Characterization of Human Health Risks from Particulate Air Pollution in Selected European Cities
Abstract
1. Introduction
2. Material and Methods
2.1. Study Areas and Field Measurements
2.2. Health Risk Assessment Methodology for Particle-Bound Metals
2.3. Health Risk Assessment Methodology for PM10 and PM2.5
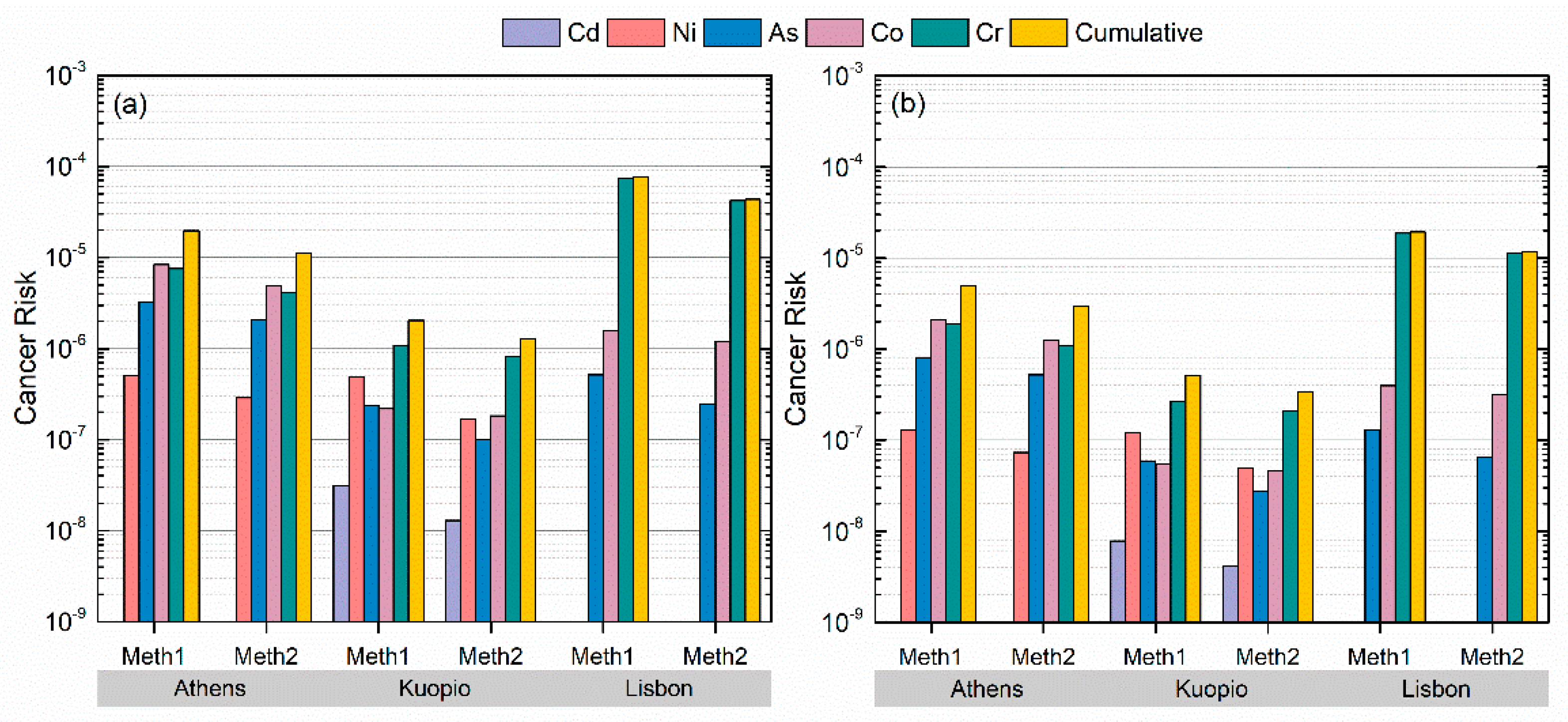
3. Results and Discussion
3.1. Ambient Concentration and Deposited Dose Rate of Particle-Bound Metals
3.2. Hazard Quotients and Cancer Risks
3.2.1. Hazard Quotients
3.2.2. Cancer Risks
3.3. Excess Risk and Attributable Mortality
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adar, S.D.; Filigrana, P.A.; Clements, N.; Peel, J.L. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr. Environ. Health Rep. 2014, 1, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US Counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, O.; Knol, A.B.; Jantunen, M.; Lim, T.A.; Conrad, A.; Rappolder, M.; Carrer, P.; Fanetti, A.C.; Kim, R.; Buekers, J.; et al. Environmental burden of disease in Europe: Assessing nine risk factors in six countries. Environ. Health Perspect. 2014, 122, 439–446. [Google Scholar] [CrossRef]
- Héroux, M.E.; Anderson, H.R.; Atkinson, R.; Brunekreef, B.; Cohen, A.; Forastiere, F.; Hurley, F.; Katsouyanni, K.; Krewski, D.; Krzyzanowski, M.; et al. Quantifying the Health Impacts of Ambient Air Pollutants: Recommendations of a WHO/Europe Project. Int. J. Public Health 2015, 60, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease; World Health Organization: Geneva, Switzerland, 2016; 121p, ISBN 978 92 4 151135 1. [Google Scholar]
- Hussain, M.; Madl, P.; Khan, A. Lung deposition predictions of airborne particles and the emergence of contemporary diseases Part-I. Health 2011, 2, 51–59. [Google Scholar]
- Oller, A.R.; Oberdörster, G. Incorporation of dosimetry in the derivation of reference concentrations for ambient or workplace air: A conceptual approach. J. Aerosol Sci. 2016, 99, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. J. Am. Med. Assoc. 2002, 287, 1132–1141. [Google Scholar] [CrossRef]
- Valavanidis, A.; Fiotakis, K.; Vlachogianni, T. Airborne Particulate Matter and Human Health: Toxicological Assessment and Importance of Size and Composition of Particles for Oxidative Damage and Carcinogenic Mechanisms. J. Environ. Sci. Health C 2008, 26, 339–362. [Google Scholar] [CrossRef]
- Lehtomäki, H.; Korhonen, A.; Asikainen, A.; Karvosenoja, N.; Kupiainen, K.; Paunu, V.V.; Savolahti, M.; Sofiev, M.; Palamarchuk, Y.; Karppinen, A.; et al. Health Impacts of Ambient Air Pollution in Finland. Int. J. Environ. Res. Public Health 2018, 15, 736. [Google Scholar] [CrossRef]
