Heterogeneous Freezing of Liquid Suspensions Including Juices and Extracts from Berries and Leaves from Perennial Plants
Abstract
1. Introduction
2. Experiments
2.1. VODCA (Vienna Optical Droplet Crystallization Analyzer)
2.2. Data Analysis
2.3. Samples
2.4. Urea and Subtilisin Treatement
2.5. Ozone Treatment
3. Results
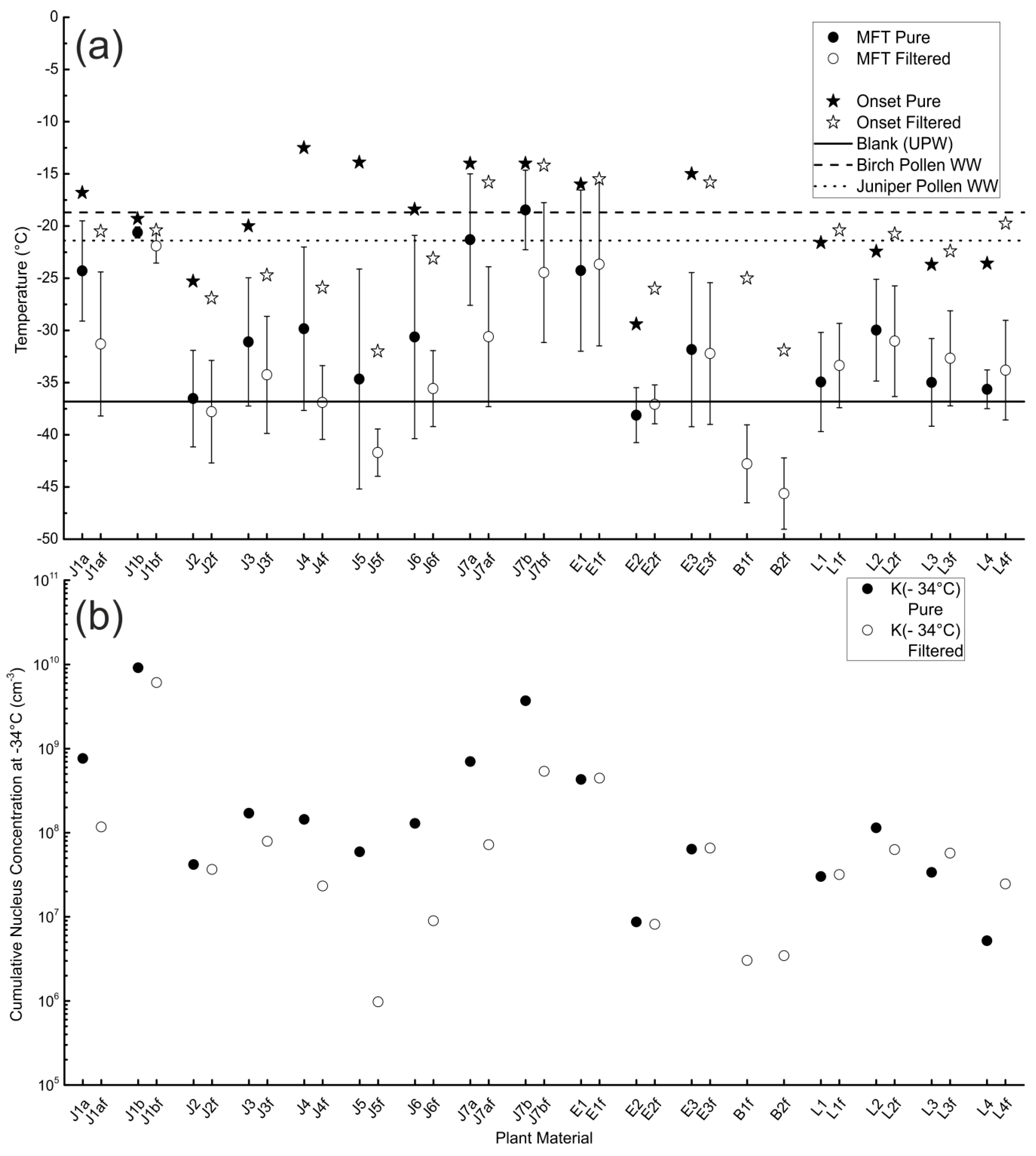
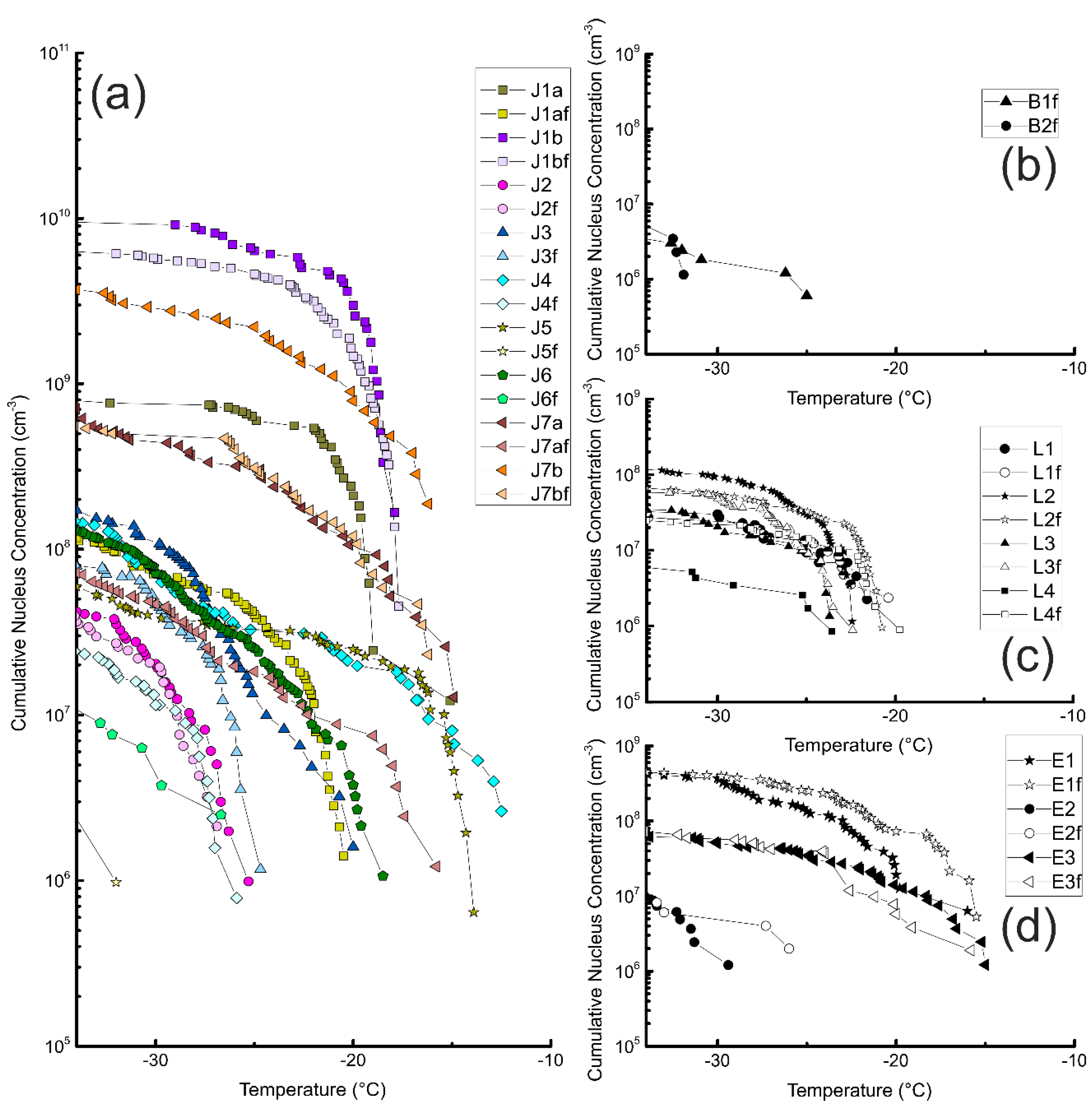
3.1. Unfiltered Samples
3.2. Filtered Samples
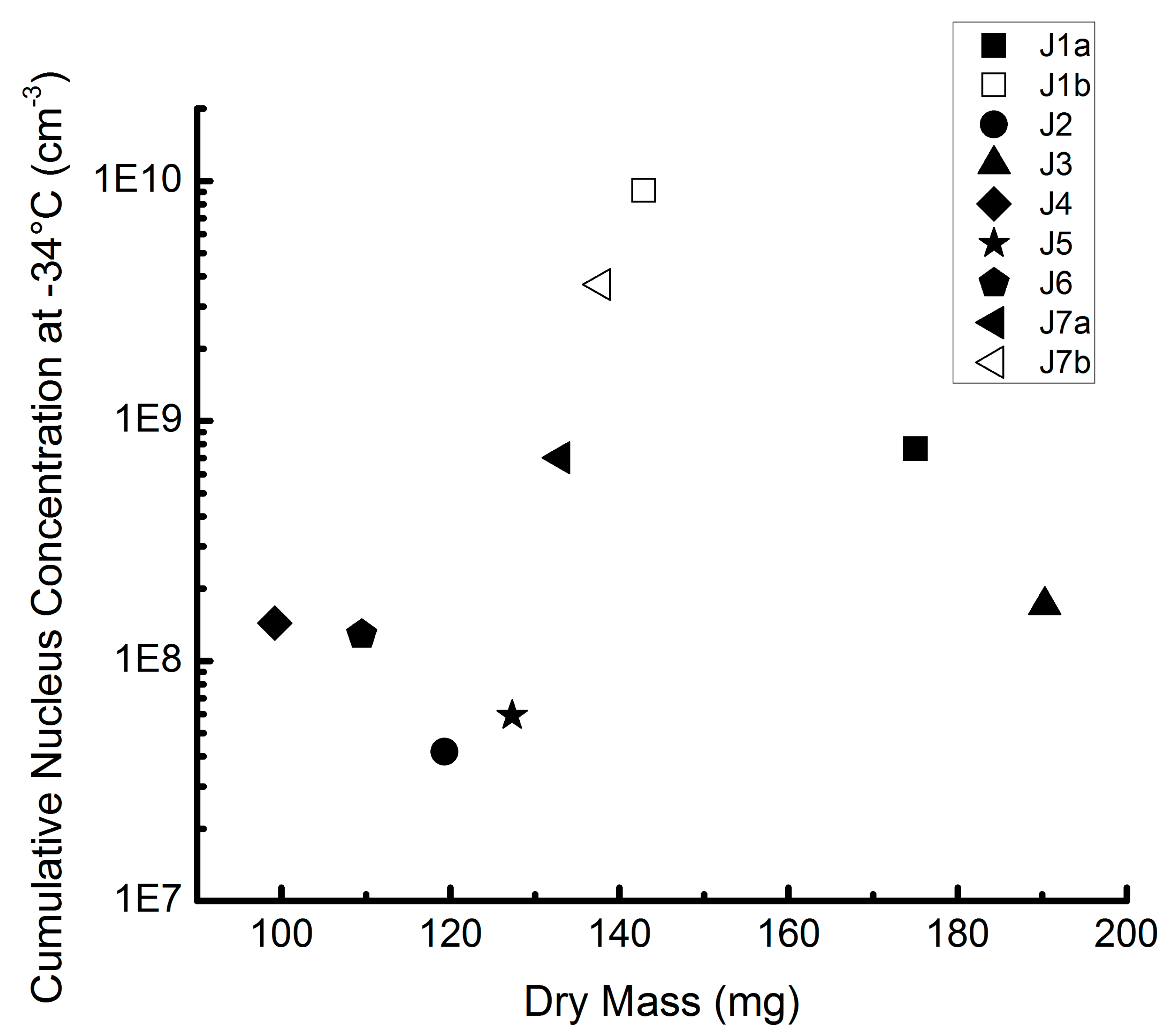
3.3. Comparison of Different Samples and Sample Classes
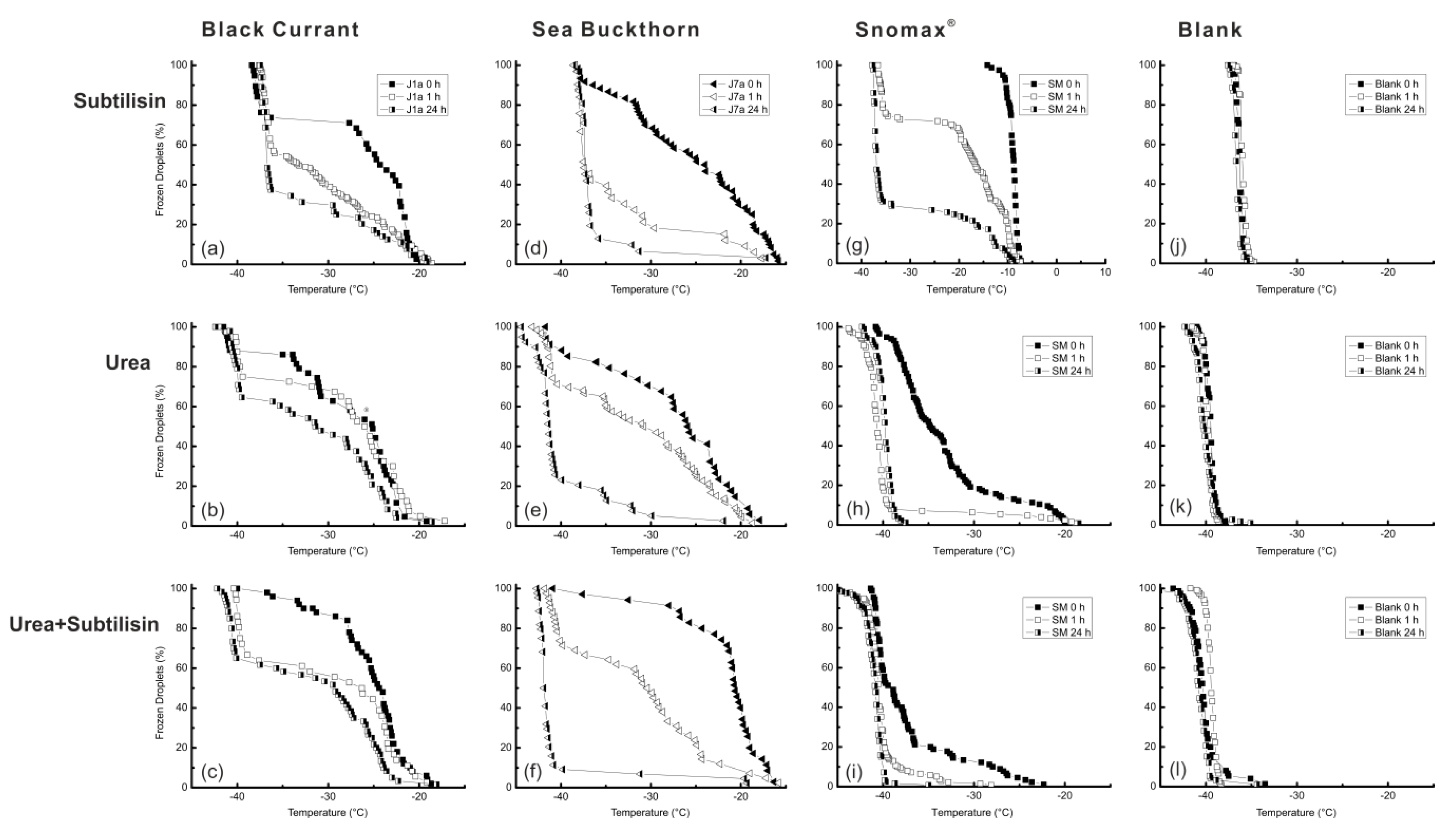
3.4. Urea and Subtilisin Treatment
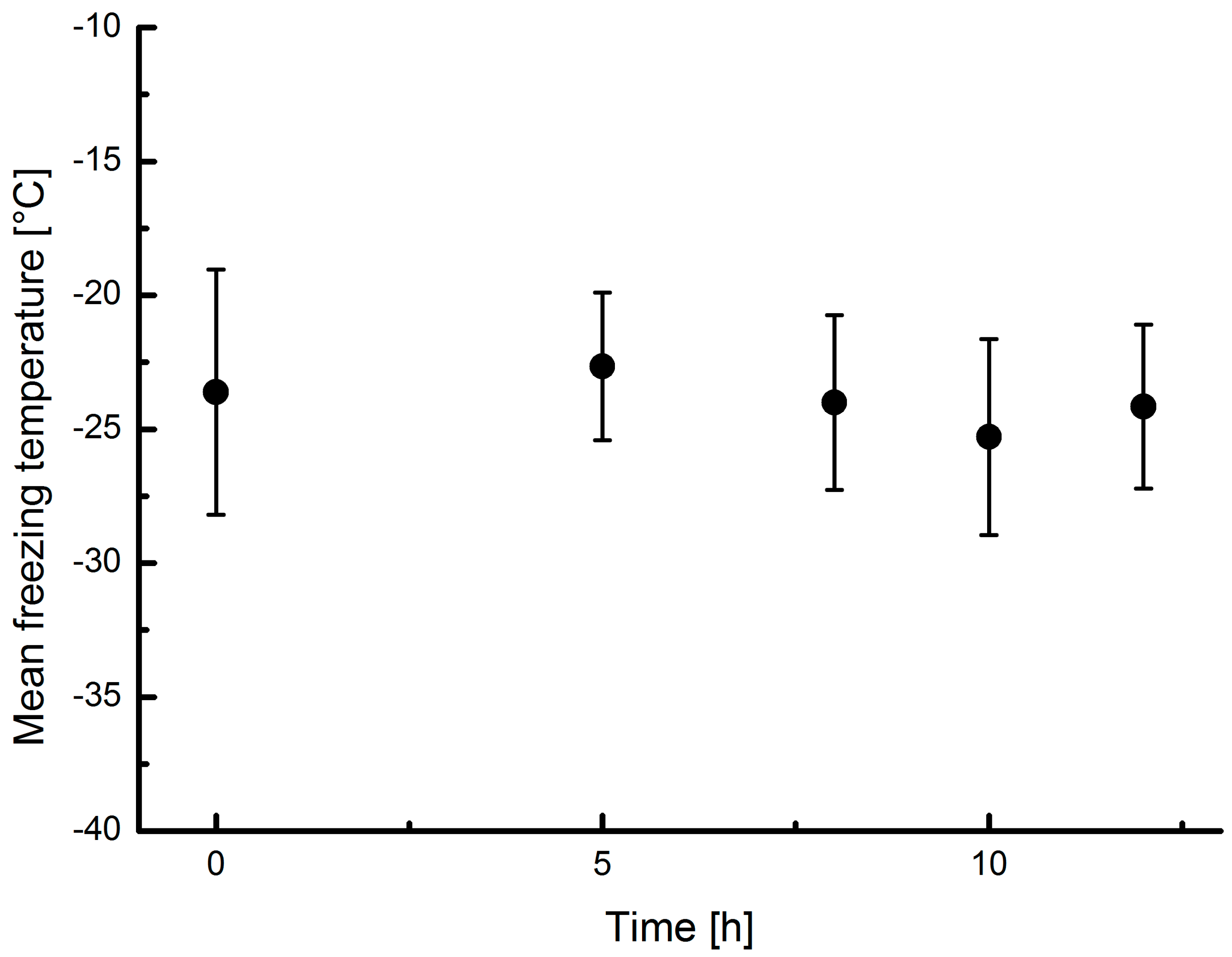
3.5. Ozone Treatment
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Plant/Material | Sample Mass | Freezing Temperature (°C) | Droplet Volume | Citation |
|---|---|---|---|---|
| Prunus tree wood | 5 cm sections | −2 a | 24 mL | [47] |
| Citrus fruits | A whole fruit per droplet | −2.5 a | 150 mL | [48] |
| Winter rye leaves (Secale cereale) | 0.1 g leaf tissue per droplet | −12–−5 b | 4 mL | [49] |
| Birch pollen I | * | −12 c | * | [50] |
| Birch pollen II | 50 mg/mL | −19 d | 0.5–4200 pL | [51] |
| Birch pollen washing water | Aqueous extract of approx. 50 mg/mL pollen, product shows approx. 2.4 wt % residue | −18 d | 0.5–4200 pL | [51] |
| Birch oil | * | −10 e | * | [53] |
| Pine pollen I | * | −12 c | * | [50] |
| Pine pollen II | 50 mg/mL | −20 d | 0.5–4200 pL | [51] |
| Pine pollen washing water | Aqueous extract of approx. 50 mg/mL pollen, product shows approx. 2.4 wt % residue | −21 d | 0.5–4200 pL | [51] |
| Pine oil | * | −12–−9 e | * | [53] |
| Decayed leaf litter | 5–100 mg per test dispersed in air | −24–−4 f | N/A | [54] |
| Ripe sea buckthorn berry juice | Tested pure | −7.9–−7.3 d | 10 µL | [55] |
| Centrifuged sea buckthorn juice from ripe berries of different origins | Pure centrifuged filtered juice | −15.1–−6.1 a | 20 µL | [56] |
| Blueberry stem | 7.5 mm long increments per droplet | −2.3 d | 0.5 mL | [57] |
| Blueberry fruits | A whole organ or half cut organ (to fit the tube) per droplet | −7.2 d | 0.5 mL | [57] |
References
