Updating Real-World Profiles of Volatile Organic Compounds and Their Reactivity Estimation in Tunnels of Mexico City
Abstract
1. Introduction
2. Experiments
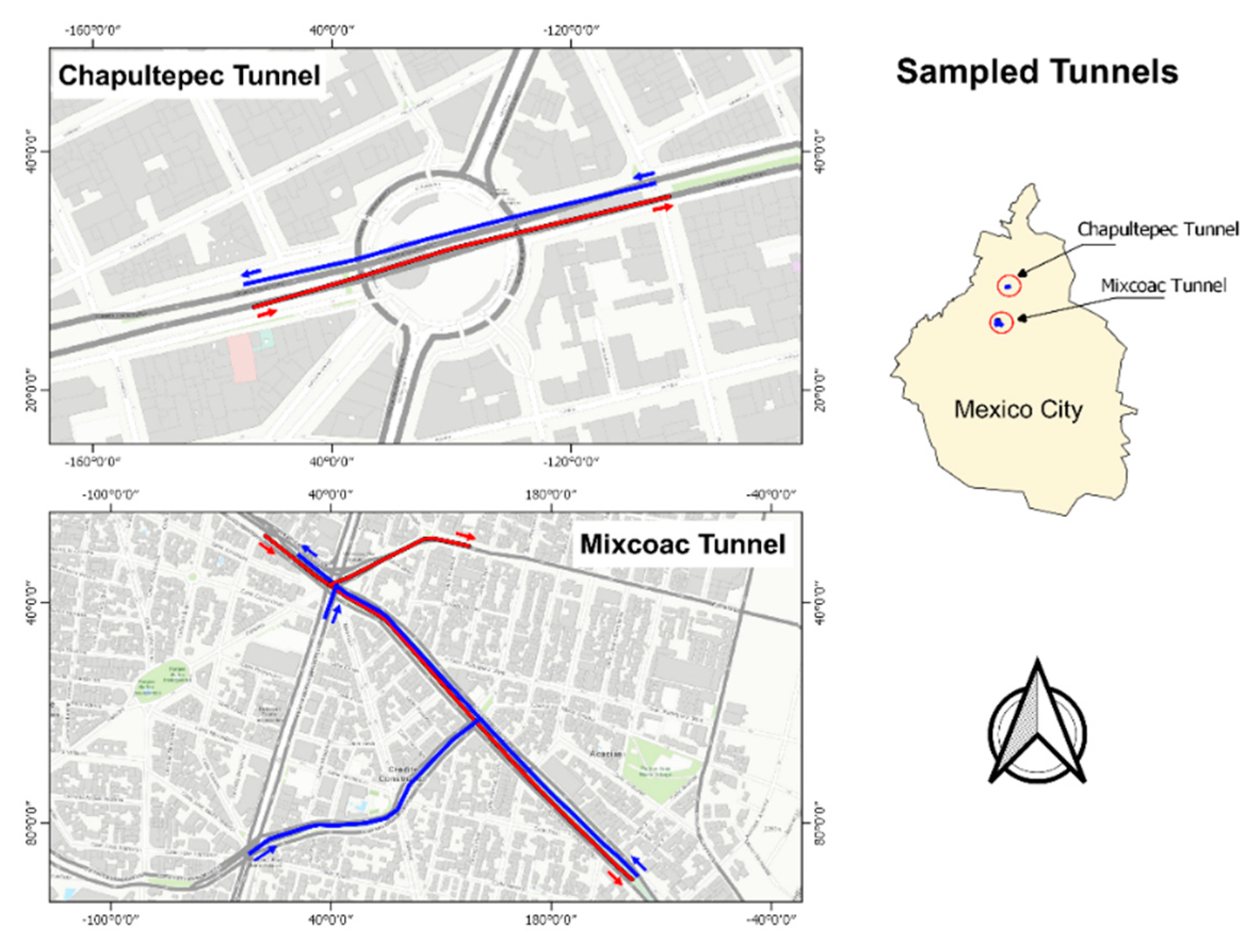
2.1. Sampling Sites
2.2. Sampling of Volatile Organic Compounds
2.3. Analyses of Volatile Organic Compounds
2.4. Gasoline and Head Space Analyses
2.5. Quality Control/Quality Assurance
2.6. Statistical Analysis
2.7. Determination of Ozone Formation Potential
3. Results
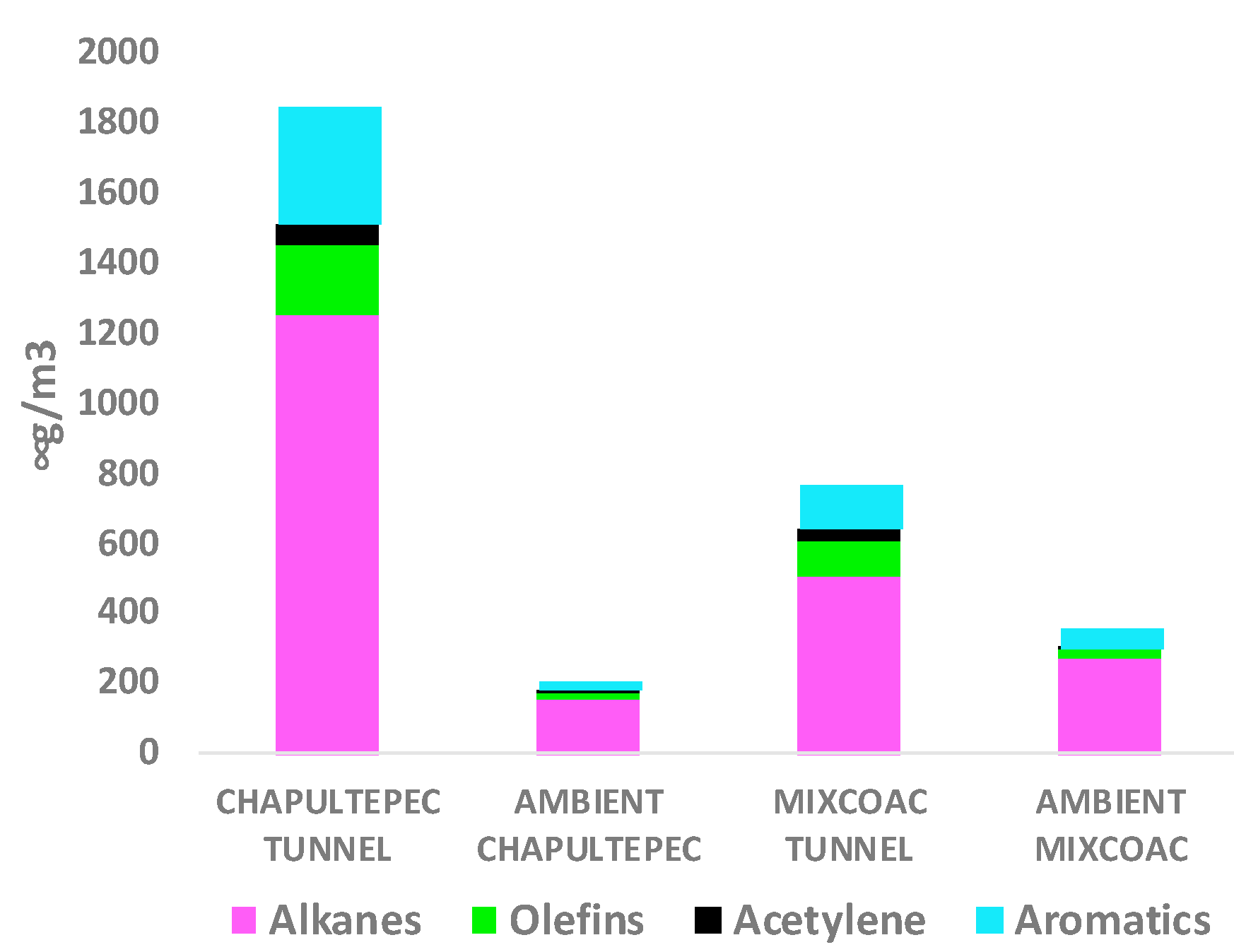
3.1. Concentrations of Volatile Organic Compounds in the Tunnel and in Ambient Air
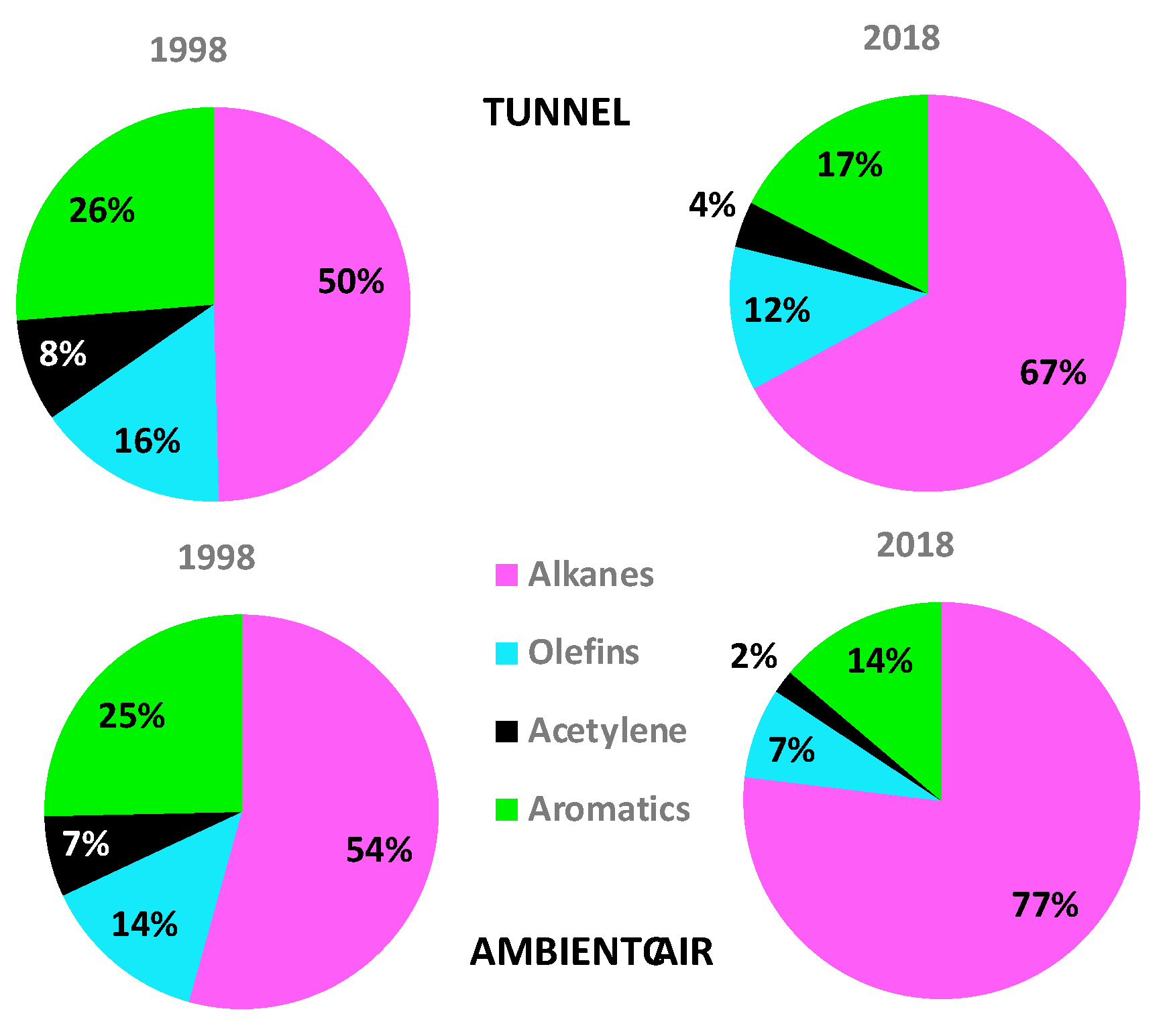
3.2. Exhaust Profiles of the Volatile Organic Compounds
3.3. Carbonyl Compounds in Exhaust Emissions
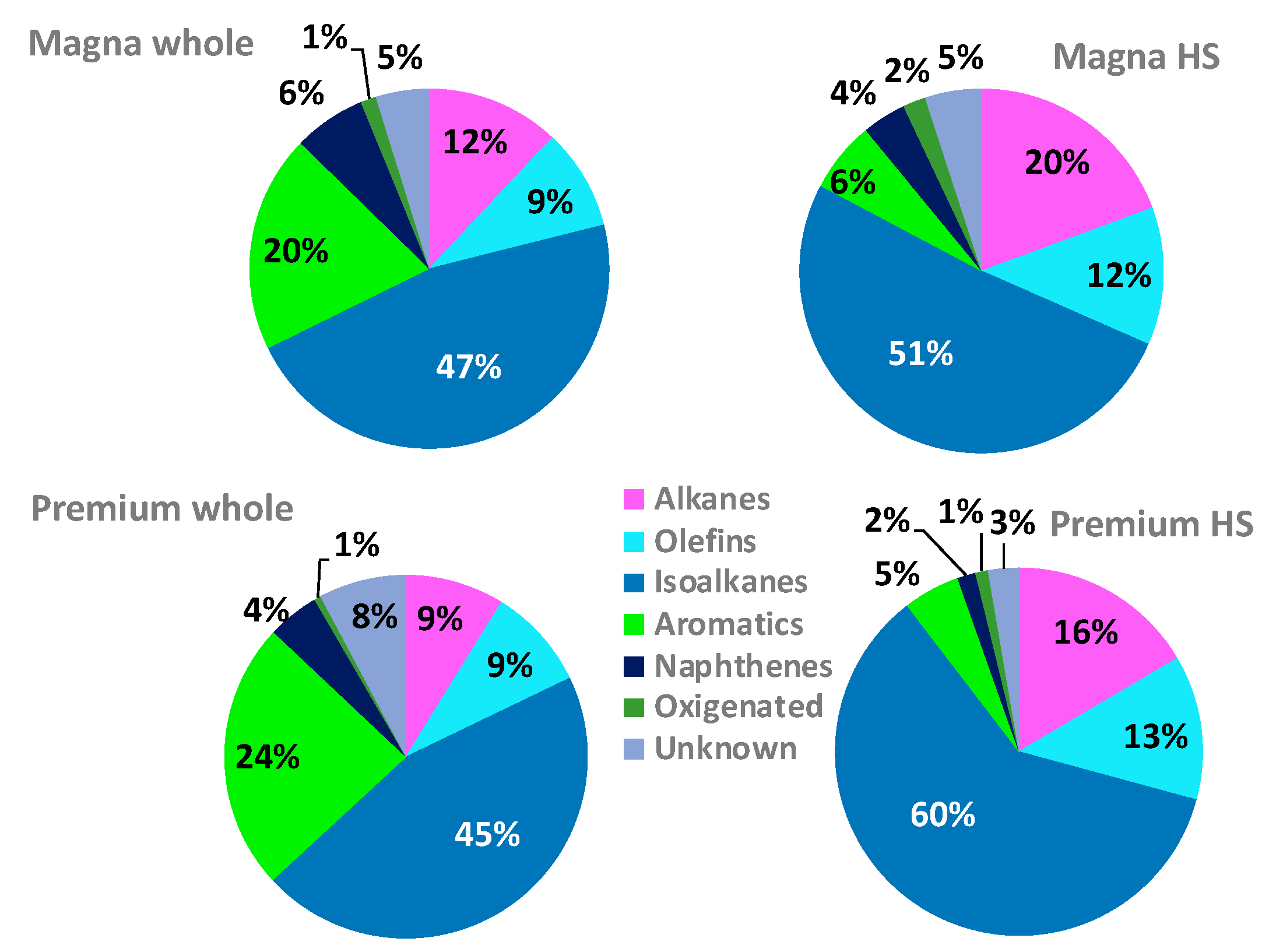
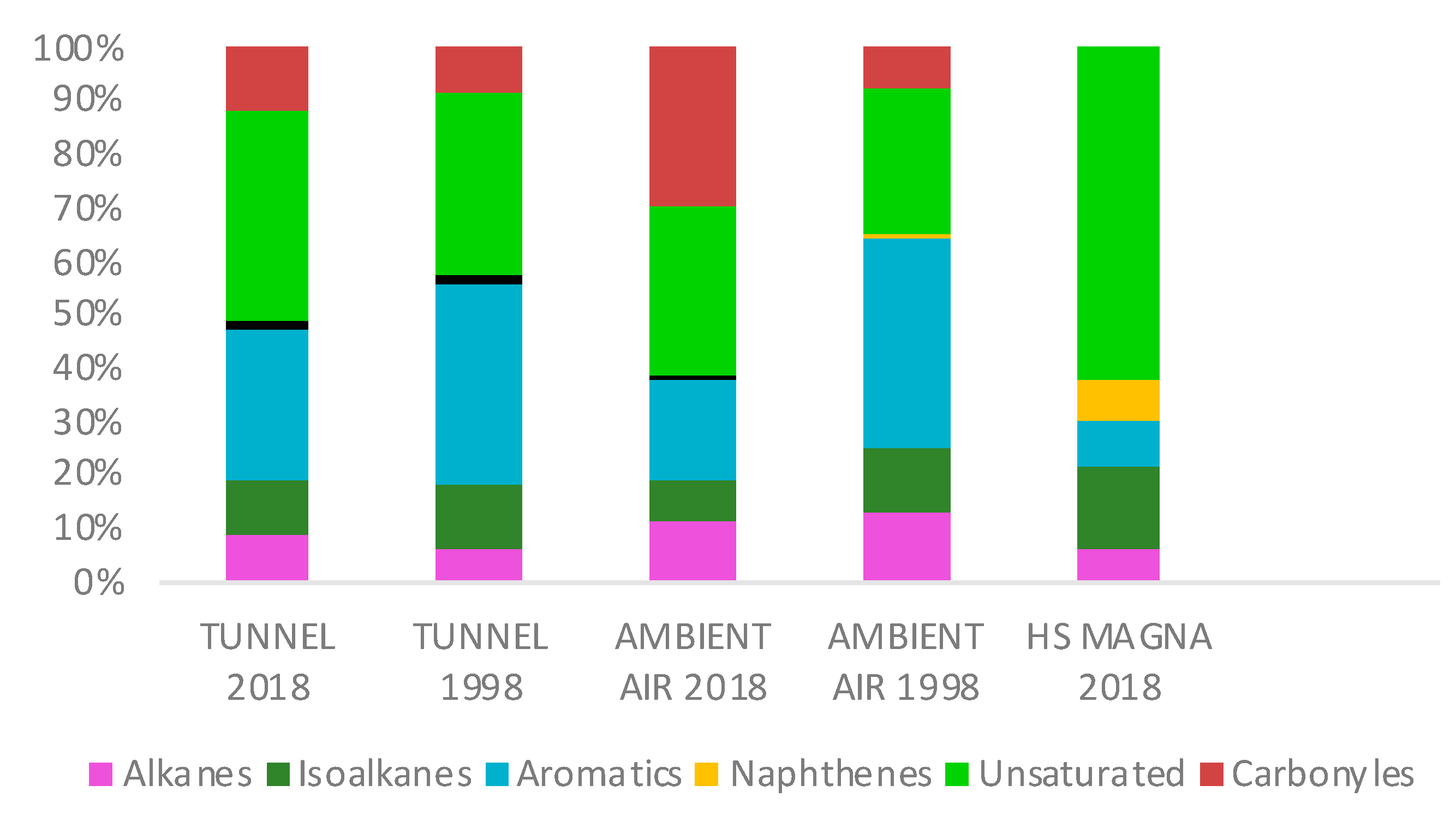
3.4. Gasoline and Head Space Profiles
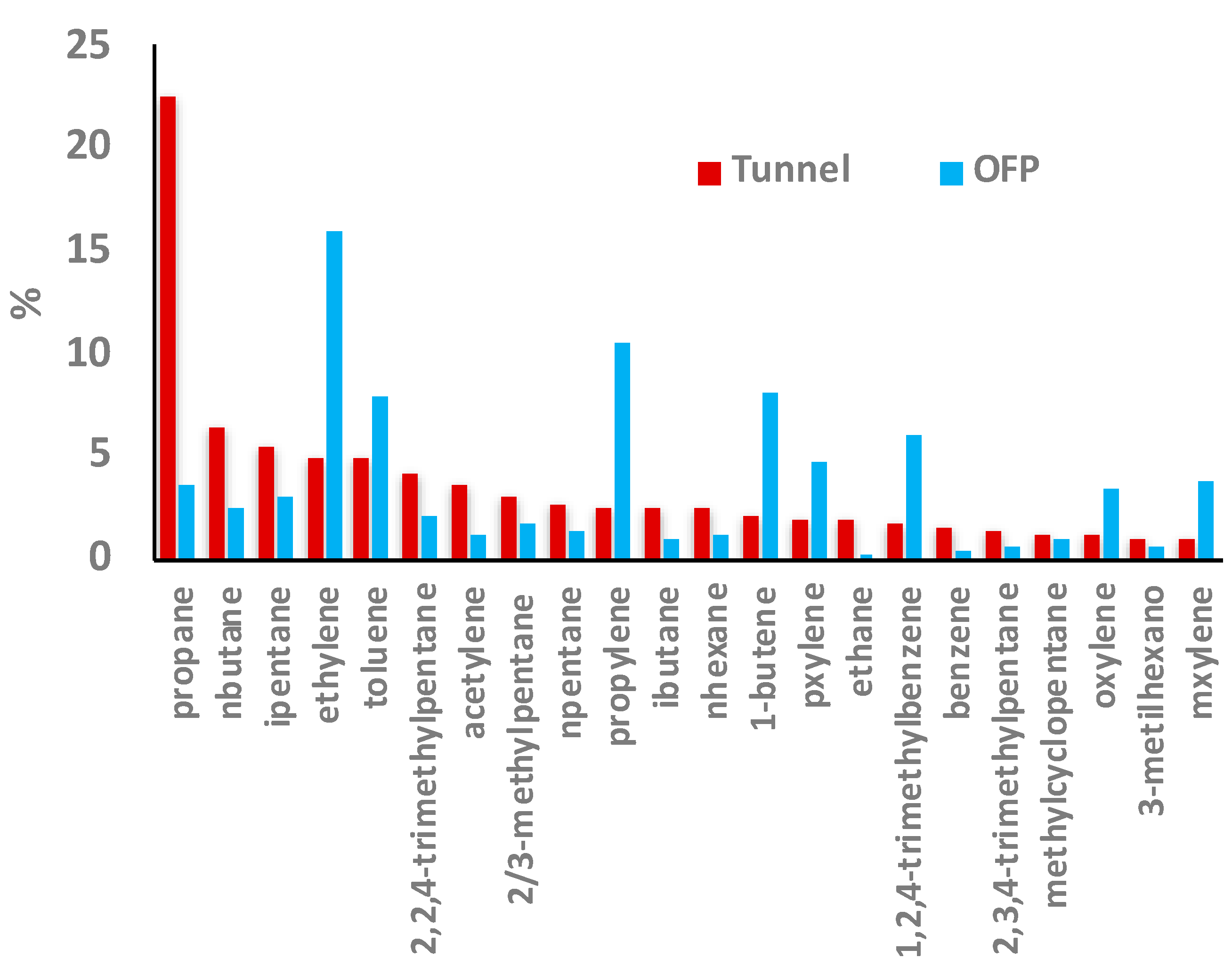
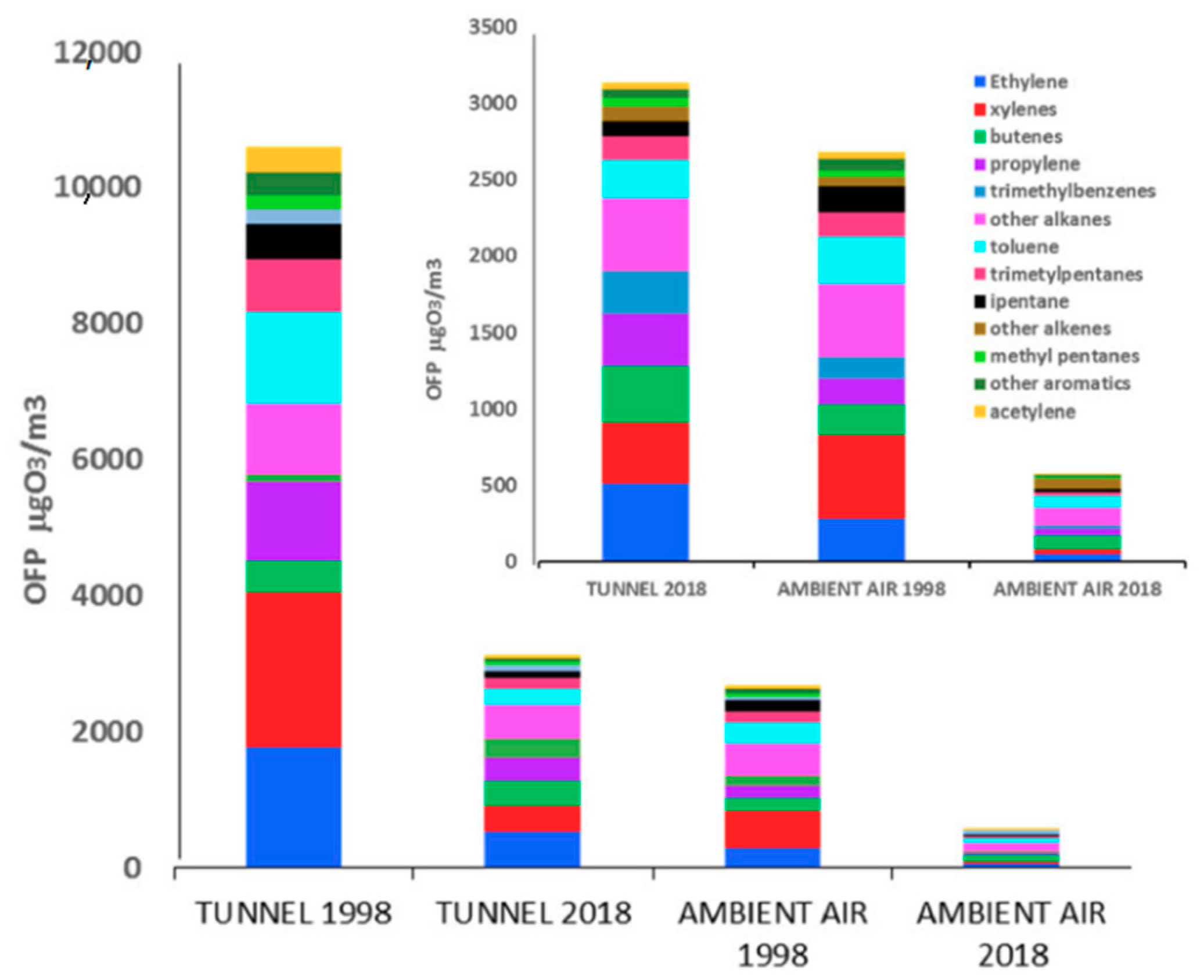
3.5. Ozone Formation Potential
4. Discussion
4.1. Volatile Organic Compounds Profiles
4.2. Gasoline and Head Space Profiles
4.3. Ozone Formation Potential
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rumchev, K.; Spickett, J.; Bulsara, M.; Phillips, M.; Stick, S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax 2004, 59, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Jerrett, M.; Burnett, R.T.; Pope, C.A., 3rd; Ito, K.; Thurston, G.; Krewski, D.; Shi, Y.; Calle, E.; Thun, M. Long-term ozone exposure and mortality. N. Engl. J. Med. 2009, 360, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Haick, H.; Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2014, 43, 1423–1449. [Google Scholar] [CrossRef] [PubMed]
- Riojas-Rodriguez, H.; Álamo-Hernández, U.; Texcalac-Sangrador, J.L.; Romieu, I. Health impact assessment of decreases in PM10 and ozone concentrations in the Mexico City Metropolitan Area. A basis for a new air quality management program. Salud Publica Mex. 2014, 56, 579–591. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, E.; Wang, X.; Jiang, L.; Liu, X. Ground-level O3 pollution and its impacts on food crops in China: A review. Environ. Pollut. 2015, 199, 42–80. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Zhong, L.; Lee, C.S.; Haghighat, F. Indoor ozone and climate change. Sustain. Cities Soc. 2017, 28, 466–472. [Google Scholar] [CrossRef]
- Maas, R.; Grennfelt, P. (Eds.) Towards Cleaner Air Scientific Assessment Report 2016 EMEP Steering Body and Working Group on Effects of the Convention on Long-Range Transboundary Air Pollution; RIVM: Oslo, Norway, 2016. [Google Scholar]