- Pope, C.A.; Ezzati, M.; Dockery, D.W. Fine-Particulate Air Pollution and Life Expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef]
- Romanazzi, V.; Casazza, M.; Malandrino, M.; Maurino, V.; Piano, A.; Schilirò, T.G.; Gilli, G. PM10 size distribution of metals and environmental-sanitary risk analysis in the city of Torino. Chemosphere 2014, 112, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.; Wang, Q.G.; Qian, X.; Qian, Y.; Yang, M.; Li, F.; Lu, H.; Wang, C. Chemical fractionation of arsenic and heavy metals in fine particle matter and its implications for risk assessment: A case study in Nanjing, China. Atmos. Environ. 2015, 103, 339–346. [Google Scholar] [CrossRef]
- Lyu, Y.; Zhang, K.; Chai, F.; Cheng, T.; Yang, Q.; Zheng, Z.; Li, X. Atmospheric size-resolved trace elements in a city affected by non-ferrous metal smelting: Indications of respiratory deposition and health risk. Environ. Pollut. 2017, 224, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.; Soares, C.; Couto, C.M.; Almeida, A. Trace Elements in Ambient Air at Porto Metropolitan Area-Checking for Compliance with New European Union (EU) Air Quality Standards. J. Toxicol. Environ. Health A 2015, 78, 848–859. [Google Scholar] [CrossRef]
- Samek, L. Overal human mortality and morbidity due to exposure to air pollution. IJOMEH 2016, 29, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Gupta, T. Source apportionment and risk assessment of PM1 bound trace metals collected during foggy and non-foggy episodes at a representative site in the Indo-Gangetic plain. Sci. Total Environ. 2016, 550, 80–94. [Google Scholar] [CrossRef]
- Niu, L.; Ye, H.; Xu, C.; Yao, Y.; Liu, W. Highly time-and size-resolved fingerprint analysis and risk assessment of airborne elements in a megacity in the Yangtze River Delta, China. Chemosphere 2015, 119, 112–121. [Google Scholar] [CrossRef]
- Rogula-Kozłowska, W.; Kozielska, B.; Klejnowski, K. Hazardous compounds in urban PM in the central part of upper Silesia (Poland) in Winter. Arch. Environ. Prot. 2013, 39, 53–65. [Google Scholar] [CrossRef]
- Bello, S.; Muhammad, B.G.; Bature, B. Total Excess Lifetime Cancer Risk Estimation from Enhanced Heavy Metals Concentrations Resulting from Tailings in Katsina Steel Rolling Mill, Nigeria. J. Mater. Sci. Eng. 2017, 6, 338. [Google Scholar] [CrossRef]
- Carpenter, D.O.; Bushkin-Bedient, S. Exposure to chemicals and radiation during childhood and risk for cancer later in life. J. Adolesc. Health 2013, 52, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.F.; Rodriguez, M.T.; Espinosa, A.J.F.; Daban, A.G. Physical speciation of arsenic, mercury, lead, cadmium and nickel in inhalable atmospheric particles. Anal. Chim. Acta 2004, 524, 33–40. [Google Scholar] [CrossRef]
