Review on Occupational Personal Solar UV Exposure Measurements
Abstract
1. Introduction
2. Literature Search
3. Results
3.1. The 1970s and 1980s
- Discovery of the suitability of PSF as chemical personal UV radiant exposure meter [18].
- Calibration of PSF in respect to the erythema response on a daily base to natural solar UVR [19].
- Introduction of the exposure ratio to ambient UVR (ERTA) [19].
- ERTA changes differently to PE with solar elevation, time of the year, etc. [24].
- ERTA derived from measurements on longer time intervals (e.g., weeks) is hardly influenced by cloudiness while PE is influenced [23].
- ERTA differs with activity [24].
- PE from manikins is not a surrogate for that of moving humans [33].
- Differentiation (e.g., analysis of personal exposure) between: Indoor, mixed-indoor-outdoor and outdoor activities [28].
- PE on weekends and holidays differs from that on working days. Both contribute significantly to total PE [19].
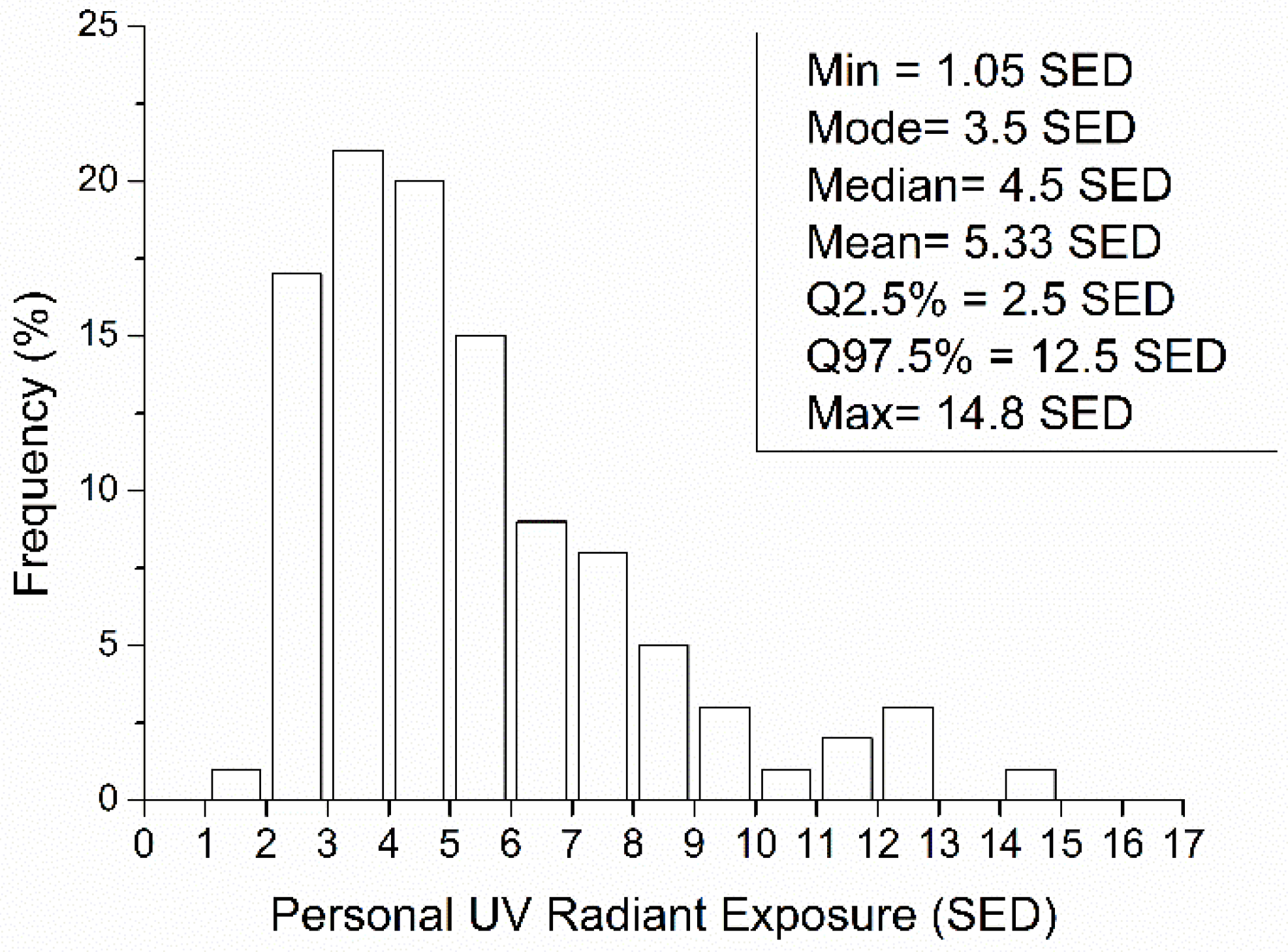
- Frequency distribution of PE in a subpopulation has positive skewness and needs appropriate statistical parameters for description (median, skewness, percentiles, etc.) [29].
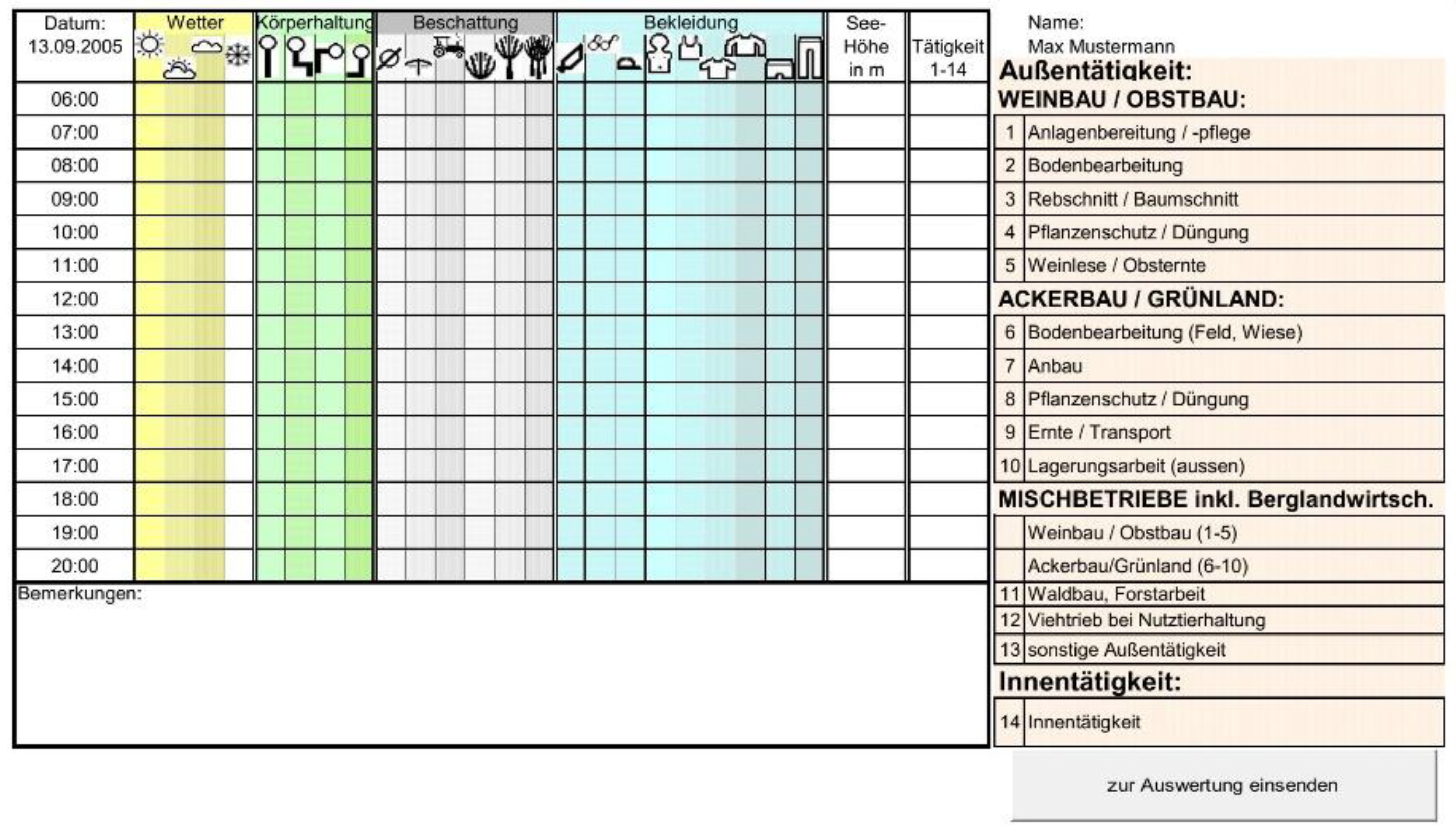
- Introduction of activity diaries [26].
