Moisture Removal Techniques for a Continuous Emission Monitoring System: A Review
Abstract
1. Introduction
HCO3− + H2O(aq) ↔ CO32− + H3O+
NH3(g) + H2O(aq) ↔ NH4+ + OH−
NH3(g) + HCO3− ↔ NH2COO− + H2O
SO2(g) + 2H2O(aq) ↔ H3O+ + HSO3−
2NO2(g) + H2O(aq) ↔ NO2- + 2H+ + NO3−
2NO2(g) + HSO3−+ H2O(aq) ↔ 3H+ + 2NO2− + SO42−
SO2(g) + H2O (aq) → H2SO3
NH3(g)+SO2(g)+H2O(g) ↔ NH4HSO3(s)
2NH3(g)+NO2(g)+H2O(g) ↔ (NH4)2NO3(s)
NH3(g) + NO2(g) + H2O(g) ↔ NH4HNO3(s)
2. Methodology
3. Results and Discussion
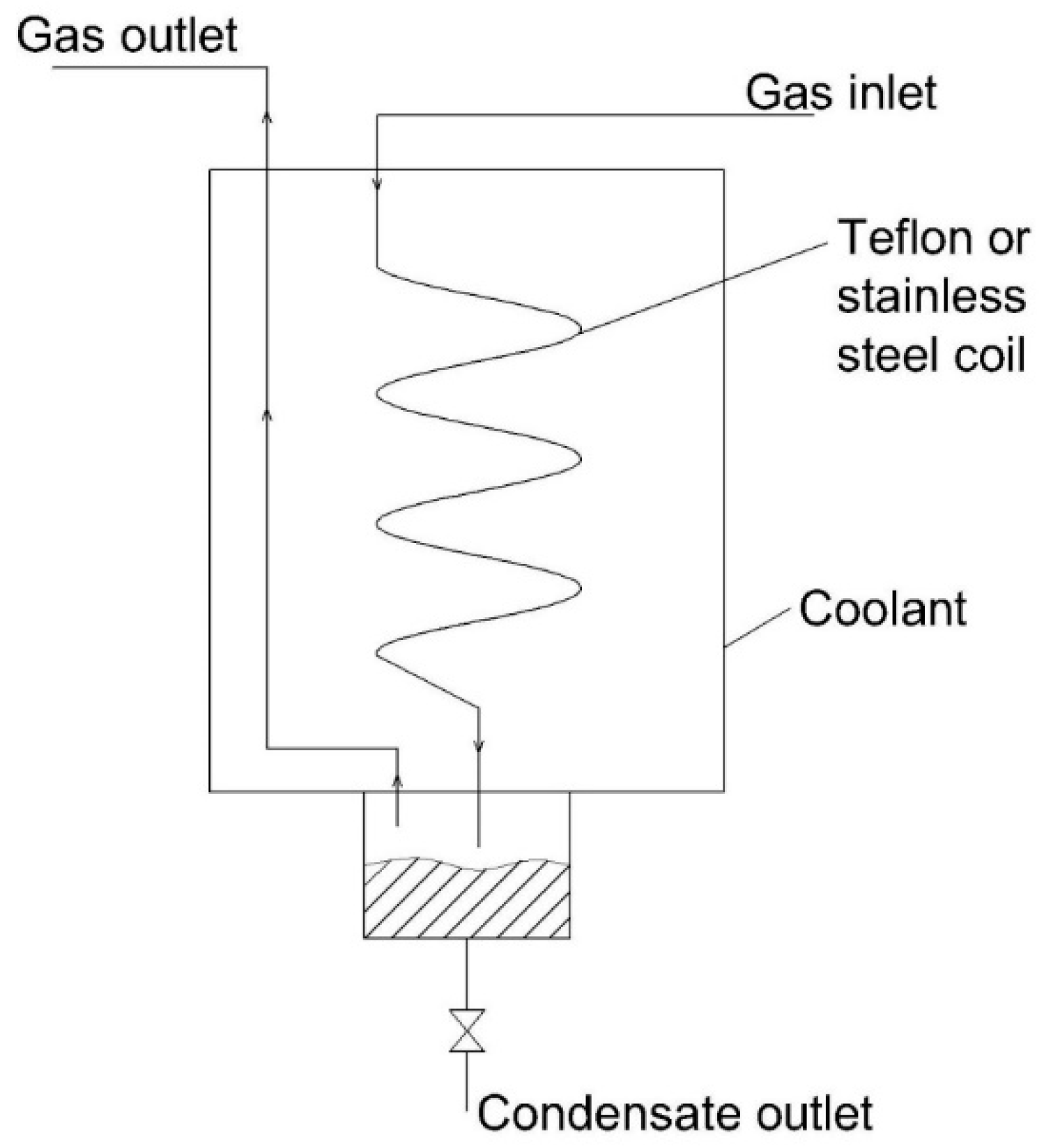
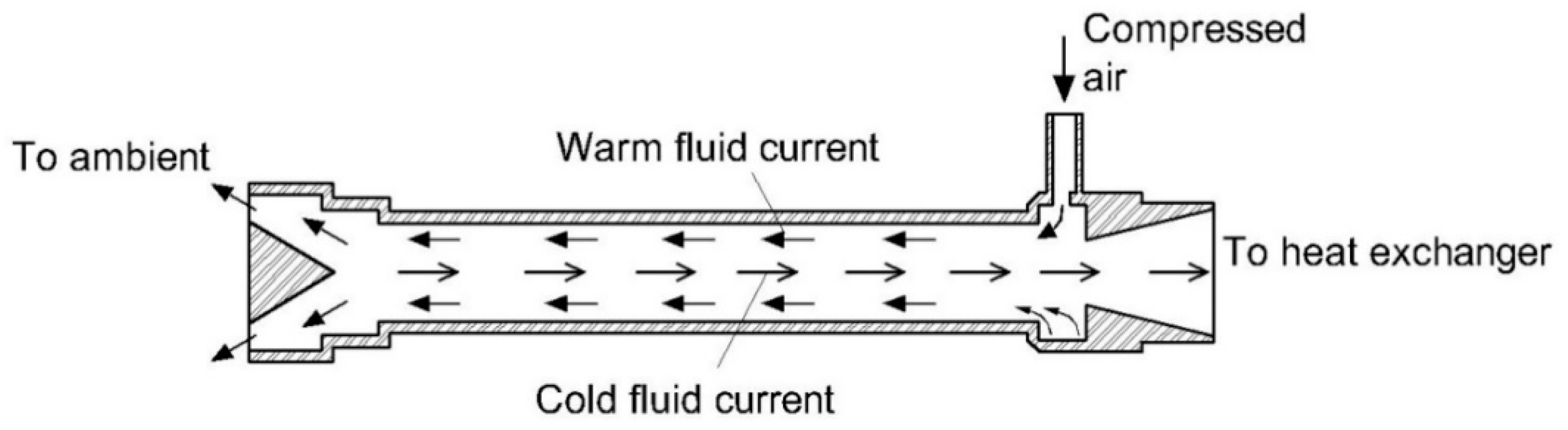
3.1. Condensation Method
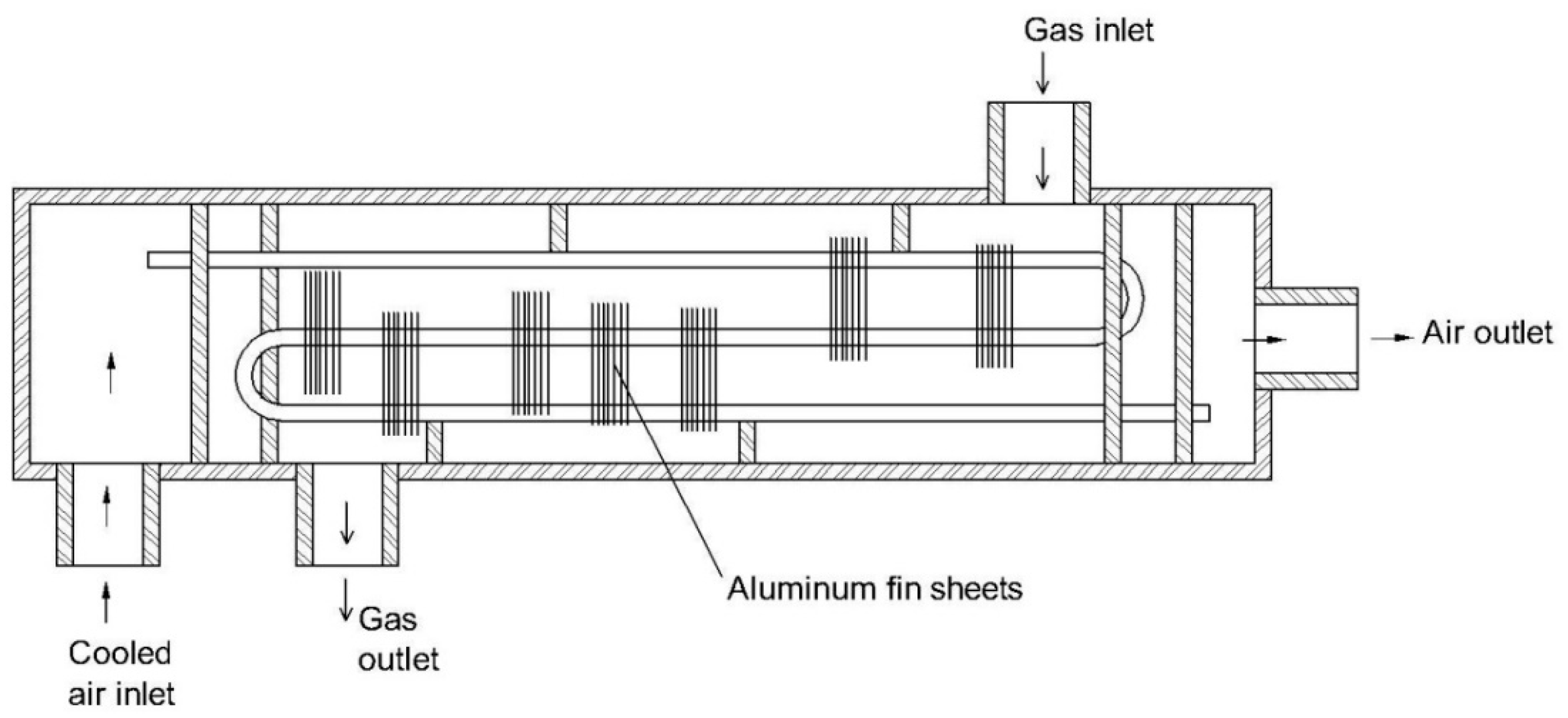
3.2. Permeation Method
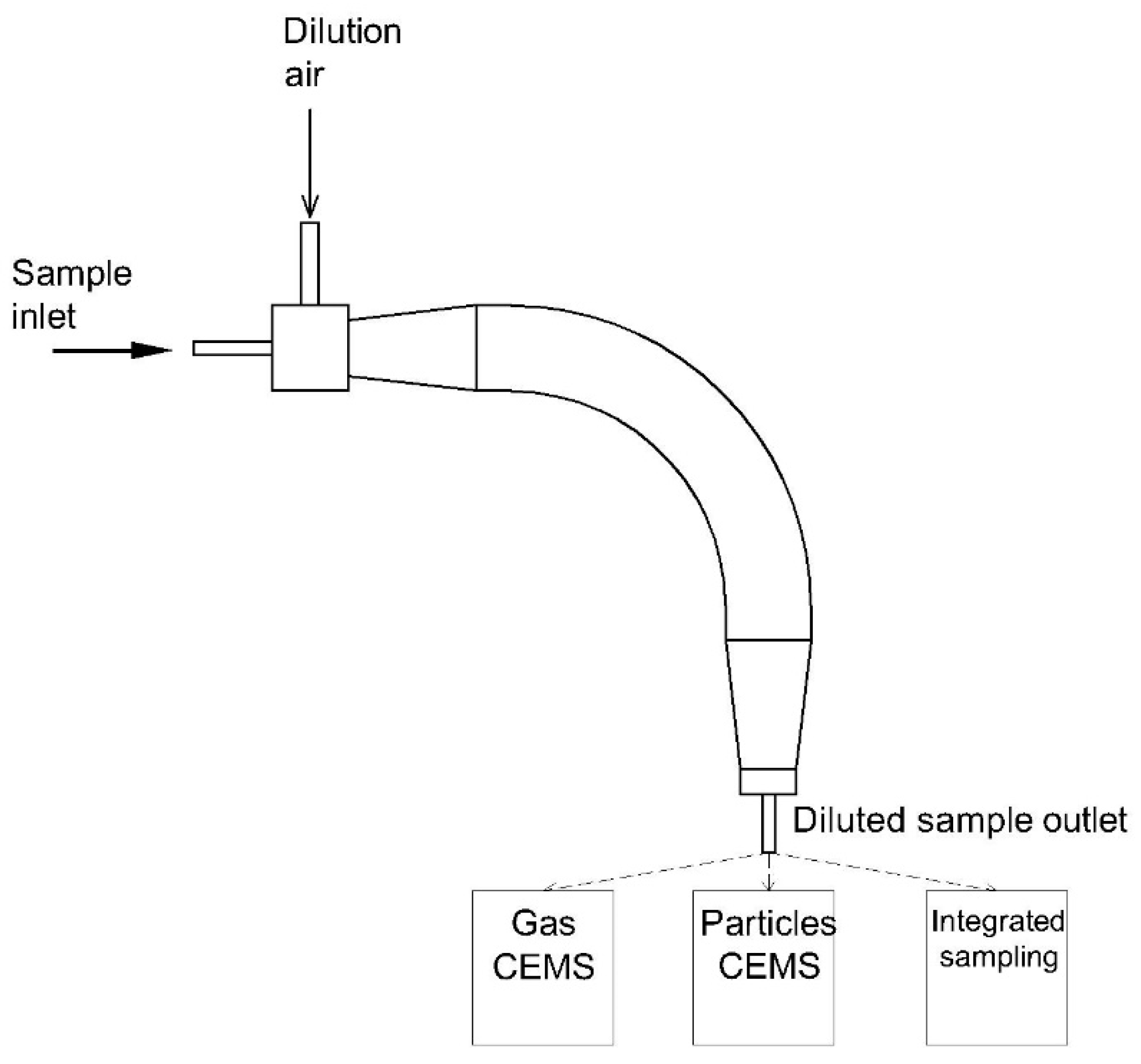
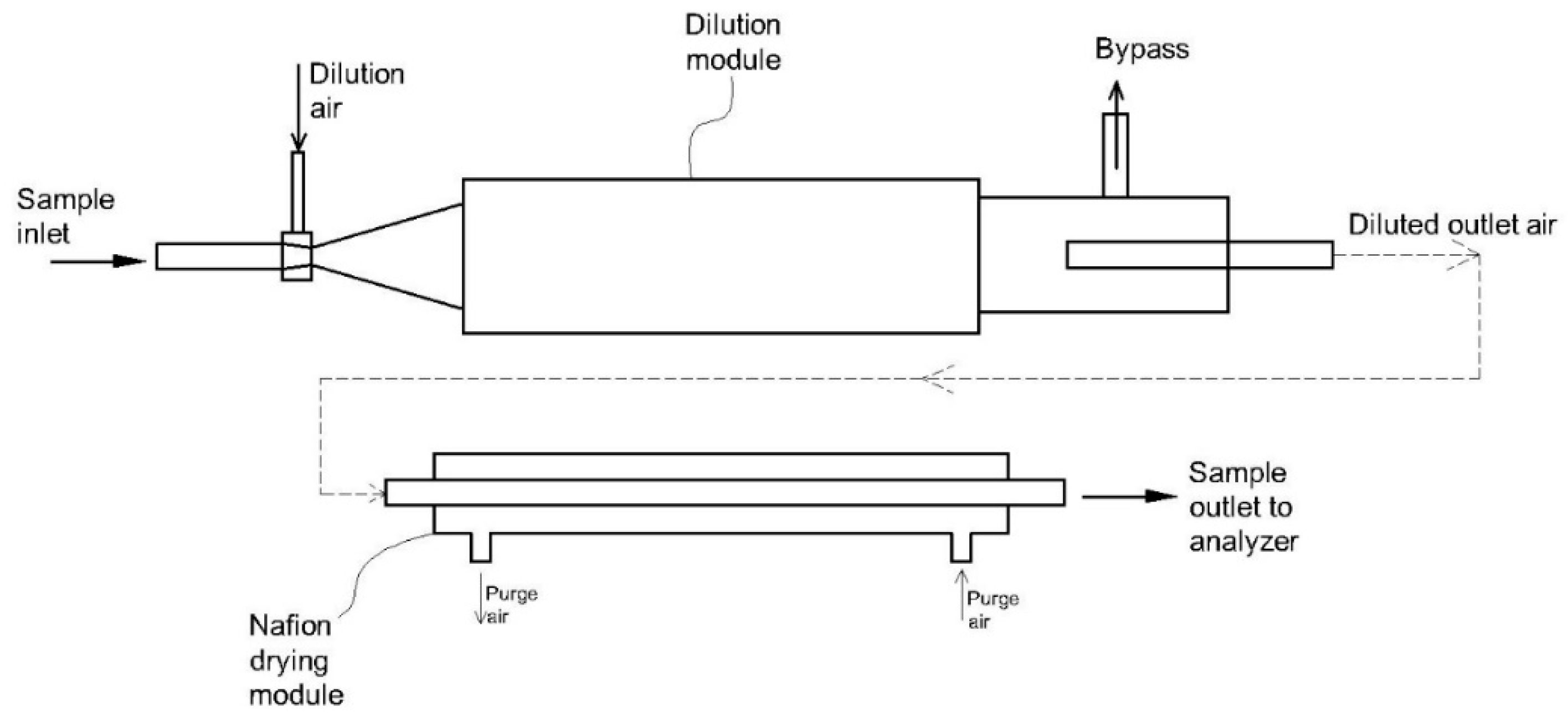
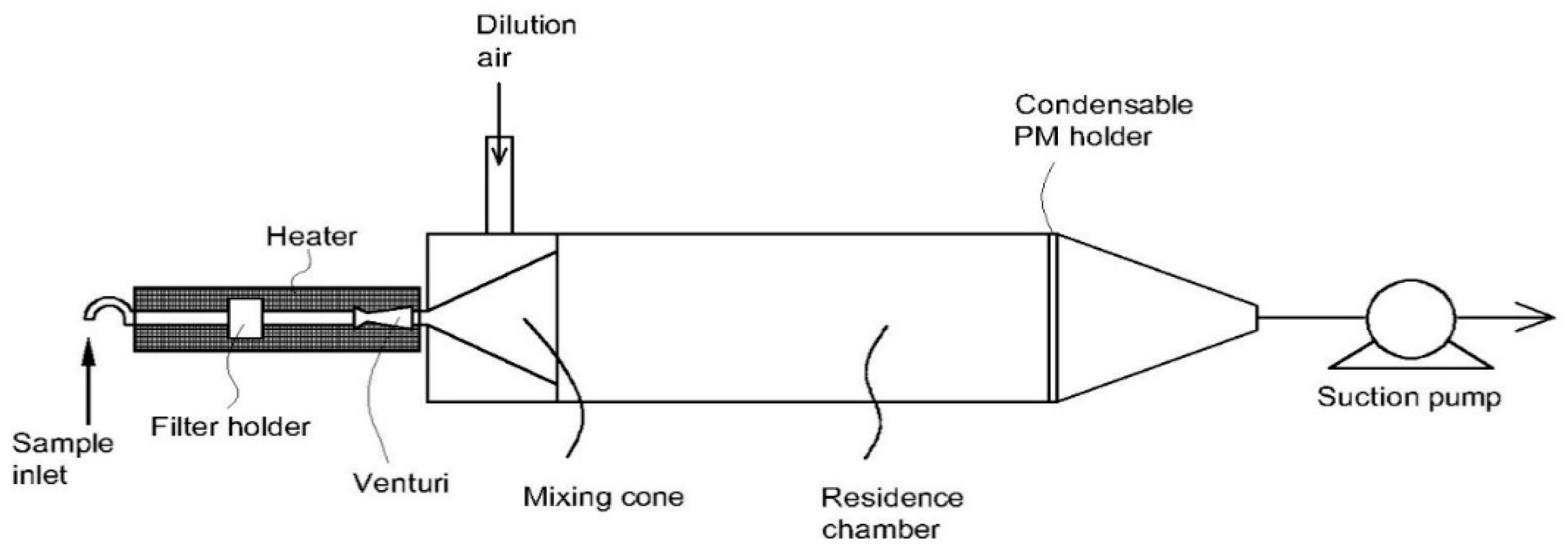
3.3. Dilution Method
4. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jahnke, J.A. Continuous Emission Monitoring, 2nd ed.; John Wiley & Sons, Inc.: Toronto, ON, Canada, 2000; ISBN 0-471-29227-3. [Google Scholar]
- Lillesand, T.; Kiefer, R.W.; Chipman, J. Remote Sensing and Image Interpretation; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Dinh, T.-V.; Choi, I.-Y.; Son, Y.-S.; Kim, J.-C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Q.; Zeng, Y.; Wang, S.-M.; Huang, S.-H.; Xie, P.; Yu, X.-M. Water Vapor Interference Correction in a Non Dispersive Infrared Multi-Gas Analyzer. Chin. Phys. Lett. 2011, 28, 073302. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Maricq, M.M.; Ntziachristos, L.; Dardiotis, C.; Wang, X.; Axmann, H.; Bergmann, A.; Schindler, W. Review of motor vehicle particulate emissions sampling and measurement: From smoke and filter mass to particle number. J. Aerosol. Sci. 2014, 67, 48–86. [Google Scholar] [CrossRef]
- Amaral, S.S., Jr.; De Carvalho, J.A.C.; Costa, M.A.M.; Pinheiro, C. An Overview of Particulate Matter Measurement Instruments. Atmosphere 2015, 6, 1327–1345. [Google Scholar] [CrossRef]
- Leskinen, J.; Tissari, J.; Uski, O.; Virén, A.; Torvela, T.; Kaivosoja, T.; Lamberg, H.; Nuutinen, I.; Kettunen, T.; Joutsensaari, J.; et al. Fine particle emissions in three different combustion conditions of a wood chip-fired appliance—Particulate physico-chemical properties and induced cell death. Atmos. Environ. 2014, 86, 129–139. [Google Scholar] [CrossRef]
- Jiang, R.; Bell, M.L. A Comparison of Particulate Matter from Biomass-Burning Rural and Non-Biomass-Burning Urban Households in Northeastern China. Environ. Health Perspect. 2008, 116, 907–914. [Google Scholar] [CrossRef]
- Torvela, T.; Tissari, J.; Sippula, O.; Kaivosoja, T.; Leskinen, J.; Virén, A.; Lahde, A.; Jokiniemi, J. Effect of wood combustion conditions on the morphology of freshly emitted fine particles. Atmos. Environ. 2014, 87, 65–76. [Google Scholar] [CrossRef]
- Krecl, P.; Ström, J.; Johansson, C. Carbon content of atmospheric aerosols in a residential area during the wood combustion season in Sweden. Atmos. Environ. 2007, 41, 6974–6985. [Google Scholar] [CrossRef]
- Mellon, D.; King, S.J.; Kim, J.; Reid, J.P.; Orr-Ewing, A.J. Measurements of Extinction by Aerosol Particles in the Near-Infrared Using Continuous Wave Cavity Ring-Down Spectroscopy. J. Phys. Chem. A 2011, 115, 774–783. [Google Scholar] [CrossRef]
- Pettersson, A.; Lovejoy, E.R.; Brock, C.A.; Brown, S.S.; Ravishankara, A. Measurement of aerosol optical extinction at with pulsed cavity ring down spectroscopy. J. Aerosol. Sci. 2004, 35, 995–1011. [Google Scholar] [CrossRef]
- Elsasser, M.; Crippa, M.; Orasche, J.; Decarlo, P.F.; Oster, M.; Pitz, M.; Cyrys, J.; Gustafson, T.L.; Pettersson, J.B.C.; Schnellekreis, J.; et al. Organic molecular markers and signature from wood combustion particles in winter ambient aerosols: Aerosol mass spectrometer (AMS) and high time-resolved GC-MS measurements in Augsburg, Germany. Atmos. Chem. Phys. Discuss. 2012, 12, 6113–6128. [Google Scholar] [CrossRef]