- Fujita, M.E.; Campbell, E.D.; Zielinska, B.; Chow, C.J.; Lindhjem, E.C.; DenBleyker, A.; Bishop, A.G.; Schuchman, G.B.; Stedman, H.D.; Lawson, D. Comparison of the MOVES2010a, MOBILE6.2, and EMFAC2007 mobile source emission models with on-road traffic tunnel and remote sensing measurements. J. Air Waste Manag. 2012, 62, 1134–1149. [Google Scholar] [CrossRef]
- Duncan, B.N.; Yoshida, Y.; Olson, J.R.; Sillman, S.; Martin, R.V.; Lamsal, L.; Hu, Y.; Pickering, K.E.; Retscher, C.; Allen, D.J.; et al. Application of OMI observations to a space-based indicator of NOx and VOC controls on surface ozone formation. Atmos. Environ. 2010, 44, 2213–2223. [Google Scholar] [CrossRef]
- Carter, W.P.L. Development of the SAPRC-7 Chemical Mechanism and Updated Ozone Reactivity Sales: Final Report to the California Air Resources Board Contract No. 03-318; University of California: Riverside, CA, USA, 2009. [Google Scholar]
- Siciliano, B.; Dantas, G.; da Silva, C.M.; Arbilla, G. Increased ozone levels during the COVID-19 lockdown: Analysis for the city of Rio de Janeiro, Brazil. Sci. Total Environ. 2020, 737, 139765. [Google Scholar] [CrossRef]
- Sicard, P.; De Marco, A.; Agathokleous, E.; Feng, Z.; Xu, X.; Paoletti, E.; Rodriguez, J.J.D.; Calatayud, V. Amplified ozone pollution in cities during the COVID-19 lockdown. Sci. Total Environ. 2020, 735, 139542. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, J.M.; Garrido-Perez, R.; García-Herrera, R. Early spring near-surface ozone in Europe during the COVID-19 shutdown: Meteorological effects outweigh emission changes. Sci. Total Environ. 2020, 747, 141322. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Brasseur, G.P. The response in air quality to the reduction of Chinese economic activities during the COVID-19 outbreak. Geophys. Res. Lett. 2020, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- SEDEMA. Secretaría del Medio Ambiente. Bases de datos—Red Automática de Monitoreo Atmosférico (RAMA). Secretaría de Medio Ambiente, Mexico City. (Mexico City Database of the Automatic Atmospheric Monitoring Network). 2020. Available online: http://www.aire.cdmx.gob.mx/default.php (accessed on 22 September 2020).
- Kanda, I.; Basaldud, R.; Magaña, M.; Retama, A.; Kubo, R. Comparison of ozone production regimes between two Mexican cities: Guadalajara and Mexico City. Atmosphere 2016, 7, 91. [Google Scholar] [CrossRef]