- Du, Y.; Gao, B.; Zhou, H.; Ju, X.; Hao, H.; Yin, S. Health Risk Assessment of Heavy Metals in Road Dusts in Urban Parks of Beijing, China. Procedia Environ. Sci. 2013, 18, 299–309. [Google Scholar] [CrossRef]
- Chalvatzaki, E.; Chatoutsidou, S.E.; Mammi-Galani, E.; Almeida, S.M.; Gini, M.I.; Eleftheriadis, K.; Diapouli, E.; Lazaridis, M. Estimation of the Personal Deposited Dose of Particulate Matter and Particle-Bound Metals Using Data from Selected European Cities. Atmosphere 2018, 9, 248. [Google Scholar] [CrossRef]
- Almeida, S.M.; Pio, C.A.; Freitas, M.C.; Reis, M.A.; Trancoso, M.A. Approaching PM2.5 and PM2.5-10 source apportionment by mass balance analysis, principal component analysis and particle size distribution. Sci Total Environ. 2006, 368, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.M.; Freitas, M.C.; Reis, M.; Pinheiro, T.; Felix, P.M.; Pio, C.A. Fifteen years of nuclear techniques application to suspended particulate matter studies. J. Radioanal. Nucl. Chem. 2013, 297, 347–356. [Google Scholar] [CrossRef]
- Sippula, O.; Rintala, H.; Happo, M. Characterization of chemical and microbial species from size-segregated indoor and outdoor particulate samples. Aerosol Air Qual. Res. 2013, 13, 1212–1230. [Google Scholar] [CrossRef]
- Chalvatzaki, E.; Lazaridis, M. Development and application of a dosimetry model (ExDoM2) for calculating internal dose of specific particle-bound metals in the human body. Inhal. Toxicol. 2015, 27, 308–320. [Google Scholar] [CrossRef]
- ICRP. Human Respiratory Tract Model for Radiological Protection. ICRP Publication 66, Ann. ICRP 24(1-3). 1994. Available online: http://www.icrp.org/publication.asp?id=icrp%20publication%2066 (accessed on 24 January 2019).
- ICRP. Occupational Intakes of Radionuclides: Part 1. ICRP Publication 130, Ann. ICRP 44(2). 2015. Available online: http://www.icrp.org/publication.asp?id=ICRP%20Publication%20130 (accessed on 24 January 2019).
- US-EPA. Risk Assessment Guidance for Superfund, Volume I: Human Health Evaluation Manual (Part A); EPA/540/1-89/002; US Environmental Protection Agency, Office of Emergency and Remedial Response: Washington, DC, USA, 1989. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf (accessed on 20 October 2018).
- US EPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment); EPA/540/R/070/002; US Environmental Protection Agency, Office of Emergency and Remedial Response: Washington, DC, USA, 2009. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/partf_200901_final.pdf (accessed on 20 September 2018).
