Hinrichs, D.; Zachariae, A.; Brockmann, D. COVID-19 Lockdown Induces Disease-Mitigating Structural Changes in Mobility Networks. Proc. Natl. Acad. Sci. USA 2020, 117, 32883–32890. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Rothman, K.J.; Ferrari, F.; Goffi, A.; Maffeis, G.; Orsini, N. Lockdown Timing and Efficacy in Controlling COVID-19 using Mobile Phone Tracking. E Clin. Med. 2020, 25, 100457. [Google Scholar] [CrossRef]
- Ran, J.; Zhao, S.; Han, L.; Liao, G.; Wang, K.; Wang, M.H.; He, D. A Re-Analysis in Exploring the Association between Temperature and COVID-19 Transmissibility: An Ecological Study with 154 Chinese Cities. Eur. Respir. J. 2020, 56, 2001253. [Google Scholar] [CrossRef] [PubMed]
- Venter, Z.S.; Aunan, K.; Chowdhury, S.; Lelieveld, J. COVID-19 Lockdowns Cause Global Air Pollution Declines. Proc. Natl. Acad. Sci. USA 2020, 117, 18984–18990. [Google Scholar] [CrossRef] [PubMed]
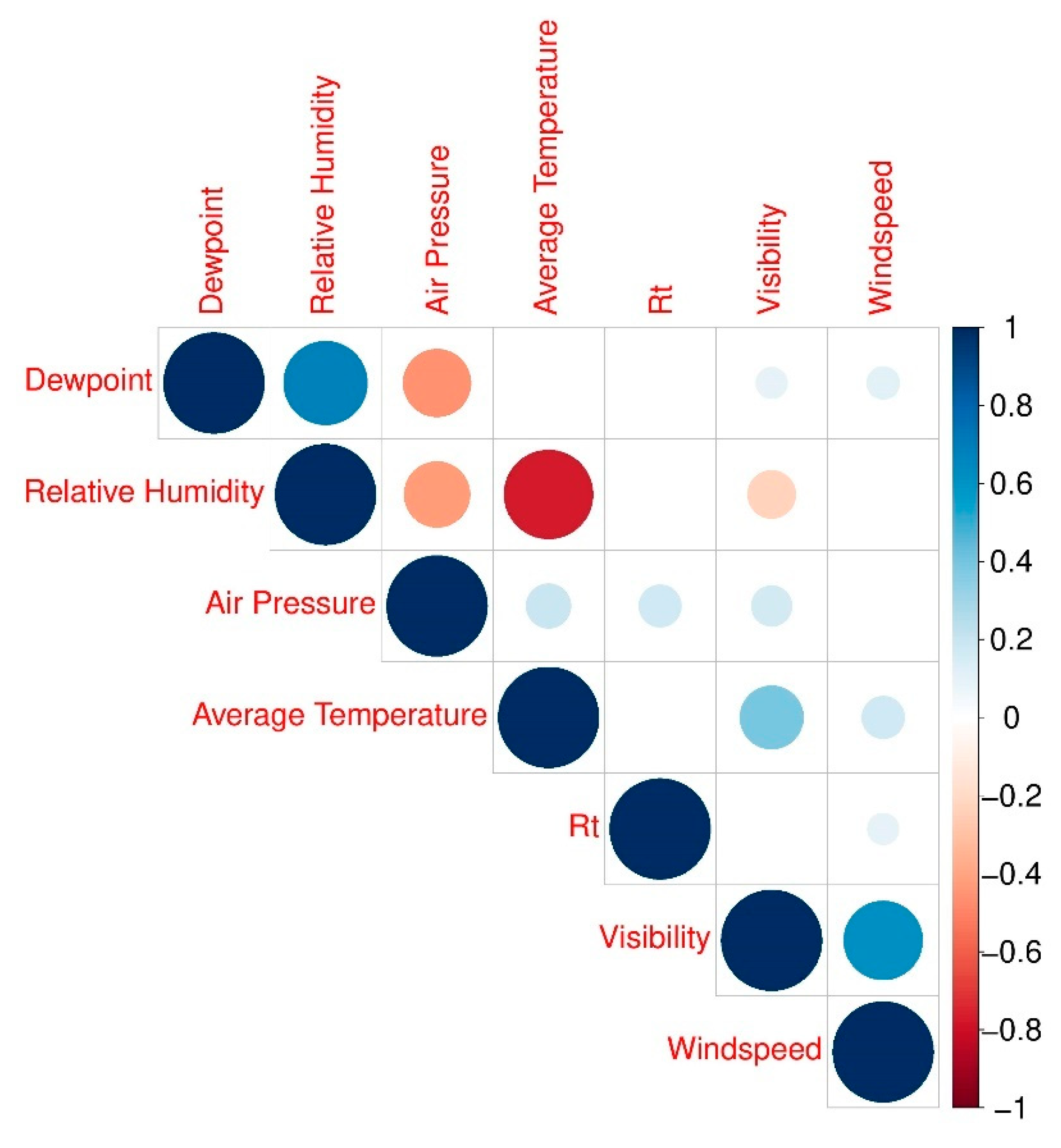
| Variables 1,2,** | Manila City | Quezon City | Cebu City | p-Value 3 |
|---|---|---|---|---|
| Daily COVID-19 cases | 101.89 (122.50) | 144.75 (182.43) | 63.37 (67.99) | <0.001 |
| Average temperature (°C) | 30.24 (1.01) | 29.13 (1.40) | 29.02 (0.99) | <0.001 |
| Dew point (°C) | 24.16 (1.15) | 23.56 (1.24) | 24.25 (0.73) | <0.001 |
| Relative Humidity (%) | 69.59 (7.62) | 72.14 (10.48) | 76.15 (5.70) | <0.001 |
| Air pressure (kPa) | 100.95 (0.15) | 100.94 (0.17) | 100.95 (0.15) | 0.351 |
| Visibility (km) | 6.48 (0.31) | 5.06 (0.43) | 6.14 (0.15) | <0.001 |
| Windspeed (m/s) | 5.18 (1.30) | 2.71 (0.60) | 5.01 (1.07) | <0.001 |
| Rt | 1.20 (0.76) | 1.18 (0.74) | 1.69 (3.09) | 0.0273 |
| RT-PCR tests (per 1000 population) 4 | 10.21 (6.74) * | 10.21 (6.74) * | 1.04 (0.73) | <0.001 |
| − | β | SE | p-Value |
|---|---|---|---|
| 1 Base model | − | − | − |
| +Air Pressure | 1.95473 | 0.69273 | 0.00500 * |
