Sensitivity of Nitrate Aerosol Production to Vehicular Emissions in an Urban Street
Abstract
1. Introduction
2. Model Description and Simulation Set-Up
2.1. Model Description
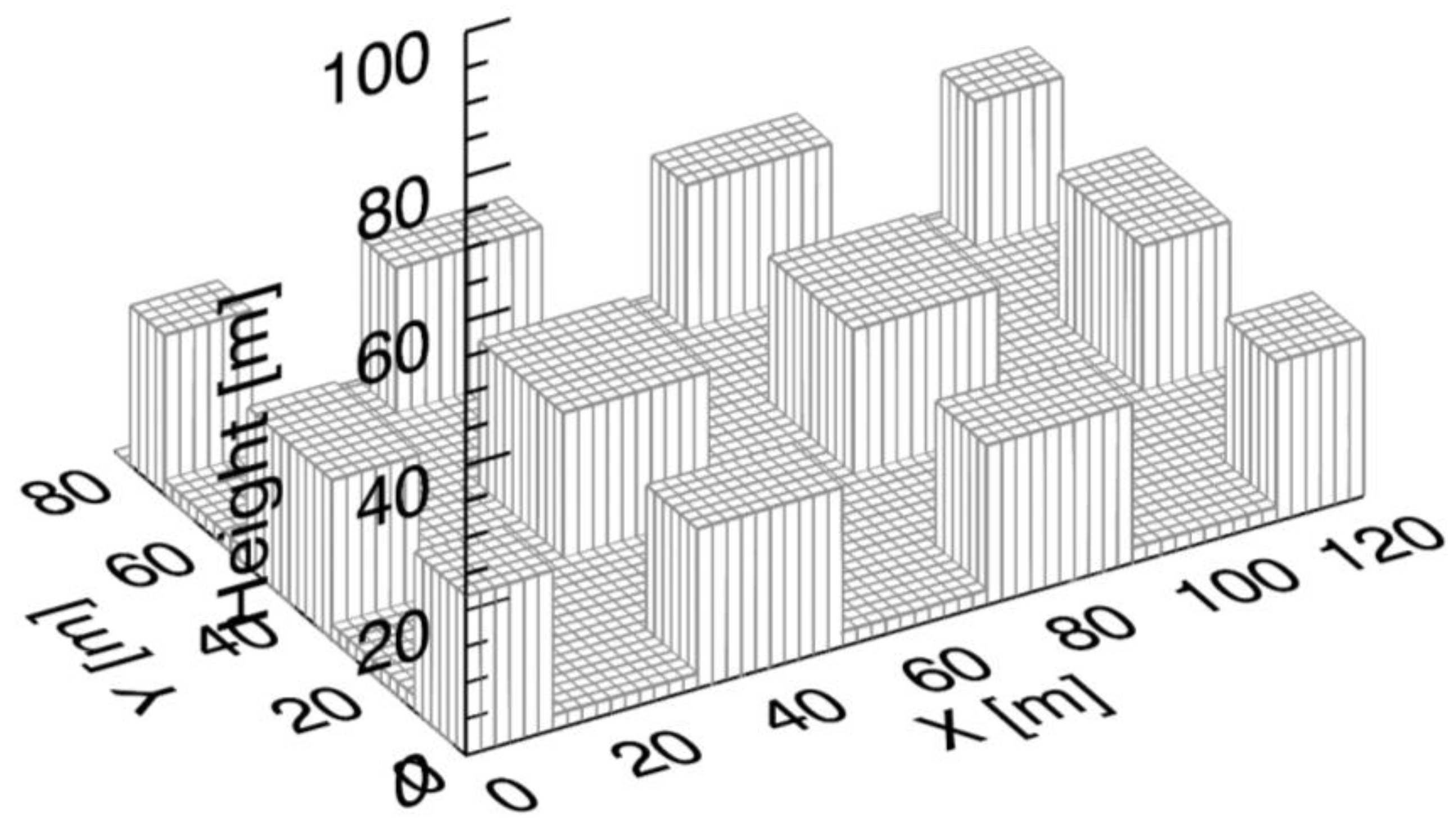
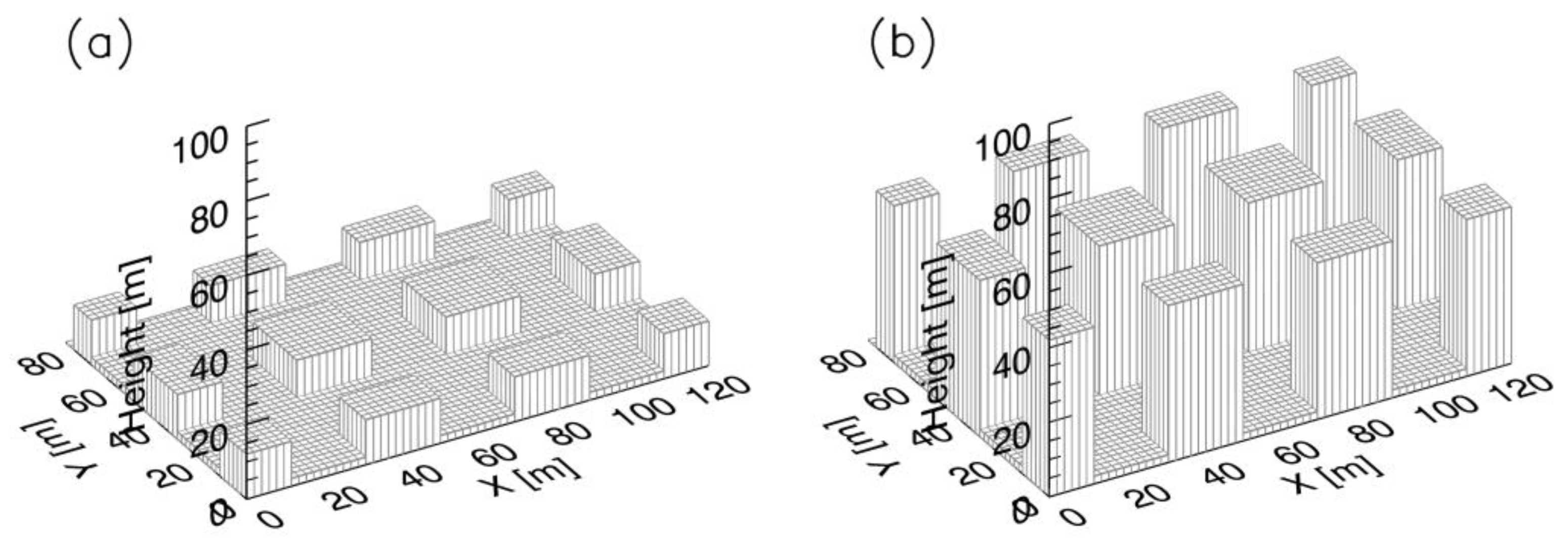
2.2. Simulation Set-Up
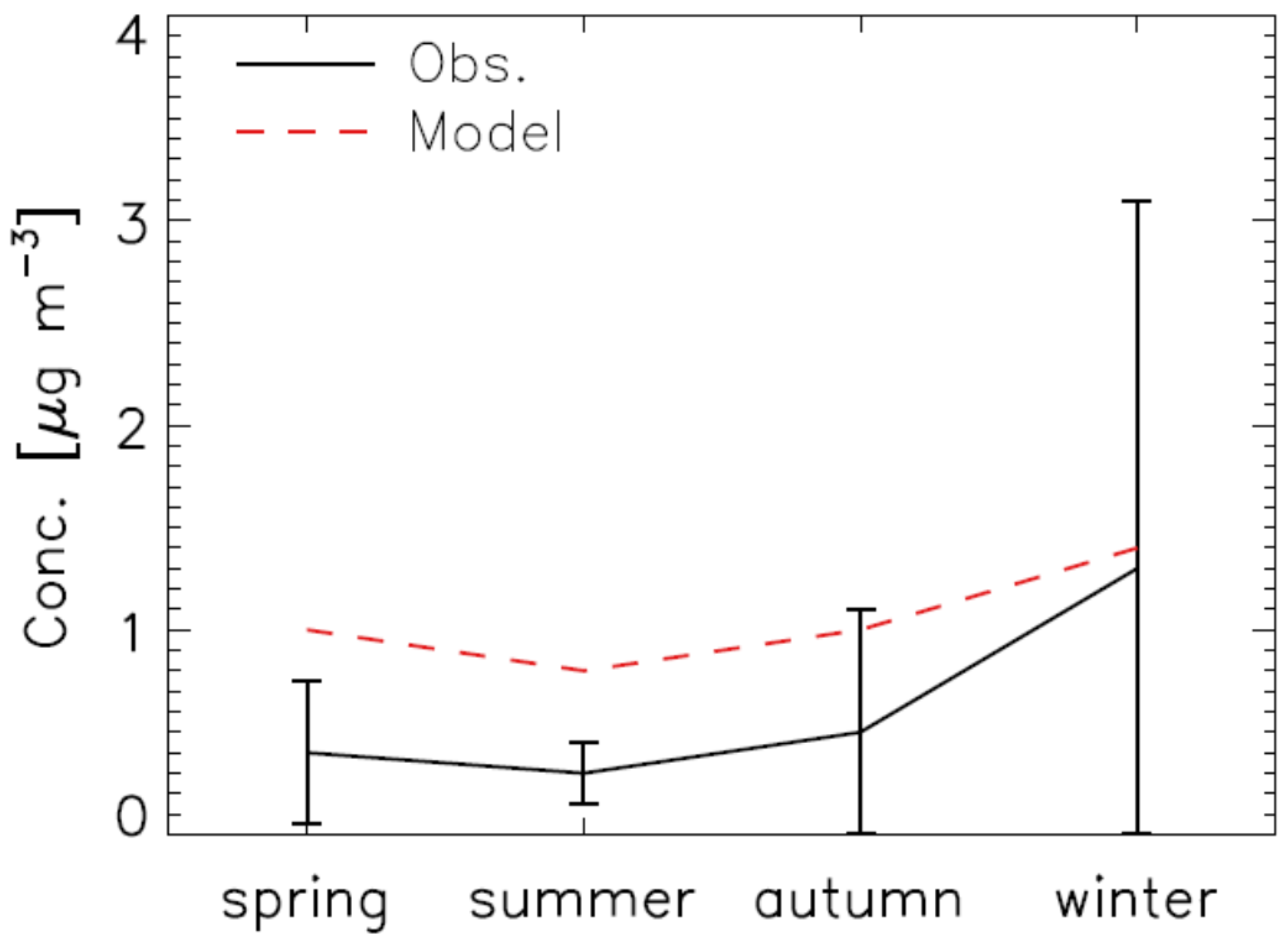
2.3. Model Validation
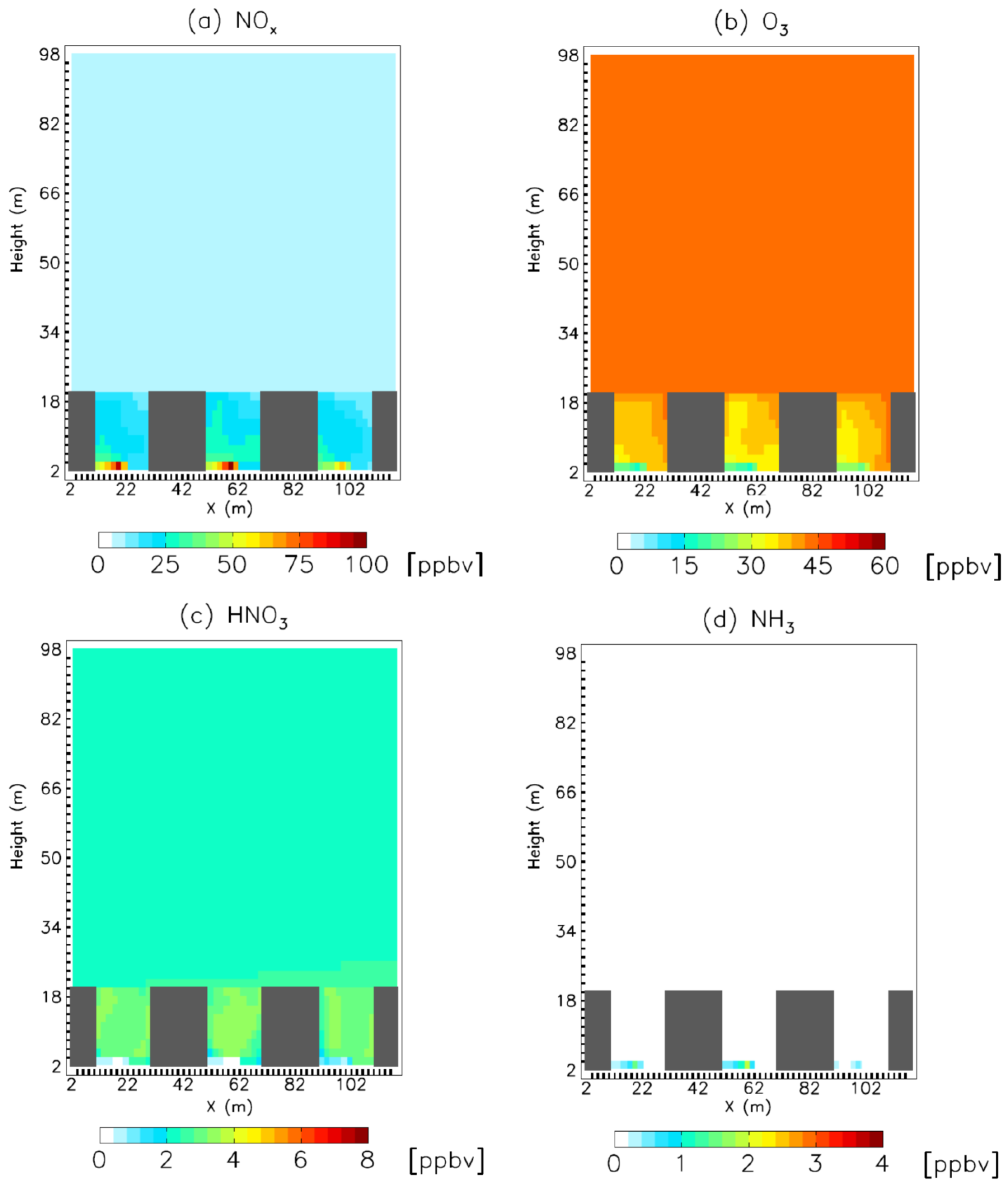
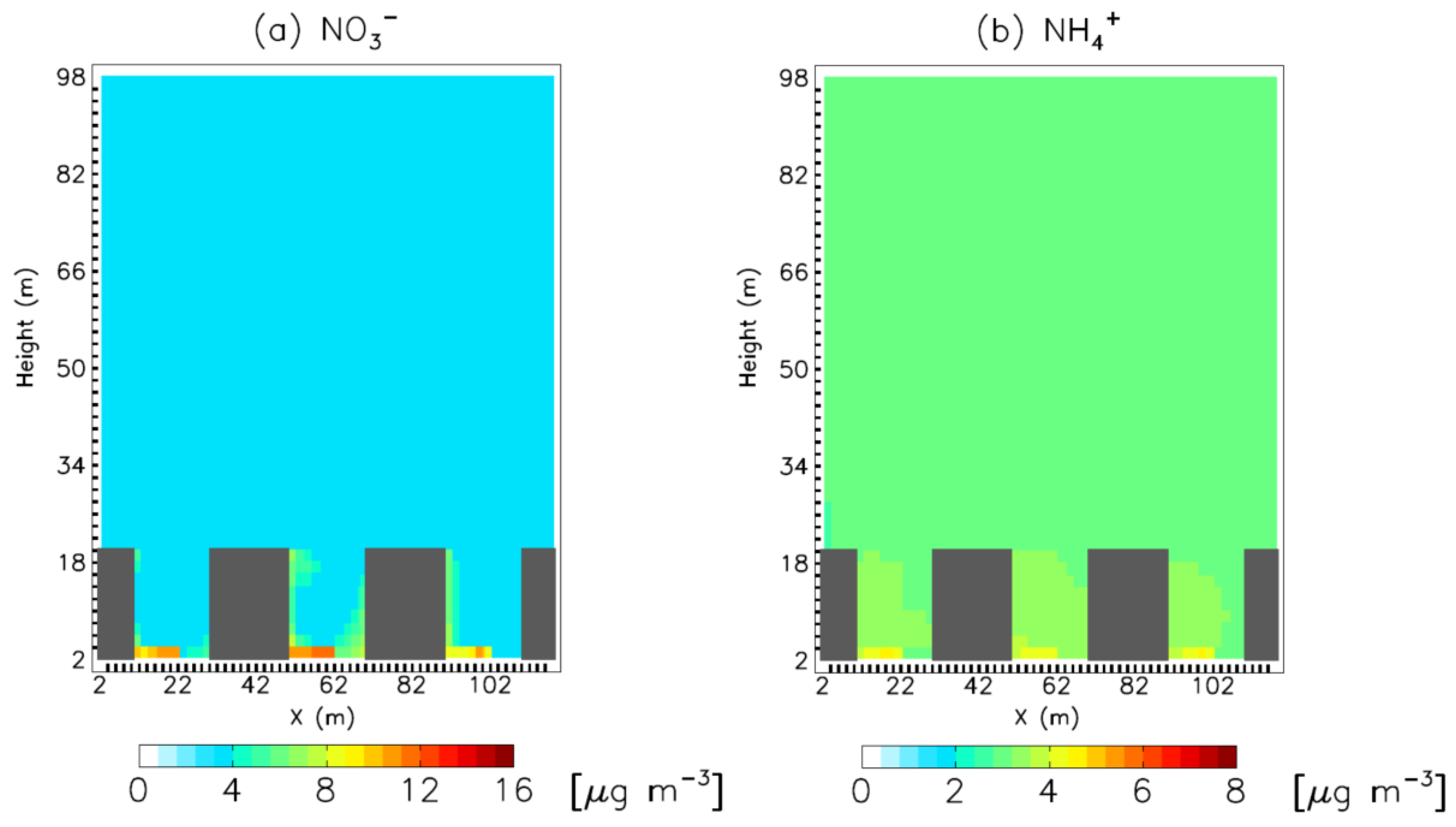
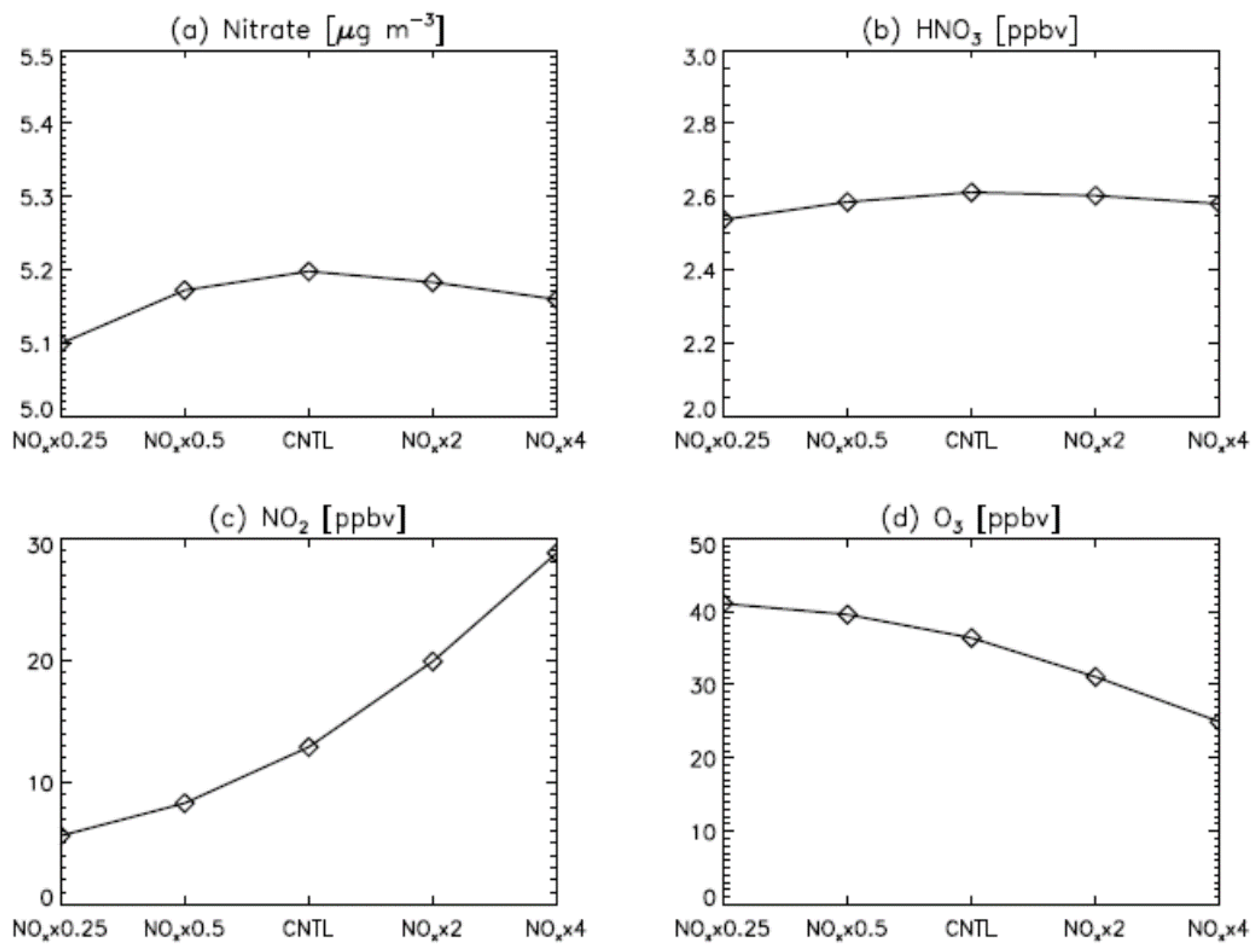
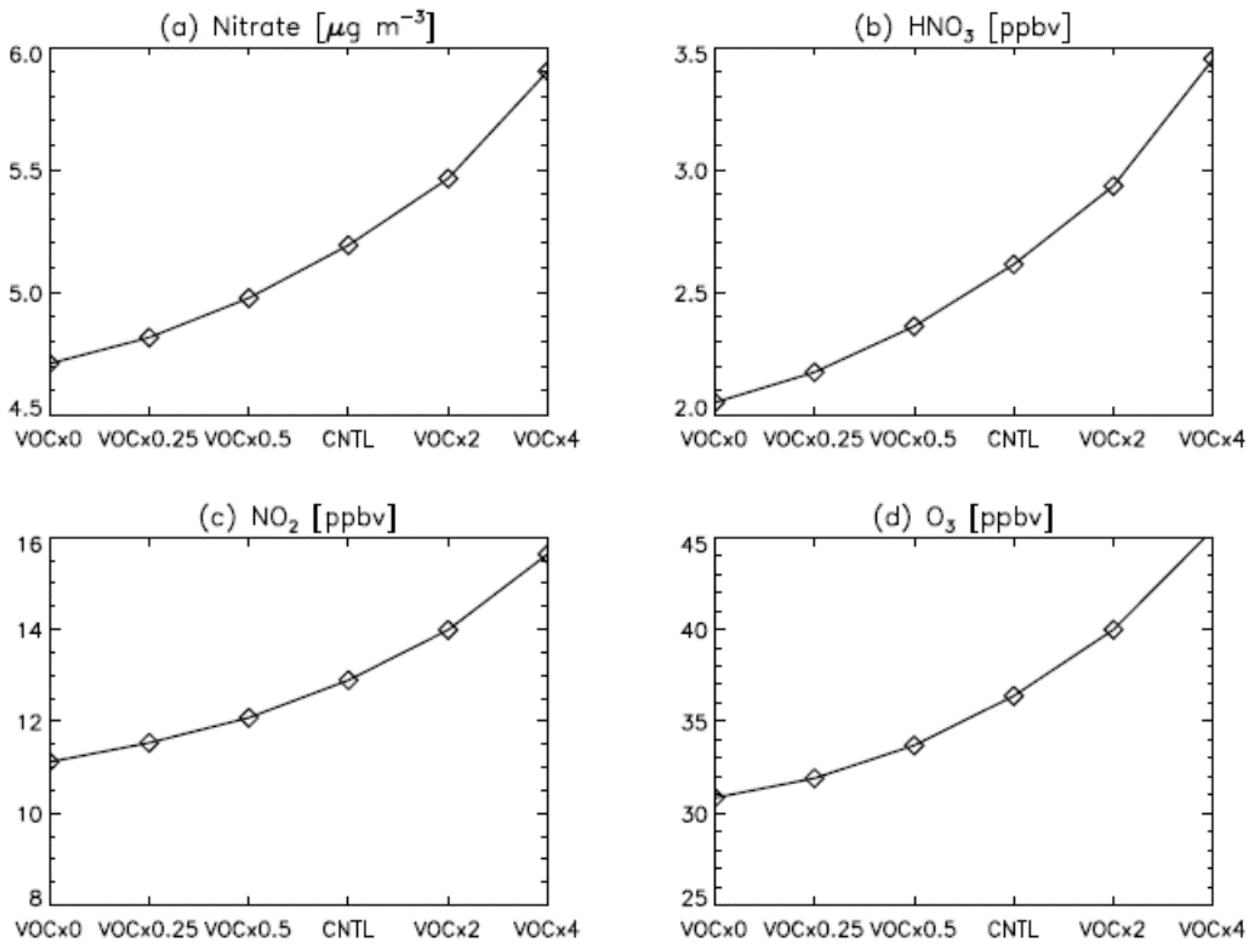
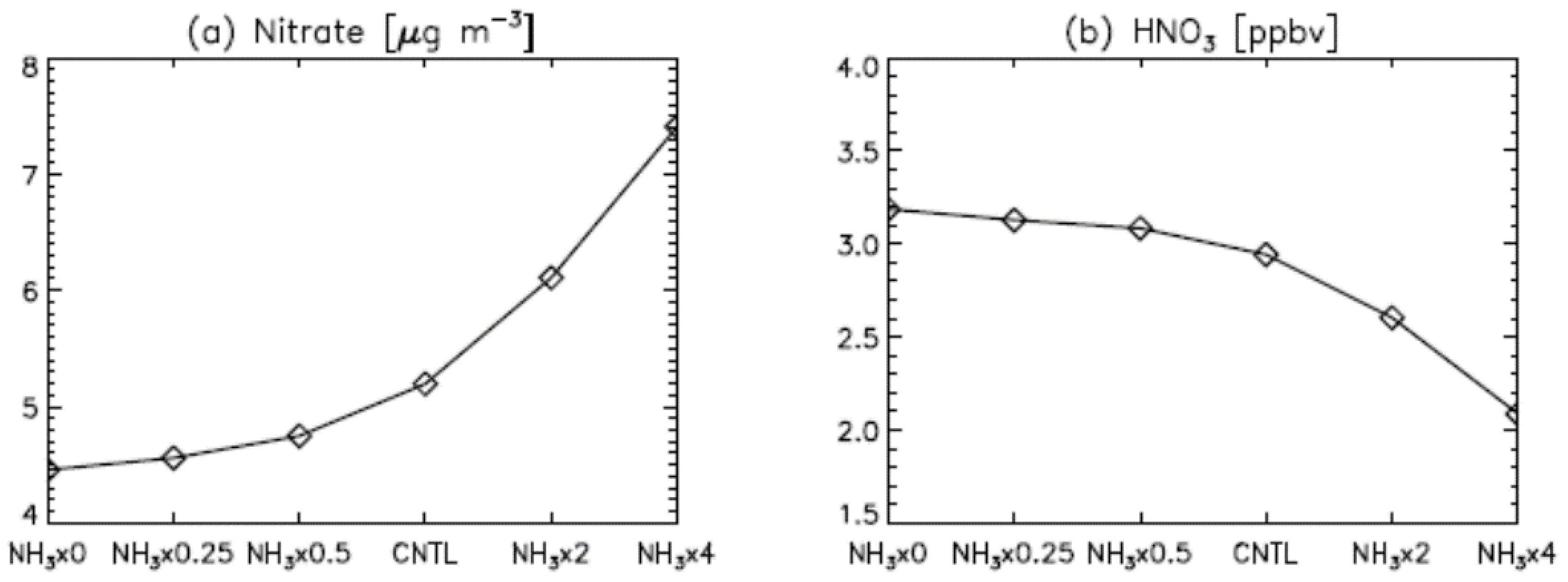
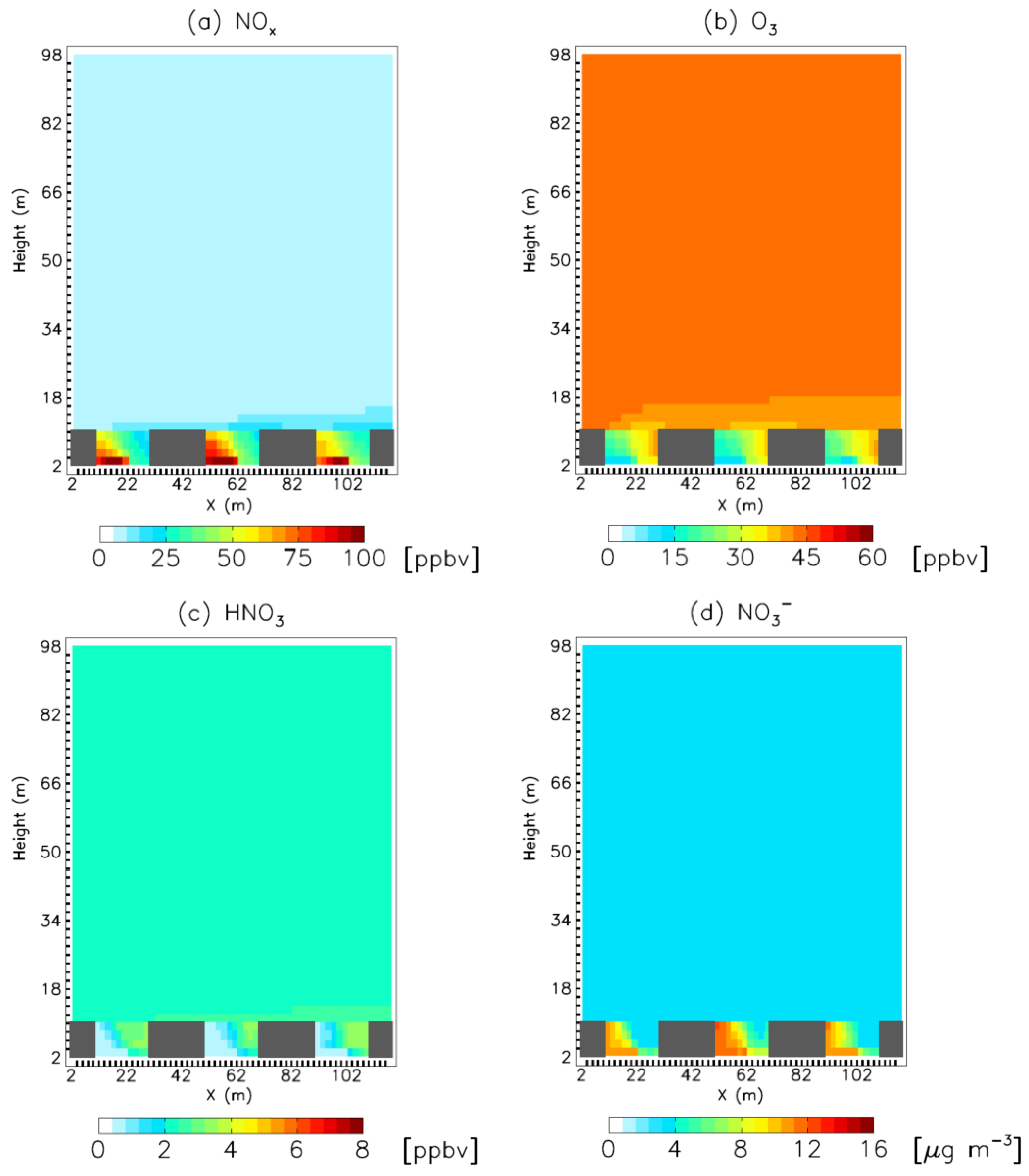
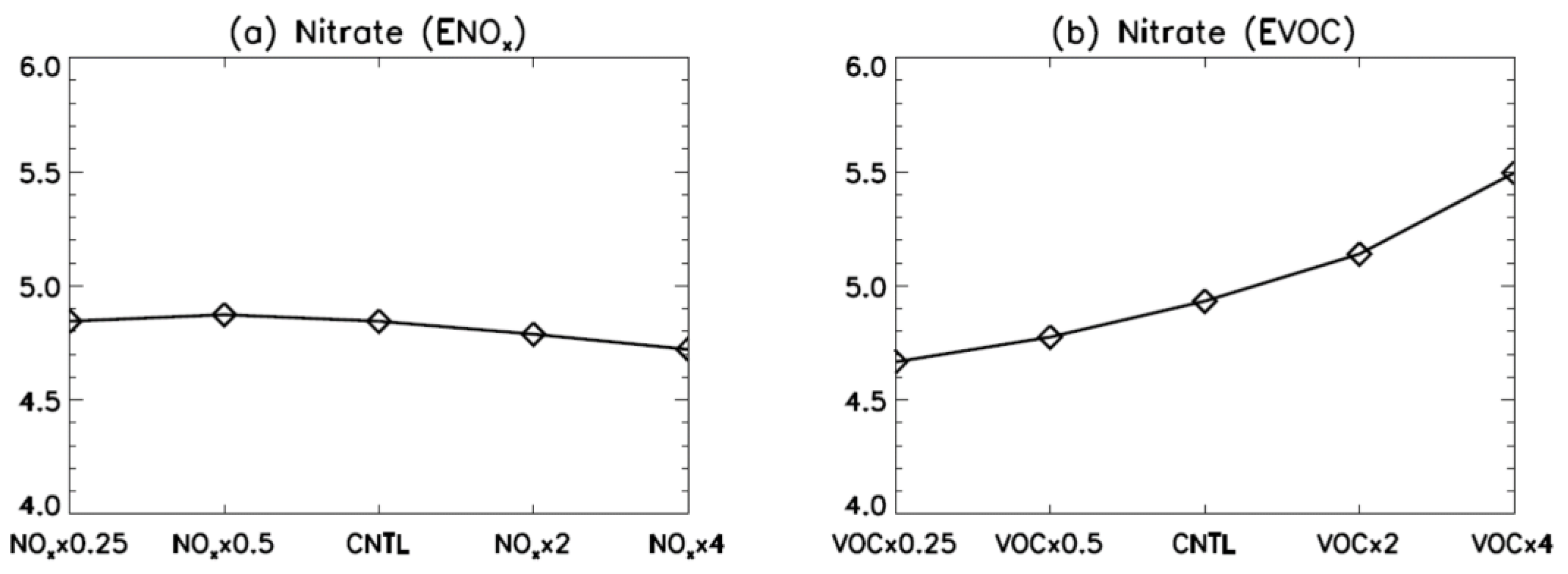
3. Results
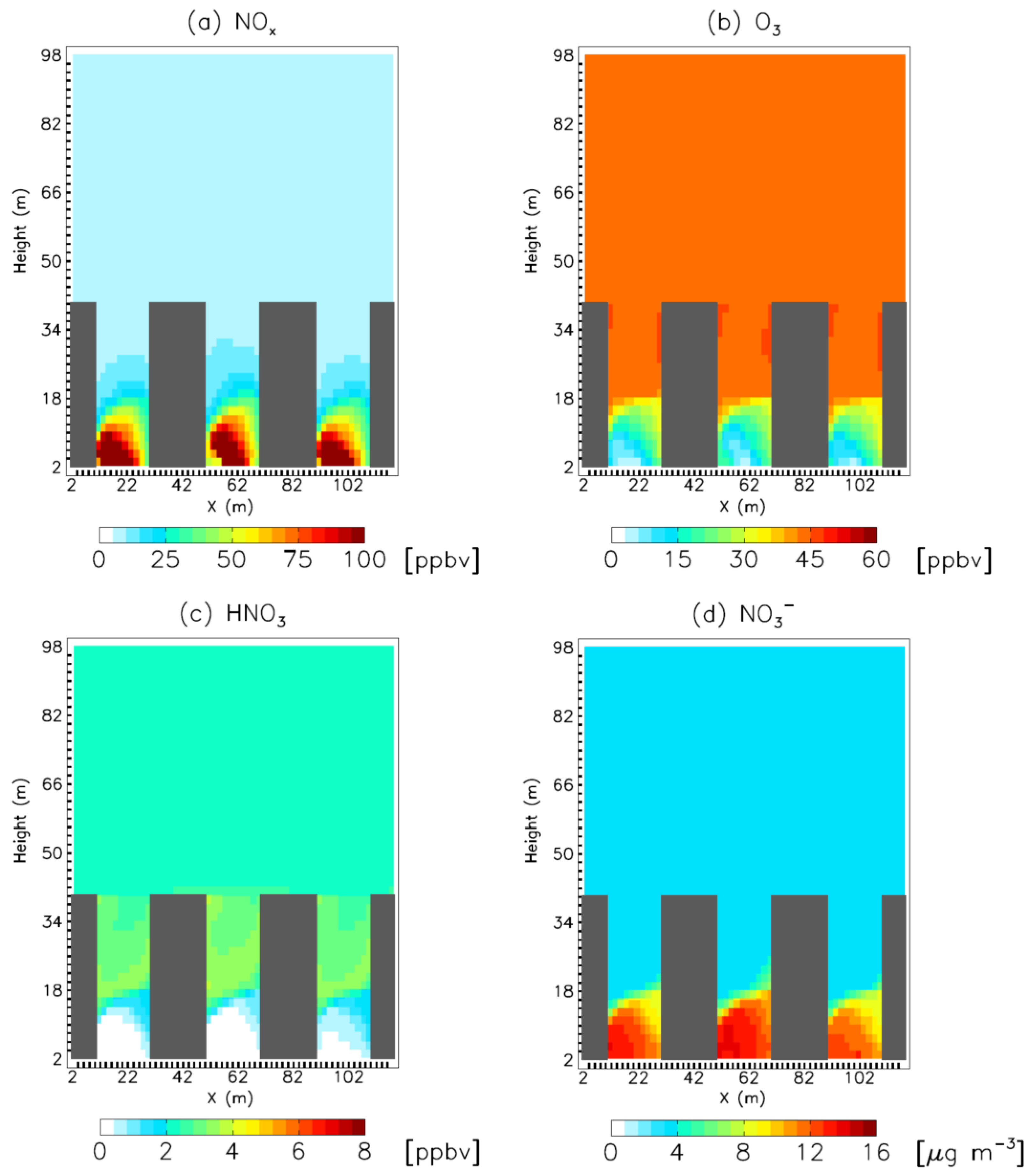
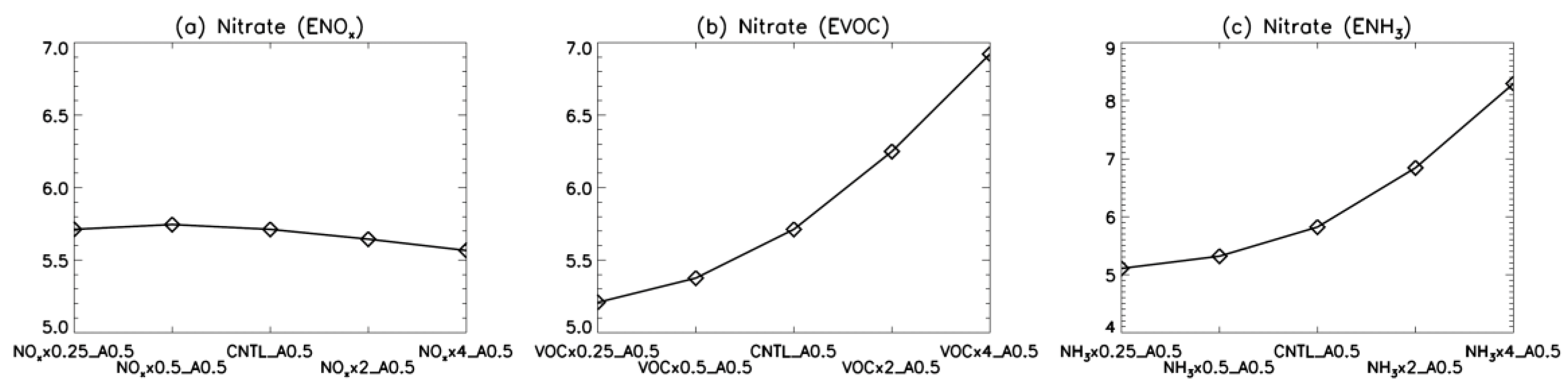
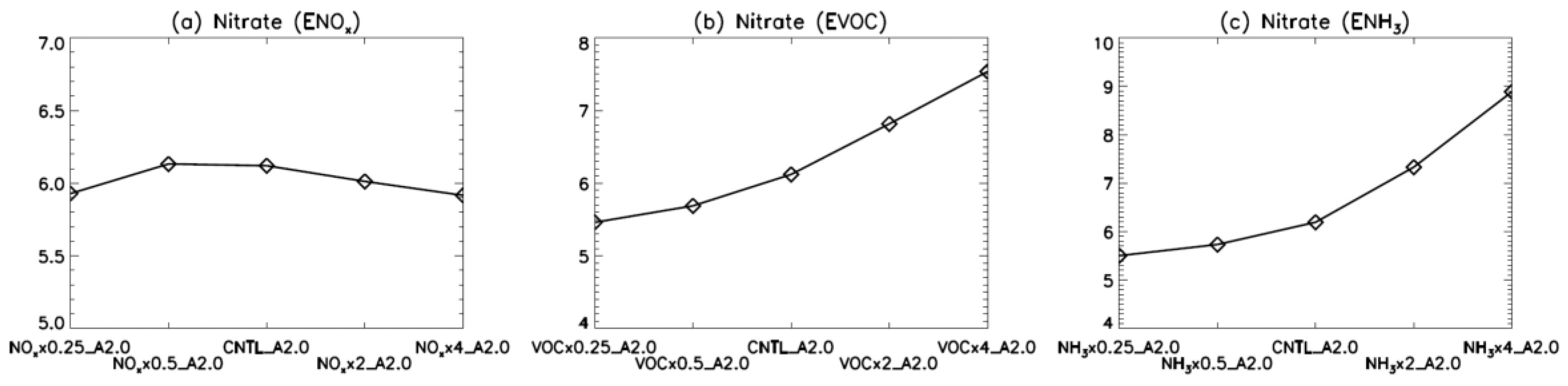
4. Model Sensitivity to Geometry and Speciation of VOC Emissions
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, R.-J.; Zhang, Y.; Bozzetti, C.; Ho, K.-F.; Cao, J.-J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218. [Google Scholar] [PubMed]
- Liu, X.; Sun, K.; Qu, Y.; Hu, M.; Sun, Y.; Zhang, F.; Zhang, Y. Secondary formation of sulfate and nitrate during a haze episode in megacity Beijing, China. Aerosol Air Qual. Res. 2015, 15, 2246–2257. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Zhang, J.; Liu, Z.; Wang, L.; Tian, S.; Tang, G.; Gao, W.; Ji, D.; Song, T. Redefining the importance of nitrate during haze pollution to help optimize an emission control strategy. Atmos. Environ. 2016, 141, 197–202. [Google Scholar] [CrossRef]
- Tan, J.; Duan, J.; He, K.; Ma, Y.; Duan, F.; Chen, Y.; Fu, J. Chemical characteristics of PM2.5 during a typical haze episode in Guangzhou. J. Environ. Sci. 2009, 21, 774–781. [Google Scholar]
- Wang, H.-J.; Chen, H.-P. Understanding the recent trend of haze pollution in eastern China: Roles of climate change. Atmos. Chem. Phys. 2016, 16, 4205–4211. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, Y.P. Long-term Trends of the Concentrations of Mass and Chemical Composition in PM 2.5 over Seoul. J. Korean Soc. Atmos. Environ. 2015, 31, 143–156. [Google Scholar] [CrossRef]