- Cantrell, W.; Heymsfield, A. Production of ice in tropospheric clouds: A review. BAMS 2005, 86, 795–807. [Google Scholar] [CrossRef]
- Hegg, D.A.; Baker, M.B. Nucleation in the atmosphere. Rep. Prog. Phys. 2009, 72, 21. [Google Scholar] [CrossRef]
- Murray, B.J.; Broadley, S.L.; Wilson, T.W.; Bull, S.J.; Wills, R.H.; Christenson, H.K.; Murray, E.J. Kinetics of the homogeneous freezing of water. Phys. Chem. Chem. Phys. 2010, 12, 10380–10387. [Google Scholar] [CrossRef] [PubMed]
- Pruppbacher, H.R.; Klett, J.D. Microphysics of Clouds and Precipitation, 2nd ed.; Kluwer Acedemic Publishers: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Koop, T.; Luo, B.; Tsias, A.; Peter, T. Water activity as the determinant for homogeneous ice nucleation in aqueous solutions. Nature 2000, 406, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Vali, G.; DeMott, P.J.; Möhler, O.; Whale, T.F. Technical Note: A proposal for ice nucleation terminology. Atmos. Chem. Phys. 2015, 15, 10263–10270. [Google Scholar] [CrossRef]
- Mishchenko, M.I.; Rossow, W.B.; Macke, A.; Lacis, A.A. Sensitivity of cirrus cloud albedo, bidirectional, reflectance and optical thickness retrieval accuracy to ice particle shape. J. Geophys. Res. 1996, 101, 16973–16985. [Google Scholar] [CrossRef]
- Baker, M.B. Cloud microphysics and climate. Science 1997, 276, 1072–1078. [Google Scholar] [CrossRef]
- Lohmann, U. A glaciation indirect aerosol effect caused by soot aerosols. Geophys. Res. Lett. 2002, 29, 11. [Google Scholar] [CrossRef]
- Forster, V.P.; Ramaswamy, P.; Artaxo, T.; Berntsen, R.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Möhler, O.; DeMott, P.J.; Vali, G.; Levin, Z. Microbiology and atmospheric processes: The role of biological particles in cloud physics. Biogeosciences 2007, 4, 1059–1071. [Google Scholar] [CrossRef]
- Després, V.R.; Huffman, A.J.; Burrows, S.M.; Hoose, C.; Safatov, A.S.; Buryak, G.; Fröhlich-Nowoisky, J.; Elbert, W.; Andreae, M.; Pöschl, U.; et al. Primary biological aerosol particles in the atmosphere: A review. Tellus B Chem. Phys. Meteorol. 2012, 64, 15598. [Google Scholar] [CrossRef]