- Hosseini, S.; Li, Q.; Cocker, D.; Weise, D.; Miller, A.; Shrivastava, M.; Miller, J.W.; Mahalingam, S.; Princevac, M.; Jung, H. Particle size distributions from laboratory-scale biomass fires using fast response instruments. Atmos. Chem. Phys. Discuss. 2010, 10, 8065–8076. [Google Scholar] [CrossRef]
- Vincent, J.H. Aerosol Sampling; John Wiley & Sons, Ltd.: Chichester, UK, 2007; ISBN 9780470060230. [Google Scholar]
- Coudray, N.; Dieterlen, A.; Roth, E.; Trouvé, G. Density measurement of fine aerosol fractions from wood combustion sources using ELPI distributions and image processing techniques. Fuel 2009, 88, 947–954. [Google Scholar] [CrossRef]
- Castellani, B.; Morini, E.; Filipponi, M.; Nicolini, A.; Palombo, M.; Cotana, F.; Rossi, F. Comparative Analysis of Monitoring Devices for Particulate Content in Exhaust Gases. Sustainability 2014, 6, 4287–4307. [Google Scholar] [CrossRef]
- Lundgren, D.A.; Cooper, D.W. Effect of Humidify on Light-Scattering Methods of Measuring Particle Concentration. J. Air Pollut. Control. Assoc. 1969, 19, 243–247. [Google Scholar] [CrossRef]
- Zieger, P.; Fierz-Schmidhauser, R.; Weingartner, E.; Baltensperger, U. Effects of relative humidity on aerosol light scattering: Results from different European sites. Atmos. Chem. Phys. Discuss. 2013, 13, 10609–10631. [Google Scholar] [CrossRef]
- Jayaratne, R.; Liu, X.; Thai, P.; Dunbabin, M.; Morawska, L. The influence of humidity on the performance of a low-cost air particle mass sensor and the effect of atmospheric fog. Atmos. Meas. Tech. 2018, 11, 4883–4890. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, H.; Zhou, H. Fine Particle Sensor Based on Multi-Angle Light Scattering and Data Fusion. Sensors 2017, 17, 1033. [Google Scholar] [CrossRef]
- US EPA. An Operator’s Guide to Eliminating Bias in CEM Systems; United States Environmental Protection Agency: Washington, DC, USA, 1994.
- US EPA. EPA Handbook–Continuous Emission. Monitoring Systems for Non-Criteria Pollutants; United States Environmental Protection Agency: Washington, DC, USA, 1997.
- US EPA. Ammonia CEMS Background; United States Environmental Protection Agency: Washington, DC, USA, 1993.
- Anglada, J.M.; Martins-Costa, M.T.C.; Francisco, J.S.; Ruiz-López, M.F. Triplet state promoted reaction of SO2 with H2O by competition between proton coupled electron transfer (pcet) and hydrogen atom transfer (hat) processes. Phys. Chem. Chem. Phys. 2019, 21, 9779–9784. [Google Scholar] [CrossRef]
- Li, K.; Yu, H.; Qi, G.; Feron, P.; Tade, M.; Yu, J.; Wang, S. Rate-based modelling of combined SO2 removal and NH3 recycling integrated with an aqueous NH3 -based CO2 capture process. Appl. Energy 2015, 148, 66–77. [Google Scholar] [CrossRef]
- Seo, J.-B.; Jeon, S.-B.; Choi, W.-J.; Kim, J.-W.; Lee, G.-H.; Oh, K.-J. The absorption rate of CO2/SO2/NO2 into a blended aqueous AMP/ammonia solution. Korean J. Chem. Eng. 2010, 28, 170–177. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, R.; Wang, T.; Yang, C. Follow-up research for integrative process of pre-oxidation and post-absorption cleaning flue gas: Absorption of NO2, NO and SO2. Chem. Eng. J. 2015, 273, 55–65. [Google Scholar] [CrossRef]
- Susianto; Pétrissans, M.; Pétrissans, A.; Zoulalian, A. Experimental study and modelling of mass transfer during simultaneous absorption of SO2 and NO2 with chemical reaction. Chem. Eng. Process. Process. Intensif. 2005, 44, 1075–1081. [Google Scholar] [CrossRef]
