CNT Parameterization Based on the Observed INP Concentration during Arctic Summer Campaigns in a Marine Environment
Abstract
1. Introduction
2. Methods
2.1. Parameterization Approach
2.2. Field Measurements
3. Results
4. Discussion and Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Comparison of CNT Approach and a Simpler Exponential Approach

References
- Hartmann, M.; Blunier, T.; Brügger, S.O.; Schmale, J.; Schwikowski, M.; Vogel, A.; Wex, H.; Stratmann, F. Variation of ice nucleating particles in the European Arctic over the last centuries. Geophys. Res. Lett. 2019, 46. [Google Scholar] [CrossRef]
- Intrieri, J.M. An annual cycle of Arctic surface cloud forcing at SHEBA. J. Geophys. Res. 2002, 107, 8039. [Google Scholar] [CrossRef]
- Baker, M.B.; Peter, T. Small-scale cloud processes and climate. Nature 2008, 451, 299–300. [Google Scholar] [CrossRef]
- DeMott, P.J.; Prenni, A.J.; Liu, X.; Kreidenweis, S.M.; Petters, M.D.; Twohy, C.H.; Richardson, M.S.; Eidhammer, T.; Rogers, D.C. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 2010, 107, 11217–11222. [Google Scholar] [CrossRef]
- Vali, G.; DeMott, P.J.; Möhler, O.; Whale, T.F. Technical note: A proposal for ice nucleation terminology. Atmos. Chem. Phys. 2015, 15, 10263–10270. [Google Scholar] [CrossRef]
- Pruppacher, H.; Klett, J. Microphysics of Clouds and Precipitation, 2nd ed.; Kluwer Academic: Boston, MA, USA, 1997. [Google Scholar]
- Fletcher, N.H. The Physics of Rain Clouds; Cambridge University Press: New York, NY, USA, 1962. [Google Scholar]
- Cooper, W.A. Ice initiation in natural clouds. In Precipitation Enhancement—A Scientific Challenge, Meteorological Monographs; Braham, R.G., Jr., Ed.; American Meteorological Society: Boston, MA, USA, 1986; Volume 21, pp. 29–32. [Google Scholar]
- Meyers, M.P.; DeMott, P.J.; Cotton, W.R. New primary ice-nucleation parameterizations in an explicit cloud model. J. Appl. Meteorol. 1992, 31, 708–721. [Google Scholar] [CrossRef]
- Prenni, A.J.; Harrington, J.Y.; Tjernstrom, M.; DeMott, P.J.; Avramov, A.; Long, C.N.; Kreidenweis, S.M.; Olsson, P.Q.; Verlinde, J. Can ice nucleating aerosols effect Arctic seasonal climate? Bull. Am. Meteorol. Soc. 2007, 88, 541–550. [Google Scholar] [CrossRef]