- Fang, W.; Yang, Y.; Xu, Z. PM10 and PM2.5 and health risk assessment for heavy metals in a typical factory for cathode ray tube television recycling. Environ Sci. Technol. 2013, 47, 12469–12476. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, X.; Yekeen, T.A.; Zhang, Y.; Chen, A.; Kim, S.S.; Dietrich, K.N.; Ho, S.M.; Lee, S.A.; Reponen, T.; et al. Ambient air heavy metals in PM2.5 and potential human health risk assessment in an informal electronic-waste recycling site of China. Aerosol Air Qual. Res. 2016, 16, 388–397. [Google Scholar] [CrossRef]
- Rissler, J.; Gudmundsson, A.; Nicklasson, H.; Swietlicki, E.; Wollmer, P.; Lӧndahl, J. Depositon efficiency of inhaled particles (15–5000 nm) related to breathing pattern and lung fuction: An experiment study in healthy children and adults. Part. Fibre Toxicol. 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S. Chapter 27—Deposition of particles. In Comparative Biology of the Normal Lung, 2nd ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 513–536. [Google Scholar] [CrossRef]
- US-EPA. Users’ Guide and Background Technical Document for USEPA Region 9—Preliminary Remediation Goals (PRG) Table. 2013. Available online: https://semspub.epa.gov/work/02/103453.pdf (accessed on 20 September 2018).
- ICRP. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values. ICRP Publication 89, Ann. ICRP 32(3-4). 2003. Available online: http://www.icrp.org/publication.asp?id=icrp%20publication%2089 (accessed on 24 January 2019).
- US-EPA. Regional Screening Level (RSL) Summary Table. 2017. Available online: https://semspub.epa.gov/work/03/2245073.pdf (accessed on 10 April 2018).
- RAIS. Risk Exposure Models for Chemicals User’s Guide. 2017. Available online: https://rais.ornl.gov/tools/rais_chemical_risk_guide.html (accessed on 20 September 2017).
- Tepanosyan, G.; Maghakyan, N.; Sahakyan, L.; Saghatelyan, A. Heavy metals pollution levels and children health risk assessment of Yerevan kindergartens soils. Ecotoxicol. Environ. Saf. 2017, 142, 257–265. [Google Scholar] [CrossRef] [PubMed]
- US EPA. IRIS Assessments. Available online: https://cfpub.epa.gov/ncea/iris2/atoz.cfm (accessed on 10 April 2018).
- Ostro, B. Outdoor Air Pollution: Assessing the Environmental Burden of Disease at National and Local Levels; Environmental Burden of Disease Series, No 5; World Health Organization: Geneva, Switzerland, 2004; Available online: https://www.who.int/quantifying_ehimpacts/publications/ebd5.pdf (accessed on 15 January 2019).
- Burnett, R.T.; Pope, C.A., III; Ezzati, M.; Olives, C.; Lim, S.S.; Mehta, S.; Shin, H.H.; Singh, G.; Hubbell, B.; Brauer, M.; et al. An integrated risk function for estimating the global burden of disease attributable to ambient fine particulate matter exposure. Environ. Health Perspect. 2014, 122, 397–403. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef]
- Hänninen, O.; Knol, A. European Perspectives on Environmental Burden of Disease Estimates for Nine Stressors in Six European Countries; National Institute for Health and Welfare (THL): Helsinki, Finland, 2011. [Google Scholar]
- WHO. WHO Methods and Data Sources for Country-Level Causes of Death 2000–2016; Global Health Estimates Technical Paper WHO/HIS/IER/GHE/2018.3; World Health Organization: Geneva, Switzerland, 2018; 65p, Available online: http://www.who.int/healthinfo/global_burden_disease/en/ (accessed on 29 October 2018).
- Querol, X.; Alastuey, A.; Ruiz, C.R.; Artinano, B.; Hansson, H.C.; Harrison, R.M.; Buringh, E.; ten Brink, H.M.; Lutz, M.; Bruckmann, P.; et al. Speciation and origin of PM10 and PM2.5 in selected European cities. Atmos. Environ. 2004, 38, 6547–6555. [Google Scholar] [CrossRef]
- IARC. Outdoor Air Pollution. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2016, Volume 109. Available online: http://monographs.iarc.fr/ENG/Monographs/vol109/index.php (accessed on 29 April 2018).