- Kong, S.; Shi, J.; Lu, B.; Qiu, W.; Zhang, B.; Peng, Y.; Zhang, B.; Bai, Z. Characterization of PAHs within PM10 fraction for ashes from coke production, iron smelt, heating station and power plant stacks in Liaoning Province, China. Atmos. Environ. 2011, 45, 3777–3785. [Google Scholar] [CrossRef]
- Yang, H.-H.; Lee, W.-J.; Chen, S.-J.; Lai, S.-O. PAH emission from various industrial stacks. J. Hazard. Mater. 1998, 60, 159–174. [Google Scholar] [CrossRef]
- Shi, J.; Deng, H.; Bai, Z.-P.; Kong, S.; Wang, X.; Hao, J.; Han, X.; Ning, P. Emission and profile characteristic of volatile organic compounds emitted from coke production, iron smelt, heating station and power plant in Liaoning Province, China. Sci. Total Environ. 2015, 515–516, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, A.; Chmielewski, A.; Licki, J.; Han, B.; Kim, J.; Kunnummal, N.; Fageeha, O.I. Pilot plant for electron beam treatment of flue gases from heavy fuel oil fired boiler. Fuel Process. Technol. 2016, 145, 123–129. [Google Scholar] [CrossRef]
- Kim, J.-C.; Kim, K.-H.; Armendariz, A.; Al-Sheikhly, M. Electron Beam Irradiation for Mercury Oxidation and Mercury Emissions Control. J. Environ. Eng. 2010, 136, 554–559. [Google Scholar] [CrossRef]
- Malik, A.U.; Al-Muaili, F.; Al-Ayashi, M.; Meroufel, A. An investigation on the corrosion of flue gas sensor in boiler stack. Case Stud. Eng. Fail. Anal. 2013, 1, 200–208. [Google Scholar] [CrossRef]
- Faghri, A.; Zhang, Y. Transport Phenomena in Multiphase Systems; Academic Press: Cambridge, MA, USA, 2006; ISBN 9780123706102. [Google Scholar]
- Qu, M.; Abdelaziz, O.; Gao, Z.; Yin, H. Isothermal membrane-based air dehumidification: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 4060–4069. [Google Scholar] [CrossRef]
- Cromer, C.J. Cooling System. U.S. Patent 623,735,4B1, 2001. Available online: https://patents.google.com/patent/US6237354B1/en (accessed on 30 January 2020).
- Curtis, M. Air Conditioning System with Moisture Control. U.S. Patent 719,160,7B2, 2007. Available online: https://patents.google.com/patent/US7191607B2/en (accessed on 30 January 2020).
- Endo, T.; Terada, H.; Katsumata, N.; Yagi, K.; Kawashima, K. Air Conditioner and Moisture Removing Device for Use with the Air Conditioner. European Patent EP0816779A4, 1998. Available online: https://patents.google.com/patent/EP0816779A4/en (accessed on 30 January 2020).
- La, D.; Dai, Y.; Li, Y.; Wang, R.; Ge, T. Technical development of rotary desiccant dehumidification and air conditioning: A review. Renew. Sustain. Energy Rev. 2010, 14, 130–147. [Google Scholar] [CrossRef]
- Mazzei, P.; Minichiello, F.; Palma, D. HVAC dehumidification systems for thermal comfort: A critical review. Appl. Therm. Eng. 2005, 25, 677–707. [Google Scholar] [CrossRef]
- Mei, L.; Dai, Y. A technical review on use of liquid-desiccant dehumidification for air-conditioning application. Renew. Sustain. Energy Rev. 2008, 12, 662–689. [Google Scholar] [CrossRef]
- Wu, G.; Cur, N.O. Squeezable Moisture Removal Device. U.S. Patent US20090158928A1, 2009. Available online: https://patents.google.com/patent/US20090158928A1/en (accessed on 30 January 2020).
- Aine, H.E. Infrared Optoacoustic Gas Analyzer Having an Improved Gas Inlet System. U.S. Patent 40,513,72A, 1977. Available online: https://patents.google.com/patent/US4051372A/en (accessed on 30 January 2020).
- Baccanti, M.; Magni, P. Process and Apparatus for Determining Total Nitrogen Content by Elemental Analysis. European Patent EP0586969A2, 1994. Available online: https://patents.google.com/patent/EP0586969A2/en (accessed on 30 January 2020).
- Jahn, M.D.; Griffin, M.T.; Popkie, N. Apparatus and Method for Water Vapor Removal in an Ion Mobility Spectrometer. U.S. Patent 7,361,206, 2008. Available online: https://patents.google.com/patent/US7361206B1/en (accessed on 30 January 2020).