- Lohmann, U.; Feichter, J.; Chuang, C.C.; Penner, J.E. Predicting the number of cloud droplets in the ECHAM GCM. J. Geophys. Res. 1999, 104, 9169–9198. [Google Scholar] [CrossRef]
- Liu, X.; Penner, J.E. Ice nucleation parameterization for global models. Meteorol. Z. 2005, 14, 499–514. [Google Scholar] [CrossRef]
- Milbrandt, J.A.; Yau, M.K. A multimoment bulk microphysics parameterization. Part I: Analysis of the role of the spectral shape parameter. J. Atmos. Sci. 2005, 62, 3051–3064. [Google Scholar] [CrossRef]
- Morrison, H.; Gettelman, A. A new two-moment bulk stratiform cloud microphysics scheme in the Community Atmosphere Model, version 3 (CAM3). Part I: Description and numerical tests. J. Clim. 2008, 21, 3642–3659. [Google Scholar] [CrossRef]
- Connolly, P.J.; Möhler, O.; Field, P.R.; Saathoff, H.; Burgess, R.; Choularton, T.; Gallagher, M. Studies of heterogeneous freezing by three different desert dust samples. Atmos. Chem. Phys. 2009, 9, 2805–2824. [Google Scholar] [CrossRef]
- Niedermeier, D.; Hartmann, S.; Shaw, R.A.; Covert, D.; Mentel, T.F.; Schneider, J.; Poulain, L.; Reitz, P.; Spindler, C.; Clauß, T.; et al. Heterogeneous freezing of droplets with immersed mineral dust particles—Measurements and parameterization. Atmos. Chem. Phys. 2010, 10, 3601–3614. [Google Scholar] [CrossRef]
- Archuleta, C.M.; DeMott, P.J.; Kreidenweis, S.M. Ice nucleation by surrogates for atmospheric mineral dust and mineral dust/sulfate particles at cirrus temperatures. Atmos. Chem. Phys. 2005, 5, 2617–2634. [Google Scholar] [CrossRef]
- Field, P.R.; Heymsfield, A.J.; Bansemer, A. Shattering and particle interarrival times measured by optical array probes in ice clouds. J. Atmos. Ocean. Technol. 2006, 23, 1357–1371. [Google Scholar] [CrossRef]
- Kanji, Z.A.; Abbatt, J.P.D. Laboratory studies of ice formation via deposition mode nucleation onto mineral dust and n-hexane soot samples. J. Geophys. Res. 2006, 111, D16204. [Google Scholar] [CrossRef]