- Noticeable contribution to PE can come from natural surface reflection (albedo) and from reflectivity of artificial surfaces [38].
3.2. The 1990s
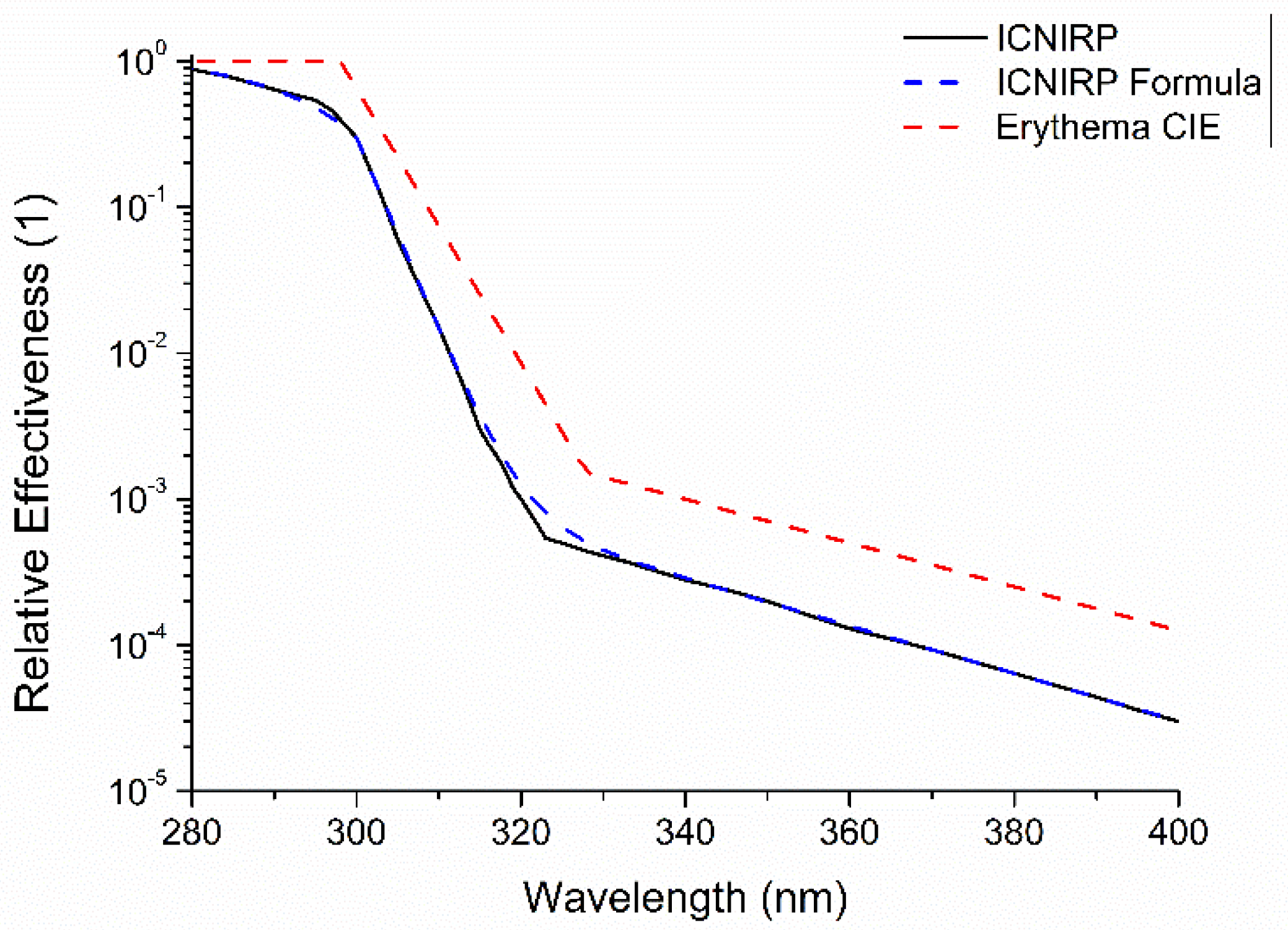
- sery(λ) = 1 for 250 nm ≤ λ ≤ 298 nm,
- sery(λ) = 10[0.094(298 − λ)] for 298 nm < λ ≤ 328 nm
- sery(λ) = 10[0.015(140 − λ)] for 328 nm < λ ≤ 400 nm
- UV Index = 1/k · 280∫ 400 nm · E(λ) · s(λ) · dλ
- k = 0.025 Wery m−2
- Guidelines for PE measurements [60].
- Alternatives to PSF from plastics [62].
- First developments of electronic personal irradiance meters [93].
- Avoidance of the Hawthorne effect [78].
- Comparison of PE with threshold limits according guidelines on limits of exposure [92].
- ERTA is not necessarily highest in summer and lowest in winter [97].
- There is a tendency that PE of outdoor workers on weekends is lower than those of non-outdoor workers [101].
- Work flow (e.g., timing, etc.), activity, behavior and attitudes play a key role for PE [102].
3.3. The 2000s
- Introduction of the UV Index into PE measurements [140].
- Connection of PE and biomarkers (skin color measurements) [140].
- Definition of “sun seekers” [120].
- Analysis in respect to a self-protection factor of 4 for sun adapted skin [120].
- ERTA larger than 100% was reported [140].
- Usage of electronic personal UV irradiance meter [120].
- Indicator cards do not provide reliable measurements [135].
3.4. The 2010s
- PE and ERTA for a certain occupation can differ more strongly between countries than by latitude [167].
- PE and ERTA for a certain occupation may differ noticeable by gender [27].
- PE in conjunction to urinary levels of thymine dimer (DNA-damage) as biomarker [158].
- PE in conjunction with vitamin D as a biomarker [168].
- Use of poly-dimethyl phenylene oxide film (PPO) badges to measure long term PE [162].
- Calibration in respect to the action spectrum according ICNIRP/ACGIH [162].
- Necessity of proper calibration procedure for electronic PE meter [27].
- Risk days (>10 SED (>4 MED for skin type II)) contribute significantly to total PE [27].
- Occupational PE (e.g., for famers) can be lower than for spouses not working outdoors [167].
- Calculation of exposed skin area due to clothing [158].
- Estimation of received radiant energy [158].
4. Summary
- The measuring position is inadequate: it should be representative for the body part(s), which is (are) most exposed to the sun or/and be selected to allow comparisons.
- Measurements of ambient UVR are missing or not described: With that, it is impossible to estimate the relative PE (Low, due to cloudiness? High, due to clear sky? etc.) and, therefore, the representativeness of the studies is questionable.
- Lack of a description of calibration or lack of a proper calibration of the PE meters: with that, an uncertainty in measured PE of ±50% has to be considered. With that, the estimated risk for non-melanoma skin cancer may vary by a factor of 9.
- Inappropriate statistical descriptors of PE values: solely from the mean value together with standard deviation (which implies a normal distribution), no further conclusions can be drawn (e.g., risk).
- Outdoor work description: without a work-task description of participants or a well-defined sub-profession division, the analysis of PE is jeopardized with respect to risk situations/tasks (very general nomenclatures like construction workers are not helpful).
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fountoulakis, I.; Diémoz, H.; Siani, A.-M.; Laschewski, G.; Filippa, G.; Arola, A.; Bais, A.F.; De Backer, H.; Lakkala, K.; Webb, A.R.; et al. Solar UV Irradiance in a Changing Climate: Trends in Europe and the Significance of Spectral Monitoring in Italy. Environments 2020, 7, 1. [Google Scholar] [CrossRef]
- Webb, A.R.; Kift, R.; Durkin, M.T.; O’Brien, S.J.; Vail, A.; Berry, J.L.; Rhodes, L.E. The role of sunlight exposure in determining the vitamin D status of the U.K. white adult population. Br. J. Dermatol. 2010, 163, 1050–1055. [Google Scholar] [CrossRef]
- Unna, P. Ueber das Pigment der menschlichen Haut. Monatsschr. Prakt. Dermatol. 1885, 4, 277–294. [Google Scholar]
- Lavker, R.M.; Geberick, G.F.; Veres, D.; Irwin, C.J.; Kaidbey, K.H. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J. Am. Acad. Dermatol. 1995, 32, 53–62. [Google Scholar] [CrossRef]
- Young, A.R.; Orchard, G.E.; Harrison, G.I.; Klock, J.L. The detrimental effects of daily sub-erythemal exposure on human skin in vivo can be prevented by a daily-care broad-spectrum sunscreen. J. Investig. Dermatol. 2007, 127, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Bech-Thomsen, N.; Wulf, H.C. Photoprotection due to pigmentation and epidermal thickness after repeated exposure to ultraviolet light and psoralen plus ultraviolet A therapy. Photodermatol. Photoimmunol. Photomed. 1995, 11, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, M.; Krebs, R.; Anders, A.; Heinrich, U.; Tronnier, H. Effect of ultraviolet adaption on the ultraviolet absorption spectra oh human skin in vivo. Photodermatol. Photoimmunol. Photomed. 2008, 24, 76–82. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Urbach, F.; Epstein, J.H.; Forbes, P.D. UV carcinogenesis. In Sunlight and Man; Fitzpatrick, T.B., Pathak, M.A., Harber, L.C., Seiji, M., Kutika, A., Eds.; University of Tokyo Press: Tokyo, Japan, 1974; pp. 259–283. [Google Scholar]
- Schmalwieser, A.W.; Siani, A.M. Review on Nonoccupational Personal Solar UV Exposure Measurements. Photochem. Photobiol. 2018, 94, 900–915. [Google Scholar] [CrossRef]
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Arnold, M.; de Vries, E.; Whiteman, D.C.; Jemal, A.; Bray, F.; Parkin, D.M.; Soerjomataram, I. Global Burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int. J. Cancer 2018, 143, 1305–1314. [Google Scholar]
- International Agency for Research on Cancer. Radiation, Volume 100D. A Review of Human Carcinogens. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012. [Google Scholar]
- Fears, T.R.; Scotto, J.; Schneiderman, M.A. Mathematical models of age and ultraviolet effects on the incidence of skin cancer among whites in the United States. Am. J. Epidemiol. 1977, 105, 420–427. [Google Scholar] [CrossRef] [PubMed]
- NOHSC. Guidance Note for the Protection of Workers from the Ultraviolet Radiation in Sunlight; NOHSC: 3012; Australian Government Publishing Service: Canberra, Australia, 1991.