- Karbiwnyk, C.M.; Mills, C.S.; Helmig, D.; Birks, J.W. Minimization of water vapor interference in the analysis of non-methane volatile organic compounds by solid adsorbent sampling. J. Chromatogr. A 2002, 958, 219–229. [Google Scholar] [CrossRef]
- Kolb, B.; Zwick, G.; Auer, M. A water trap for static cryo-headspace gas chromatography. J. High Resolut. Chromatogr. 1996, 19, 37–42. [Google Scholar] [CrossRef]
- Legendre, M.G.; Fisher, G.S. Inlet System for Direct Gas Chromatographic and Combined Gas Chromatographic/Mass Spectrometric Analysis of Food Volatiles. U.S. Patent 42,454,94A, 1981. Available online: https://patents.google.com/patent/US4245494A/en (accessed on 30 January 2020).
- Namieśnik, J.; Wardencki, W. Water Vapour Removal from Gaseous Samples Used for Analytical Purposes. A Review. Int. J. Environ. Anal. Chem. 1999, 73, 269–280. [Google Scholar] [CrossRef]
- Son, Y.-S.; Lee, G.; Kim, J.-C.; Han, J.-S. Development of a Pretreatment System for the Analysis of Atmospheric Reduced Sulfur Compounds. Anal. Chem. 2013, 85, 10134–10141. [Google Scholar] [CrossRef]
- Ahn, S.; Moon, J.; Kim, D.; Baek, S.; Ryoo, B. Clothes Dryer with a Dehumidifier. U.S. Patent 20060117593A1, 2006. Available online: https://patents.google.com/patent/US20060117593A1/en (accessed on 30 January 2020).
- Cromer, C.J. Heat Pump Dryer with Desciccant Enhanced Moisture Removal. U.S. Patent 60,948,35A, 2000. Available online: https://patents.google.com/patent/US6094835A/en (accessed on 30 January 2020).
- Lentz, L.E. Dehumidifier Clothes Dryer Apparatus. U.S. Patent 745,817,1B1, 2008. Available online: https://patents.google.com/patent/US7458171B1/en (accessed on 30 January 2020).
- Naboulsi, Z.A. System to Reduce Moisture Within a Clothes Dryer. U.S. Patent 20150059201A1, 2015. Available online: https://patents.google.com/patent/US20150059201A1/en (accessed on 30 January 2020).
- Sieben, F.; Volkel, T. Air Dehumidifier for Use in a Dryer. U.S. Patent 20120137535A1, 2012. Available online: https://patents.google.com/patent/US20120137535A1/en (accessed on 30 January 2020).
- Araki, H.; Shibata, T.; Hatamiya, S.; Tsukamoto, M. Cooling Apparatus, Gas Turbine System Using Cooling Apparatus, Heat Pump System Using Cooling System, Cooling Method, and Method for Operating Cooling Apparatus. U.S. Patent 840,273,5B2, 2013. Available online: https://patents.google.com/patent/US8402735B2/en (accessed on 30 January 2020).
- Baudat, N.; Richardson, F. Direct Turbine Air Chiller/Scrubber System. U.S. Patent 20010054354A1, 2001. Available online: https://patents.google.com/patent/US20010054354A1/en (accessed on 30 January 2020).
- Kulkarni, A.M.; Ashley Mass, R.M.; Davies, J.C. Mist/Moisture Removal Using Fixed Bed Trickle Columns. U.S. Patent 841,984,4B1, 2013. Available online: https://patents.google.com/patent/US8419844B1/en (accessed on 30 January 2020).
- Balinski, B.A.; Jackson, F.G.; McAllister, K.D. Controlled Moisture Removal in a Laundry Treating Appliance. U.S. Patent 1,063,377,6B2, 2020. Available online: https://patents.google.com/patent/US10633776B2/en (accessed on 30 May 2020).
- Kim, Y.-J. Washing Machine Having Dehumidifying Apparatus and Dehumidifying Method Thereof. U.S. Patent 20080295696A1, 2008. Available online: https://patents.google.com/patent/US20080295696A1/en (accessed on 30 January 2020).
- Saukahanian, G.; Kruger, M. Dehumidifier for Air Utilized in Laundry Drying. U.S. Patent 38,663,33A, 1975. Available online: https://patents.google.com/patent/US3866333A/en (accessed on 30 January 2020).
- Downling, R.O. Compressed Air Dryer. U.S. Patent 42,350,81A, 1980. Available online: https://patents.google.com/patent/US4235081A/en (accessed on 30 January 2020).
- Nanaumi, K.; Baba, K. Heat Exchanger for Cooling System Compressed Air Dehumidifiers. U.S. Patent 41,934,43A, 1980. Available online: https://patents.google.com/patent/US4193443A/en (accessed on 30 January 2020).
- Basseen, S.K.; Harlan, R.A. High Efficiency Air Drying System. U.S. Patent 47,619,68A, 1988. Available online: https://patents.google.com/patent/US4761968A/en (accessed on 30 January 2020).
- Tosi, S. Gas Analyzer with Cooling Apparatus. European Patent EP2124049A1, 2009. Available online: https://patents.google.com/patent/EP2124049A1/en (accessed on 30 January 2020).
- Chapman, R.L.; Harman, J.N. Thermoelectric Gas Dryer. U.S. Patent 42,312,56A, 1980. Available online: https://patents.google.com/patent/US4231256A/en (accessed on 1 January 2021).
- Groeger, H. Process and Apparatus for Pretreatment of a Gas to Be Analysed. European Patent EP0291630A3, 1988. Available online: https://patents.google.com/patent/EP0291630A3/en (accessed on 30 January 2020).
- Groeger, C.-M.; Groeger, H. Apparatus for Separating Vaporous Compounds from a Gas Stream. European Patent EP0361068A3, 1989. Available online: https://patents.google.com/patent/EP0361068A3/en (accessed on 30 January 2020).
- Bacharach, Inc. Technical Note: Flue Gas Sample Conditioning System (P/N 0024-7224). 2010. Available online: http://www.mybacharach.com/wp-content/uploads/2015/08/Flue-Gas-Conditioner-Manual.pdf (accessed on 11 November 2019).
- Kim, D.-J.; Dinh, T.-V.; Lee, J.-Y.; Choi, I.-Y.; Son, D.-J.; Kim, I.-Y.; Sunwoo, Y.; Kim, J.-C. Effects of Water Removal Devices on Ambient Inorganic Air Pollutant Measurements. Int. J. Environ. Res. Public Health 2019, 16, 3446. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Dinh, T.-V.; Kim, D.-J.; Choi, I.-Y.; Ahn, J.-W.; Park, S.-Y.; Jung, Y.-J.; Kim, J.-C. Comparison of Water Pretreatment Devices for the Measurement of Polar Odorous Compounds. Appl. Sci. 2019, 9, 4045. [Google Scholar] [CrossRef]
- Kondo, M.; Kawanowa, H.; Gotoh, Y.; Souda, R. Hydration of the HCl and NH3 molecules adsorbed on amorphous water–ice surface. Appl. Surf. Sci. 2004, 237, 510–514. [Google Scholar] [CrossRef]
- Woittequand, S.; Toubin, C.; Monnerville, M.; Pouilly, B.; Briquez, S.; Picaud, S. Photodissociation of a HCl molecule adsorbed on ice at T=210K. Surf. Sci. 2007, 601, 3034–3041. [Google Scholar] [CrossRef]
- Makino, H.; Nakagawa, K. Method for Removing Water Vapor from Water Vapor-Containing Gas. U.S. Patent 47,189,21A, 1988. Available online: https://patents.google.com/patent/US4718921A/en (accessed on 30 January 2020).
- Norlien, J.A.; Michler, K.J.; Crawford, A.G. Drying Sample Line. U.S. Patent 50,425,00A, 1991. Available online: https://patents.google.com/patent/US5042500A/en (accessed on 30 January 2020).
- Favre, E. Polymeric Membranes for Gas Separation. In Comprehensive Membrane Science and Engineering; Elsevier BV: Amsterdam, The Netherlands, 2010; pp. 155–212. [Google Scholar]
- Prasad, R. Membrane Drying Process and System. U.S. Patent 50,840,73A, 1992. Available online: https://patents.google.com/patent/US5084073A/en (accessed on 30 January 2020).
- Bolto, B.; Hoang, M.; Xie, Z. A review of water recovery by vapour permeation through membranes. Water Res. 2012, 46, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Li, N.N. Membrane Separation. U.S. Patent 3,566,580, 1971. Available online: https://patents.google.com/patent/US3566580A/en (accessed on 30 January 2020).
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Keyser, L.A.; Nelson, D.A.; Thornton, D.C. Dehumidifier for Waveguide System. U.S. Patent 4,944,776, 1990. Available online: https://patents.google.com/patent/US4944776A/en (accessed on 30 January 2020).
- Lovelock, J.E. Water-Vapour Permeable Material. U.S. Patent 51,605,11A, 1992. Available online: https://patents.google.com/patent/US5160511A/en (accessed on 30 January 2020).
- Bartholomew, S.A.; Bikson, B.; Giglia, S.; Johnson, B.Q. Integral Hollow Fiber Membrane Gas Dryer and Filtration Device. European Patent EP1275432A1, 2003. Available online: https://patents.google.com/patent/EP1275432A1/en (accessed on 30 January 2020).
- Eckerbom, A. Trouble-Free Sidestream Gas Analysis; Masimo Sweden AB: Danderyd, Sweden, 2009; Available online: https://www.masimo.com/siteassets/us/documents/pdf/nomoline-trouble-free-sidestream-gas-analysis.pdf (accessed on 11 November 2019).