- Eastwood, M.L.; Cremel, S.; Gehrke, C.; Girard, E.; Bertram, A.K. Ice nucleation on mineral dust particles: Onset conditions, nucleation rates and contact angles. J. Geophys. Res. 2008, 113, D22203. [Google Scholar] [CrossRef]
- Welti, A.; Lüönd, F.; Lohmann, U. Influence of particle size on the ice nucleating ability of mineral dusts. Atmos. Chem. Phys. 2009, 9, 6705–6715. [Google Scholar] [CrossRef]
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854. [Google Scholar] [CrossRef]
- Murray, B.J.; O’Sullivan, D.; Atkinson, J.D.; Webb, M.E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef]
- Möhler, O.; Field, P.R.; Connolly, P.; Benz, S.; Saathoff, H.; Schnaiter, M.; Wagner, R.; Cotton, R.; Krämer, M.; Mangold, A.; et al. Efficiency of the deposition mode ice nucleation on mineral dust particles. Atmos. Chem. Phys. 2006, 6, 3007–3021. [Google Scholar] [CrossRef]
- Pratt, K.A.; Demott, P.J.; French, J.R.; Wang, Z.; Westphal, D.L.; Heymsfield, A.J.; Twohy, C.H.; Prenni, A.J.; Prather, K.A. In situ detection of biological particles in cloud ice-crystals. Nat. Geosci. 2009, 2, 398–401. [Google Scholar] [CrossRef]
- Sullivan, R.C.; Guazzotti, S.A.; Sodeman, D.A.; Prather, K.A. Direct observations of the atmospheric processing of Asian mineral dust. Atmos. Chem. Phys. 2007, 7, 1213–1236. [Google Scholar] [CrossRef]
- Cziczo, D.J.; Froyd, K.D.; Gallavardin, S.J.; Moehler, O.; Benz, S.; Saathoff, H.; Murphy, D.M. Deactivation of ice nuclei due to atmospherically relevant surface coatings. Environ. Res. Lett. 2009, 4, 044013. [Google Scholar] [CrossRef]
- Eastwood, M.L.; Cremel, S.; Wheeler, M.; Murray, B.J.; Girard, E.; Bertram, A.K. Effects of sulfuric acid and ammonium sulfate coatings on the ice nucleation properties of kaolinite particles. Geophys. Res. Lett. 2009, 36, L02811. [Google Scholar] [CrossRef]
- Phillips, V.T.J.; DeMott, P.J.; Andronache, C. An empirical parameterization of heterogeneous ice nucleation for multiple chemical species of aerosol. J. Atmos. Sci. 2008, 65, 2757–2783. [Google Scholar] [CrossRef]
- Steinke, I.; Hoose, C.; Möhler, O.; Connolly, P.; Leisner, T. A new temperature- and humidity-dependent surface site density approach for deposition ice nucleation. Atmos. Chem. Phys. 2015, 15, 3703–3717. [Google Scholar] [CrossRef]