- Megido, L.; Suarez-Pena, B.; Negral, L.; Castrillon, L.; Fernandez-Nava, Y. Suburban air quality: Human health hazard assessment of potentially toxic elements in PM10. Chemosphere 2017, 177, 284–291. [Google Scholar] [CrossRef]
- Wang, X.; He, S.; Chen, S.; Zhang, Y.; Wang, A.; Luo, J.; Ye, X.; Mo, Z.; Wu, L.; Xu, P.; et al. Spatiotemporal Characteristics and Health Risk Assessment of Heavy Metals in PM2.5 in Zhejiang Province. Int. J. Environ. Res. Public Health 2018, 15, 583. [Google Scholar] [CrossRef]
- Martins, V.; Moreno, T.; Minguillón, M.; Amato, F.; de Miguel, E.; Capdevila, M.; Querol, X. Exposure to airborne particulate matter in the subway system. Sci. Total Environ. 2015, 511, 711–722. [Google Scholar] [CrossRef]
- Wang, C.S. Inhaled particles. In Interface Science and Technology; Elsevier, Academic Press: Cambridge, MA, USA, 2005; Volume 5, ISBN 0-12088579-4. [Google Scholar]
- Cardaba Arranz, M.; Muñoz Moreno, M.F.; Armentia Medina, A.; Alonso Capitán, M.; Carreras Vaquer, F.; Almaraz Gómez, A. Health impact assessment of air pollution in Valladolid, Spain. BMJ Open 2014, 4, e005999. [Google Scholar] [CrossRef]
- Pokorski, M. Respiratory Treatment and Prevention; Springer International Publishing: Cham, Switzeralnd, 2017. [Google Scholar]
- Evans, J.; van Donkelaar, A.; Martin, R.V.; Burnett, R.; Rainham, D.G.; Birkett, N.J.; Krewski, D. Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environ. Res. 2013, 12, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Rumrich, I.; Kukec, A.; Rejc, T.; Pasetto, R.; Iavarone, I.; Hänninen, O. Methods of health risk and impact assessment at industrially contaminated sites: Systematic review. Epidemiol. Prev. 2018, 42 (Suppl. 1), 49–58. [Google Scholar] [PubMed]
| Athens | Kuopio | Lisbon | |||
|---|---|---|---|---|---|
| PM1.77 | PM6.8 | PM2.5 | PM10 | PM2 | PM8 |
| 11.1 | 19.6 | 6.2 | 10.3 | 21.3 | 36.6 |
| Athens (PM6.8) | Kuopio (PM10) | Lisbon (PM8) | |
|---|---|---|---|
| As | 2.87 | 0.21 | 0.46 |
| Cd | - | 0.07 | - |
| Co | 3.53 * | 0.09 | 0.67 |
| Cr | 2.39 | 0.34 | 23.75 |
| Mn | 14.96 | 4.19 | - |
| Ni | 7.47 * | 7.12 | - |
| Pb | 6.01 | 1.56 | - |
| Athens | Kuopio | Lisbon | ||||
|---|---|---|---|---|---|---|
| Average | min–max | Average | min–max | Average | min–max | |
| As | 1.45 | 0.50–2.60 | 0.07 | 0.02–0.13 | 0.18 | 0.06–0.32 |
| Cd | - | - | 0.02 | 0.01–0.04 | - | - |
| Co | 1.64 | 0.55–2.97 | 0.06 | 0.02–0.11 | 0.40 | 0.12–0.75 |
| Cr | 1.03 | 0.31–1.89 | 0.21 | 0.07–0.37 | 10.60 | 3.29–19.46 |
| Mn | 7.46 | 2.60–13.38 | 2.36 | 0.81–4.22 | - | - |