- Zhao, B.; Peng, N.; Liang, C.; Yong, W.F.; Chung, T.-S. Hollow Fiber Membrane Dehumidification Device for Air Conditioning System. Membranes 2015, 5, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Z.; Chung, T.-S. Robust thin film composite PDMS/PAN hollow fiber membranes for water vapor removal from humid air and gases. Sep. Purif. Technol. 2018, 202, 345–356. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Kil Choi, W.; Park, S.R.; Kang, E.C.; Lee, H.K. Development of carboxylated TiO2 incorporated thin film nanocomposite hollow fiber membranes for flue gas dehydration. J. Membr. Sci. 2016, 514, 622–635. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Kil Choi, W.; Park, S.R.; Kang, E.C.; Lee, H.-K. Water vapor permeation behavior of interfacially polymerized polyamide thin film on hollow fiber membrane substrate. J. Taiwan Inst. Chem. Eng. 2016, 60, 623–635. [Google Scholar] [CrossRef]
- An, X.; Ingole, P.G.; Choi, W.-K.; Lee, H.-K.; Hong, S.U.; Jeon, J.-D. Enhancement of water vapor separation using ETS-4 incorporated thin film nanocomposite membranes prepared by interfacial polymerization. J. Membr. Sci. 2017, 531, 77–85. [Google Scholar] [CrossRef]
- Ingole, P.G.; Choi, W.K.; Lee, G.B.; Lee, H.K. Thin-film-composite hollow-fiber membranes for water vapor separation. Desalination 2017, 403, 12–23. [Google Scholar] [CrossRef]
- Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Selective water vapor permeation from steam/non-condensable gas mixtures via organosilica membranes at moderate-to-high temperatures. J. Membr. Sci. 2019, 589, 117254. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Vovushua, H.; Villalobos, L.F.; Shevate, R.; Kumar, M.; Nunes, S.P.; Schwingenschlögl, U.; Peinemann, K.-V. Highways for water molecules: Interplay between nanostructure and water vapor transport in block copolymer membranes. J. Membr. Sci. 2019, 572, 641–649. [Google Scholar] [CrossRef]
- Leoga, A.J.K.; Roualdès, S.; Rouessac, V.; Follain, N.; Marais, S. Sorption and permeation of water through Plasma Enhanced Chemical Vapour Deposited phosphonic acid-based membranes. Thin Solid Films 2020, 700, 137918. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, P.; Shi, R.; Hong, Z.; Zhu, X.; Gao, B.; Gu, X. Blocking defects of zeolite membranes with WS2 nanosheets for vapor permeation dehydration of low water content isopropanol. J. Membr. Sci. 2020, 597, 117625. [Google Scholar] [CrossRef]
- Deimede, V.; Vroulias, D.; Kallitsis, J.; Ioannides, T. Pyridinium based Poly(Ionic Liquids) membranes with exceptional high water vapor permeability and selectivity. Sep. Purif. Technol. 2020, 251, 117412. [Google Scholar] [CrossRef]
- Perma Pure LLC GASSTM-2040 Gas Sampling System. Available online: https://www.permapure.com/scientific-emissions/products/gas-drying-systems/nafion-based-sample-conditioning-systems/gass-2040-sampling-system/ (accessed on 8 February 2020).
- Wang, X.; Peng, L.; Li, J.; Qu, T.; Wang, A.; Li, H. Gas Dewatering Device and Using Method Thereof. Chinese Patent CN104707448A, 2013. Available online: https://patents.google.com/patent/CN104707448A/en (accessed on 30 January 2020).
- Jetter, J.J.; Maeshiro, S.; Yamazaki, K. System for Removing Water from a Gaseous Sample. US Patent US6346142B1, 2002. Available online: https://patents.google.com/patent/US6346142B1/en (accessed on 30 January 2020).
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Dinh, T.-V.; Kim, D.-J.; Choi, I.-Y.; Ahn, J.-W.; Park, S.-Y.; Jung, Y.-J.; Kim, J.-C. Effect of Conventional Water Pretreatment Devices on Polar Compound Analysis. Asian J. Atmos. Environ. 2019, 13, 249–258. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Chung, S.-H.; Baek, S.-O. Current Status and Prospective of Hazardous VOC in Ambient Air. J. Korean Soc. Atmos. Environ. 2011, 27, 734–745. [Google Scholar] [CrossRef]