- Jaimes-Palomera, M. Diseño del Monitoreo de Compuestos Precursores de Ozono en la Atmósfera de la Ciudad de México y su Área Metropolitana. Ph.D. Thesis, UNAM, Mexico City, Mexico, 18 January 2017. [Google Scholar]
- Zavala, M.; Brune, W.H.; Velasco, E.; Retama, A.; Cruz-Alavez, L.A.; Molina, L.T. Changes in ozone production and VOC reactivity in the atmosphere of the Mexico City Metropolitan area. Atmos. Environ. 2020, 238, 117747. [Google Scholar] [CrossRef]
- Garzón, J.P.; Huertas, J.I.; Magaña, M.; Huertas, M.E.; Cárdenas, B.; Watanabe, T.; Maeda, T.; Wakamatsu, S.; Blanco, S. Volatile organic compounds in the atmosphere of Mexico City. Atmos. Environ. 2015, 119, 415–429. [Google Scholar] [CrossRef]
- SEDEMA. Secretaría del Medio Ambiente. Calidad del Aire en la Ciudad de México. Informe 2017. (Air Quality Report in Mexico City). 2017. Available online: http://www.aire.cdmx.gob.mx/descargas/publicaciones/flippingbook/informe_anual_calidad_aire_2017/mobile/#p=8 (accessed on 12 August 2020).
- Velasco, E.; Retama, A. Ozone’s threat hits back Mexico City. Sustain. Cities Soc. 2017, 31, 260–263. [Google Scholar] [CrossRef]
- Jaimes-Palomera, M.; Retama, A.; Elias-Castro, G.; Neria-Hernández, A.; Rivera-Hernández, O.; Velasco, E. Non-methane hydrocarbons in the atmosphere of Mexico City: Results of the 2012 ozone-season campaign. Atmos. Environ. 2016, 132, 258–275. [Google Scholar] [CrossRef]
- Watson, J.G.; Chow, J.; Pace, T.G. Chemical mass balance. In Receptor Modeling for Air Quality Management, Data Handling; Science and Technology; Hopke, P.K., Ed.; Elsevier Science: New York, NY, USA, 1991. [Google Scholar]
- Song, C.; Liu, Y.; Sun, L.; Zhang, Q.; Mao, H. Emissions of volatile organic compounds (VOCs) from gasoline- and liquified natural gas (LNG)-fueled vehicles in tunnel studies. Atmos. Environ. 2020, 234, 117626. [Google Scholar] [CrossRef]
- Lonneman, W.A.; Seila, R.L.; Meeks, S.A. Non-methane organic composition in the Lincoln Tunnel. Environ. Sci. Technol. 1986, 20, 790. [Google Scholar] [CrossRef]
- McLaren, R.; Gertler, A.W.; Wittorff, D.N.; Belzer, W.; Dann, T.; Singleton, D.L. Real world measurements of exhaust and evaporative emissions in the Cassiar Tunnel predicted by chemical mass balance modeling. Environ. Sci. Technol. 1996, 30, 300–310. [Google Scholar] [CrossRef][Green Version]
- Barrefors, G.; Petersson, G. Volatile hazardous hydrocarbons in a Scandinavian urban road tunnel. Chemosphere 1992, 25, 691–696. [Google Scholar] [CrossRef]