- Diffey, B. The Early Days of Personal Solar Ultraviolet Dosimetry. Atmosphere 2020, 11, 125. [Google Scholar] [CrossRef]
- Urbach, F. Geographic pathology of skin cancer. In The Biologic Effects of Ultraviolet Radiation with Emphasis on the Skin; Urbach, F., Ed.; Pergamon Press: Oxford, UK, 1969; pp. 635–650. [Google Scholar]
- Davis, A.; Deane, G.H.W.; Diffey, B.L. A preliminary study of a dosimeter for ultraviolet radiation. Nature 1976, 261, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Challoner, A.V.J.; Corless, D.; Davis, A.; Deane, G.H.W.; Diffey, B.L.; Gupta, S.P.; Magnus, I.A. Personnel monitoring of exposure to ultraviolet radiation. Clin. Exp. Dermatol. 1976, 1, 175–179. [Google Scholar] [CrossRef]
- Mackenzie, L.A.; Frain-Bell, W. The construction and development of grating monochromator and its application to the study of the reaction of the skin to light. Br. J. Dermatol. 1973, 89, 251–264. [Google Scholar] [CrossRef]
- Roberston, D.F. Long-term field measurements of erythemally effective natural ultraviolet radiation. In The Biologic Effects of Ultraviolet Radiation with Emphasis on the Skin; Urbach, F., Ed.; Pergamon Press: Oxford, UK, 1969; pp. 433–436. [Google Scholar]
- Berger, D. The sunburning ultraviolet meter: Design and performance. Photochem. Photobiol. 1976, 24, 587–593. [Google Scholar] [CrossRef]
- Diffey, B.L.; Kerwin, M.; Davis, A. The anatomical distribution of sunlight. Br. J. Dermatol. 1977, 97, 407–410. [Google Scholar] [CrossRef]
- Leach, J.F.; McLeod, V.E.; Pingstone, A.R.; Davis, A.; Deane, G.H.W. Measurement of the ultraviolet doses received by office workers. Clin. Exp. Dermatol. 1978, 3, 77–79. [Google Scholar] [CrossRef]
- Diffey, B.L.; Tate, T.J.; Davis, A. Solar dosimetry of the face: The relationship of natural ultraviolet radiation exposure to basal cell carcinoma localisation. Phys. Med. Biol. 1979, 24, 931–939. [Google Scholar] [CrossRef]
- Corbett, M.F.; Davis, A.; Magnus, I.A. Personnel radiation dosimetry in drug photosensitivity: Field study of patients on phenothiazine therapy. Br. J. Dermatol. 1978, 98, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Schmalwieser, A.W.; Cabaj, A.; Schauberger, G.; Rohn, H.; Maier, B.; Maier, H. Facial solar UV exposure of Austrian farmers during occupation. Photochem. Photobiol. 2010, 86, 1404–1430. [Google Scholar] [PubMed]
- Diffey, B.L.; Larko, O.; Swanbeck, G. UV-B Doses received during different outdoor activities and UV-B treatment of Psoriasis. Br. J. Dermatol. 1982, 106, 33–41. [Google Scholar] [CrossRef]
- Larkö, O.; Diffey, B.L. Natural UV-B radiation received by people with outdoor, indoor, and mixed occupations and UV-B treatment of psoriasis. Clin. Exp. Dermatol. 1983, 8, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Schothorst, A.A.; Slaper, H.; Schouten, R.; Suurmond, D. UVB doses in maintenance psoriasis phototherapy versus solar UVB exposure. Photodermatology 1985, 2, 213–220. [Google Scholar]
- Schothorst, A.; Slaper, H.; Telgt, D.; Alhadi, B.; Suurmond, D. Amounts of ultraviolet b (UVB) received from sunlight or artificial sources by various population groups in The Netherlands. In Human Exposure to Ultraviolet Radiation: Risks and Regulations; Passchier, W.F., Bosnjakovic, B.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 269–273. [Google Scholar]
- CIE (Commission Internationale de l’Eclairage). Erythema Reference Action Spectrum and Standard Erythema Dose; CIE S007E-1998; CIE Central Bureau: Vienna, Austria, 1998. [Google Scholar]
- Holman, C.D.J.; Gibson, I.M.; Stephenson, M.; Armstrong, B.K. Ultraviolet irradiation of human body sites in relation to occupation and outdoor activity: Field studies using personal UVR dosimeters. Clin. Exp. Dermatol. 1983, 8, 269–277. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Wallisch, S.; Diffey, B. A library of action spectra for erythema and pigmentation. Photochem. Photobiol. Sci. 2012, 11, 251–268. [Google Scholar]
- McKinlay, A.F.; Diffey, B.L. A reference action spectrum for ultra-violet induced erythemal in human skin. In Human Exposure to Ultraviolet Radiation: Risks and Regulations; Passchier, W.F., Bosnjakovic, B.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 83–87. [Google Scholar]
- McKinlay, A.F.; Diffey, B.L. A reference action spectrum for ultraviolet induced erythemal in human skin. CIE J. 1987, 6, 17–22. [Google Scholar]
- Webb, A.R.; Slaper, H.; Koepke, P.; Schmalwieser, A.W. Know your standard: Clarifying the CIE erythema action spectrum. Photochem. Photobiol. 2011, 87, 483–486. [Google Scholar] [CrossRef]
- Rosenthal, F.S.; Phoon, C.; Bakalian, A.E.; Taylor, H.R. The ocular dose of ultraviolet radiation to outdoor workers. Investig. Ophthal. Vis. Sci. 1988, 29, 649–656. [Google Scholar]
- Diffey, B.L.; Cheesman, J. Sun protection with hats. Br. J. Dermatol. 1992, 127, 10–12. [Google Scholar] [CrossRef] [PubMed]
- UNEP; ICNIRP; WHO. Ultraviolet Radiation, Environmental Health Criteria (EHC) 160; WHO: Geneva, Switzerland, 1994. [Google Scholar]
- Krueger, A.; Schoeberl, M.; Newman, P.; Stolarski, R. The 1991 Antarctic ozone hole: TOMS observations. Geophys. Res. Lett. 1992, 19, 1215–1218. [Google Scholar] [CrossRef]
- Stolarski, R.S.; Bloomfield, P.; McPeters, R.D.; Herman, J.R. Total ozone trends deduced from NMBUS 7 TOMS Data. Geophys. Res. Lett. 1991, 18, 1015–1018. [Google Scholar] [CrossRef]