- DeMott, P.; Prenni, A.; McMeeking, G.; Sullivan, R.; Petters, M.; Tobo, Y.; Niemand, M.; Möhler, O.; Snider, J.; Wang, Z.; et al. Integrating laboratory and field data to quantify the immersion freezing ice nucleation activity of mineral dust particles. Atmos. Chem. Phys. 2015, 15, 393–409. [Google Scholar] [CrossRef]
- DeMott, P.J.; Hill, T.C.J.; McCluskey, C.S.; Prather, K.A.; Collins, D.B.; Sullivan, R.C.; Ruppel, M.J.; Mason, R.H.; Irish, V.E.; Lee, T.; et al. Sea spray aerosol as a unique source of ice nucleating particles. Proc. Natl. Acad. Sci. USA 2016, 113, 5797–5803. [Google Scholar] [CrossRef]
- Harrison, A.D.; Whale, T.F.; Carpenter, M.A.; Holden, M.A.; Neve, L.; O’Sullivan, D.; Vergara-Temprado, J.; Murray, B.J. Not all feldspars are equal: A survey of ice nucleating properties across the feldspar group of minerals. Atmos. Chem. Phys. 2016, 16, 10927–10940. [Google Scholar] [CrossRef]
- Ullrich, R.; Hoose, C.; Möhler, O.; Niem, M.; Wagner, R.; Höhler, K.; Hiranuma, N.; Saathoff, H.; Leisner, T. A new ice nucleation active site parameterization for desert dust and soot. J. Atmos. Sci. 2017, 74, 699–717. [Google Scholar] [CrossRef]
- Girard, E.; Dueymes, G.; Du, P.; Bertram, A.K. Assessment of the effects of acid-coated ice nuclei on the Arctic cloud microstructure, atmospheric dehydration, radiation and temperature during winter. Int. J. Climatol. 2013, 33, 599–614. [Google Scholar] [CrossRef]
- Keita, S.A.; Girard, E. Importance of Chemical Composition of Ice Nuclei on the Formation of Arctic Ice Clouds. Pure Appl. Geophys. 2016, 173, 3141–3163. [Google Scholar] [CrossRef]
- Abbatt, J.P.D.; Leaitch, W.R.; Aliabadi, A.A.; Bertram, A.K.; Blanchet, J.-P.; Boivin-Rioux, A.; Bozem, H.; Burkart, J.; Chang, R.Y.W.; Charette, J.; et al. Overview paper: New insights into aerosol and climate in the Arctic. Atmos. Chem. Phys. 2019, 19, 2527–2560. [Google Scholar] [CrossRef]
- Westbrook, C.D.; Illingworth, A.J. Evidence that ice forms primarily in supercooled liquid clouds at temperatures > −27 °C. Geophys. Res. Lett. 2011, 38, L14808. [Google Scholar] [CrossRef]