| Ni | 3.31 | 1.12–5.97 | 1.96 | 0.55–3.68 | - | - |
| Pb | 1.70 | 0.61–3.02 | 0.64 | 0.20–1.16 | - | - |
| Athens | Kuopio | Lisbon | ||||
|---|---|---|---|---|---|---|
| Average | min–max | Average | min–max | Average | min–max | |
| As | 0.85 | 0.31–1.49 | 0.04 | 0.01–0.08 | 0.11 | 0.04–0.19 |
| Cd | - | - | 0.02 | 0.01–0.03 | - | - |
| Co | 0.98 | 0.35–1.73 | 0.04 | 0.01–0.06 | 0.25 | 0.08–0.44 |
| Cr | 0.64 | 0.21–1.15 | 0.12 | 0.05–0.21 | 6.53 | 2.19–11.72 |
| Mn | 4.35 | 1.62–7.64 | 1.42 | 0.51–2.49 | - | - |
| Ni | 1.97 | 0.71–3.48 | 1.33 | 0.38–2.46 | - | - |
| Pb | 1.04 | 0.39–1.82 | 0.40 | 0.13–0.71 | - | - |
| City | Metal | Methodology 1 (US EPA) | Methodology 2 (Lyu et al. [15]) |
|---|---|---|---|
| Athens | As | 1.47 × 10−1 | 9.32 × 10−2 |
| Cd | - | - | |
| Co | 4.51 × 10−1 | 2.64 × 10−1 | |
| Cr | 1.83 × 10−2 | 9.92 × 10−3 | |
| Mn | 2.30 × 10−1 | 1.44 × 10−1 | |
| Ni | 2.87 × 10−1 | 1.60 × 10−1 | |
| Pb | 2.31 × 10−2 | 8.20 × 10−3 | |
| Cumulative | 1.16 | 6.79 × 10−1 | |
| Kuopio | As | 1.06 × 10−2 | 4.52 × 10−3 |
| Cd | 5.06 × 10−3 | 2.11 × 10−3 | |
| Co | 1.18 × 10−2 | 9.87 × 10−3 | |
| Cr | 2.59 × 10−3 | 1.99 × 10−3 | |
| Mn | 6.43 × 10−2 | 4.55 × 10−2 | |
| Ni | 2.73 × 10−1 | 9.47 × 10−2 | |
| Pb | 5.99 × 10−3 | 3.07 × 10−3 | |
| Cumulative | 3.73 × 10−1 | 1.62 × 10−1 | |
| Lisbon | As | 2.34 × 10−2 | 1.13 × 10−2 |
| Cd | - | - | |
| Co | 8.55 × 10−2 | 6.50 × 10−2 | |
| Cr | 1.82 × 10−1 | 1.02 × 10−1 | |
| Mn | - | - | |
| Ni | - | - | |
| Pb | - | - | |
| Cumulative | 2.91 × 10−1 | 1.79 × 10−1 |
| City | Metal | Methodology 1 (US EPA) | Methodology 2 (Lyu et al. [15]) |
|---|---|---|---|
| Athens | As | 1.47 × 10−1 | 9.42 × 10−2 |
| Cd | - | - | |
| Co | 4.51 × 10−1 | 2.72 × 10−1 | |
| Cr | 1.83 × 10−2 | 1.07 × 10−2 | |
| Mn | 2.30 × 10−1 | 1.45 × 10−1 | |
| Ni | 2.87 × 10−1 | 1.64 × 10−1 | |
| Pb | 2.31 × 10−2 | 8.64 × 10−3 | |
| Cumulative | 1.16 | 6.95 × 10−1 | |
| Kuopio | As | 1.06 × 10−2 | 4.96 × 10−3 |
| Cd | 5.06 × 10−3 | 2.66 × 10−3 | |
| Co | 1.18 × 10−2 | 1.00 × 10−2 | |
| Cr | 2.59 × 10−3 | 2.05 × 10−3 | |
| Mn | 6.43 × 10−2 | 4.72 × 10−2 | |
| Ni | 2.73 × 10−1 | 1.10 × 10−1 | |
| Pb | 5.99 × 10−3 | 3.30 × 10−3 | |
| Cumulative | 3.73 × 10−1 | 1.81 × 10−1 | |
| Lisbon | As | 2.34 × 10−2 | 1.17 × 10−2 |
| Cd | - | - | |
| Co | 8.55 × 10−2 | 6.83 × 10−2 | |
| Cr | 1.82 × 10−1 | 1.09 × 10−1 | |
| Mn | - | - | |
| Ni | - | - | |
| Pb | - | - | |
| Cumulative | 2.91 × 10−1 | 1.89 × 10−1 |
| Cancer Cases per Lifetime (70 Years) | ||||||
|---|---|---|---|---|---|---|