- Na, K.; Kim, Y.P.; Moon, I.; Moon, K.C. Chemical composition of major VOC emission sources in the Seoul atmosphere. Chemosphere 2004, 55, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Peng, Y.P. Volatile hydrocarbon emissions from vehicles and vertical ventilations in the Hsuehshan traffic tunnel, Taiwan. Environ. Monit. Assess. 2012, 184, 4015–4028. [Google Scholar] [CrossRef]
- Araizaga, A.; Mancilla, Y.; Mendoza, A. Volatile organic compound emissions from light-duty vehicles in Monterrey, Mexico: A tunnel study. Int. J. Environ. Res. 2013, 7, 277–292. [Google Scholar]
- Salameh, T.; Afif, C.; Sauvage, S.; Borbon, A.; Locoge, N. Speciation of non-methane hydrocarbons (NMHCs) from anthropogenic sources in Beirut, Lebanon. Environ. Sci. Pollut. Res. 2014, 21, 10867–10877. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, M.; Liu, X.; Yu, Z. Emission characteristics of VOCs from on-road vehicles in an urban tunnel in eastern china and predictions for 2017–2026. Aerosol Air Qual. Res. 2018, 18, 3025–3034. [Google Scholar] [CrossRef]
- Huang, C.; Tao, S.S.; Lou, Q.; Hu, H.; Wang, Q.; Wang, L.; Li, H.; Wang, J.; Liu, Q.; Zhou, L. Evaluation of emission factors for light-duty gasoline vehicles based on chassis dynamometer and tunnel studies in Shanghai, China. Atmos. Environ. 2017, 169, 203. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, W.; Simpson, I.; Huang, X.; Yu, J.; Huang, Z.; Wang, Z.; Zhang, Z.; Liu, D.; Huang, Z.; et al. Decadal changes in emissions of volatile organic compounds (VOCs) from on-road vehicles with intensified automobile pollution control: Case study in a busy urban tunnel in south China. Environ. Pollut. 2018, 233, 806–819. [Google Scholar] [CrossRef]
- Marinello, S.; Lolli, F.; Gamberini, R. Roadway tunnels: A critical review of air pollutant concentrations and vehicular emissions. Transp. Res. D 2020, 86, 102478. [Google Scholar] [CrossRef]
- Mugica, V.; Vega, E.; Arriaga, J.L.; Ruiz, M.E. Determination of motor vehicle profiles for non-methane organic compounds in the Mexico City Metropolitan Area. J. Air Waste Manag. 1998, 48, 1060–1068. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mugica, V.; Vega, E.; Ruiz, H.; Sánchez, G.; Reyes, E.C.A. Photochemical reactivity and sources of individual VOCS in Mexico City. Air Pollut. 2002, X, 209–217. [Google Scholar]
- USEPA. Compendium Method TO-11A. Determination of Formaldehyde in Ambient Air Using Adsorbent Cartridge Followed by High Performance Liquid Chromatography (HPLC); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999.