- Guo, H.; Ding, A.J.; So, K.L.; Ayoko, G.; Li, Y.S.; Hung, W.T. Receptor modeling of source apportionment of Hong Kong aerosols and the implication of urban and regional contribution. Atmos. Environ. 2009, 43, 1159–1169. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Hu, Q.; Deng, W.; Zhang, Y.; Ding, X.; Fu, X.; Bernard, F.; Zhang, Z.; Lü, S. Formation of secondary aerosols from gasoline vehicle exhaust when mixing with SO2. Atmos. Chem. Phys. 2016, 16, 675–689. [Google Scholar] [CrossRef]
- Nowak, J.B.; Neuman, J.A.; Bahreini, R.; Middlebrook, A.M.; Holloway, J.S.; McKeen, S.A.; Parrish, D.D.; Ryerson, T.B.; Trainer, M. Ammonia sources in the California South Coast Air Basin and their impact on ammonium nitrate formation. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Durant, J.L.; Ash, C.A.; Wood, E.C.; Herndon, S.C.; Jayne, J.T.; Knighton, W.B.; Canagaratna, M.R.; Trull, J.B.; Brugge, D.; Zamore, W. Short-term variation in near-highway air pollutant gradients on a winter morning. Atmos. Chem. Phys. 2010, 10, 5599. [Google Scholar] [CrossRef] [PubMed]
- Hagler, G.S.W.; Baldauf, R.W.; Thoma, E.D.; Long, T.R.; Snow, R.F.; Kinsey, J.S.; Oudejans, L.; Gullett, B.K. Ultrafine particles near a major roadway in Raleigh, North Carolina: Downwind attenuation and correlation with traffic-related pollutants. Atmos. Environ. 2009, 43, 1229–1234. [Google Scholar] [CrossRef]
- Hu, S.; Fruin, S.; Kozawa, K.; Mara, S.; Paulson, S.E.; Winer, A.M. A wide area of air pollutant impact downwind of a freeway during pre-sunrise hours. Atmos. Environ. 2009, 43, 2541–2549. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Y.; Qi, L.; Tang, X. Spatial distribution of traffic-related pollutant concentrations in street canyons. Atmos. Environ. 2003, 37, 3213–3224. [Google Scholar] [CrossRef]