- Murray, B.J.; O’Sullivan, D.; Atkinson, J.D.; Webb, M.E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. [Google Scholar] [CrossRef] [PubMed]
- DeMott, P.J.; Prenni, A.J.; Liu, X.; Kreidenweis, S.M.; Petters, M.D.; Twohy, C.H.; Richardson, M.S.; Eidhammer, T.; Rodgers, D.C. Predicting global atmospheric ice nuclei distributions and their impacts on climate. Proc. Natl. Acad. Sci. USA 2010, 107, 11217–11222. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kreidenweis, S.M.; McInnes, L.M.; Rogers, D.C.; DeMott, P.J. Single particle analyses of ice nucleating aerosols in the upper troposphere and lower stratosphere. Geophys. Res. Lett. 1998, 25, 1391–1394. [Google Scholar] [CrossRef]
- Sesartic, A.; Lohmann, U.; Storelvmo, T. Modelling the impact of fungal spore ice nuclei on clouds and precipitation. Environ. Res. Lett. 2013, 8, 014029. [Google Scholar] [CrossRef]
- Sesartic, A.; Lohmann, U.; Storelvmo, T. Bacteria in the ECHAM5-HAM global climate model. Atmos. Chem. Phys. 2012, 12, 8645–8661. [Google Scholar] [CrossRef]
- Hoose, C.; Kristjánsson, J.E.; Burrows, S.M. How important is biological ice nucleation in clouds on a global scale? Environ. Res. Lett. 2010, 5, 024009. [Google Scholar] [CrossRef]
- Burrows, S.M.; Hoose, C.; Pöschl, U.; Lawrence, M.G. Ice nuclei in marine air: Biogenic particles or dust? Atmos. Chem. Phys. 2013, 13, 245–267. [Google Scholar] [CrossRef]
- Pöschl, U.; Martin, S.T.; Sinha, B.; Chen, Q.; Gunthe, S.S.; Huffman, J.A.; Borrmann, S.; Farmer, D.K.; Garland, R.M.; Helas, G.; et al. Rainforest Aerosols as Biogenic Nuclei of Clouds and Precipitation in the Amazon. Science 2010, 329, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M. Laboratory and Field Measurements of Immersion Freezing Utilizing a Newly Developed Cloud Chamber. Ph.D. Thesis, ETH Zürich, Zürich, Switzerland, 2016. [Google Scholar]
- Huffman, J.A.; Prenni, A.J.; Demott, P.J.; Pöhlker, C.; Mason, R.H.; Robinson, N.H.; Fröhlich-Nowoisky, J.; Tobo, Y.; Després, V.R.; Garcia, E.; et al. High concentrations of biological aerosol particles and ice nuclei during and after rain. Atmos. Chem. Phys. 2013, 13, 6151–6164. [Google Scholar] [CrossRef]