- USEPA. Compendium Method TO-14A. Determination of Volatile Organic Compounds (VOCs) in Ambient Air Using Specially Prepared Canisters with Subsequent Analysis by Gas Chromatography; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999.
- USEPA. Compendium Method TO-15. Determination of Volatile Organic Compounds (VOCs) in Air Collected in Specially-Prepared Canisters and Analyzed by Gas Chromatography/Mass Spectrometry (GC/MS); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1999.
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Wagner, T.; Wyszyński, M.L. Aldehydes and ketones in engine exhaust emissions—A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 1996, 210, 109–122. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Tsai, W.Y. Carbonyl compounds in the roadside environment of Hong Kong. J. Hazard. Mater. 2006, 133, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kanjanasiranont, N.; Prueksasit, T.; Morknoy, D. Inhalation exposure and health risk levels to BTEX and carbonyl compounds of traffic policeman working in the inner city of Bangkok, Thailand. Atmos. Environ. 2017, 152, 111–120. [Google Scholar] [CrossRef]
- Grutter, M.; Flores, E.; Andraca-Ayala, G.; Báez, A. Formaldehyde levels in downtown Mexico City during 2003. Atmos. Environ. 2005, 39, 1027–1034. [Google Scholar] [CrossRef]
- Mugica-Álvarez, V.; Martínez-Reyes, C.A.; Santiago-Tello, N.M.; Martínez-Rodríguez, I.; Gutiérrez-Arzaluz, M.; Figueroa-Lara, J.J. Evaporative volatile organic compounds from gasoline in Mexico City: Characterization and atmospheric reactivity. Energy Rep. 2020, 6, 825–830. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, B.; Wang, S.; Hao, J. Ozone and secondary organic aerosol formation potential from anthropogenic volatile organic compounds emissions in China. J. Environ. Sci. 2017, 53, 224–237. [Google Scholar] [CrossRef]
- SEDEMA. Secretaría del Medio Ambiente. Inventario de Emisiones de la CDMX 2016. Secretaría de Medio Ambiente. 2016. Available online: http://www.aire.cdmx.gob.mx/descargas/publicaciones/flippingbook/inventario-emisiones-2016/mobile/ (accessed on 15 March 2020).
- INEGI. Parque Vehicular. Instituto Nacional de Estadística, Geografía e Informática. 2019. Available online: https://www.inegi.org.mx/temas/vehiculos (accessed on 10 October 2019).
- Cui, L.; Wang, X.L.; Ho, K.F.; Gao, Y.; Liu, C.; Hang, H.S.S.; Li, H.W.; Lee, S.C.; Wang, X.M.; Jiang, B.Q.; et al. Decrease of VOC emissions from vehicular emissions in Hong Kong from 2003 to 2015: Results from a tunnel study. Atmos. Environ. 2018, 177, 64–74. [Google Scholar] [CrossRef]
- Guo, H.; Zou, S.C.; Tsai, W.Y.; Chan, L.Y.; Blake, D.R. Emission characteristics of nonmethane hydrocarbons from private cars and taxis at different driving speeds in Hong Kong. Atmos. Environ. 2011, 45, 2711–2721. [Google Scholar] [CrossRef]
- Kean, A.J.; Harley, R.A.; Kendall, G.R. Effects of vehicle speed and engine. Load on motor vehicle emissions. Environ. Sci. Technol. 2003, 37, 3739–3746. [Google Scholar] [CrossRef] [PubMed]
- Sosa, E.R.; Bravo, A.H.; Mugica, A.V.; Sanchez, A.P.; Bueno, L.E.; Krupa, S. Levels and source apportionment of volatile organic compounds in Southwestern area of Mexico City. Environ. Pollut. 2009, 157, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Durrenberger, C.J.; Spinhirne, J.P.; McGaughey, G.; McDonald-Buller, E.C. VOC Monitoring in the Austin Area: 2012; Center for Energy and Environmental Resources of the University of Texas: Austin, TX, USA, 2013. [Google Scholar]
- Aung, W.Y.; Noguchi, M.; Yi, E.E.P.N.; Thant, Z.; Uchiyama, S.; Shwe, T.T.W.; Kunugita, N.; Mar, O. Preliminary assessment of outdoor and indoor air quality in Yangon city, Myanmar. Atmos. Pollut. Res. 2019, 10, 722–730. [Google Scholar] [CrossRef]
- Possanzini, M.; Di Palo, V.; Petricca, M.; Fratarcangeli, R.; Brocco, D. Measurement of formaldehyde and acetaldehyde in the urban ambient air. Atmos. Environ. 1996, 30, 3757–3764. [Google Scholar] [CrossRef]
- Grosjean, D.; Grosjean, E. Airborne carbonyls from motor vehicle emissions in two highway tunnels. Res. Rep. Health Eff. Inst. 2002, 107, 5778. [Google Scholar]
- Nogueira, T.; de Souza, K.F.; Fornaro, A.; de Fatima Andrade, M.; de Carvalho, L.R.F. On-road emissions of carbonyls from vehicles powered by biofuel blends in traffic tunnels in the Metropolitan Area of Sao Paulo, Brazil. Atmos. Environ. 2015, 108, 88–97. [Google Scholar] [CrossRef]
- Kean, A.J.; Grosjean, E.; Grosjean, D.; Harley, R.A. On-road measurement of carbonyls in California light-duty vehicle emissions. Environ. Sci. Technol. 2001, 35, 4198–4204. [Google Scholar] [CrossRef]
- Gentner, D.R.; Worton, D.R.; Isaacman, G.; Davis, L.C.; Dallmann, T.R.; Wood, E.C.; Herndon, S.C.; Goldstein, A.H.; Harley, R.A. Chemical composition of gas phase organic carbon emissions from motor vehicles and implications for ozone production. Environ. Sci. Technol. 2013, 47, 11837–11848. [Google Scholar] [CrossRef]