| +Air temperature | 0.02125 | 0.07804 | 0.78600 |
| +Dew point | 0.09836 | 0.09914 | 0.32169 |
| +Relative Humidity | 0.01002 | 0.01346 | 0.45692 |
| +Visibility | −0.02081 | 0.19820 | 0.91643 |
| +Windspeed | 0.10784 | 0.07467 | 0.14950 |
| +RT-PCR tests | 0.08219 | 0.02551 | 0.00137 * |
| +CQ:Time | |||
| CQ 1:Time | −0.00999 | 0.00774 | 0.19739 |
| CQ 2:Time | −0.00799 | 0.00226 | 0.00045 * |
| CQ 3:Time | −0.00617 | 0.00271 | 0.02324 * |
| CQ 4:Time | −0.01546 | 0.00458 | 0.00079 * |
| Covariate | β | SE | p-Value |
|---|---|---|---|
| Air Pressure (Lag 0) | 2.59425 | 0.68426 | <0.001 |
| Air Pressure (Lag 7) | 2.25998 | 0.63500 | <0.001 |
| RT-PCR tests (Lag 0) | 0.14491 | 0.03068 | <0.001 |
| CQ:Time | |||
| CQ 1:Time | −0.01496 | 0.00807 | 0.06366 |
| CQ 2:Time | −0.01671 | 0.00417 | <0.001 |
| CQ 3:Time | −0.01385 | 0.00486 | 0.00437 |
| CQ 4:Time | −0.02743 | 0.00616 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seposo, X.; Ng, C.F.S.; Madaniyazi, L. Immediate and Delayed Meteorological Effects on COVID-19 Time-Varying Infectiousness in Tropical Cities. Atmosphere 2021, 12, 513. https://doi.org/10.3390/atmos12040513
Seposo X, Ng CFS, Madaniyazi L. Immediate and Delayed Meteorological Effects on COVID-19 Time-Varying Infectiousness in Tropical Cities. Atmosphere. 2021; 12(4):513. https://doi.org/10.3390/atmos12040513
Chicago/Turabian StyleSeposo, Xerxes, Chris Fook Sheng Ng, and Lina Madaniyazi. 2021. "Immediate and Delayed Meteorological Effects on COVID-19 Time-Varying Infectiousness in Tropical Cities" Atmosphere 12, no. 4: 513. https://doi.org/10.3390/atmos12040513
APA StyleSeposo, X., Ng, C. F. S., & Madaniyazi, L. (2021). Immediate and Delayed Meteorological Effects on COVID-19 Time-Varying Infectiousness in Tropical Cities. Atmosphere, 12(4), 513. https://doi.org/10.3390/atmos12040513