- Maki, L.R.; Galyan, E.L.; Chang-Chien, M.M.; Caldwell, D.R. Ice Nucleation Induced by Pseudomonas syringae. Appl. Environ. Microbiol. 1974, 28, 456–459. [Google Scholar]
- Morris, C.E.; Sands, D.C.; Bardin, M.; Jaenicke, R.; Vogel, B.; Leyronas, C.; Ariya, P.A.; Psenner, R. Microbiology and atmospheric processes: Research challenges concerning the impact of airborne micro-organisms on the atmosphere and climate. Biogeosciences 2011, 8, 17–25. [Google Scholar] [CrossRef]
- Attard, E.; Yang, H.; Delort, A.M.; Amato, P.; Pöschl, U.; Glaux, C.; Koop, T.; Morris, C.E. Effects of atmospheric conditions on ice nucleation activity of Pseudomonas. Atmos. Chem. Phys. 2012, 12, 10667–10677. [Google Scholar] [CrossRef]
- Stephens, E.R. Chemistry of atmospheric oxidants. J. Air Pollut. Control Assoc. 1969, 19, 181–185. [Google Scholar] [CrossRef]
- Fitzner, M.; Sosso, G.C.; Cox, S.J.; Michaelides, A. The Many Faces of Heterogeneous Ice Nucleation: Interplay between Surface Morphology and Hydrophobicity. J. Am. Chem. Soc. 2015, 137, 13658–13669. [Google Scholar] [CrossRef] [PubMed]
- Zachariassen, K.E.; Kristiansen, E. Ice nucleation and antinucleation in nature. Cryobiology 2000, 41, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Kajava, A.V.; Lindow, S.E. A model of the three-dimensional structure of ice nucleation proteins. J. Mol. Biol. 1993, 232, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.; Wolber, P. Molecular aspects of microbial ice nucleation. Mol. Microbiol. 1991, 5, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Pridmore, D.; Capitani, G.; Battistutta, R.; Neeser, J.R.; Jann, A. Molecular organisation of the ice nucleation protein InaV from Pseudomonas syringae. FEBS Lett. 1997, 414, 590–594. [Google Scholar] [CrossRef]
- Garnham, C.P.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Novel dimeric β-helical model of an ice nucleation protein with bridged active sites. BMC Struct. Biol. 2011, 11, 36. [Google Scholar] [CrossRef]
- Pratt, K.A.; Demottm, P.J.; French, J.R.; Wang, Z.; Westphal, D.L.; Heymsfield, A.J.; Twohy, C.H.; Prenni, A.J.; Prather, K.A. In situ detection of biological particles in cloud ice-crystals. Nat. Geosci. 2009, 2, 398–401. [Google Scholar] [CrossRef]