- Diffey, B.L.; Roscoe, A.H. Exposure to solar ultraviolet radiation in flight. Aviat. Space Environ. Med. 1990, 61, 1032–1035. [Google Scholar] [PubMed]
- Rosenthal, F.S.; West, S.K.; Munoz, B.; Emmett, E.A.; Strickland, P.T.; Taylor, H.R. Ocular and facial skin exposure to ultraviolet radiation in sunlight: A personal exposure model with application to a worker population. Health Phys. 1991, 61, 77–86. [Google Scholar] [CrossRef]
- Diffey, B.L. Stratospheric ozone depletion and the risk of non-melanoma skin cancer in a British population. Phys. Med. Biol. 1992, 37, 2267–2279. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Gröbner, J.; Blumthaler, M.; Klotz, B.; De Backer, H.; Bolsée, D.; Werner, R.; Tomsic, D.; Metelka, L.; Eriksen, P.; et al. UV Index monitoring in Europe. Photochem. Photobiol. Sci. 2017, 16, 1349–1370. [Google Scholar] [CrossRef]
- Weatherhead, E. Worldwide monitoring and trend analysis. In Measurements and Trends of Terrestrial UVB Radiation in Europe; Diffey, B.L., Ed.; OEMF: Milano, Italy, 1996; pp. 34–39. [Google Scholar]
- Berger, D.S.; Urbach, F.A. A climatology of sunburning ultraviolet radiation. Photochem. Photobiol. 1982, 35, 187–192. [Google Scholar] [CrossRef]
- Diffey, B.L. The calculation of the spectral distribution of natural ultraviolet radiation under clear day conditions. Phys. Med. Biol. 1977, 22, 309–316. [Google Scholar] [CrossRef]
- Green, A.E.S.; Cross, K.R.; South, L.A. Improved analytical characterization of ultraviolet skylight. Photochem. Photobiol. 1979, 31, 59–65. [Google Scholar] [CrossRef]
- Stamnes, K.; Tsay, S.-C.; Wiscombe, W.; Jayaweera, K. Numerically stable algorithm for discrete-ordinate-method radiative transfer in multiple scattering and emitting layered media. Appl. Opt. 1988, 27, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Kneizys, F.X.; Shettle, E.P.; Abreu, L.W.; Chetwynd, J.H.; Anderson, G.P.; Gallery, W.; Selby, J.E.A.; Clough, S.A. LOWTRAN 7, AFGL-TR-88-0177. Environmental Research Papers No. 1010; Air Force Geophysical Laboratory: Hanscom, MA, USA, 1988. [Google Scholar]
- Koepke, P.; Bais, A.; Balis, D.; Buchwitz, M.; De Backer, H.; de Cabo, X.; Eckert, P.; Eriksen, P.; Gillotay, D.; Heikkilä, A.; et al. Comparison of models used for UV index calculations. Photochem. Photobiol. 1998, 67, 657–662. [Google Scholar] [CrossRef] [PubMed]
- De Backer, H.; Koepke, P.; Bais, A.; De Cabo, X.; Frei, T.; Gillotay, D.; Haite, C.; Heikkilä, A.; Kazantzidis, A.; Koskela, T.; et al. Comparison of measured and modelled uv indices for the assessment of health risks. Meteorol. Appl. 2001, 8, 267–277. [Google Scholar] [CrossRef]
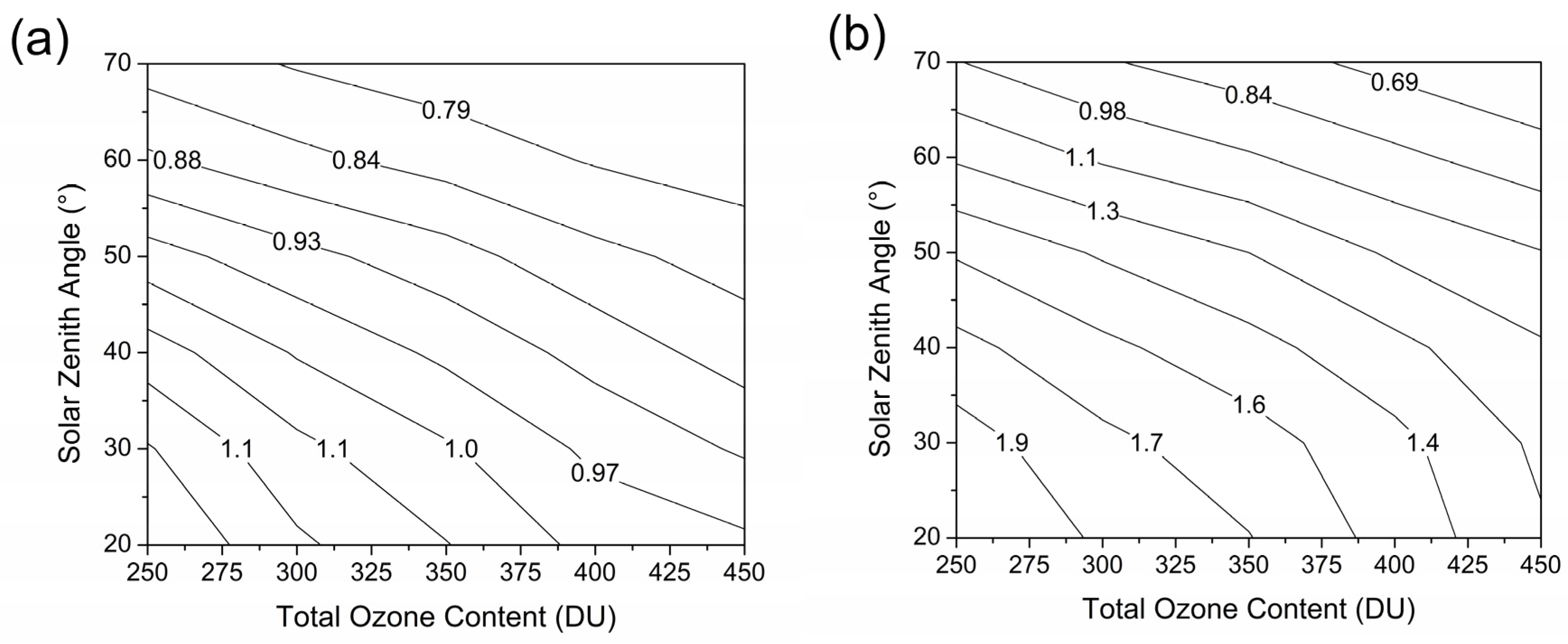
- Schmalwieser, A.W.; Schauberger, G.; Erbertseder, T.; Janouch, M.; Coetzee, G.J.; Weihs, P. Sensitivity of erythemally effective UV irradiance and daily exposure to uncertainties in measured total ozone. Photochem. Photobiol. 2006, 83, 433–443. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Erbertseder, T.; Schauberger, G.; Weihs, P. Sensitivity of UV erythemally effective irradiance and daily dose to spatial variability in total ozone. Photochem. Photobiol. 2008, 84, 1149–1163. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Erbertseder, T.; Schauberger, G.; Weihs, P. Sensitivity of erythemally effective UV irradiance and daily exposure to temporal variability in total ozone. Photochem. Photobiol. 2008, 85, 261–271. [Google Scholar] [CrossRef][Green Version]
- Diffey, B. Personal ultraviolet radiation dosimetry with polysulphone film badges. Photodermatology 1984, 1, 151–157. [Google Scholar]
- Diffey, B.L. A Comparison of dosimeters used for solar ultraviolet radiometry. Photochem. Photobiol. 1987, 46, 55–60. [Google Scholar] [CrossRef]
- CIE (Commission Internationale d’Eclairage). Personal Dosimetry of UV Radiation; Technical Report CIE 98-1992; CIE Central Bureau: Vienna, Austria, 1992. [Google Scholar]
- Wong, C.F.; Fleming, R.; Carter, S.A. A new dosimeter for ultraviolet-B radiation. Photochem. Photobiol. 1989, 50, 611–615. [Google Scholar] [CrossRef]
- Wong, C.F.; Fleming, R.A.; Carter, S.J.; Ring, I.T.; Vishvakarman, D. Measurement of human exposure to ultraviolet-B solar radiation using a CR- 39 dosimeter. Health Phys. 1992, 63, 457–461. [Google Scholar] [CrossRef]
- Berces, A.; Fekete, A.; Gaspar, S.; Grof, P.; Rettberg, P.; Horneck, G.; Ronto, G. Biological UV dosimeters in the assessment of the biological hazard from environmental radiation. J. Photochem. Photobiol. B 1999, 53, 36–43. [Google Scholar] [CrossRef]
- Rettberg, P.; Horneck, G.; Baumstark-Khan, C.; Amanatidis, G.T. (Eds.) Biological UV Dosimetry, a Tool for Assessing the Impact of UV Radiation on Health and Ecosystems; Air Pollution Research Report 7; Office for Official Publications of the European Communities: Luxembourg, 2000. [Google Scholar]
- BWDSUVR (Biologically Weighted Dosimetry of Solar UV Radiation), FP3-ENV 1C, EV5V0342, European Communities, 1994–1995. Available online: https://cordis.europa.eu/project/id/EV5V0342 (accessed on 22 January 2021).
- BIODOS (Development of Biological Dosimetry Systems for Monitoring the Impact of Solar UVB Radiation on the Biosphere and Human Health), FP4-ENV 2C, ENV4950044, European Communities, 1996–1998. Available online: https://cordis.europa.eu/project/id/ENV4950044/de (accessed on 22 January 2021).