- Son, E.-S.; Seo, Y.-K.; Lee, N.-H.; Lee, M.-D.; Han, J.-S.; Baek, S.-O. A Study on the Performance Optimization of a Continuous Monitoring Method for Hazardous VOCs in the Ambient Atmosphere. J. Korean Soc. Atmos. Environ. 2009, 25, 523–538. [Google Scholar] [CrossRef]
- US EPA. Dry Impinger Method for Determining Condensable Particulate Emissions from Stationary Sources; United States Environmental Protection Agency: Washington, DC, USA, 2017.
- Chen, Y.; Li, G.; Sun, Z.; Guo, H.; Liu, H.; Chen, H.; Du, S.; Yang, Y. Review of Monitoring Methods for Submicronsized Particulates Emission in Coal-fired Power Plants. In Proceedings of the 2016 International Conference on Engineering Science and Management, Zhengzhou, China, 13–14 August 2016. [Google Scholar] [CrossRef]
- ISO Stationary Source Emissions. Test Method for Determining PM2.5 and PM10 Mass in Stack Gases Using Cyclone Samplers and Sample Dilution; ISO Stationary Source Emissions: Geneva, Switzerland, 2013; p. 75. [Google Scholar]
- US EPA. Measurement of PM2.5 and PM10 Emissions by Dilution Sampling (Constant Sampling Rate Procedures); United States Environmental Protection Agency: Washington, DC, USA, 2004; p. 77.
- Feng, Y.; Li, Y.; Cui, L. Critical review of condensable particulate matter. Fuel 2018, 224, 801–813. [Google Scholar] [CrossRef]
- Hildemann, L.M.; Cass, G.R.; Markowski, G.R. A Dilution Stack Sampler for Collection of Organic Aerosol Emissions: Design, Characterization and Field Tests. Aerosol Sci. Technol. 1989, 10, 193–204. [Google Scholar] [CrossRef]
- Lipsky, E.M.; Robinson, A.L. Design and Evaluation of a Portable Dilution Sampling System for Measuring Fine Particle Emissions. Aerosol Sci. Technol. 2005, 39, 542–553. [Google Scholar] [CrossRef]
- England, G.C.; Watson, J.G.; Chow, J.C.; Zielinska, B.; Chang, M.-C.O.; Loos, K.R.; Hidy, G.M. Dilution-Based Emissions Sampling from Stationary Sources: Part 2—Gas-Fired Combustors Compared with Other Fuel-Fired Systems. J. Air Waste Manag. Assoc. 2007, 57, 65–78. [Google Scholar] [CrossRef]
- Wang, X.; Watson, J.G.; Chow, J.; Kohl, S.; Chen, L.A.; Sodeman, D.; Legge, A.; Percy, K. Measurement of Real-World Stack Emissions with a Dilution Sampling System. Dev. Environ. Sci. 2012, 11, 171–192. [Google Scholar] [CrossRef]
- Wang, X.; Watson, J.G.; Chow, J.C.; Gronstal, S.; Kohl, S.D. An Efficient Multipollutant System for Measuring Real-World Emissions from Stationary and Mobile Sources. Aerosol Air Qual. Res. 2012, 12, 145–160. [Google Scholar] [CrossRef]
- Pei, B.; Wang, X.; Zhang, Y.; Hu, M.; Sun, Y.; Deng, J.; Dong, L.; Fu, Q.; Yan, N. Emissions and source profiles of PM2.5 for coal-fired boilers in the Shanghai megacity, China. Atmos. Pollut. Res. 2016, 7, 577–584. [Google Scholar] [CrossRef]
- Peng, Y.; Sui, Z.; Zhang, Y.; Wang, T.; Norris, P.; Pan, W.-P. The effect of moisture on particulate matter measurements in an ultra-low emission power plant. Fuel 2019, 238, 430–439. [Google Scholar] [CrossRef]
- Cano, M.; Vega, F.; Navarrete, B.; Plumed, A.; Camino, J.A.; Mercedes, M. Characterization of Emissions of Condensable Particulate Matter in Clinker Kilns Using a Dilution Sampling System. Energy Fuels 2017, 31, 7831–7838. [Google Scholar] [CrossRef]
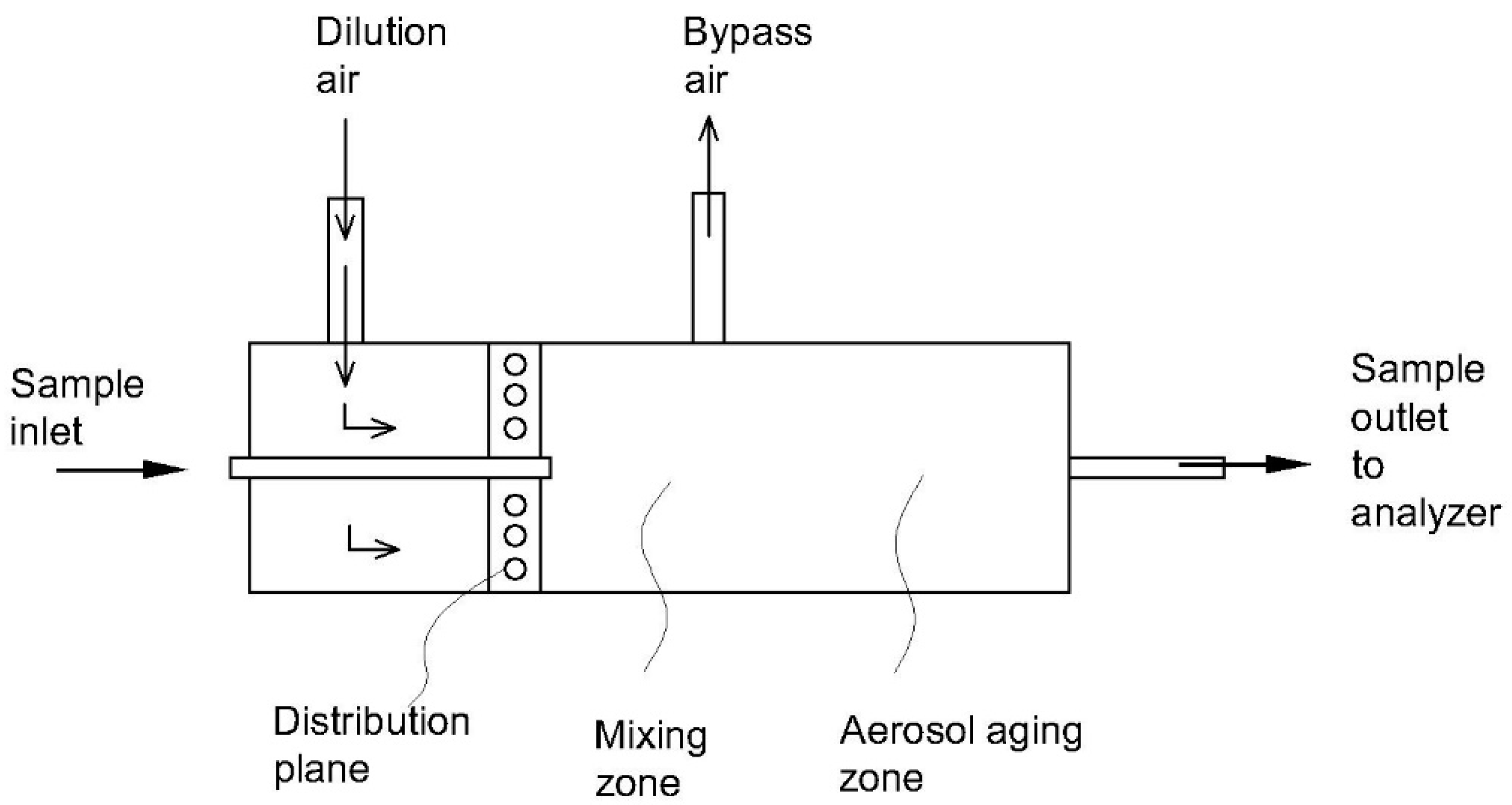
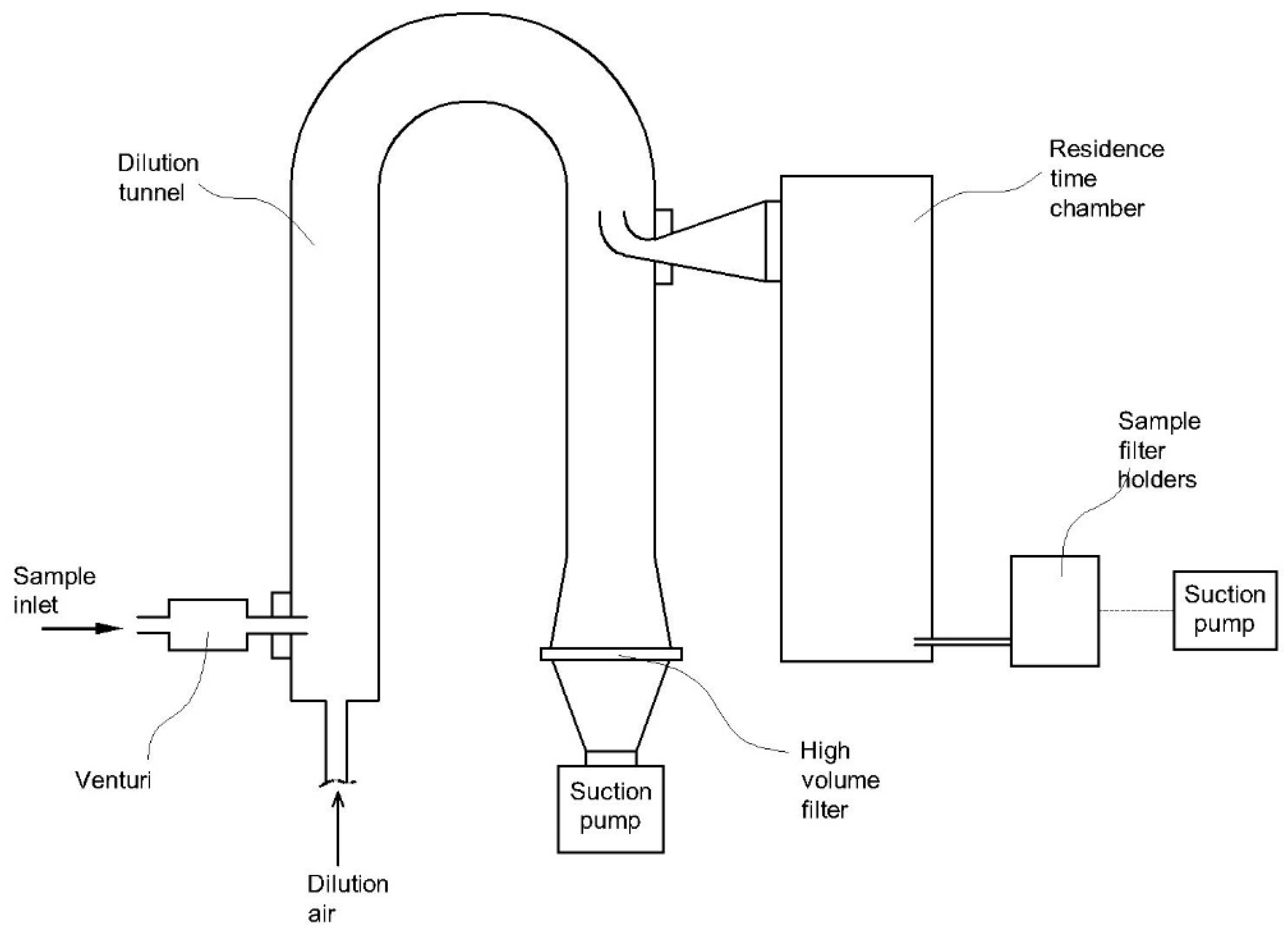
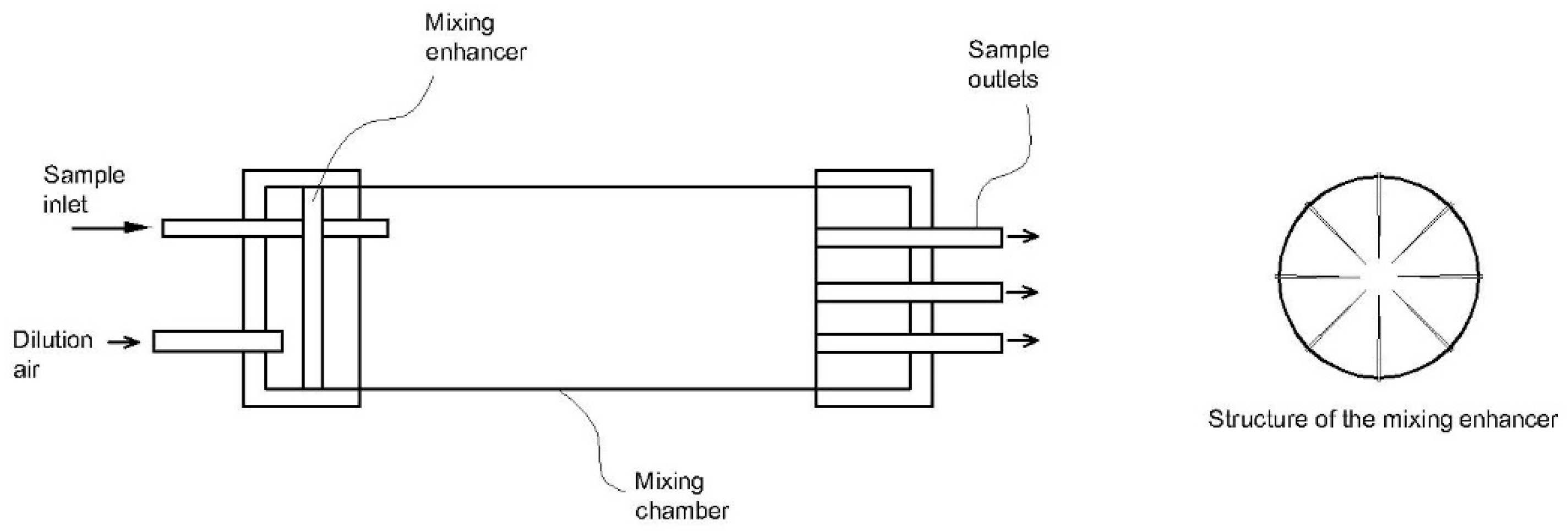
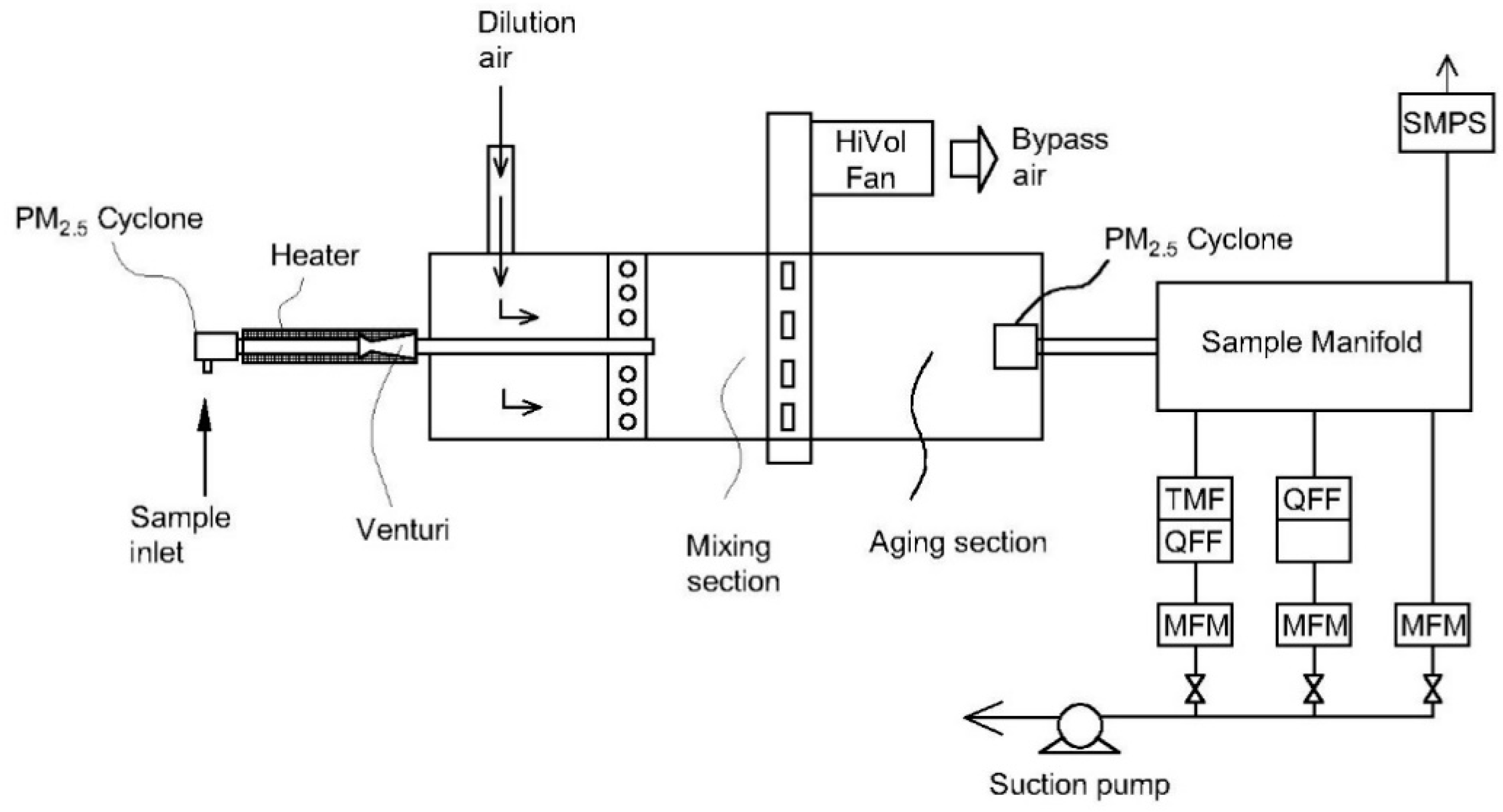
| Emission Source | Temperature (°C) | Humidity (vol%) |
|---|---|---|
| Blast furnace (coal fuel) [30,31] | 72 | 5.6–10 |
| Heating station [32] | 150 | 0.9 |
| Basic oxygen furnace [31] | 54 | 5.7 |
| Coke oven [31,32] | 79–150 | 0.7–5.1 |
| Electric arc furnace [31] | 86.7 | 4.2 |
| Heavy oil plant [31] | 247 | 7.3 |
| Cement (drying) [31] | 103 | 19.4 |
| Cement (pryo-processing) [31] | 142 | 3.5 |
| Boiler (heavy oil fuel) [33] | 310 | 10 |
| Power plant (coal fuel) [30,31,32,34,35] | 100–177 | 0.9–8.7 |