- Baustian, K.J.; Cziczo, D.J.; Wise, M.E.; Pratt, K.A.; Kulkarni, G.; Hallar, A.G.; Tolbert, M.A. Importance of aerosol composition, mixing state, and morphology for heterogeneous ice nucleation: A combined field and laboratory approach. J. Geophys. Res. Atmos. 2012, 117, D06217. [Google Scholar] [CrossRef]
- Möhler, O.; Benz, S.; Saathoff, H.; Schnaiter, M.; Wagner, R.; Schneider, J.; Walter, S.; Ebert, V.; Wagner, S. The effect of organic coating on the heterogeneous ice nucleation efficiency of mineral dust aerosols. Environ. Res. Lett. 2008, 3, 025007. [Google Scholar] [CrossRef]
- Sands, D.C.; Langham, V.E.; Scharen, A.L.; de Smet, C. The association between bacteria and rain and possible resultant meteorological implications. Idojaras 1982, 86, 148–152. [Google Scholar]
- Morris, C.E.; Conen, F.; Huffman, A.J.; Phillips, V.; Pöschl, U.; Sands, D.C. Bioprecipitation: A feedback cycle linking Earth history, ecosystem dynamics and land use through biological ice nucleators in the atmosphere. Glob. Chang. Biol. 2014, 20, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Lindow, S.E.; Arny, D.C.; Upper, C.D. Bacterial Ice Nucleation: A Factor in Frost Injury to Plants. Plant Physiol. 1982, 70, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Wolber, P.; Deininger, C.; Southworth, M.; Vandekerckhove, J.; van Montagu, M.; Warren, G. Identification and purification of a bacterial ice-nucleation protein. Proc. Natl. Acad. Sci. USA 1986, 83, 7256–7260. [Google Scholar] [CrossRef]
- Pouleur, S.; Richard, C.; Martin, J.G.; Antoun, H. Ice Nucleation Activity in Fusarium-Acuminatum and Fusarium-Avenaceum. Appl. Environ. Microbiol. 1992, 58, 2960–2964. [Google Scholar]
- Fröhlich-Nowoisky, J.; Hill, T.C.J.; Pummer, B.G.; Yordanova, P.; Franc, G.D.; Pöschl, U. Ice nucleation activity in the widespread soil fungus Mortierella alpina. Biogeosciences 2015, 12, 1057–1071. [Google Scholar] [CrossRef]