- Schifter, I.; Díaz, L.; González, U.; González-Macías, C. Fuel formulation for recent model light duty vehicles in Mexico base on a model for predicting gasoline emissions. Fuel 2013, 107, 371–381. [Google Scholar] [CrossRef]
| Light Duty Cars | Vans and Pick Ups | Motorcycles | Buses and Diesel Trucks | Total Vehicles | |||||
|---|---|---|---|---|---|---|---|---|---|
| Chapultepec 2018 | Number | % | Number | % | Number | % | Number | % | |
| January 17 | 3728 | 69.6 | 1004 | 18.7 | 560 | 10.45 | 68 | 1.3 | 5360 |
| January 18 | 3728 | 67.6 | 1200 | 21.8 | 500 | 9.1 | 88 | 1.6 | 5516 |
| January 19 | 3010 | 65.78 | 878 | 19.19 | 598 | 11.61 | 90 | 2.1 | 4576 |
| Average Chap | 3488.6 | 1027.4 | 553 | 82 | 5151 | ||||
| Mixcoac 2018 | |||||||||
| March 26 | 2227 | 66.0 | 598 | 17.7 | 544 | 16.1 | 3 | 0.1 | 3372 |
| March 27 | 2177 | 68.4 | 746 | 22.9 | 273 | 8.6 | 4 | 0.13 | 3200 |
| Average Mix | 2202 | 67.2 | 672 | 20.3 | 408.5 | 12.4 | 3.5 | 0.1 | 3286 |
| 2018. Total Average | 2974 | 67.5 | 885.2 | 20 | 495 | 11.2 | 51 | 1 | 4405 |
| 1998. Total Average (3 days) | 1588 | 87.7 | 173 | 9.6 | 44 | 2.4 | 6 | 0.3 | 1811 |
| Compound | Tunnel 2018 Concentration Percent | Ambient Air 2018 Concentration Percent | Tunnel 1998 a Concentration Percent | Ambient Air 1998 a Concentration Percent | ||||
|---|---|---|---|---|---|---|---|---|
| µg/m3 | % | µg/m3 | % | µg/m3 | % | µg/m3 | % | |
| ethane | 21.8 ± 9.4 | 1.9 ± 0.3 | 6.9 ± 0.9 | 2.6 ± 1.0 | 48.0 ± 12.1 | 1.3 ± 0.1 | 16.5 ± 7.2 | 1.4 ± 0.4 |
| ethylene | 59.1 ± 23.3 | 5.1 ± 1.2 | 6.1 ± 3.3 | 2.1 ± 0.3 | 201.4 ± 98.7 | 5.3 ± 0.4 | 32.4 ± 17.1 | 2.8 ± 0.3 |
| propane | 258.6 ± 126.5 | 23.1 ± 6.5 | 91.0 ± 57.1 | 35.5 ± 10.5 | 215.8 ± 106.7 | 5.7 ± 0.7 | 258.0 ± 147 | 22.2 ± 7.7 |
| propylene | 30.2 ± 12.1 | 2.6 ± 0.7 | 3.9 ± 1.8 | 1.4 ± 0.3 | 103.5 ± 23.6 | 2.7 ± 0.2 | 14.9 ± 3.6 | 1.3 ± 0.5 |
| ibutane | 29.4 ± 11.2 | 2.5 ± 0.6 | 10.8 ± 4.7 | 4.1 ± 0.5 | 58.9 ± 13.2 | 1.6 ± 0.3 | 58.0 ± 13.7 | 4.9 ± 0.4 |
| nbutane | 75.3 ± 27.6 | 6.5 ± 1.7 | 21.5 ± 7.4 | 8.3 ± 0.7 | 180.0 ± 41.3 | 4.8 ± 0.7 | 133.1 ± 61.9 | 11.4 ± 1.1 |
| acetylene | 44.6 ± 19.2 | 3.8 ± 0.8 | 5.2 ± 1.9 | 1.9 ± 0.4 | 380.8 ± 171.4 | 10.1 ± 1.2 | 48.7 ± 10.8 | 4.2 ± 0.3 |
| 1-butene | 27.6 ± 18.1 | 2.4 ± 0.8 | 7.2 ± 5.1 | 4.4 ± 3.6 | 10.6 ± 3.15 | 0.3 ± 0.0 | 10.6 ± 2.8 | 0.9 ± 0.3 |
| trans-2-butene | 4.2 ± 2.5 | 0.4 ± 0.1 | 0.8±0.3 | 0.3±0.2 | 13.5 ± 2.9 | 0.4 ± 0.0 | 3.3 ± 6.0 | 0.3 ± 0.0 |
| cis-2-butene | 3.4 ± 2.1 | 0.3 ± 0.1 | 0.8 ± 0.1 | 0.3 ± 0.2 | 11.5 ± 1.7 | 0.3 ± 0.0 | 3.9 ± 0.9 | 0.3 ± 0.0 |
| ipentane | 73.2 ± 44.2 | 6.3 ± 1.3 | 18.9 ± 6.8 | 7.0 ± 4.9 | 379.9 ± 192 | 10.1 ± 1.2 | 125.8 ± 71.4 | 10.8 ± 1.0 |
| 1-pentene | 4.3 ± 1.9 | 0.4 ± 0.1 | 1.2 ± 1.1 | 0.4 ± 0.3 | 11.4 ± 1.8 | 0.3 ± 0.0 | 2.9 ± 0.7 | 0.3 ± 0.0 |
| nPentane | 36.0 ± 20.6 | 3.1 ± 0.6 | 8.9 ± 2.3 | 3.2 ± 2.0 | 152.2 ± 65.7 | 4.0 ± 0.4 | 43.3 ± 9.9 | 3.7 ± 0.0 |
| trans-2-pentene | 5.6 ± 3.1 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 21.1 ± 4.6 | 0.6 ± 0.1 | 4.5 ± 0.9 | 0.4 ± 0.2 |
| isoprene | 3.6 ± 1.0 | 0.3 ± 0.2 | 5.6 ± 3.9 | 1.8 ± 1.8 | 1.6 ± 0.5 | 0.1 ± 0.9 | 1.6 ± 0.4 | 0.1 ± 0.0 |
| cis-2-pentene | 2.7 ± 1.4 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 11.4 ± 3.9 | 0.3 ± 2.8 | 2.4 ± 0.5 | 0.2 ± 0.0 |
| 2,2-dimethylbutane | 3.3 ± 1.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 3.2 ± 0.7 | 0.1 ± 0.0 | 5.6 ± 1.2 | 0.5 ± 0.0 |
| cyclopentane | 12.2 ± 7.3 | 1.1 ± 0.2 | 2.2 ± 0.5 | 0.8 ± 0.4 | 11.6 ± 2.6 | 0.3 ± 0.3 | 2.4 ± 0.5 | 0.2 ± 0.0 |
| 2/3-methylpentane | 41.0 ± 26.0 | 3.5 ± 0.7 | 8.4 ± 3.1 | 2.9 ± 2.0 | 155.7 ± 72.3 | 4.1 ± 0.2 | 30.0 ± 7.2 | 2.6 ± 0.0 |
| nhexane | 33.1 ± 22.3 | 2.8 ± 0.6 | 10.0 ± 5.0 | 3.7 ± 2.7 | 120.7 ± 48.7 | 3.2 ± 0.1 | 31.2 ± 7.1 | 2.7 ± 0.7 |
| 2,4-dimethylpentane | 10.5 ± 7.8 | 0.9 ± 0.3 | 0.5 ± 0.5 | 0.1 ± 0.1 | 38.8 ± 9.5 | 1.0 ± 0.0 | 3.4 ± 0.8 | 0.3 ± 0.0 |
| methylcyclopentane | 16.0 ± 10.9 | 1.4 ± 0.3 | 2.3 ± 1.4 | 0.8 ± 0.6 | 5.5 ± 1.3 | 0.2 ± 0.0 | 6.8 ± 1.3 | 0.6 ± 0.1 |
| benzene | 19.4 ± 10.5 | 1.7 ± 0.2 | 3.0 ± 0.7 | 1.0 ± 0.3 | 119.7 ± 51.6 | 3.2 ± 0.2 | 17.7 ± 4.8 | 1.5 ± 0.1 |
| 2-methylhexane | 13.5 ± 9.1 | 1.2 ± 0.2 | 0.8 ± 0.3 | 0.3 ± 0.1 | 51.2 ± 12.6 | 1.4 ± 0.0 | 9.0 ± 2.1 | 0.8 ± 0.1 |
| cyclohexane | 5.6 ± 3.3 | 0.5 ± 0.1 | 0.3 ± 0.3 | 0.1 ± 0.2 | 43.9 ± 16.5 | 1.2 ± 0.0 | 2.0 ± 0.5 | 0.2 ± 0.0 |
| 2,3-dimethylpentane | 11.9 ± 9.1 | 1.0 ± 0.3 | 0.4 ± 0.4 | 0.1 ± 0.1 | 45.2 ± 9.8 | 1.2 ± 0.0 | 4.3 ± 0.8 | 0.4 ± 0.0 |
| 3-methylhexane | 14.5 ± 9.9 | 1.2 ± 0.3 | 0.8 ± 0.4 | 0.2 ± 0.2 | 56.7 ± 7.5 | 1.5 ± 0.0 | 11.4 ± 2.7 | 1.0 ± 0.1 |
| 2,2,4-trimethylpentane | 58.1 ± 47.8 | 5.0 ± 1.7 | 5.1 ± 1.0 | 1.9 ± 0.8 | 233.7 ± 114.3 | 6.2 ± 0.4 | 22.6 ± 5.3 | 1.9 ± 0.2 |
| nheptane | 12.5 ± 8.2 | 1.1 ± 0.2 | 1.3 ± 1.2 | 0.5 ± 0.3 | 49.2 ± 12.0 | 1.3 ± 0.0 | 10.3 ± 3.0 | 0.9 ± 0.1 |
| methylcyclohexane | 7.4 ± 5.3 | 0.6 ± 0.2 | 0.4 ± 0.4 | 0.1 ± 0.1 | 19.1 ± 2.4 | 0.5 ± 0.0 | 4.1 ± 1.3 | 0.3 ± 0.0 |
| 2,3,4-trimethylpentane | 19.0 ± 15.4 | 1.6 ± 0.5 | 0.8 ± 0.4 | 0.2 ± 0.2 | 93.9 ± 3.7 | 2.5 ± 0.1 | 11.0 ± 3.6 | 0.9 ± 0.1 |