- Myhre, G.; Grini, A.; Metzger, S. Modelling of nitrate and ammonium-containing aerosols in presence of sea salt. Atmos. Chem. Phys. 2006, 6, 4809–4821. [Google Scholar] [CrossRef]
- Riemer, N.; Vogel, H.; Vogel, B.; Schell, B.; Ackermann, I.; Kessler, C.; Hass, H. Impact of the heterogeneous hydrolysis of N2O5 on chemistry and nitrate aerosol formation in the lower troposphere under photosmog conditions. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar]
- Schaap, M.; Cuvelier, C.; Hendriks, C.; Bessagnet, B.; Baldasano, J.M.; Colette, A.; Thunis, P.; Karam, D.; Fagerli, H.; Graff, A. Performance of European chemistry transport models as function of horizontal resolution. Atmos. Environ. 2015, 112, 90–105. [Google Scholar] [CrossRef]
- Tewari, M.; Kusaka, H.; Chen, F.; Coirier, W.J.; Kim, S.; Wyszogrodzki, A.A.; Warner, T.T. Impact of coupling a microscale computational fluid dynamics model with a mesoscale model on urban scale contaminant transport and dispersion. Atmos. Res. 2010, 96, 656–664. [Google Scholar] [CrossRef]
- Wehner, M.F.; Reed, K.A.; Li, F.; Bacmeister, J.; Chen, C.-T.; Paciorek, C.; Gleckler, P.J.; Sperber, K.R.; Collins, W.D.; Gettelman, A. The effect of horizontal resolution on simulation quality in the Community Atmospheric Model, CAM5.1. J. Adv. Model. Earth Syst. 2014, 6, 980–997. [Google Scholar] [CrossRef]
- Prasad, R.; Bella, V.R. A review on diesel soot emission, its effect and control. Bull. Chem. React. Eng. Catal. 2011, 5, 69–86. [Google Scholar] [CrossRef]
- Zhang, Q.; He, K.; Huo, H. Policy: Cleaning China’s air. Nature 2012, 484, 161. [Google Scholar]
- Link, M.F.; Kim, J.; Park, G.; Lee, T.; Park, T.; Babar, Z.B.; Sung, K.; Kim, P.; Kang, S.; Kim, J.S. Elevated production of NH4NO3 from the photochemical processing of vehicle exhaust: Implications for air quality in the Seoul Metropolitan Region. Atmos. Environ. 2017, 156, 95–101. [Google Scholar] [CrossRef]