- Quintern, L.E.; Horneck, G.; Eschweiler, U.; Buecker, H. A biofilm used as ultraviolet-dosimeter. Photochem. Photobiol. 1992, 55, 389–395. [Google Scholar] [CrossRef]
- Munakata, N. Killing and mutagenic action of sunlight upon Bacillus subtilis spores: A dosimetric system. Mutat. Res. 1981, 82, 263–268. [Google Scholar] [CrossRef]
- Munakata, N.; Kazadzis, S.; Bais, A.F.; Hieda, K.; Rontó, G.; Rettberg, P.; Horneck, G. Comparisons of spore dosimetry and spectral photometry of solar-UV radiation at four sites in Japan and Europe. Photochem. Photobiol. 2000, 72, 739–745. [Google Scholar] [CrossRef]
- Munakata, N.; Cornain, S.; Kanoko, M.; Mulyadi, K.; Lestari, S.; Wirohadidjojo, W.; Bolseé, D.; Kazadzis, S.; Meyer-Rochow, V.; Schuch, N.; et al. Biological Monitoring of Solar-UV Radiation at 17 Sites in Asia, Europe and South America from 1999 to 2004. Photochem. Photobiol. 2006, 82, 689–695. [Google Scholar] [PubMed]
- Munakata, N.; Kazadzis, S.; Bolseé, D.; Schuch, N.; Koskela, T.; Karpetchko, A.; Meleti, C.; Casiccia, C.; Barcellos da Rosa, M.; Saida, T.; et al. Variations and trends of biologically effective doses of solar ultraviolet radiation in Asia, Europe and South America from 1999 to 2007. Photochem. Photobiol. Sci. 2009, 8, 1117–1124. [Google Scholar] [CrossRef]
- Munakata, N. Blologlcally-effective dose of solar ultraviolet radiation estimated by spore dosimetry in Tokyo since 1980. Photochem. Photobiol. 1993, 58, 386–392. [Google Scholar] [CrossRef]
- Quintern, L.E.; Puskeppeleit, M.; Rainer, P.; Weber, S.; el Naggar, S.; Eschweiler, U.; Horneck, G. Continuous dosimetry of the biologically harmful UV-radiation in Antarctica with the biofilm technique. J. Photochem. Photobiol. B 1994, 22, 59–66. [Google Scholar] [CrossRef]
- Horneck, G. Quantification of the biological effectiveness of environmental UV radiation. J. Photochem. Photobiol. B 1995, 31, 43–49. [Google Scholar] [CrossRef]
- Quintern, L.E.; Furusawa, K.; Fukutsu, K.; Holtschmidt, H. Characterization and application of UV detector spore films: The sensitivity curve of a new detector system provides good similarity to the action spectrum for UV-induced erythema in human skin. J. Photochem. Photobiol. B 1997, 37, 158–166. [Google Scholar] [CrossRef]
- Galkin, O.N.; Terenetskaya, I.P. Vitamin D’ biodosimeter: Basic characteristics and potential applications. J. Photochem. Photobiol. B 1999, 53, 12–19. [Google Scholar] [CrossRef]
- Knapp, R.G.; Miller, M.C. Clinical Epidemiology and Biostatistics; Williams and Wilkins: Baltimore, MD, USA, 1992. [Google Scholar]
- Herlihy, E.; Gies, H.P.; Roy, C.R.; Jones, M. Personal dosimetry of solar UVR for different outdoor activities. Photochem. Photobiol. 1994, 60, 288–294. [Google Scholar] [CrossRef] [PubMed]
- ICNIRP (International Commission on Non-Ionizing Radiation Protection). Global Solar UV-Index—WHO⁄WMO⁄ICNIRP Recommendation; ICNIRP publication No.1/95; ICNIRP: Oberschleissheim, Germany, 1995. [Google Scholar]
- CIE (Commission Internationale d’Eclairage). Erythema Reference Action Spectrum and Standard Erythema Dose; ISO/CIE 17166:2019(E); CIE Central Bureau: Vienna, Austria, 2019. [Google Scholar]
- Wester, U. Analytic expressions to represent the hazard ultra-violet action spectrum of ICNIRP and ACGIH. Radiat. Protect. Dosim. 2000, 91, 231–232. [Google Scholar] [CrossRef]
- ICNIRP (International Commission on Non-Ionizing Radiation Protection). Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm ad 400 nm (incoherent optical radiation). Health Phys. 2004, 87, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Vanicek, K.; Frei, T.; Litynska, Z.; Schmalwieser, A. UV Index for the Public; COST-713; European Communities: Brussels, Belgium, 2000. [Google Scholar]
- WHO (World Health Organization). Global Solar UV Index: A Practical User Guide; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Saxebøl, G. UVIh—A proposal for a practical unit for biological effective dose for ultraviolet radiation exposure. Radiat. Prot. Dosim. 2000, 88, 261–264. [Google Scholar] [CrossRef]
- Schmalwieser, A.W. Possibilities to estimate the personal UV radiation exposure from ambient UV radiation measurements. Photochem. Photobiol. Sci. 2020, 19, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- ACGIH (American Conference of Governmental Industrial Hygienists). Threshold Limit Values of Physical Agents Adopted by ACGIH for 1971; ACGIH: Cincinnati, OH, USA, 1971. [Google Scholar]
- Sliney, D.H. The merits of an envelope action spectrum for ultraviolet radiation exposure criteria. Am. Ind. Hyg. Assoc. J. 1972, 33, 644–653. [Google Scholar] [CrossRef]
- IRPA. Guidelines on limits of exposure to ultraviolet radiation of wavelengths between 180 nm and 400 nm (incoherent optical radiation). Health Phys. 1985, 49, 331–340. [Google Scholar]
- IRPA/INIRC. Proposed change to the IRPA 1985 guidelines on limits of exposure to ultraviolet radiation. Health Phys. 1989, 56, 971–972. [Google Scholar]
- National Health and Medical Research Council. Occupational Standard for Exposure to Ultraviolet Radiation; Radiation Health Series No.29; NHMRC: Canberra, Australia, 1989.
- Gies, H.P.; Roy, C.R.; Toomey, S.; MacLennan, R.; Watson, M. Solar UVR exposures of three groups of outdoor workers on the Sunshine Coast, Queensland. Photochem. Photobiol. 1995, 62, 1015–1021. [Google Scholar] [CrossRef]
- Diffey, B.L.; Saunders, P.J. Behavior outdoors and its effects on personal ultraviolet exposure rate measured using an ambulatory datalogging dosimeter. Photochem. Photobiol. 1995, 61, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Gniadecka, M. CaF2: Dy and CaF2 crystal-based UV dosimeters. Skin Res. Technol. 1996, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Gniadecka, M. Electronic UV dosimeters. Skin Res. Technol. 1996, 2, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Naggar, S.E.; Gustat, H.; Magister, H.; Rochlitzer, R. An electronic personal UV-B-dosimeter. J. Photochem. Photobiol. B 1995, 31, 83–86. [Google Scholar] [CrossRef]
- Knuschke, P.; Barth, J. Biologically weighted personal UV dosimetry. J. Photochem. Photobiol. B 1996, 36, 77–83. [Google Scholar] [CrossRef]
- Kimlin, M.G.; Wong, J.C.F.; Parisi, A.V. A simultaneous comparison of the personal UV exposure of two human groups at different altitudes. Health Physics 1998, 74, 429–434. [Google Scholar] [CrossRef][Green Version]
- Blumthaler, M.; Ambach, W.; Rehwald, W. Solar UV-A and UV-B radiation fluxes at two Alpine stations at different altitudes. Theor. Appl. Climatol. 1992, 46, 39–44. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Klotz, B.; Schwarzmann, M.; Baumgartner, D.J.; Schreder, J.; Schauberger, G.; Blumthaler, M. The Austrian UVA-Network. Photochem. Photobiol. 2019, 95, 1258–1266. [Google Scholar] [CrossRef]
- Kimlin, M.G.; Parisi, A.V.; Wong, J.C.F. Quantification of personal solar UV exposure of outdoor workers, indoor workers and adolescents at two locations in Southeast Queensland. Photodermatol. Photoimmunol. Photomed. 1998, 14, 7–11. [Google Scholar] [CrossRef]
- Parisi, A.V.; Meldrum, L.R.; Kimlin, M.G.; Wong, J.C.F.; Aitken, J.; Mainstone, J.S. Evaluation of differences in ultraviolet exposure during weekend and weekday activities. Phys. Med. Biol. 2000, 45, 2253–2262. [Google Scholar] [CrossRef]
- Diffey, B.L.; Gibson, C.J.; Haylock, R.; McKinlay, A.F. Outdoor ultraviolet exposure of children and adolescents. Br. J. Dermatol. 1996, 134, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Kimlin, M.G.; Mainstone, J.S. Variations in solar erythemal ultraviolet occupational exposure due to daylight saving time in Australia. Radiat. Prot. Australas. 1999, 16, 13–20. [Google Scholar]