- Mason, R.H.; Chou, C.; McCluskey, C.S.; Levin, E.J.T.; Schiller, C.L.; Hill, T.C.J.; Huffman, J.A.; DeMott, P.J.; Bertram, A.K. The micro-orifice uniform deposit impactor-droplet freezing technique (MOUDI-DFT) for measuring concentrations of ice nucleating particles as a function of size: Improvements and initial validation. Atmos. Meas. Tech. 2015, 8, 2449–2462. [Google Scholar] [CrossRef]
- Irish, V.E.; Hanna, S.J.; Willis, M.D.; China, S.; Thomas, J.L.; Wentzell, J.J.B.; Cirisan, A.; Si, M.; Leaitch, W.R.; Murphy, J.G.; et al. Ice nucleating particles in the marine boundary layer in the Canadian Arctic during summer 2014. Atmos. Chem. Phys. 2019, 19, 1027–1039. [Google Scholar] [CrossRef]
- Chen, J.P.; Hazra, A.; Levin, Z. Parameterizing ice nucleation rates using contact angle and activation energy derived from laboratory data. Atmos. Chem. Phys. 2008, 8, 7431–7449. [Google Scholar] [CrossRef]
- Marcolli, C.; Gedamke, S.; Peter, T.; Zobrist, B. Efficiency of immersion mode ice nucleation on surrogates of mineral dust. Atmos. Chem. Phys. 2007, 7, 5081–5091. [Google Scholar] [CrossRef]
- Kulkarni, G.; Dobbie, S. Ice nucleation properties of mineral dust particles: Determination of onset RHi, IN active fraction, nucleation time-lag, and the effect of active sites on contact angles. Atmos. Chem. Phys. 2010, 10, 95–105. [Google Scholar] [CrossRef]
- Chernoff, D.I.; Bertram, A.K. Effects of sulfate coatings on the ice nucleation properties of a biological ice nucleus and several types of minerals. J. Geophys. Res. 2010, 115, D20205. [Google Scholar] [CrossRef]
- Lüönd, F.; Stetzer, O.; Welti, A.; Lohmann, U. Experimental study on the ice nucleation ability of size selected kaolinite particles in the immersion mode. J. Geophys. Res. 2010, 115, D14201. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Bertram, A.K. Deposition nucleation on mineral dust particles: A case against classical nucleation theory with the assumption of a single contact angle. Atmos. Chem. Phys. 2012, 12, 1189–1201. [Google Scholar] [CrossRef]
- Welti, A.; Lüönd, F.; Kanji, Z.A.; Stetzer, O.; Lohmann, U. Time dependence of immersion freezing: An experimental study on size selected kaolinite particles. Atmos. Chem. Phys. 2012, 12, 9893–9907. [Google Scholar] [CrossRef]