- Pathak, R.K.; Wu, W.S.; Wang, T. Summertime PM 2.5 ionic species in four major cities of China: Nitrate formation in an ammonia-deficient atmosphere. Atmos. Chem. Phys. 2009, 9, 1711–1722. [Google Scholar] [CrossRef]
- Wen, L.; Chen, J.; Yang, L.; Wang, X.; Xu, C.; Sui, X.; Yao, L.; Zhu, Y.; Zhang, J.; Zhu, T. Enhanced formation of fine particulate nitrate at a rural site on the North China Plain in summer: The important roles of ammonia and ozone. Atmos. Environ. 2015, 101, 294–302. [Google Scholar] [CrossRef]
- Pun, B.K.; Seigneur, C. Sensitivity of particulate matter nitrate formation to precursor emissions in the California San Joaquin Valley. Environ. Sci. Technol. 2001, 35, 2979–2987. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, R.J.; Kim, J.-J. Urban air quality modeling with full O3–NOx–VOC chemistry: Implications for O3 and PM air quality in a street canyon. Atmos. Environ. 2012, 47, 330–340. [Google Scholar] [CrossRef]
- Kim, J.-J.; Baik, J.-J. A numerical study of the effects of ambient wind direction on flow and dispersion in urban street canyons using the RNG k–ε turbulence model. Atmos. Environ. 2004, 38, 3039–3048. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, J.-J.; Kim, M.J.; Park, R.J.; Cheong, H.-B. Characteristics of flow and reactive pollutant dispersion in urban street canyons. Atmos. Environ. 2015, 108, 20–31. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, R.J.; Kim, J.-J.; Park, S.H.; Chang, L.-S.; Lee, D.-G.; Choi, J.-Y. A computational fluid dynamics simulation of reactive fine particulate matter in street canyons. Atmos. Environ. 2019, 209, 54–66. [Google Scholar] [CrossRef]
- Bey, I.; Jacob, D.J.; Yantosca, R.M.; Logan, J.A.; Field, B.D.; Fiore, A.M.; Li, Q.; Liu, H.Y.; Mickley, L.J.; Schultz, M.G. Global modeling of tropospheric chemistry with assimilated meteorology: Model description and evaluation. J. Geophys. Res. Atmos. 2001, 106, 23073–23095. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Jiang, J.; Zhou, W.; Wang, B.; He, K.; Duan, F.; Zhang, Q.; Philip, S.; Xie, Y. Enhanced sulfate formation during China’s severe winter haze episode in January 2013 missing from current models. J. Geophys. Res. Atmos. 2014, 119, 10–425. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, H.; Lou, S. Increase in winter haze over eastern China in recent decades: Roles of variations in meteorological parameters and anthropogenic emissions. J. Geophys. Res. Atmos. 2016, 121, 13050–13065. [Google Scholar] [CrossRef]
- Neu, J.L.; Prather, M.J.; Penner, J.E. Global atmospheric chemistry: Integrating over fractional cloud cover. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Wild, O.; Zhu, X.; Prather, M.J. Fast-J: Accurate simulation of in-and below-cloud photolysis in tropospheric chemical models. J. Atmos. Chem. 2000, 37, 245–282. [Google Scholar] [CrossRef]
- Park, R.J.; Jacob, D.J.; Field, B.D.; Yantosca, R.M.; Chin, M. Natural and transboundary pollution influences on sulfate-nitrate-ammonium aerosols in the United States: Implications for policy. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Park, R.J.; Jacob, D.J.; Chin, M.; Martin, R.V. Sources of carbonaceous aerosols over the United States and implications for natural visibility. J. Geophys. Res. Atmos. 2003, 108. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+–Ca2+–Mg2+–NH4+–Na+–SO42–NO3–Cl–H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef]
- Nenes, A.; Pandis, S.N.; Pilinis, C. ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols. Aquat. Geochem. 1998, 4, 123–152. [Google Scholar] [CrossRef]
- Jacob, D.J. Heterogeneous chemistry and tropospheric ozone. Atmos. Environ. 2000, 34, 2131–2159. [Google Scholar] [CrossRef]
- Zhang, L.; Jacob, D.J.; Knipping, E.M.; Kumar, N.; Munger, J.W.; Carouge, C.C.; Van Donkelaar, A.; Wang, Y.X.; Chen, D. Nitrogen deposition to the United States: Distribution, sources, and processes. Atmos. Chem. Phys. 2012, 12, 4539–4554. [Google Scholar] [CrossRef]