- Krins, A.; Bolsee, D.; Doerschel, B.; Gillotay, D.; Knuschke, P. Angular dependence of the efficiency of the UV sensor polysulphone film. Radiat. Protect. Dosim. 2000, 87, 261–266. [Google Scholar] [CrossRef]
- Krins, A.; Dorschel, B.; Knuschke, P.; Seidlitz, H.K.; Thiel, S. Determination of the calibration factor of polysulfone film UV dosimeters for terrestrial solar-radiation. Radiat. Protect. Dosim. 2001, 95, 345–352. [Google Scholar] [CrossRef]
- Casale, G.R.; Borra, M.; Colosimo, A.; Colucci, M.; Militello, A.; Siani, A.M.; Sisto, R. Variability among polysulphone calibration curves. Phys. Med. Biol. 2006, 51, 4413–4427. [Google Scholar] [CrossRef]
- Siani, A.M.; Casale, G.R.; Modesti, S.; Parisi, A.V.; Colosimo, A. Investigation on the capability of polysulphone for measuring biologically effective solar UV exposures. Photochem. Photobiol. Sci. 2014, 13, 521–530. [Google Scholar] [CrossRef]
- CIE (Commission Internationale de l’Eclairage). Action Spectrum for the Production of Previtamin D3 in Human Skin; CIE 174:2006; CIE Central Bureau: Vienna, Austria, 2006. [Google Scholar]
- Moehrle, M.; Korn, M.; Garbe, C. Bacillus subtilis spore film dosimeters in personal dosimetry for occupational solar ultraviolet exposure. Int. Arch. Occup. Environ. Health 2000, 73, 575–580. [Google Scholar] [CrossRef]
- Moehrle, M.; Garbe, C. Personal UV dosimetry by Bacillus subtilis spore films. Dermatology 2000, 200, 1–5. [Google Scholar] [CrossRef]
- Moehrle, M.; Dennenmoser, B.; Garbe, C. Continous long-term monitoring of UV radiation in professional mountain guides reveals extreme high exposure. Int. J. Cancer 2003, 103, 775–778. [Google Scholar] [CrossRef]
- Thieden, E.; Ågren, M.S.; Wulf, H.C. Solar UVR exposures of indoor workers in a Working and a Holiday Period assessed by personal dosimeters and sun exposure diaries. Photodermatol. Photoimmunol. Photomed. 2001, 17, 249–255. [Google Scholar] [CrossRef]
- Thieden, E.; Agren, M.S.; Wulf, H.C. The wrist is a reliable body site for personal dosimetry of ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2000, 16, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Vishvakarman, D.; Wong, J.C.F.; Boreham, B.W. Annual occupational exposure to ultraviolet radiation in central Queensland. Health Phys. 2001, 81, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.; Horneck, G.; Rettberg, P.; Arendt, J.; Scherer, K.; Facius, R.; Gugg-Helminger, A. Human exposure to ultraviolet radiation at the, Antipodes—A comparison between an Antarctic (67° S) and Arctic (75° N) location. Polar Biol. 2002, 25, 492–499. [Google Scholar] [CrossRef]
- Cockell, C.S.; Scherer, K.; Horneck, G.; Rettberg, P.; Facius, R.; Gugg-Helminger, A.; Driscoll, C.; Lee, P. Exposure of Arctic Field Scientists to Ultraviolet Radiation Evaluated Using Personal Dosimeters. Photochem. Photobiol. 2001, 74, 570–578. [Google Scholar] [CrossRef]
- Gies, P.; Wright, J. Measured Solar Ultraviolet Radiation Exposures of Outdoor Workers in Queensland in the Building and Construction Industry. Photochem. Photobiol. 2003, 78, 342–348. [Google Scholar] [CrossRef]
- Roy, C.R.; Gies, H.P.; Toomey, S. The solar UV radiation environment: Measurement techniques and results. J. Photochem. Photobiol. B 1995, 31, 21–27. [Google Scholar] [CrossRef]
- Thieden, E.; Philipsen, P.A.; Heydenreich, J.; Wulf, H.C. UV Radiation Exposure Related to Age, Sex, Occupation, and Sun Behavior Based on Time-Stamped Personal Dosimeter Readings. Arch. Dermatol. 2004, 140, 197–203. [Google Scholar] [CrossRef]
- Thieden, E.; Philipsen, P.A.; Wulf, H.C. Compliance and data reliability in sun exposure studies with diaries and personal, electronic UV dosimeters. Photodermatol. Photoimmunol. Photomed. 2006, 22, 93–99. [Google Scholar] [CrossRef]
- Thieden, E.; Philipsen, P.A.; Sandby-Møller, J.; Heydenreich, J.; Wulf, H.C. Proportion of Lifetime UV Dose Received by Children, Teenagers and Adults Based on Time-Stamped Personal Dosimetry. J. Investig. Dermatol. 2004, 123, 1147–1150. [Google Scholar]
- Thieden, E.; Philipsen, P.A.; Sandby-Møller, J.; Wulf, H.C. Sunburn Related to UV Radiation Exposure, Age, Sex, Occupation, and Sun Bed Use Based on Time-Stamped Personal Dosimetry and Sun Behavior Diaries. Arch. Dermatol. 2005, 141, 482–488. [Google Scholar] [CrossRef]
- Thieden, E.; Philipsen, P.A.; Sandby-Møller, J.; Wulf, H.C. Sunscreen Use Related to UV Exposure, Age, Sex, and Occupation Based on Personal Dosimeter Readings and Sun-Exposure Behavior Diaries. Arch. Dermatol. 2005, 141, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Heydenreich, J.; Wulf, H.C. Miniature personal electronic UVR dosimeter with erythema response and time-stamped readings in a wristwatch. Photochem. Photobiol. 2005, 81, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Heydenreich, J.; Wulf, H.C. Personal electronic UVR dosimeter measurements: Specific and general uncertainties. Photochem. Photobiol. Sci. 2019, 18, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B.; Pathak, M.; Parrish, J.A. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In Sunlight and Man: Normal and Abnormal Photobiologic Responses; Pathak, M.A., Harber, L.C., Seiji, M., Kukita, A., Eds.; University of Tokyo Press: Tokyo, Japan, 1974; p. 751. [Google Scholar]
- Thieden, E.; Collins, S.M.; Philipsen, P.A.; Murphy, G.M.; Wulf, H.C. Ultraviolet exposure patterns of Irish and Danish gardeners during work and leisure. Br. J. Dermatol. 2005, 153, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Thieden, E.; Philipsen, P.A.; Wulf, H.C. Ultraviolet radiation exposure pattern in winter compared with summer based on time-stamped personal dosimeter readings. Br. J. Dermatol. 2006, 154, 133–138. [Google Scholar] [CrossRef]
- Weber, M.; Graber, F.; Schulmeister, K.; Brusl, H.; Hann, H.; Kindl, P.; Knuschke, P. Solar UVR exposure of outdoor workers (tinsmiths) in Austria. UV radiation and its effects—An update. J. R. Soc. N. Z. 2006, 68, 99–100. [Google Scholar]
- Turner, J.; Parisi, A.V. Ultraviolet Radiation Albedo and Reflectance in Review: The Influence to Ultraviolet Exposure in Occupational Settings. Int. J. Environ. Res. Public Health 2018, 15, 1507. [Google Scholar] [CrossRef]
- Schmalwieser, A.W.; Schauberger, G. A monitoring network for erythemally-effective solar ultraviolet radiation in Austria: Determination of the measuring sites and visualisation of the spatial distribution. Theor. Appl. Climatol. 2001, 69, 221–229. [Google Scholar] [CrossRef]
- Blumthaler, M.; Klotz, B.; Schwarzmann, M.; Schreder, J. The Austrian UV Monitoring Network. AIP Conf. Proc. 2017, 1810, 110001. [Google Scholar]
- Weber, M.; Uller, A.; Schulmeister, K.; Brusl, H.; Hann, H.; Kindl, P. Outdoor Workers’ Acceptance of Personal Protective Measures Against Solar Ultraviolet Radiation. Photochem. Photobiol. 2007, 83, 1471–1480. [Google Scholar] [CrossRef]
- Weber, M.; Schulmeister, K.; Brusl, H. Parameters influencing the accuracy and practical applicability of UV indicator cards. Photochem. Photobiol. Sci. 2006, 5, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Milon, A.; Sottas, P.-E.; Bulliard, J.-L.; Vernez, D. Effective exposure to solar UV in building workers: Influence of local and individual factors. J. Exp. Sci. Env. Epidem. 2007, 17, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Bener, P. Investigation of the Spectral Intensity of Ultraviolet Sky and Sun + Sky Radiation (between 297.5 and 370 nm) under Different Conditions of Cloudless Weather at 1590 ma.s.l.; Contract AF61(052)-54, Technical Summary n.1; Physikalisch-Meteorologisches Observatorium Davos: Davos Platz, Switzerland, 1960. [Google Scholar]