| Methodology 1 (US EPA) | Methodology 2 (Lyu et al. [15]) | |||||
| Athens | Kuopio | Lisbon | Athens | Kuopio | Lisbon | |
| As | 12.2 | 0.020 | 1.5 | 7.7 | 0.008 | 0.7 |
| Cd | - | 0.003 | - | - | 0.001 | - |
| Co | 31.3 | 0.018 | 4.4 | 18.4 | 0.015 | 3.4 |
| Cr | 28.3 | 0.090 | 210.8 | 15.3 | 0.069 | 118.3 |
| Ni | 1.9 | 0.041 | - | 1.1 | 0.014 | - |
| Cumulative | 73.7 | 0.172 | 216.7 | 42.5 | 0.107 | 122.4 |
| Athens | Kuopio | Lisbon | ||||
|---|---|---|---|---|---|---|
| (a) All-cause mortality (PM10) | ||||||
| ER (95% CI) | 0.77 | (0.58–0.96) | 0.02 | (0.02–0.03) | 2.2 | (1.6–2.7) |
| AF (95% CI) | 0.77 | (0.57–0.96) | 0.02 | (0.02–0.03) | 2.1 | (1.58–2.62) |
| Deaths (95% CI) | 320 | (236–398) | 0.2 | (0.2–0.3) | 730 | (546–905) |
| (b) Cardiopulmonary mortality (PM2.5) | ||||||
| ER (95% CI) | 19 | (6.4–33) | 9.5 | (3.4–16) | 31 | (10–55) |
| AF (95% CI) | 15.8 | (6.0–24.5) | 8.7 | (3.2–13.9) | 23.4 | (9.2–35.4) |
| Deaths (95% CI) | 3450 | (1311–5353) | 38 | (14–61) | 2450 | (962–3702) |
| (c) Lung cancer mortality (PM2.5) | ||||||
| ER (95% CI) | 29 | (9.9–52) | 15 | (5.2–25) | 49 | (16–92) |
| AF (95% CI) | 22.7 | (9.0–34.2) | 12.8 | (4.9–20.0) | 32.9 | (13.7–47.8) |
| Deaths (95% CI) | 480 | (190–721) | 6.1 | (2.3–9.5) | 370 | (155–540) |
| Sum of deaths (b+c) | 3930 | 44.1 | 2820 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalvatzaki, E.; Chatoutsidou, S.E.; Lehtomäki, H.; Almeida, S.M.; Eleftheriadis, K.; Hänninen, O.; Lazaridis, M. Characterization of Human Health Risks from Particulate Air Pollution in Selected European Cities. Atmosphere 2019, 10, 96. https://doi.org/10.3390/atmos10020096
Chalvatzaki E, Chatoutsidou SE, Lehtomäki H, Almeida SM, Eleftheriadis K, Hänninen O, Lazaridis M. Characterization of Human Health Risks from Particulate Air Pollution in Selected European Cities. Atmosphere. 2019; 10(2):96. https://doi.org/10.3390/atmos10020096
Chicago/Turabian StyleChalvatzaki, Eleftheria, Sofia Eirini Chatoutsidou, Heli Lehtomäki, Susana Marta Almeida, Konstantinos Eleftheriadis, Otto Hänninen, and Mihalis Lazaridis. 2019. "Characterization of Human Health Risks from Particulate Air Pollution in Selected European Cities" Atmosphere 10, no. 2: 96. https://doi.org/10.3390/atmos10020096
APA StyleChalvatzaki, E., Chatoutsidou, S. E., Lehtomäki, H., Almeida, S. M., Eleftheriadis, K., Hänninen, O., & Lazaridis, M. (2019). Characterization of Human Health Risks from Particulate Air Pollution in Selected European Cities. Atmosphere, 10(2), 96. https://doi.org/10.3390/atmos10020096