- Walker, J.M.; Philip, S.; Martin, R.V.; Seinfeld, J.H. Simulation of nitrate, sulfate, and ammonium aerosols over the United States. Atmos. Chem. Phys. 2012, 12, 11213–11227. [Google Scholar] [CrossRef]
- Cooke, W.F.; Liousse, C.; Cachier, H.; Feichter, J. Construction of a 1 × 1 fossil fuel emission data set for carbonaceous aerosol and implementation and radiative impact in the ECHAM4 model. J. Geophys. Res. Atmos. 1999, 104, 22137–22162. [Google Scholar] [CrossRef]
- Wesely, M.L. Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical models. Atmos. Environ. (1967) 1989, 23, 1293–1304. [Google Scholar] [CrossRef]
- Lee, D.-G.; Lee, Y.-M.; Jang, K.-W.; Yoo, C.; Kang, K.-H.; Lee, J.-H.; Jung, S.-W.; Park, J.-M.; Lee, S.-B.; Han, J.-S. Korean national emissions inventory system and 2007 air pollutant emissions. Asian J. Atmos. Environ. 2011, 5, 278–291. [Google Scholar] [CrossRef]
- Carslaw, D.C.; Beevers, S.D. Estimations of road vehicle primary NO2 exhaust emission fractions using monitoring data in London. Atmos. Environ. 2005, 39, 167–177. [Google Scholar] [CrossRef]
- Amon, B.; Hutchings, N.; Dämmgen, U.; Webb, J. EMEP/EEA Air Pollutant Emission Inventory Guidebook—2016; European Environment Agency: Copenhagen, Denmark, 2016. [Google Scholar]
- Heo, J.-B.; Hopke, P.K.; Yi, S.-M. Source apportionment of PM 2.5 in Seoul, Korea. Atmos. Chem. Phys. 2009, 9, 4957–4971. [Google Scholar] [CrossRef]
- Shin, H.J.; Roh, S.A.; Kim, J.C.; Lee, S.J.; Kim, Y.P. Temporal variation of volatile organic compounds and their major emission sources in Seoul, Korea. Environ. Sci. Pollut. Res. 2013, 20, 8717–8728. [Google Scholar] [CrossRef]
- Shon, Z.-H.; Kim, K.-H.; Song, S.-K.; Jung, K.; Kim, N.-J.; Lee, J.-B. Relationship between water-soluble ions in PM2.5 and their precursor gases in Seoul megacity. Atmos. Environ. 2012, 59, 540–550. [Google Scholar] [CrossRef]