- Blumthaler, M.; Ambach, W. Human solar ultraviolet radiant exposure in high mountains. Atmos. Environ. 1988, 22, 749–753. [Google Scholar] [CrossRef]
- Simic, S.; Fitzka, M.; Schmalwieser, A.; Weihs, P.; Hadzimustafic, J. Factors affecting UV irradiance at selected wavelengths at Hoher Sonnblick. Atmos. Res. 2011, 101, 869–878. [Google Scholar] [CrossRef]
- Siani, A.M.; Casale, G.R.; Diémoz, H.; Agnesod, G.; Kimlin, M.G.; Lang, C.A.; Colosimo, A. Personal UV exposure in high albedo alpine sites. Atmos. Chem. Phys. 2008, 8, 3749–3760. [Google Scholar] [CrossRef]
- CIE (Commission Internationale d’Eclairage). CIE 1976 Uniform Color Spaces, Colorimetry; CIE Publication: Vienna, Austria, 1976; pp. 29–32. [Google Scholar]
- Casale, G.R.; Siani, A.M.; Diémoz, H.; Agnesod, G.; Parisi, A.V.; Colosimo, A. Extreme UV index and solar exposures at plateau rosà (3500 ma.s.l.) in valle d’aosta Region, Italy. Sci. Total Environ. 2015, 512–513, 622–630. [Google Scholar] [CrossRef]
- Gies, P.; Glanz, K.; O’Riordan, D.; Elliott, T.; Nehl, E. Measured Occupational Solar UVR Exposures of Lifeguards in Pool Settings. Am. J. Ind. Med. 2009, 52, 645–653. [Google Scholar] [CrossRef]
- O’Riordan, D.L.; Glanz, K.; Gies, P.; Elliott, T. A Pilot Study of the Validity of Self-reported Ultraviolet Radiation Exposure and Sun Protection Practices Among Lifeguards, Parents and Children. Photochem. Photobiol. 2008, 84, 774–778. [Google Scholar] [CrossRef]
- Serrano, M.A.; Canada, J.; Moreno, J.C. Erythemal Ultraviolet Exposure in Two Groups of Outdoor Workers in Valencia, Spain. Photochem. Photobiol. 2009, 85, 1468–1473. [Google Scholar] [CrossRef]
- Gies, P.; Watzl, R.; Javorniczky, J.; Roy, C.; Henderson, S.; Kingston, J.A.M. Measurement of the UVR Exposures of Expeditioners on Antarctic Resupply Voyages. Photochem. Photobiol. 2009, 85, 1485–1490. [Google Scholar] [CrossRef]
- Glanz, K.; Gies, P.; O’Riordan, D.L.; Elliott, T.; Nehl, E.; McCarty, F.; Davis, E. Validity of self-reported solar UVR exposure compared with objectively measured UVR exposure. Cancer Epidemiol. Biomark. Prev. 2010, 19, 3005–3012. [Google Scholar] [CrossRef] [PubMed]
- Hammond, V.; Reeder, A.I.; Gray, A. Patterns of real-time occupational ultraviolet radiation exposure among a sample of outdoor workers in New Zealand. Public Health 2009, 123, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.; McKenzie, R. Enhanced UV exposure on a ski-field compared with exposures at sea level. Photochem. Photobiol. Sci. 2005, 4, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.W.; Swift, N.; Nield, K.M.; Liley, B.; McKenzie, R.L. Use of Electronic UV Dosimeters in Measuring Personal UV Exposures and Public Health Education. Atmosphere 2020, 11, 744. [Google Scholar]
- Schmid-Kubista, K.E.; Kellner, L.; Maier, H.; Felke, S.; Wanka, A.; El Modeir, A.; Schmidt, J.B.; Cabaj, A.; Schmalwieser, A.; Rohn, H.; et al. Effect of work-related ultraviolet exposure and ophthalmic changes in Austrian farmers: The SVB-UV study. Ophthalmic Res. 2010, 43, 201–207. [Google Scholar] [CrossRef]
- Maier, H.; Schmalwieser, A.W. Sunscreens and occupation: The Austrian experience. Photochem. Photobiol. Sci. 2010, 9, 510–515. [Google Scholar] [PubMed]
- Siani, A.M.; Casale, G.R.; Sisto, R.; Colosimo, A.; Lang, C.A.; Kimlin, M.G. Occupational Exposures to Solar Ultraviolet Radiation of Vineyard Workers in Tuscany (Italy). Photochem. Photobiol. 2011, 87, 925–934. [Google Scholar] [PubMed]
- Serrano, M.-A.; Cañada, J.; Moreno, J.C. Solar UV exposure in construction workers in Valencia, Spain. J. Expo. Sci. Environ. Epidemiol. 2012, 23, 525–530. [Google Scholar]
- Feister, U.; Meyer, G.; Kirst, U. Solar UV radiation exposure of seamen—Measurements, calibration and model calculations of erythemal irradiance along ship routes. AIP Conf. Proc. 2013, 1531, 860–863. [Google Scholar]
- Feister, U.; Meyer, G.; Kirst, U. Solar UV exposure of seafarers along subtropical and tropical shipping Routes. Photochem. Photobiol. 2013, 89, 1497–1506. [Google Scholar]
- Cheng, I.; Kiss, A.; Lilge, L. An Observational Study of Personal Ultraviolet Dosimetry and Acute Diffuse Reflectance Skin Changes at Extreme Altitude. Wilderness Environ. Med. 2013, 24, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Sandberg Liljendahl, T.; Blomqvist, A.; Andersson, E.M.; Barregard, L.; Segerbäck, D. Urinary levels of thymine dimer as a biomarker of exposure to ultraviolet radiation in humans during outdoor activities in the summer. Mutagenesis 2013, 28, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. 1916, 17, 863–871. [Google Scholar] [CrossRef]
- Landelius, T.; Josefsson, W.; Persson, T. SMHI (STRÅNG): A System for Modelling Solar Radiation Parameters with Mesoscale Spatial Resolution; SMHI Reports, RMK. No. 96; SMHI: Norrkoping, Sweden, 2001.
- Wolska, A. Occupational exposure to solar ultraviolet radiation of polish outdoor workers: Risk estimation method and criterion. Int. J. Occup. Saf. Ergon. 2013, 19, 107–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Downs, N.J.; Parisi, A.V.; Igoe, D. Measurements of occupational ultraviolet exposure and the implications of timetabled yard duty for school teachers in Queensland, Australia: Preliminary results. J. Photochem. Photobiol. B 2014, 31, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lester, R.A.; Parisi, A.V.; Kimlin, M.G.; Sabburg, J. Optical Properties of poly(2,6-dimethyl-1,4-phenylene Oxide) Film and Its Potential for a Long-Term Solar Ultraviolet Dosimeter. Phys. Med. Biol. 2003, 22, 3685–3698. [Google Scholar] [CrossRef][Green Version]
- Downs, N.J.; Harrison, S.L.; Garzon Chavez, D.R.; Parisi, A.V. Solar ultraviolet and the occupational radiant exposure of Queensland school teachers: A comparative study between teaching classifications and behavior patterns. J. Photochem. Photobiol. B 2016, 158, 105–112. [Google Scholar] [CrossRef]
- Downs, N.J.; Igoe, D.P.; Parisi, A.V.; Taylor, O.; Lazzaroni, S.L.; Rawlings, A.; Garzon-Chavez, D.R.; Harrison, S.L. Seasonal Minimum and Maximum Solar Ultraviolet Exposure Measurements of Classroom Teachers Residing in Tropical North Queensland, Australia. Photochem. Photobiol. 2019, 95, 1083–1093. [Google Scholar] [CrossRef]
- Serrano, M.-A.; Cañada, J.; Moreno, J.C.; Gurrea, G. Occupational UV exposure of environmental agents in Valencia, Spain. Photochem. Photobiol. 2014, 90, 911–918. [Google Scholar] [CrossRef]
- Bodekær, M.; Harrison, G.I.; Philipsen, P.; Petersen, B.; Triguero-Mas, M.; Schmalwieser, A.W.; Rogowski-Tylman, M.; Dadvand, P.; Lesiak, A.; Narbutt, J.; et al. Personal UVR exposure of farming families in four European countries. J. Photochem. Photobiol. B 2015, 153, 267–275. [Google Scholar] [CrossRef]
- Bodekær, M.; Petersen, B.; Thieden, E.; Philipsen, P.; Heydenreich, J.; Olsen, P.; Wulf, H.C. UVR exposure and vitamin D in a rural population. A study of outdoor working farmers, their spouses and children. Photochem. Photobiol. Sci. 2014, 13, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Makgabutlane, M.; Wright, C.Y. Real-time measurements of outdoor worker’s exposure to solar ultraviolet radiation in Pretoria, South Africa. South Afr. J. Sci. 2015, 111, 1–7. [Google Scholar]