- Iannone, R.; Chernoff, D.I.; Pringle, A.; Martin, S.T.; Bertram, A.K. The ice nucleation ability of one of the most abundant types of fungal spores found in the atmosphere. Atmos. Chem. Phys. 2011, 11, 1191–1201. [Google Scholar] [CrossRef]
- Haga, D.I.; Iannone, R.; Wheeler, M.J.; Mason, R.; Polishchuk, E.A.; Fetch, T.; van der Kamp, B.J.; McKendry, L.G.; Bertram, A.K. Ice nucleation properties of rust and bunt fungal spores and their transport to high altitudes, where they can cause heterogeneous freezing. J. Geophys. Res. Atmos. 2013, 118, 7260–7272. [Google Scholar] [CrossRef]
- Weber, C.F. Polytrichum commune spores nucleate ice and associated microorganisms increase the temperature of ice nucleation activity onset. Aerobiologia 2016, 32, 353–361. [Google Scholar] [CrossRef]
- Kieft, T.L. Ice nucleation activity in lichens. Appl. Environ. Microbiol. 1988, 54, 1678–1681. [Google Scholar] [PubMed]
- Jaenicke, R. Abundance of cellular material and proteins in the atmosphere. Science 2005, 308, 73. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.C.; Proebsting, E.L.; Maccrindle-zimmerman, H. Development, Distribution, and Characteristics of Intrinsic, Nonbacterial Ice Nuclei in Prunus Wood. Plant Physiol. 1988, 88, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Constantinidou, H.A.; Menkissoglu, O. Characteristics and importance of heterogeneous ice nuclei associated with citrus fruits. J. Exp. Bot. 1992, 43, 585–591. [Google Scholar] [CrossRef]
- Brush, R.A.; Griffith, M.; Mlynarz, A. Characterization and Quantification of Intrinsic Ice Nucleators in Winter Rye (Secale-Cereale) Leaves. Plant Physiol. 1994, 104, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Diehl, K.; Quick, C.; Matthias-Maser, S.; Mitra, S.K.; Jaenicke, R. The ice nucleating ability of pollen Part I: Laboratory studies in deposition and condensation freezing modes. Atmos. Res. 2001, 58, 75–87. [Google Scholar] [CrossRef]
- Pummer, B.G.; Bauer, H.; Bernardi, J.; Bleicher, S.; Grothe, H. Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen. Atmos. Chem. Phys. 2012, 12, 2541–2550. [Google Scholar] [CrossRef]
- Macromolecule. Available online: https://www.britannica.com/science/macromolecule (accessed on 16 December 2018).