- Kim, Y.; Guldmann, J.-M. Impact of traffic flows and wind directions on air pollution concentrations in Seoul, Korea. Atmos. Environ. 2011, 45, 2803–2810. [Google Scholar] [CrossRef]
- Inness, A.; Baier, F.; Benedetti, A.; Bouarar, I.; Chabrillat, S.; Clark, H.; Clerbaux, C.; Coheur, P.; Engelen, R.J.; Errera, Q. The MACC reanalysis: An 8 yr data set of atmospheric composition. Atmos. Chem. Phys. 2013, 13, 4073–4109. [Google Scholar] [CrossRef]
- Huang, M.; Carmichael, G.R.; Pierce, R.B.; Jo, D.S.; Park, R.J.; Flemming, J.; Emmons, L.K.; Bowman, K.W.; Henze, D.K.; Davila, Y. Impact of intercontinental pollution transport on North American ozone air pollution: An HTAP phase 2 multi-model study. Atmos. Chem. Phys. 2017, 17, 5721–5750. [Google Scholar] [CrossRef]
- Kim, J.-J.; Baik, J.-J. Effects of street-bottom and building-roof heating on flow in three-dimensional street canyons. Adv. Atmos. Sci. 2010, 27, 513–527. [Google Scholar] [CrossRef]
- Baker, J.; Walker, H.L.; Cai, X. A study of the dispersion and transport of reactive pollutants in and above street canyons—A large eddy simulation. Atmos. Environ. 2004, 38, 6883–6892. [Google Scholar] [CrossRef]
- Yubero, E.; Galindo, N.; Nicolás, J.F.; Crespo, J.; Calzolai, G.; Lucarelli, F. Temporal variations of PM1 major components in an urban street canyon. Environ. Sci. Pollut. Res. 2015, 22, 13328–13335. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Streets, D.G.; Zhang, Q.; Wang, S.; Carmichael, G.R.; Cheng, Y.F.; Wei, C.; Chin, M.; Diehl, T.; Tan, Q. Sulfur dioxide emissions in China and sulfur trends in East Asia since 2000. Atmos. Chem. Phys. 2010, 10, 6311–6331. [Google Scholar] [CrossRef]
- WHO. Review of Evidence on Health Aspects of Air Pollution–REVIHAAP Project. Available online: https://scholar.google.com/scholar_lookup?title=Review%20of%20evidence%20on%20health%20aspects%20of%20air%20pollution%20%E2%80%94%20REVIHAAP%20project%20technical%20report&publication_year=2013&author=WHOhttps://scholar.google.co.kr (accessed on 2 February 2019).