- Murray, B.J.; Broadley, S.L.; Wilson, T.W.; Atkinson, J.D.; Wills, R.H. Heterogeneous freezing of water droplets containing kaolinite particles. Atmos. Chem. Phys. 2011, 11, 4191–4207. [Google Scholar] [CrossRef]
- Keita, S.A.; Girard, É.; Raut, J.-C.; Leriche, M.; Pelon, J.; Onishi, T.; Blanchet, J.-P.; Cirisan, A. A new parameterization of ice heterogeneous nucleation coupled to aerosol chemistry in WRF-Chem model version 3.5.1: Evaluation through the ISDAC measurements. Geosci. Model Dev. Discuss. 2020. [Google Scholar] [CrossRef]
- Zobrist, B.; Koop, T.; Luo, B.P.; Marcolli, C.; Peter, T. Heterogeneous ice nucleation rate coefficient of water droplets coated by a nonadecanol monolayer. J. Phys. Chem. C 2007, 111, 2149–2155. [Google Scholar] [CrossRef]
- Smith, R.S.; Kay, B.D. The existence of supercooled liquid water at 150 K. Nature 1999, 398, 788–791. [Google Scholar] [CrossRef]
- Eadie, W.J. A Molecular Theory of the Homogeneous Nucleation of Ice in Supercooled Water. Ph.D. Thesis, University of Chicago, Cloud Physics Lab, Chicago, IL, USA, 1971. [Google Scholar]
- DeMott, P.J.; Rogers, D.C. Freezing nucleation rates of dilute solution droplets measured between −30 °C and −40 °C in laboratory simulations of natural clouds. J. Artnos. Sci. 1990, 47, 1056–1064. [Google Scholar] [CrossRef]
- Reinhardt, A.; Doye, J.P.K. Homogeneous TIP4P/2005 ice nucleation at low supercooling. J. Chem. Phys. 2013, 139, 096102. [Google Scholar] [CrossRef]
- Ickes, L.; Welti, A.; Hoose, C.; Lohmann, U. Classical nucleation theory of homogeneous freezing of water: Thermodynamic and kinetic parameters. Phys. Chem. Chem. Phys. 2015, 17, 5514–5537. [Google Scholar] [CrossRef] [PubMed]
- Trainer, M.G.; Toon, O.B.; Tolbert, M.A. Measurements of depositional ice nucleation on insoluble substrates at low temperatures: Implications for Earth and Mars. J. Phys. Chem. C. 2009, 113, 2036–2040. [Google Scholar] [CrossRef]
- Ladino, L.A.; Abbatt, J.P.D. Laboratory investigation of Martian water ice cloud formation using dust aerosol stimulants. J. Geophys. Res. 2013, 118, 14–25. [Google Scholar] [CrossRef]