- Rosinski, J.; Parungo, F. Terpene-Iodine Compounds as Ice Nuclei. J. Appl. Meteorol. 1966, 5, 119–123. [Google Scholar] [CrossRef]
- Schnell, R.C.; Vali, G. Biogenic Ice Nuclei: Part I. Terrestrial and Marine Sources. J. Atmos. Sci. 1976, 33, 1554–1564. [Google Scholar] [CrossRef]
- Jann, A.; Lundheim, R.; Niederberger, P.; Richard, M. Increasing Freezing Point of Food with Sea Buckthorn Ice Nucleating Agent. U.S. Patent 5,665,361, 9 September 1997. [Google Scholar]
- Lundheim, R.; Wahlberg, K. Ice nucleation in fruit juice from different varieties of sea buckthorn Hippophaë rhamnoides L. Euphytica 1998, 102, 117–124. [Google Scholar] [CrossRef]
- Kishimoto, T.; Yamazaki, H.; Saruwatari, A.; Murakawa, H.; Sekozawa, Y.; Kuchitsu, K.; Price, W.S.; Ishikawa, M. High ice nucleation activity located in blueberry stem bark is linked to primary freeze initiation and adaptive freezing behaviour of the bark. AoB Plants 2014, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dreischmeier, K.; Budke, C.; Wiehemeier, L.; Kottke, T.; Koop, T. Boreal pollen contain ice-nucleating as well as ice-binding “antifreeze” polysaccharides. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.J.; Ouyang, B.; Nikolovski, N.; Lienhard, D.M.; Pope, F.D.; Kalberer, M. A new electrodynamic balance (EDB) design for low-temperature studies: Application to immersion freezing of pollen extract bioaerosols. Atmos. Meas. Tech. 2015, 8, 1183–1195. [Google Scholar] [CrossRef]
- Wisniewski, M.; Lindow, S.E.; Ashworth, E.N. Observations of Ice Nucleation and Propagation in Plants Using Infrared Video Thermography. Plant Physiol. 1997, 113, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, E.N. Xylem development in prunus flower buds and the relationship to deep supercooling. Plant Physiol. 1984, 74, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Pummer, B.G.; Budke, C.; Augustin-Bauditz, S.; Niedermeier, D.; Felgitsch, L.; Kampf, C.J.; Huber, R.G.; Liedl, K.R.; Loerting, T.; Moschen, T.; et al. Ice nucleation by water-soluble macromolecules. Atmos. Chem. Phys. 2015, 15, 4077–4091. [Google Scholar] [CrossRef]
- Hauptmann, A.; Handle, K.F.; Baloh, P.; Grothe, H.; Loerting, T. Does the emulsification procedure influence freezing and thawing of aqueous droplets? J. Chem. Phys. 2016, 145, 211923. [Google Scholar] [CrossRef] [PubMed]
- Vali, G. Quantitative Evaluation of Experimental Results an the Heterogeneous Freezing Nucleation of Supercooled Liquids. J. Atmos. Sci. 1971, 28, 402–409. [Google Scholar] [CrossRef]
- Govindarajan, A.G.; Lindow, S.E. Size of bacterial ice-nucleation sites measured in situ by radiation inactivation analysis. Proc. Natl. Acad. Sci. USA 1988, 85, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.M.; Storey, K.B. Cold Hardiness and Freeze Tolerance. In Functional Metabolism: Regulation and Adaptation; Storey, K.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; pp. 470–503. [Google Scholar] [CrossRef]
- Hedstrom, L. Serine protease mechanism and specificity. Chem. Rev. 2002, 102, 4501–4523. [Google Scholar] [CrossRef] [PubMed]
- Wallqvist, A.; Covell, D.G.; Thirumalai, D. Hydrophobic interactions in aqueous urea solutions with implications for the mechanism of protein denaturation. J. Am. Chem. Soc. 1998, 120, 427–428. [Google Scholar] [CrossRef]
- Salvi, G.; Rios, P.D.L.; Vendruscolo, M. Effective Interactions between Chaotropic Agents and Proteins. Proteins Struct. Funct. Bioinform. 2005, 499, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Clark, D.S. Activation of enzymes for nonaqueous biocatalysis by denaturing concentrations of urea. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2001, 1546, 406–411. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Murray, B.J.; Ross, J.F.; Whale, T.F.; Price, H.C.; Atkinson, J.D.; Umo, N.S.; Webb, M.E. The relevance of nanoscale biological fragments for ice nucleation in clouds. Sci. Rep. 2015, 5, 8082. [Google Scholar] [CrossRef]
- O’Sullivan, D.; Murray, B.J.; Ross, J.F.; Webb, M.E. The adsorption of fungal ice-nucleating proteins on mineral dusts: A terrestrial reservoir of atmospheric ice-nucleating particles. Atmos. Chem. Phys. 2016, 16, 7879–7887. [Google Scholar] [CrossRef]
- Conen, F.; Morris, C.E.; Leifeld, J.; Yakutin, M.V.; Alewell, C. Biological residues define the ice nucleation properties of soil dust. Atmos. Chem. Phys. 2011, 11, 9643–9648. [Google Scholar] [CrossRef]