- Nkogatse, M.M.; Ramotsehoa, M.C.; Eloff, F.C.; Wright, C.Y. Solar Ultraviolet Radiation Exposure and Sun Protection Behaviors and Knowledge Among a High-Risk and Overlooked Group of Outdoor Workers in South Africa. Photochem. Photobiol. 2019, 95, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Rascon, D.S.; Ferreira, A.D.; da Silva, M.G. Cumulative and momentary skin exposures to solar radiation in central receiver solar systems. Energy 2017, 137, 336–349. [Google Scholar] [CrossRef]
- ICNIRP (International Commission on Non-Ionizing Radiation Protection). Protecting Workers from Ultraviolet Radiation; ICNIRP: Oberschleißheim, Germany, 2007. [Google Scholar]
- Grandahl, K.; Eriksen, P.; Ibler, K.S.; Bonde, J.P.; Mortensen, O.S. Measurements of Solar Ultraviolet Radiation Exposure at Work and at Leisure in Danish Workers. Photochem. Photobiol. 2018, 94, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Grandahl, K.; Mortensen, O.S.; Sherman, D.Z.; Køster, B.; Lund, P.-A.; Ibler, K.S.; Eriksen, P. Solar UV exposure among outdoor workers in Denmark measured with personal UV-B dosimeters: Technical and practical feasibility. BioMed. Eng. OnLine 2017, 16, 119. [Google Scholar] [CrossRef]
- Peters, C.E.; Demers, P.A.; Kalia, S.; Nicol, A.-M.; Koehoorn, M.W. Levels of Occupational Exposure to Solar Ultraviolet Radiation in Vancouver, Canada. Occup. Hyg. 2016, 60, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.E.; Pasko, E.; Strahlendorf, P.; Holness, D.L.; Tenkate, T. Solar Ultraviolet Radiation Exposure Among Outdoor Workers in Three Canadian Provinces. Ann. Work Expo. Health 2019, 63, 679–688. [Google Scholar] [CrossRef]
- Rydz, E.; Harper, A.; Leong, B.; Arrandale, V.H.; Kalia, S.; Forsman-Phillips, L.; Holness, D.L.; Tenkate, T.; Peters, C.E. Solar ultraviolet radiation exposure among outdoor workers in Alberta, Canada. Environ. Res. 2020, 189, 109902. [Google Scholar] [CrossRef]
- Alshurafa, N.; Jain, J.; Stump, T.K.; Spring, B.; Robinson, J.K. Assessing recall of personal sun exposure by integrating UV dosimeter and selfreported data with a network flow framework. PLoS ONE 2019, 14, e0225371. [Google Scholar] [CrossRef]
- Schoder, D.; Heydenreich, J.; Schmalwieser, A.W. First Results From Personal UV Exposure Measurements In Kenya. In Proceedings of the ESP-IUBP World Congress, Barcelona, Spain, 25–30 August 2019; p. 477. [Google Scholar]
- Wittlich, M.; Westerhausen, S.; Kleinespel, P.; Rifer, G.; Stöppelmann, W.J. An approximation of occupational lifetime UVR exposure: Algorithm for retrospective assessment and current measurements. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 3), 27–33. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, H.R.; Wittlich, M.; John, S.M.; Brans, R.; Tiplica, G.S.; Salavastru, C.; Toader, S.; Voidazan, S.T.; Duca, R.C.; Fugulyan, E.; et al. Exposure to solar UV radiation in outdoor construction workers using personal dosimetry. Environ. Res. 2020, 181, 108967. [Google Scholar] [CrossRef] [PubMed]
- Kovačić, J.; Wittlich, M.; John, S.M.; Macan, J. Personal ultraviolet radiation dosimetry and its relationship with environmental data: A longitudinal pilot study in Croatian construction workers. J. Photochem. Photobiol. B 2020, 207, 111866. [Google Scholar] [CrossRef] [PubMed]
- Wittlich, M.; John, S.M.; Tiplica, G.S.; Sălăvăstru, C.M.; Butacu, A.I.; Modenese, A.; Paolucci, V.; D’Hauw, G.; Gobba, F.; Sartorelli, P.; et al. Personal solar ultraviolet radiation dosimetry in an occupational setting across Europe. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Baczynska, K.A.; Brown, S.; Chorley, A.C.; Lyachev, A.; Wittlich, M.; Khazova, M. Me asurements of UV—A Exposure of Commercial Pilots Using Genesis-UV Dosimeters. Atmosphere 2020, 11, 475. [Google Scholar] [CrossRef]
- Baczynska, K.A.; Brown, S.; Chorley, A.C.; O’Hagan, J.B.; Khazova, M.; Lyachev, A.; Wittlich, M. In-flight UV-A exposure of commercial airline pilots. Aerosp. Med. Hum. Perform. 2020, 91, 1–10. [Google Scholar] [CrossRef]
- Parisi, A.V.; Turnbull, D.J.; Kimlin, M.G. Dosimetric and spectroradiometric investigations of glass filtered solar UV. Photochem. Photobiol. 2007, 83, 777–781. [Google Scholar] [CrossRef]
- Parisi, A.V.; Wong, J.C. The erythemal ultraviolet exposure for humans in greenhouses. Phys. Med. Biol. 1997, 42, 2331–2339. [Google Scholar] [CrossRef][Green Version]
- García-Ruiz, R.A.; López-Martínez, J.; Blanco-Claraco, J.L.; Pérez-Alonso, J.; Callejón-Ferre, Á.J. Ultraviolet Index (UVI) inside an Almería-Type Greenhouse (Southeastern Spain). Agronomy 2020, 10, 145. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Nioi, A.; Wendelboe-Nelson, C.; Cowan, S.; Cherrie, M.; Rashid, S.; Cowie, H.; Ritchie, P.; Lansdown, T.C. Exposure to Solar UV During Outdoor Construction Work in Britain. Ann. Work Expo. Health 2020, wxaa028. [Google Scholar] [CrossRef]
- IUPAC (International Union of Pure and Applied Chemistry). Glossary of Terms Used in Photochemistry 3rd Edition (IUPAC Recommendations 2006). Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- EN 14255-3:2008 Measurement and Assessment of Personal Exposures to Incoherent Optical Radiation—Part 3: UV-Radiation Emitted by the Sun; European Communities: Brussels, Belgium, 2008.
- EN 14255-4:2006 Measurement and Assessment of Personal Exposures to Incoherent Optical Radiation—Part 4: Terminology and Quantities Used in UV-, Visible and IR-Exposure Measurements; European Communities: Brussels, Belgium, 2008.
- Knuschke, P. UV Exposure. In Kanerva’s Occupational Dermatology; John, S., Johansen, J., Rustemeyer, T., Elsner, P., Maibach, H., Eds.; Springer: Cham, Switzerland, 2018; p. 36. [Google Scholar]
- Sliney, D.H. Radiometric Quantities and Units Used in Photobiology and Photochemistry: Recommendations of the Commission Internationale de l’Eclairage (International Commission on Illumination). Photochem. Photobiol. 2007, 83, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Thomas Tenkate, T.; Adam, B.; Al-Rifai, R.H.; Chou, B.R.; Gobba, F.; Ivanov, I.D.; Leppink, N.; Loney, T.; Pega, F.; Peters, C.E.; et al. WHO/ILO work-related burden of disease and injury: Protocol for systematic reviews of occupational exposure to solar ultraviolet radiation and of the effect of occupational exposure to solar ultraviolet radiation on cataract. Environ. Int. 2019, 125, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Silva Paulo, M.; Adam, B.; Akagwu, C.; Akparibo, I.; Al-Rifaia, R.H.; Bazrafshane, S.; Gobbaf, F.; Green, A.C.; Ivanov, I.; Kezic, S.; et al. WHO/ILO work-related burden of disease and injury: Protocol for systematic reviews of occupational exposure to solar ultraviolet radiation and of the effect of occupational exposure to solar ultraviolet radiation on melanoma and non-melanoma skin cancer. Environ. Int. 2019, 126, 804–815. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmalwieser, A.W.; Casale, G.R.; Colosimo, A.; Schmalwieser, S.S.; Siani, A.M. Review on Occupational Personal Solar UV Exposure Measurements. Atmosphere 2021, 12, 142. https://doi.org/10.3390/atmos12020142
Schmalwieser AW, Casale GR, Colosimo A, Schmalwieser SS, Siani AM. Review on Occupational Personal Solar UV Exposure Measurements. Atmosphere. 2021; 12(2):142. https://doi.org/10.3390/atmos12020142
Chicago/Turabian StyleSchmalwieser, Alois W., Giuseppe R. Casale, Alfredo Colosimo, Susanne S. Schmalwieser, and Anna Maria Siani. 2021. "Review on Occupational Personal Solar UV Exposure Measurements" Atmosphere 12, no. 2: 142. https://doi.org/10.3390/atmos12020142
APA StyleSchmalwieser, A. W., Casale, G. R., Colosimo, A., Schmalwieser, S. S., & Siani, A. M. (2021). Review on Occupational Personal Solar UV Exposure Measurements. Atmosphere, 12(2), 142. https://doi.org/10.3390/atmos12020142