- Nguyen, K.; Dabdub, D. NOx and VOC control and its effects on the formation of aerosols. Aerosol Sci. Technol. 2002, 36, 560–572. [Google Scholar] [CrossRef]
- Jin, X.; Fiore, A.M.; Murray, L.T.; Valin, L.C.; Lamsal, L.N.; Duncan, B.; Folkert Boersma, K.; De Smedt, I.; Abad, G.G.; Chance, K. Evaluating a Space-Based Indicator of Surface Ozone-NOx-VOC Sensitivity Over Midlatitude Source Regions and Application to Decadal Trends. J. Geophys. Res. Atmos. 2017, 122. [Google Scholar]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef]
- Chang, W.; Zhan, J.; Zhang, Y.; Li, Z.; Xing, J.; Li, J. Emission-driven changes in anthropogenic aerosol concentrations in China during 1970–2010 and its implications for PM2.5 control policy. Atmos. Res. 2018, 212, 106–119. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Y.; Ma, N.; Wolke, R.; Nordmann, S.; Schüttauf, S.; Ran, L.; Wehner, B.; Birmili, W.; Gon, H.A. Sea salt emission, transport and influence on size-segregated nitrate simulation: A case study in northwestern Europe by WRF-Chem. Atmos. Chem. Phys. 2016, 16, 12081–12097. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, J.H.; Park, H.-S.; Sun, Y.S.; Kim, H.-S.; Choi, K.H.; Yi, J. Emission inventory of VOCs from mobile sources in a metropolitan region. Korean J. Chem. Eng. 2006, 23, 919–924. [Google Scholar] [CrossRef]
| [g km−1] | CO | NOx | VOC | NH3 |
|---|---|---|---|---|
| PC (diesel) | 0.010 | 0.041 | 0.006 | 0.002 |
| PC (gasoline) | 0.085 | 0.004 | 0.002 | 0.023 |
| PC (gas) | 0.104 | 0.002 | 0.001 | 0.001 |
| LDV (diesel) | 0.087 | 0.063 | 0.009 | 0.002 |
| LDV (gas) | 0.163 | 0.026 | 0.002 | 0.002 |
| HDV (diesel) | 0.543 | 0.959 | 0.104 | 0.002 |
| HDV (gas) | 0.457 | 0.045 | 0.009 | 0.003 |
| Bus (diesel) | 1.850 | 0.613 | 0.150 | 0.002 |
| Bus (gas) | 1.736 | 0.346 | 0.191 | 0.000 |
| [%] | Passenger Cars | Light-Duty Vehicles | Heavy-Duty Vehicles | Bus |
|---|---|---|---|---|
| Diesel | 20.6 | 17.6 | 7.3 | 2.2 |
| Gasoline | 43.3 | - | - | - |
| Gas | 8.3 | 0.9 | 0.3 | 0.4 |
| Total | 72.2 | 18.5 | 7.6 | 2.6 |
| Tracer Name | Formula | Emission Rate [mg km−1] | Ratio [%] |
|---|---|---|---|
| ALK4 | ≥C4 alkanes | 3.60 | 24.0 |
| ISOP | CH2 = C(CH3)CH = CH2 | - | - |
| ACET | CH3C(O)CH3 | 0.44 | 2.9 |
| MEK | RC(O)R | 0.18 | 1.2 |
| ALD2 | CH3CHO | 0.98 | 6.5 |
| RCHO | CH3CH2CHO | 1.89 | 12.6 |
| MVK | CH2 = CHC(=O)CH3 | - | - |
| MACR | CH2 = C(CH3)CHO | - | - |
| PRPE | ≥C3 alkenes | 2.58 | 17.2 |
| C3H8 | C3H8 | 0.02 | 0.1 |
| CH2O | HCHO | 1.80 | 12.0 |
| C2H6 | C2H6 | 0.05 | 0.3 |
| Unspeciated | - | - | 23.2 |
| Hour | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 |
| Temperature [K] | −3.1 | −3.4 | −3.6 | −4.0 | −4.4 | −4.6 | −4.8 | −5.1 | −4.5 | −2.7 | −0.8 | 0.6 |
| Relative Humidity [%] | 37.7 | 38.3 | 37.8 | 36.8 | 37.4 | 36.9 | 37.5 | 37.6 | 32.2 | 26.4 | 21.3 | 17.9 |
| Hour | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| Temperature [K] | 1.4 | 2.2 | 2.7 | 2.6 | 1.6 | 0.3 | −0.5 | −1.2 | −1.7 | −2.1 | −2.4 | −2.8 |
| Relative Humidity [%] | 16.5 | 14.4 | 13.7 | 14.0 | 18.0 | 21.6 | 26.0 | 30.2 | 32.6 | 34.5 | 34.7 | 35.8 |
| Tracer Name | Formula | Emission Rate [mg km−1] | Ratio [%] |
|---|---|---|---|
| ALK4 | ≥C4 alkanes | 6.03 | 40.2 |
| ISOP | CH2 = C(CH3)CH = CH2 | - | - |
| ACET | CH3C(O)CH3 | 0.15 | 1.0 |
| MEK | RC(O)R | 0.0 | 0.0 |
| ALD2 | CH3CHO | 0.08 | 0.5 |
| RCHO | CH3CH2CHO | 0.08 | 0.5 |
| MVK | CH2 = CHC(=O)CH3 | - | - |
| MACR | CH2 = C(CH3)CHO | - | - |
| PRPE | ≥C3 alkenes | 2.10 | 14.0 |
| C3H8 | C3H8 | 0.15 | 1.0 |
| CH2O | HCHO | 0.17 | 1.1 |
| C2H6 | C2H6 | 0.27 | 1.8 |
| Unspeciated | - | - | 39.9 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J. Sensitivity of Nitrate Aerosol Production to Vehicular Emissions in an Urban Street. Atmosphere 2019, 10, 212. https://doi.org/10.3390/atmos10040212
Kim MJ. Sensitivity of Nitrate Aerosol Production to Vehicular Emissions in an Urban Street. Atmosphere. 2019; 10(4):212. https://doi.org/10.3390/atmos10040212
Chicago/Turabian StyleKim, Minjoong J. 2019. "Sensitivity of Nitrate Aerosol Production to Vehicular Emissions in an Urban Street" Atmosphere 10, no. 4: 212. https://doi.org/10.3390/atmos10040212
APA StyleKim, M. J. (2019). Sensitivity of Nitrate Aerosol Production to Vehicular Emissions in an Urban Street. Atmosphere, 10(4), 212. https://doi.org/10.3390/atmos10040212