- Ming, Y.; Russell, L. Predicted hygroscopic growth of sea salt aerosol. J. Geophys. Res. 2001, 106, 28259–28274. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Mason, R.H.; Steunenberg, K.; Wagstaff, M.; Chou, C.; Bertram, A.K. Immersion freezing of supermicron mineral dust particles: Freezing results, testing different schemes for describing ice nucleation, and ice nucleation active site densities. J. Phys. Chem. A 2015, 119, 4358–4372. [Google Scholar] [CrossRef]
- Lagarias, J.C.; Reeds, J.A.; Wright, M.H.; Wright, P.E. Convergence Properties of the Nelder-Mead Simplex Method in Low Dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef]
- McCluskey, C.S.; Hill, T.C.J.; Sultana, C.M.; Laskina, O.; Trueblood, J.; Santander, M.V.; Beall, C.M.; Michaud, J.M.; Kreidenweis, S.M.; Prather, K.A.; et al. A mesocosm double feature: Insights into the chemical makeup of marine ice nucleating particles. J. Atmos. Sci. 2018, 75, 2405–2423. [Google Scholar] [CrossRef]
- Murphy, D.M.; Koop, T. Review of the vapour pressures of ice and supercooled water for atmospheric applications. Q. J. Roy. Meteor. Soc. 2005, 131, 1539–1565. [Google Scholar] [CrossRef]
- Ickes, L.; Welti, A.; Lohmann, U. Classical nucleation theory of immersion freezing: Sensitivity of contact angle schemes to thermodynamic and kinetic parameters. Atmos. Chem. Phys. 2017, 17, 1713–1739. [Google Scholar] [CrossRef]
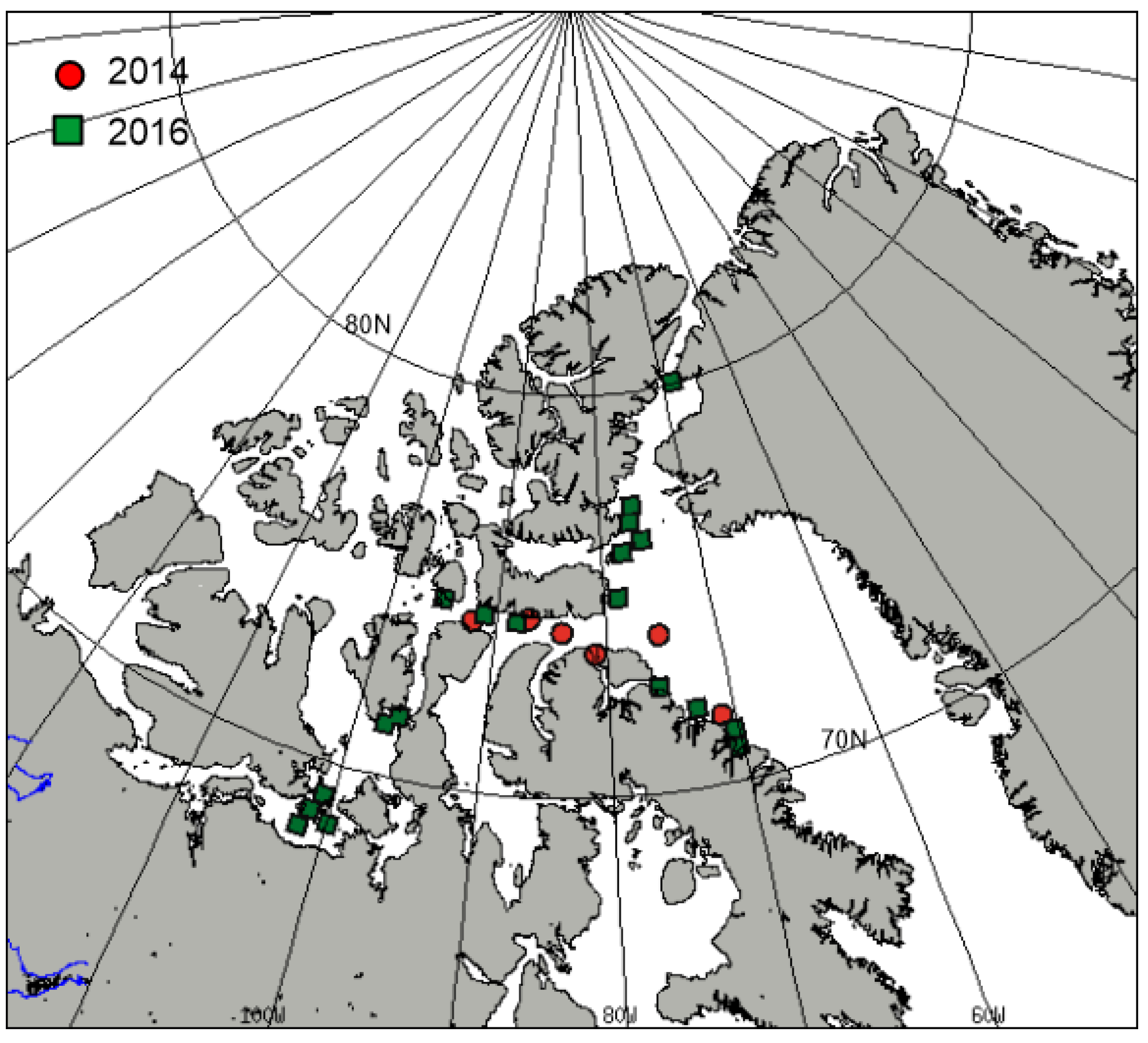
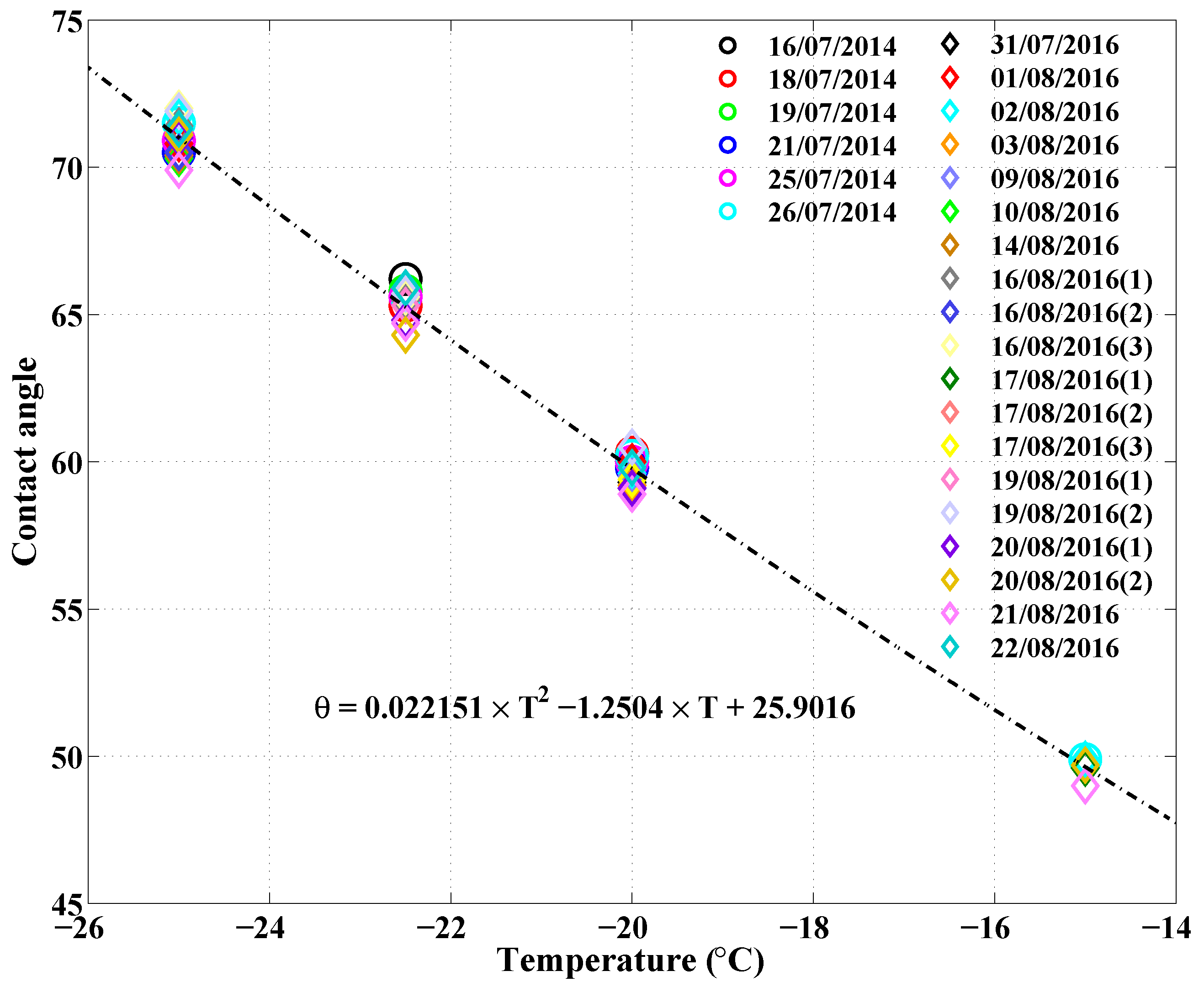
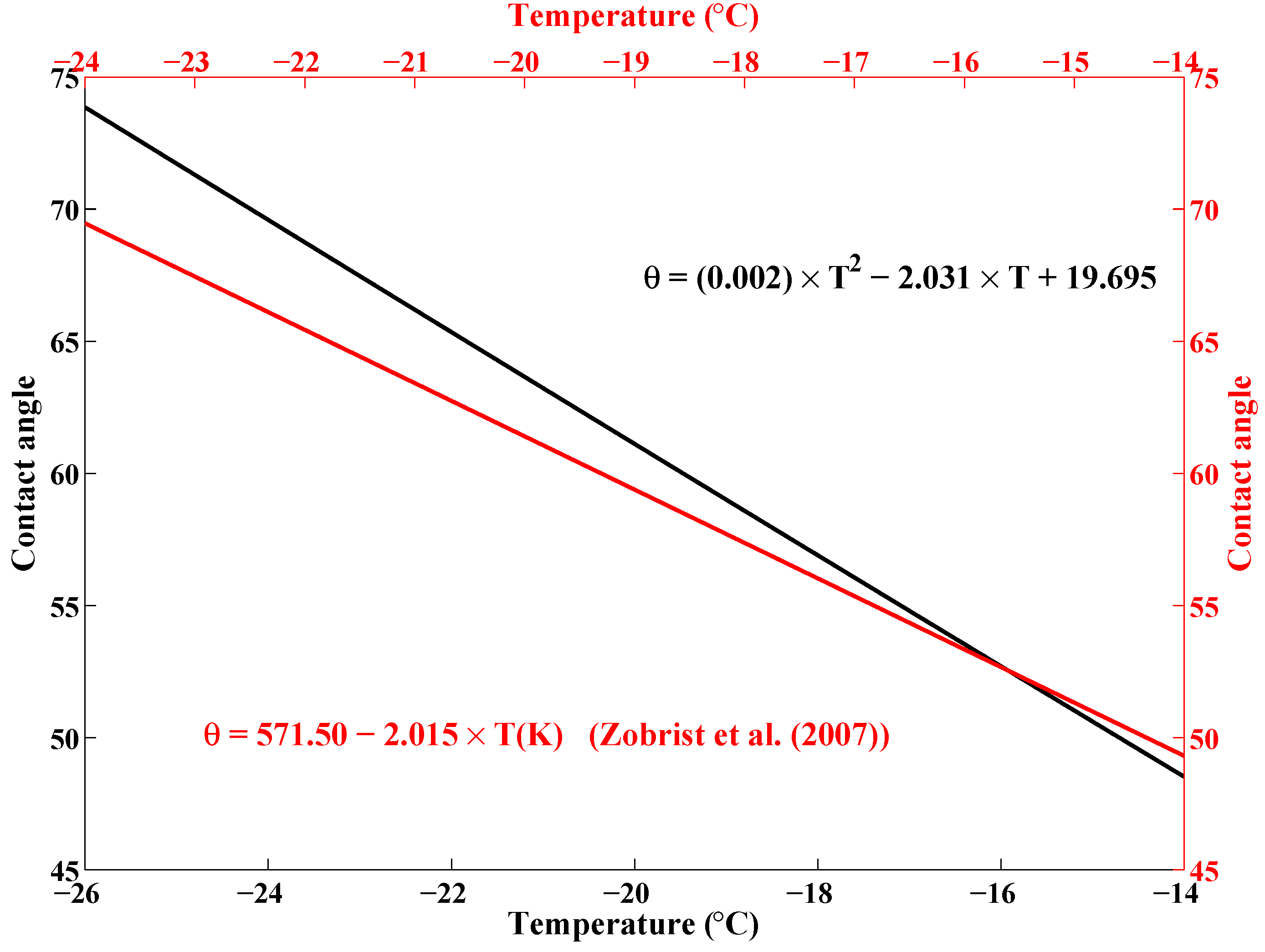
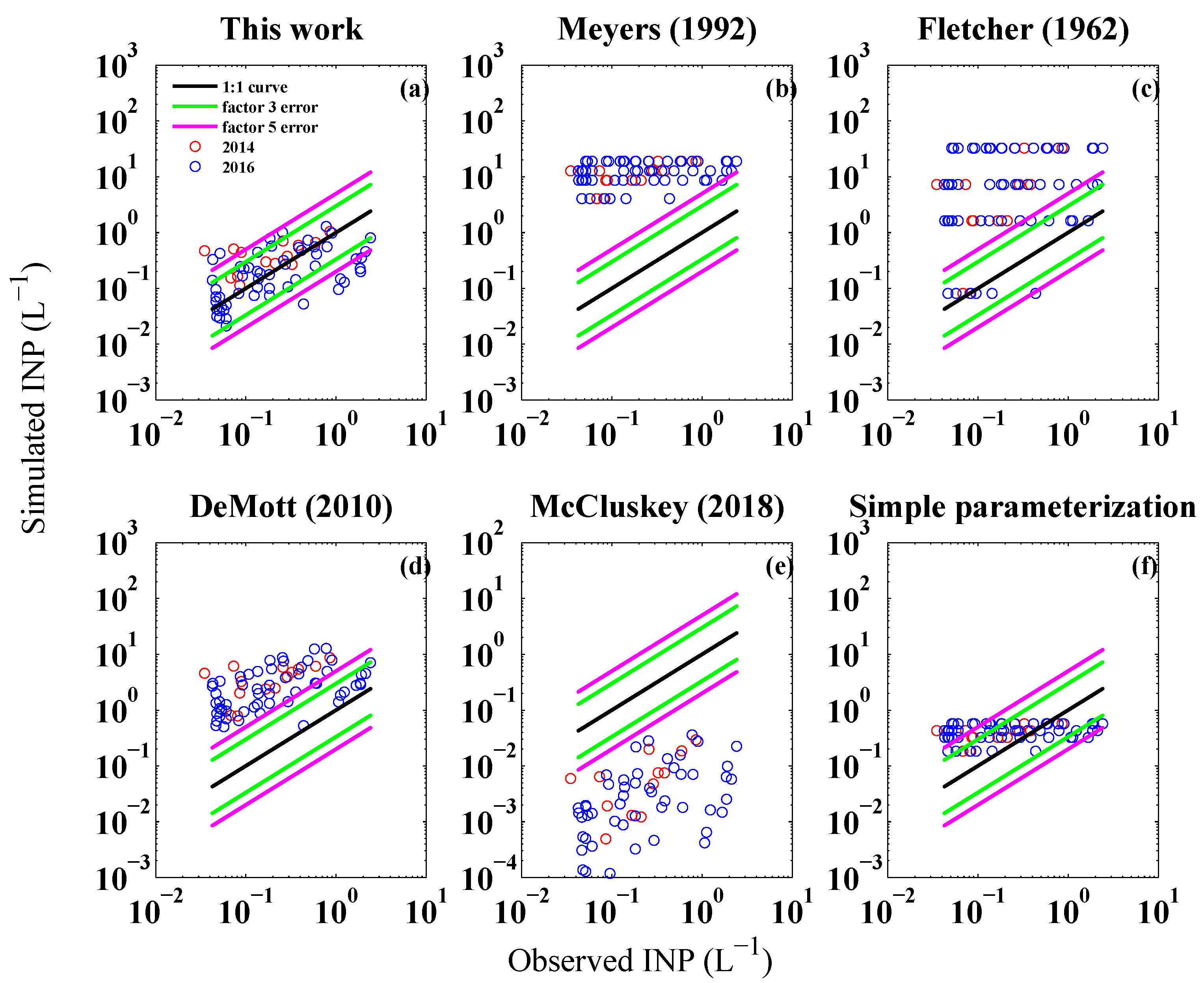
| Date | Time Mid-Sample (UTC) | Longitude () | Latitude () |
|---|---|---|---|
| 16/07/2014 | 21:52 | −71.117 | 71.702 |
| 18/07/2014 | 20:37 | −81.018 | 73.569 |
| 19/07/2014 | 16:18 | −83.976 | 74.110 |
| 21/07/2014 | 14:21 | −92.225 | 74.237 |
| 25/07/2014 | 19:50 | −86.998 | 74.428 |
| 26/07/2014 | 17:13 | −75.270 | 73.926 |
| 31/07/2016 | 19:42 | −70.350 | 70.846 |
| 01/08/2016 | 22:08 | −70.353 | 71.292 |
| 02/08/2016 | 13:02 | −72.960 | 71.977 |
| 03/08/2016 | 13:46 | −75.762 | 72.638 |
| 09/08/2016 | 13:14 | −75.757 | 76.317 |