| 2-methylheptane | 5.8 ± 4.6 | 0.5 ± 0.2 | 0.5 ±0.4 | 0.1 ± 0.1 | 21.9 ± 4.2 | 0.6 ± 0.1 | 4.1 ± 1.0 | 0.4 ± 0.0 |
| toluene | 65.5 ± 41.10 | 5.6 ± 1.1 | 20.8 ± 15.1 | 7.1 ± 6.3 | 347.7 ± 164.1 | 9.2 ± 0.2 | 79.9 ± 22.9 | 6.9 ± 0.2 |
| 3-methylheptane | 9.1 ± 6.7 | 0.8 ± 0.2 | 0.5 ± 0.4 | 0.1 ± 0.1 | 25.0 ± 6.4 | 0.7 ± 0.0 | 4.1 ± 0.8 | 0.4 ± 0.0 |
| noctane | 6.5 ± 3.9 | 0.6 ± 0.2 | 0.8 ± 0.4 | 0.3 ± 0.2 | 28.1 ± 6.6 | 0.7 ± 0.4 | 6.2 ± 1.8 | 0.5 ± 0.0 |
| ethylbenzene | 12.9 ± 7.4 | 1.1 ± 0.2 | 1.2 ± 1.0 | 0.3 ± 0.2 | 69.7 ± 15.0 | 1.9 ± 0.1 | 15.5 ± 4.6 | 1.3 ± 0.1 |
| m/p-xylene | 40.5 ± 18.9 | 3.5 ± 0.6 | 2.8 ± 1.3 | 0.5 ± 0.5 | 233.5 ± 123.7 | 6.2 ± 0.3 | 56.0 ± 18.1 | 4.8 ± 0.2 |
| nnonane | 2.9 ± 1.2 | 0.3 ± 0.3 | 0.6 ± 0.6 | 0.2 ± 0.1 | 2.3 ± 0.6 | 0.1 ± 0.0 | 4.4 ± 1.5 | 0.4 ± 0.0 |
| styrene | 2.9 ± 1.4 | 0.3 ± 0.3 | 1.0 ± 0.6 | 0.2 ± 0.2 | 13.3 ± 3.6 | 0.4 ± 0.0 | 3.6 ± 0.9 | 0.3 ± 0.0 |
| o-xylene | 14.9 ± 9.5 | 1.3 ± 0.3 | 1.2 ± 1.1 | 0.3 ± 0.2 | 90.2 ± 23.7 | 2.4 ± 0.4 | 21.1 ± 6.8 | 1.8 ± 0.1 |
| iprophylbenzene | 0.9 ± 0.7 | 0.1 ± 0.1 | 0.5 ± 0.5 | 0.1 ± 0.1 | 8.4 ± 2.0 | 0.2 ± 0.0 | 4.6 ± 2.3 | 0.4 ± 0.0 |
| nprophylbenzene | 2.0 ± 1.8 | 0.2 ± 0.3 | 0.8 ± 0.4 | 0.2 ± 0.2 | 18.8 ± 4.3 | 0.5 ± 0.0 | 3.1 ± 2.0 | 0.3 ± 0.0 |
| 1,3,5 trimethylbenzene | 5.4 ± 3.3 | 0.46 ± 0.32 | 0.8 ± 0.7 | 0.2 ± 0.1 | 35.2 ± 7.7 | 0.9 ± 1.0 | 10.2 ± 3.6 | 0.9 ± 1.0 |
| ndecane | 3.1 ± 2.9 | 0.26 ± 0.54 | 0.9 ± 0.5 | 0.3 ± 0.3 | 10.0 ± 2.6 | 0.3 ± 0.1 | 2.8 ± 1.5 | 0.2 ± 0.0 |
| 1,2,4-trimethylbenzene | 22.9 ± 16.6 | 2.0 ± 0.6 | 1.8 ± 1.1 | 0.5 ± 0.3 | 10.5 ± 3.6 | 0.3 ± 0.0 | 16.3 ± 4.9 | 1.4 ± 1.0 |
| nundecane | 1.9 ± 1.5 | 0.2 ± 0.5 | 3.1 ± 2.3 | 1.5 ± 1.9 | 7.6 ± 1.6 | 0.2 ± 0.1 | 1.3 ± 0.6 | 0.1 ± 0.0 |
| Total | 1156.6 | 262.7 | 3771.5 | 1165.3 |
| City | Country | Abundance Ranking 1 | Abundance Ranking 2 | Abundance Ranking 3 | Abundance Ranking 4 | Abundance Ranking 5 | Reference and Publication Year |
|---|---|---|---|---|---|---|---|
| Seoul | Korea | nbutane | ethylene | toluene | ibutane | m/p xylene | [29] 2004 |
| Taipei | Taiwan | ethylene | ipentane | propylene | acetylene | 1-butene | [30] 2012 |
| Monterrey | Mexico | ethylene | acethylene | ipentane | toluene | nbutane | [31] 2013 |
| Beirut | Lebanon | ipentane | nbutane | toluene | m/p xylene | ibutane | [32] 2014 |
| Nanjing | China | ethane | ethylene | propane | ipentane | acetylene | [35] 2018 |
| Beijing | China | ethylene | ipentane | toluene | acethylene | nbutane | [25] 2020 |
| Mexico City | Mexico | acethylene | ipentane | toluene | 2,2,4 trimethylpentane | m/p xylene | [37] 1998 |
| Mexico City | Mexico | propane | nbutane | ipentane | toluene | ethylene | This study |
| Compound | Tunnel Concentrations µg m−3 | Composition Tunnel | Ambient air Concentrations µg m−3 | Composition Tunnel | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Min–Max | wt% | Mean ± SD | Min–Max | wt% | |
| Formaldehyde | 30.3 ± 15.8 | 10.8–58.6 | 51.5 ± 6.1 | 11.3 ± 5.8 | 4.7–21.4 | 39.4 ± 10.5 |
| Acetaldehyde | 19.2 ± 8.5 | 8.5–40.3 | 35.1 ± 2.6 | 16.2 ± 14.4 | 3.2–47.3 | 41.9 ± 1.3 |
| 2-Propenal | 3.8 ± 12.0 | 1.5–8.5 | 6.4 ± 0.8 | 3.4 ± 2.9 | 1–8.7 | 8.2 ± 4.7 |
| Acetone | 1.1 ± 0.7 | 0.4–3.1 | 1.8 ± 0.05 | 0.4 ± 0.4 | 0.1–1.3 | 1.2 ± 0.7 |
| Propanal | 1.8 ± 0.4 | 0.4–2.6 | 4.1 ± 0.21 | 1.6 ± 1.4 | 0.1–4.6 | 7.9 ± 6.2 |
| 2-Butenal | 0.7 ± 0.5 | 0.2–2.1 | 1.1 ± 0.15 | 0.5 ± 1.0 | 0.0–4.4 | 1.3 ± 1.5 |
| ∑carbonyls | 58.9 ± 33 | 25.8–148.3 | 33.4 ± 22.6 | 9.9–81.8 | ||
| Compounds | wt% Magna | wt% Premium | wt% Magna HS | wt% Premium HS |
|---|---|---|---|---|
| nbutane | 0.9 ± 0.4 | 0.8 ± 0.3 | 5.21 ± 2.9 | 5.5 ± 2.7 |
| trans-2-butene | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.1 ± 0.1 |
| ipentane | 7.1 ± 1.8 | 9.3 ± 3.6 | 21.1 ± 8.7 | 34.5 ± 9.1 |
| 2-methyl-1-butene | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.0 ± 0.0 | 1.1 ± 0.3 |
| 1-pentene | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.9 ± 0.5 | 0.5 ± 0.2 |
| npentane | 4.1 ± 1.2 | 3.0 ± 1.0 | 9.3 ± 3.8 | 7.9 ± 2.8 |
| trans-2-pentene | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.8 ± 0.4 | 1.7 ± 0.3 |
| cis-2-pentene | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.2 | 0.7 ± 0.2 |
| trans-2-butene | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.3 ± 0.2 | 0.4 ± 0.3 |
| 2-methyl-2-butene | 0.7±0.2 | 0.9±0.4 | 1.1±0.6 | 2.1±0.5 |
| 2,2-dimethylbutane | 0.7±0.5 | 0.7±0.3 | 1.6±1.4 | 0.9±0.4 |
| cyclopentene | 0.1±0.0 | 0.1±0.0 | 0.3±0.2 | 0.2±0.1 |
| 2-methylpentane | 9.4 ± 1.9 | 9.4 ± 2.6 | 12.7 ± 4.4 | 14.0 ± 3.4 |
| 4-methyl-cis 2-pentene | 3.5 ± 0.7 | 3.1 ± 1.0 | 3.9 ± 1.5 | 3.7 ± 1.0 |
| 3-methylpentane | 2.2 ± 0.4 | 1.8 ± 0.6 | 2.2 ± 0.8 | 1.8 ± 0.6 |
| nhexane | 2.8 ± 0.7 | 1.7 ± 0.8 | 2.3 ± 0.8 | 1.5 ± 0.6 |
| 3-methyl-cIs-2-pentene | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.2 | 0.2 ± 0.1 |
| 3,3-dimethylpentene-1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 |
| methylcyclopentane | 1.5 ± 0.3 | 1.0 ± 0.4 | 1.2 ± 0.4 | 0.8 ± 0.3 |