| Hazardous waste incinerator [23] | – | 50 |
| Portland cement kiln [23] | – | 10–35 |
| No. | Method | Condensation Type | Advantages | Disadvantages | Recommendation of Applicable Analytes |
|---|---|---|---|---|---|
| 1 | Refrigerated moisture removal device with improvement of heat exchanger [64] | Heterogeneous | - Reduced dimension of the device due to the absence of a water separator - High cooling efficiency - Less foiling - Allowable for high loading of moisture. | - Complicated structure - Potential loss of highly water-soluble compounds | - Poorly water-soluble gases such as CO, NO, CO2, and CH4 due to water droplets absorbing highly water-soluble compounds |
| 2 | Refrigerated moisture removal device with an extra heat exchanger [65] | Heterogeneous | - High cooling efficiency - Allowable for high temperature of the inlet gas - Allowable for high loading of moisture. | - Bulky device - Potential loss of highly water-soluble compounds | - Poorly water-soluble gases such as CO, NO, CO2, and CH4 due to water droplets absorbing highly water-soluble compounds |
| 3 | Two stages: refrigeration at −40 °C and desiccant bed [66] | Heterogeneous | -High cooling efficiency - Allowable for high loading of moisture - Suitable for highly water-soluble compounds. | - Complex and bulky structure - Potential loss of certain compounds due to the adsorption of desiccants. | - Highly water-soluble gases such as SO2 or NO2 under a solid phase |
| 4 | Vortex tube [67] | Homogeneous | - Low energy consumption - Less maintenance required | - Potential loss of highly water-soluble compounds | - Poorly water-soluble gases such as CO, NO, CO2, and CH4 due to water droplets absorbing highly water-soluble compounds. |
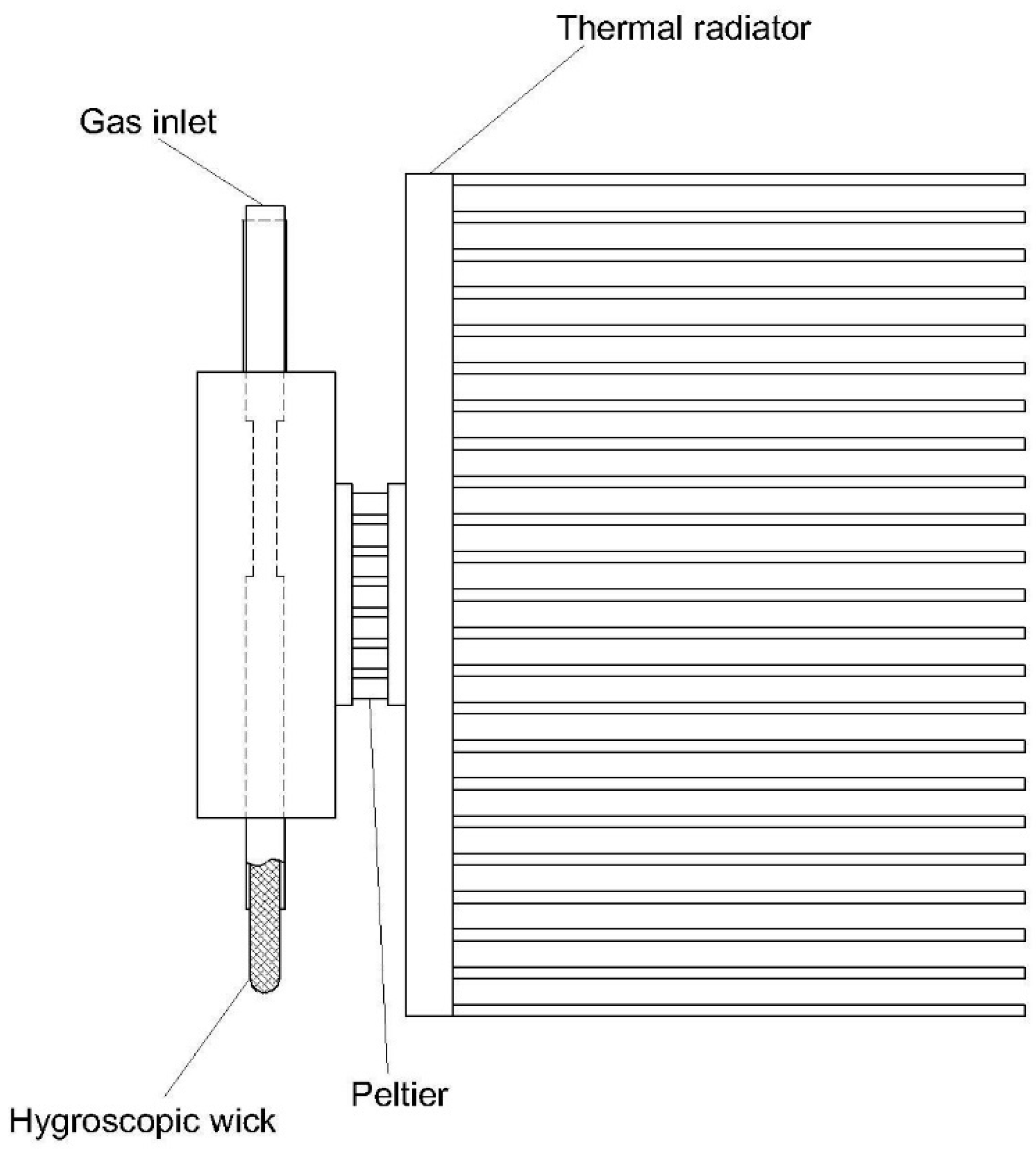
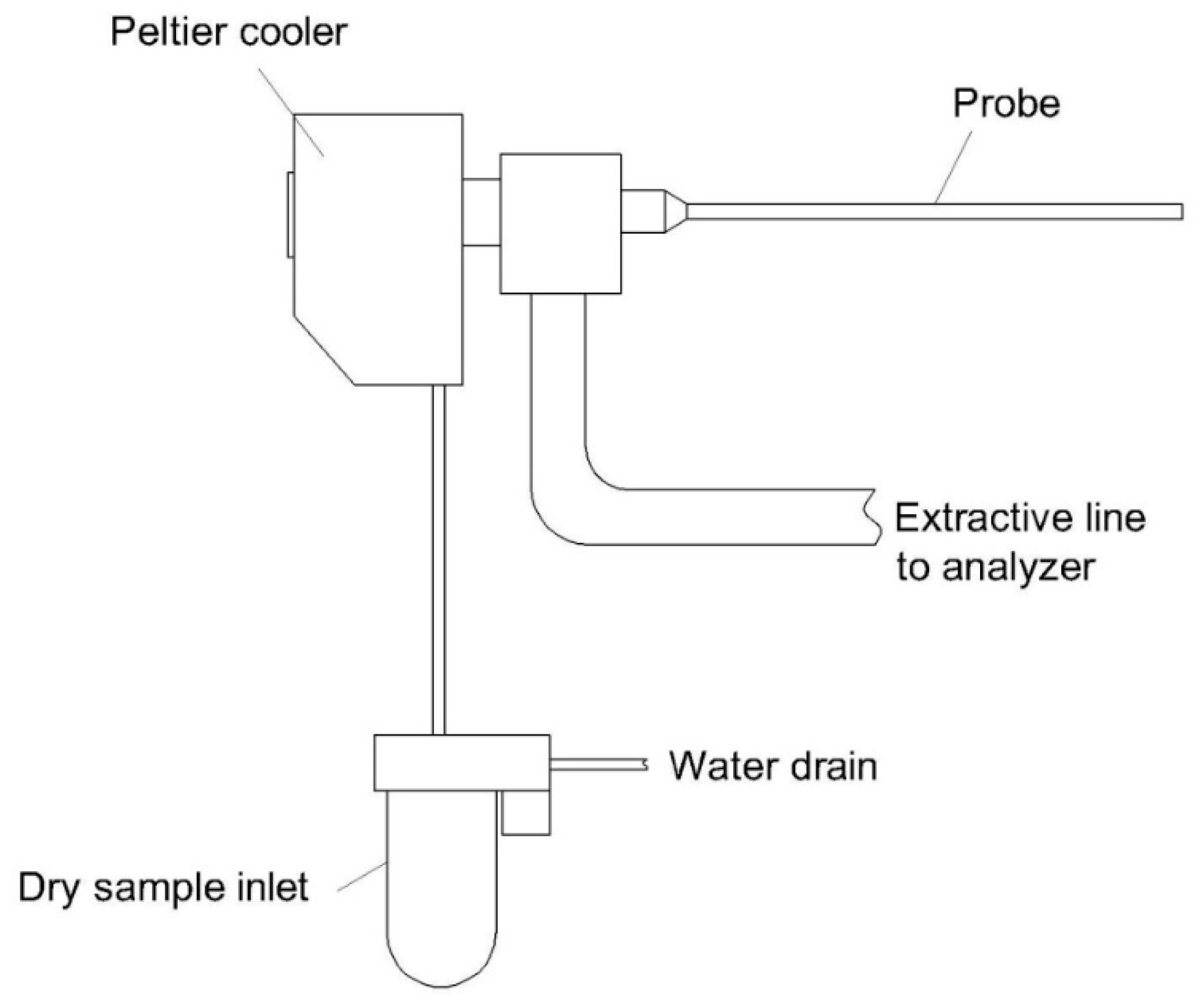
| 5 | Peltier moisture removal device [68] | Heterogeneous | - Compact size - Low energy consumption - Suitable for highly water-soluble compounds | - Not operatable with a high loading amount of moisture - Unsuitable for a high temperature of the inlet gas | - Highly water-soluble gases such as SO2 or NO2 under a solid phase. |
| 6 | Two stages: Peltier and membrane [69] | Heterogeneous | - Allowable for high loading of moisture. | - Potential loss of certain compounds due to the selectivity of the membrane - Potential loss of highly water-soluble compounds due to water droplets. | - Poorly water-soluble gases such as CO, NO, CO2, CH4 due to water droplets absorbing highly water-soluble compounds. |
| 7 | Conical cooling tube using a Peltier [70] | Heterogeneous | - Compact size - Large contact surface - Easy manufacturing due to its simple structure. | - Potential loss of highly water-soluble compounds due to water droplets. | Poorly water-soluble gases such as CO, NO, CO2, and CH4 due to water droplets absorbing highly water-soluble compounds |
| 8 | Peltier probe [71] | Heterogeneous | - Easy reduction in the temperature of an extractive line - Saving energy for a CEMS. | - Hard to maintain due to in situ location - Potential loss of highly water-soluble compounds due to water droplets. | Poorly water-soluble gases such as CO, NO, CO2, and CH4 due to water droplets absorbing highly water-soluble compounds. |
| No. | Device | Membrane Type | Materials | Advantages | Disadvantages | Recommendation of Applicable Analytes |
|---|---|---|---|---|---|---|
| 1 | Heater cartridge [83] | Any commercial membrane | Any commercial membrane | - A dry gas tank is unnecessary. - The condensation does not occur on the membrane surface. | - The device is bulky - It consumes a lot of energy due to the compressor and heater. | Target gases depended on membrane’s specification. |
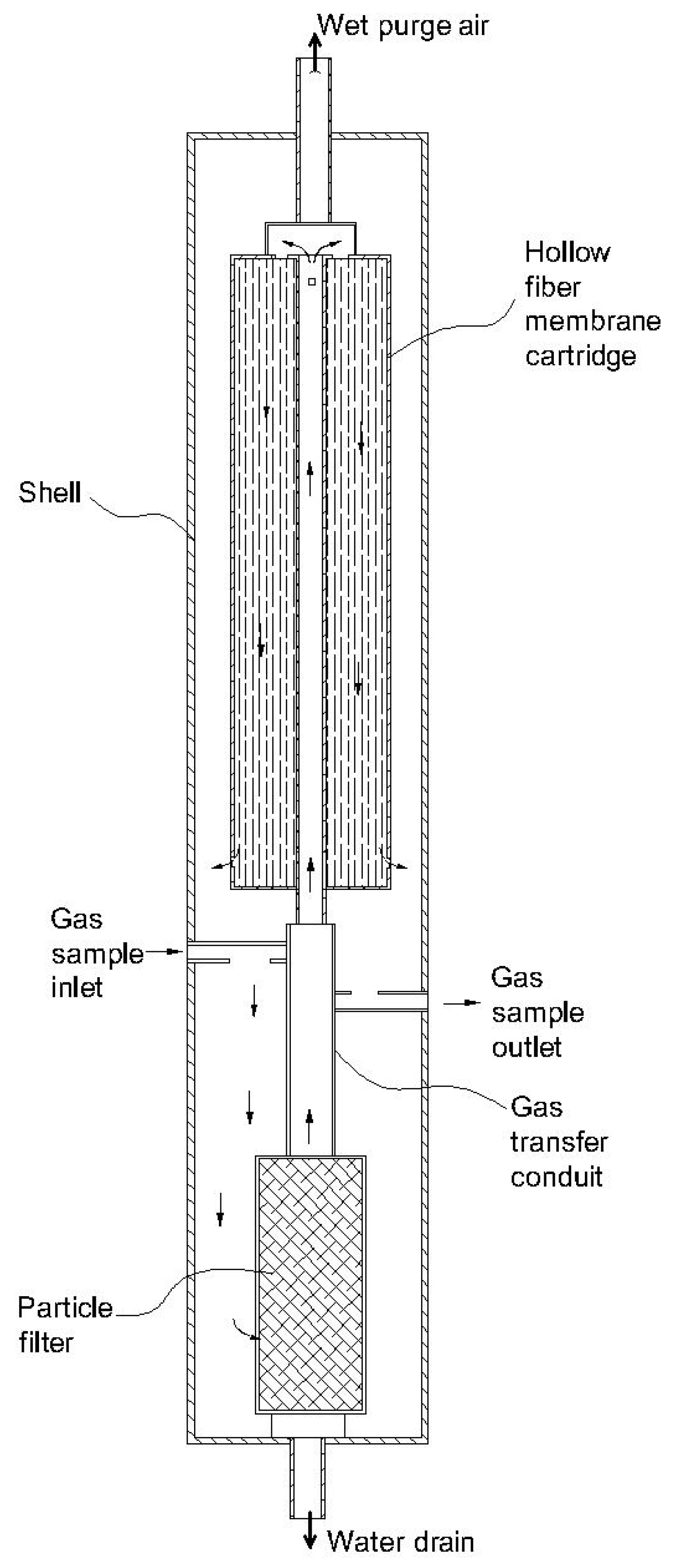
| 2 | Integral moisture membrane and particle filter [85] | Polymer | Any commercial product | - The effect of particles on the membrane is reduced. - The particle filter helps to enhance the lifetime of the membrane. | -Maintaining the filters is costly. | Target gases depended on membrane’s specification. |