| 10/08/2016 | 16:44 | −76.436 | 77.192 |
| 14/08/2016 | 12:54 | −68.535 | 80.047 |
| 16/08/2016(1) | 12:30 | −76.838 | 76.794 |
| 16/08/2016(2) | 18:30 | −77.875 | 76.053 |
| 16/08/2016(3) | 23:40 | −78.633 | 74.940 |
| 17/08/2016(1) | 12:42 | −88.119 | 74.328 |
| 17/08/2016(2) | 16:28 | −91.054 | 74.430 |
| 17/08/2016(3) | 22:08 | −95.155 | 74.659 |
| 19/08/2016(1) | 12:56 | −96.763 | 71.557 |
| 19/08/2016(2) | 19:06 | −97.699 | 71.294 |
| 20/08/2016(1) | 13:48 | −100.703 | 69.172 |
| 20/08/2016(2) | 19:10 | −101.320 | 68.723 |
| 21/08/2016 | 15:02 | −101.802 | 68.246 |
| 22/08/2016 | 13:18 | −99.888 | 68.492 |
| Scheme | Bias | RMSE | MRE |
|---|---|---|---|
| this work | −0.10 | 0.55 | 1.16 |
| Meyers et al. [9] | 12.26 | 13.24 | 97.52 |
| Fletcher [7] | 12.86 | 18.80 | 85.30 |
| DeMott et al. [4] | 3.01 | 4.00 | 19.01 |
| McCluskey et al. [61] | −0.41 | 0.69 | 0.98 |
| simple parameterization | 0.15 | 0.23 | 2.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirisan, A.; Girard, E.; Blanchet, J.-P.; Keita, S.A.; Gong, W.; Irish, V.; Bertram, A.K. CNT Parameterization Based on the Observed INP Concentration during Arctic Summer Campaigns in a Marine Environment. Atmosphere 2020, 11, 916. https://doi.org/10.3390/atmos11090916
Cirisan A, Girard E, Blanchet J-P, Keita SA, Gong W, Irish V, Bertram AK. CNT Parameterization Based on the Observed INP Concentration during Arctic Summer Campaigns in a Marine Environment. Atmosphere. 2020; 11(9):916. https://doi.org/10.3390/atmos11090916
Chicago/Turabian StyleCirisan, Ana, Eric Girard, Jean-Pierre Blanchet, Setigui Aboubacar Keita, Wanmin Gong, Vickie Irish, and Allan K. Bertram. 2020. "CNT Parameterization Based on the Observed INP Concentration during Arctic Summer Campaigns in a Marine Environment" Atmosphere 11, no. 9: 916. https://doi.org/10.3390/atmos11090916
APA StyleCirisan, A., Girard, E., Blanchet, J.-P., Keita, S. A., Gong, W., Irish, V., & Bertram, A. K. (2020). CNT Parameterization Based on the Observed INP Concentration during Arctic Summer Campaigns in a Marine Environment. Atmosphere, 11(9), 916. https://doi.org/10.3390/atmos11090916