| 2,4-dimethylpentane | 1.1 ± 0.3 | 0.9 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.2 |
| 1-methylcyclopentene | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.0 |
| benzene | 0.6 ± 0.1 | 0.6 ± 0.3 | 0.5 ± 0.2 | 0.4 ± 0.1 |
| 2-methylhexane | 1.6 ± 0.3 | 1.1 ± 0.5 | 0.8 ± 0.4 | 0.5 ± 0.2 |
| cyclohexane | 0.8 ± 0.3 | 0.4 ± 0.2 | 0.6 ± 0.3 | 0.2 ± 0.1 |
| t-amylmethyether | 0.5 ± 0.5 | 0.4 ± 0.4 | 0.4 ± 0.4 | 0.2 ± 0.1 |
| 2,3-dimethylpentane | 1.3 ± 0.4 | 0.9 ± 0.3 | 0.8 ± 0.4 | 0.4 ± 0.2 |
| 1t,3,dimethylcyclopentane | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.1 ± 0.0 |
| 3-methylhexane | 1.8 ± 0.3 | 1.2 ± 0.5 | 0.9 ± 0.4 | 0.5 ± 0.2 |
| 1c,3,dimethylcyclopentane | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.9 | 0.1 ± 0.0 |
| 2,2,4-trimethylpentane | 7.4 ± 3.9 | 6.8 ± 2.7 | 3.8 ± 2.3 | 2.5 ± 1.3 |
| nheptane | 1.8 ± 0.3 | 1.0 ± 0.4 | 0.8 ± 0.4 | 0.3 ± 0.2 |
| 1,2-dimethylbenzene | 1.7 ± 0.4 | 2.2 ± 0.6 | 0.5 ± 0.7 | 0.3 ± 0.3 |
| methylcyclohexane | 1.1 ± 0.4 | 0.6 ± 0.2 | 0.4 ± 0.3 | 0.2 ± 0.1 |
| 2,5-dimethylhexane | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.4 ± 0.2 | 0.3 ± 0.2 |
| 2,4-dimethylhexane | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.5 ± 0.3 | 0.3 ± 0.3 |
| 2,3,3-trimethylpentane | 2.2 ± 0.6 | 3.2 ± 1.6 | 0.9 ± 1.2 | 0.8 ± 0.7 |
| 2,3,4- trimethylpentane | 2.2 ± 0.6 | 2.7 ± 1.1 | 0.8 ± 0.6 | 0.7 ± 0.6 |
| 2-methyheptane | 0.8 ± 0.2 | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.1 ± 0.1 |
| 2,2,5-trimethylhexane | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.3 ± 0.3 | 0.2 ± 0.2 |
| toluene | 5.0 ± 1.0 | 5.9 ± 1.7 | 1.9 ± 1.3 | 1.8 ± 1.3 |
| 2,3-dimethyhexane | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.2 ± 0.2 | 0.2 ± 0.1 |
| 3-methyheptane | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.3 ± 0.2 | 0.1 ± 0.1 |
| noctane | 0.9 ± 0.2 | 0.6 ± 0.2 | 0.3 ± 0.3 | 0.1 ± 0.1 |
| ethylbenzene | 1.2 ± 0.3 | 1.7 ± 0.5 | 0.4 ± 0.4 | 0.3 ± 0.3 |
| 1,3-dimethylbenzene | 3.0 ± 0.7 | 3.9 ± 1.2 | 1.0 ± 1.0 | 0.8 ± 0.8 |
| 1,4-dimethylbencene | 1.2 ± 0.3 | 1.4 ± 0.5 | 0.4 ± 0.4 | 0.4 ± 0.4 |
| nnonane | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 |
| 1,2-dimethylbencene | 0.6 ± 0.2 | 2.2 ± 0.6 | 0.5 ± 0.3 | 0.5 ± 0.5 |
| ipropylbenzene | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| npropylbenzene | 0.5 ± 0.1 | 0.5 ± 0.2 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| m-xylene | 1.6 ± 0.4 | 1.7 ± 0.8 | 0.5 ± 0.7 | 0.3 ± 0.3 |
| p-xylene | 0.7 ± 0.2 | 0.8 ± 0.3 | 0.3 ± 0.3 | 0.2 ± 0.2 |
| o-xylene | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.2 ± 0.2 | 0.1 ± 0.1 |
| 1,3,5-tri-MeBenzene | 0.7 ± 0.2 | 0.8 ± 0.4 | 0.3 ± 0.3 | 0.2 ± 0.2 |
| 2-methylnonane | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.1 ± 0.1 |
| ndecane | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.0 ± 0.0 |
| 1,2,4-trimethylbenzene | 2.5 ± 0.6 | 2.8 ± 1.4 | 0.7 ± 4 | 0.5 ± 0.6 |
| 1,2,3-trimethylbenzene | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.2 ± 0.2 | 0.1 ± 0.1 |
| 1,3-diethylbenzene | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| 1,4-diethylbenzene | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.1 ± 0.1 | 0.1 ± 0.0 |
| FA | AA | 2P | A | PA | 2B | |
|---|---|---|---|---|---|---|
| Formaldehyde (FA) | 1.00 | |||||
| Acetaldehyde (AA) | 0.86 | 1.00 | ||||
| 2-propenal (2P) | 0.92 | 0.83 | 1.00 | |||
| Acetone (A) | 0.88 | 0.74 | 0.85 | 1.00 | ||
| Propanal (PA) | 0.11 | 0.34 | 0.13 | 0.29 | 1.00 | |
| 2-butenal (2B) | 0.92 | 0.83 | 0.89 | 0.82 | 0.28 | 1.00 |
| Acetylene | 0.91 | 0.87 | 0.76 | 0.78 | 0.11 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugica-Álvarez, V.; Magaña-Reyes, M.; Martínez-Reyes, A.; Figueroa-Lara, J.; Blanco-Jiménez, S.; Goytia-Leal, V.; Páramo-Figueroa, V.H.; García-Martínez, R. Updating Real-World Profiles of Volatile Organic Compounds and Their Reactivity Estimation in Tunnels of Mexico City. Atmosphere 2020, 11, 1339. https://doi.org/10.3390/atmos11121339
Mugica-Álvarez V, Magaña-Reyes M, Martínez-Reyes A, Figueroa-Lara J, Blanco-Jiménez S, Goytia-Leal V, Páramo-Figueroa VH, García-Martínez R. Updating Real-World Profiles of Volatile Organic Compounds and Their Reactivity Estimation in Tunnels of Mexico City. Atmosphere. 2020; 11(12):1339. https://doi.org/10.3390/atmos11121339
Chicago/Turabian StyleMugica-Álvarez, Violeta, Miguel Magaña-Reyes, Adriana Martínez-Reyes, Jesús Figueroa-Lara, Salvador Blanco-Jiménez, Valia Goytia-Leal, Victor H. Páramo-Figueroa, and Rocío García-Martínez. 2020. "Updating Real-World Profiles of Volatile Organic Compounds and Their Reactivity Estimation in Tunnels of Mexico City" Atmosphere 11, no. 12: 1339. https://doi.org/10.3390/atmos11121339
APA StyleMugica-Álvarez, V., Magaña-Reyes, M., Martínez-Reyes, A., Figueroa-Lara, J., Blanco-Jiménez, S., Goytia-Leal, V., Páramo-Figueroa, V. H., & García-Martínez, R. (2020). Updating Real-World Profiles of Volatile Organic Compounds and Their Reactivity Estimation in Tunnels of Mexico City. Atmosphere, 11(12), 1339. https://doi.org/10.3390/atmos11121339