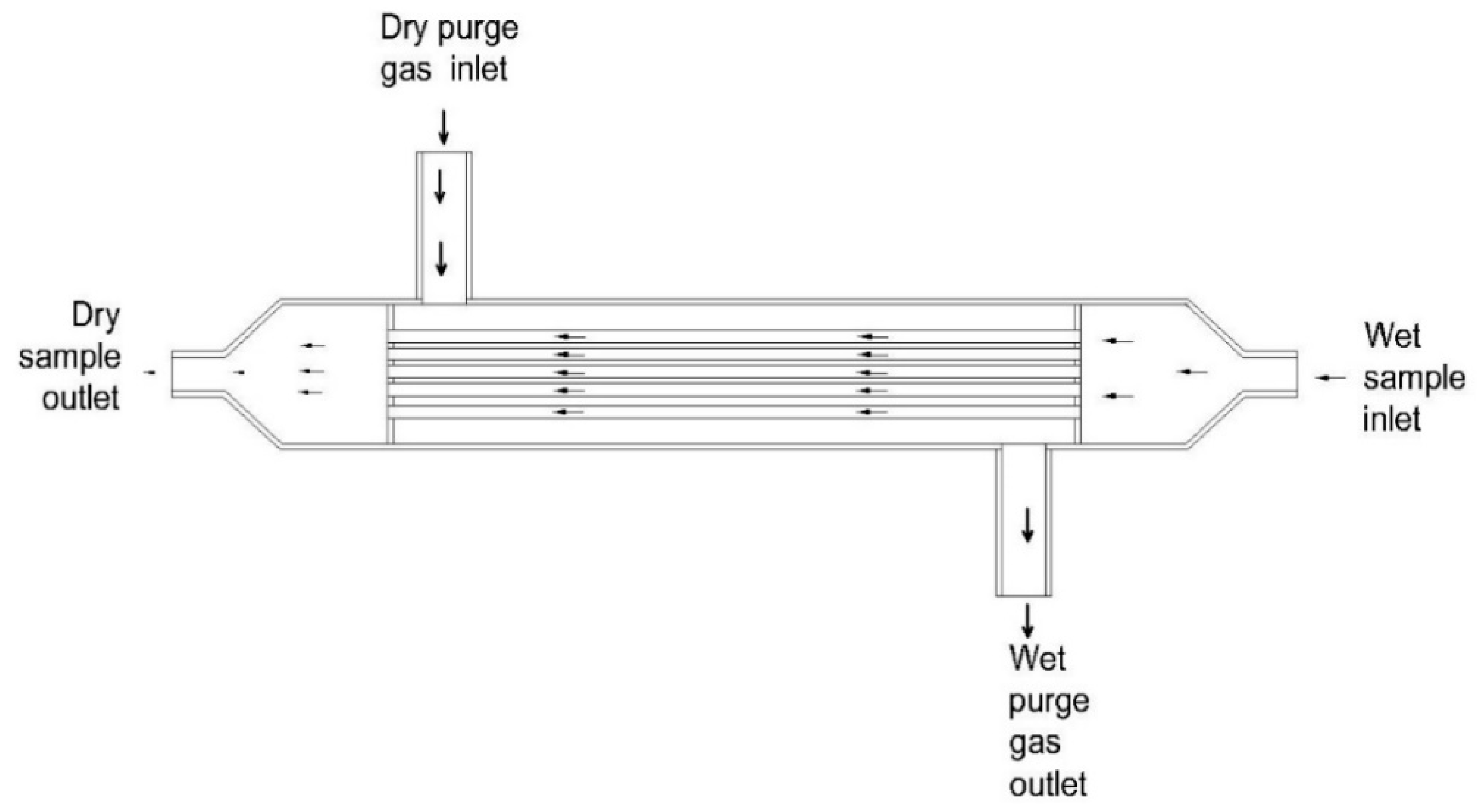
| 3 | Two-stage permeable tubes [100] | Polymer | Perfluorinated polymer plastic | - The device has a high moisture removal efficiency. - Condensation cannot occur on the membrane surface. - It shows a high loading amount of moisture. | - The device may consume a lot of energy - Particles may damage the membrane surface due to the absence of a filter. | The authors recommend the device be used for an FTIR analyzer. |
| 4 | Two-stage winding cylinder device [99] | Polymer | Nafion® | - The device has a high moisture removal efficiency. - Condensation cannot occur on the membrane surface. - It has high loading of moisture. - It shows a high selectivity. | - The device may consume a lot of energy - Nafion® membrane is sensitive to particles. | - Nafion® membrane has been widely applied for CO, CO2, NO, NO2, HCl, NH3, H2S, and SO2 measurements. |
| No. | Method | Characteristics | Advantages | Disadvantages | Application |
|---|---|---|---|---|---|
| 1 | U-shaped dilution system [110] | - Flow rate: 30 L/min - Gas temperature: 150 °C - Dilution ratio: 25–100-fold | Able to improve the collection of organic carbon | - Bulky system - Significant loss of particle in the size range of 1–2 µm | Condensable plus filterable particulate matter |
| 2 | Compact dilution system [111] | - Flow rate: 174 L/min - Gas temperature: 150–300 °C - Dilution ratio: 20–350-fold | - Compact size due to absence of residence time tank - Retention time of sample gas < 1 s - Able to operate at a high and wide temperature range of inlet gas | - Potential change of particle shape according to dilution ratio - Potential for significant bias | Filterable particulate matter |
| 3 | Compact dilution system [112] | - Flow rate: 113 L/min - Gas temperature: <175 °C - Dilution ratio: 20:1 | - Small dimensions of the system - Low minimum detection limit of particles compared to a standard method | - Potential for significant bias (27–34%) | Condensable plus filterable particulate matter |
| 4 | 90° elbow dilution chamber [113,114,115] | - Flow rate: 125 L/min - Gas temperature: 146–174 °C - Dilution ratio: 20–50-fold | - Compact size - Various functions for particulate and gaseous measurements- Low measurement bias | - Complicated structure - Hard to maintain | Condensable plus filterable particulate matter |
| 5 | Hybrid dilution system coupled with Nafion® dryer [116] | - Gas temperature: 50 °C - Dilution ratio: 8:1 | - Suitable for a wide range of particles, especially ultra-low particulate matter - Low measurement bias caused by humidity | - Costly due to Nafion® dryer - Hard to maintain | Filterable particulate matter |
| 6 | Dilution system with a filter for filterable particulate matter [117] | - Gas temperature: 190–200 °C - Dilution ratio: 10–40-fold | - Highly accurate for condensable particulate matter | - Hard to maintain - Potential bias due to pore filter size | Condensable particulate matter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinh, T.-V.; Kim, J.-C. Moisture Removal Techniques for a Continuous Emission Monitoring System: A Review. Atmosphere 2021, 12, 61. https://doi.org/10.3390/atmos12010061
Dinh T-V, Kim J-C. Moisture Removal Techniques for a Continuous Emission Monitoring System: A Review. Atmosphere. 2021; 12(1):61. https://doi.org/10.3390/atmos12010061
Chicago/Turabian StyleDinh, Trieu-Vuong, and Jo-Chun Kim. 2021. "Moisture Removal Techniques for a Continuous Emission Monitoring System: A Review" Atmosphere 12, no. 1: 61. https://doi.org/10.3390/atmos12010061
APA StyleDinh, T.-V., & Kim, J.-C. (2021). Moisture Removal Techniques for a Continuous Emission Monitoring System: A Review. Atmosphere, 12(1), 61. https://doi.org/10.3390/atmos12010061