- Tobo, Y.; Demott, P.J.; Hill, T.C.J.; Prenni, A.J.; Swoboda-Colberg, N.G.; Franc, G.D.; Kreidenweis, S.M. Organic matter matters for ice nuclei of agricultural soil origin. Atmos. Chem. Phys. 2014, 14, 8521–8531. [Google Scholar] [CrossRef]
- Hill, T.C.J.; Demott, P.J.; Tobo, Y.; Fröhlich-Nowoisky, J.; Moffett, B.F.; Franc, G.D.; Kreidenweis, S.M. Sources of organic ice nucleating particles in soils. Atmos. Chem. Phys. 2016, 16, 7195–7211. [Google Scholar] [CrossRef]
- Vali, G. Interpretation of freezing nucleation experiments: Singular and stochastic; Sites and surfaces. Atmos. Chem. Phys. 2014, 14, 5271–5294. [Google Scholar] [CrossRef]
- Niedermeier, D.; Shaw, R.A.; Hartmann, S.; Wex, H.; Clauss, T.; Voigtländer, J.; Stratmann, F. Heterogeneous ice nucleation: Exploring the transition from stochastic to singular freezing behavior. Atmos. Chem. Phys. 2011, 11, 8767–8775. [Google Scholar] [CrossRef]
- Murray, B.J.; Broadley, S.L.; Wilson, T.W.; Atkinson, J.D.; Wills, R.H. Heterogeneous freezing of water droplets containing kaolinite particles. Atmos. Chem. Phys. 2011, 11, 4191–4207. [Google Scholar] [CrossRef]
- Welti, A.; Lüönd, F.; Kanji, Z.A.; Stetzer, O.; Lohmann, U. Time dependence of immersion freezing: An experimental study on size selected kaolinite particles. Atmos. Chem. Phys. 2012, 12, 9893–9907. [Google Scholar] [CrossRef]
- Broadley, S.L.; Murray, B.J.; Herbert, R.J.; Atkinson, J.D.; Dobbie, S.; Malkin, T.L.; Condliffe, E.; Neve, L. Immersion mode heterogeneous ice nucleation by an illite rich powder representative of atmospheric mineral dust. Atmos. Chem. Phys. 2012, 12, 287–307. [Google Scholar] [CrossRef]
- DeMott, P.J. An Exploratory Study of Ice Nucleation by Soot Aerosols. J. Appl. Meteorol. 1990, 29, 1072–1079. [Google Scholar] [CrossRef]
- Wright, T.P.; Petters, M.D.; Hader, J.D.; Morton, T.; Holder, A.L. Minimal cooling rate dependence of ice nuclei activity in the immersion mode. J. Geophys Res. Atmos 2013, 118, 10535–10543. [Google Scholar] [CrossRef]
- Mazur, P. Freezing Injury in Plants. Annu. Rev. Plant Physiol. 1969, 20, 419–448. [Google Scholar] [CrossRef]
- Burke, M.J.; Gusta, L.V.; Quamme, H.A.; Weiser, C.J.; Li, P.H. Freezing and Injury in Plants. Annu. Rev. Plant Physiol. 1976, 27, 507–528. [Google Scholar] [CrossRef]
- Pearce, R. Plant Freezing and Damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ide, H.; Price, W.S.; Arata, Y.; Nakamura, T.; Kishimoto, T. Freezing Behaviours in Plant Tissues: Visualization using NMR Micro-imaging and Biochemical Regulatory Factors Invovled. In Plant Cold Hardiness: From the Laboratory to the Field; Gusta, L.V., Wisniewski, M., Tanino, K.K., Eds.; CAB International: Wallingford, UK, 2009; pp. 19–28. [Google Scholar] [CrossRef]
- Griffith, M.; Ala, P.; Yang, D.S.C.; Hon, W.-C.; Moffatt, B.A. Antifreeze Protein Produced Endogenously in Winter Rye Leaves. Plant Physiol. 1992, 100, 593–596. [Google Scholar] [CrossRef]
- Urrutia, M.E.; Duman, J.G.; Knight, C.A. Plant thermal hysteresis proteins. Biochim. Biophys. Acta Protein Struct. Mol. 1992, 1121, 199–206. [Google Scholar] [CrossRef]
- Kasuga, J.; Arakawa, K.; Fujikawa, S. High accumulation of soluble sugars in deep supercooling Japanese white birch xylem parenchyma cells. New Phytol. 2007, 174, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelski, E. Formation of cyanide as a product of decomposition of cyanogenic glucosides in the treatment of elderberry fruit (Sambucus nigra). J. Sci. Food Agric. 1982, 33, 496–498. [Google Scholar] [CrossRef]
- Felgitsch, L.; Baloh, P.; Burkart, J.; Mayr, M.; Momken, M.E.; Seifried, T.M.; Winkler, P.; Schmale, D.G., III; Grothe, H. Birch leaves and branches as a source of ice-nucleating macromolecules. Atmos. Chem. Phys. 2018, 18, 16063–16079. [Google Scholar] [CrossRef]
- Bull, H.B.; Breese, K.; Ferguson, G.L.; Swenson, C.A. The pH of Urea Solutions. Arch. Biochem. Biophys. 1964, 104, 297–304. [Google Scholar] [CrossRef]
- Abdelmonem, A.; Backus, E.H.; Hoffmann, N.; Sánchez, M.A.; Cyran, J.D.; Kiselev, A.; Bonn, M. Surface-charge-induced orientation of interfacial water suppresses heterogeneous ice nucleation on α-alumina (0001). Atmos. Chem. Phys. 2017, 17, 7827–7837. [Google Scholar] [CrossRef]
- Lindow, S.E.; Arny, D.C.; Upper, C.D. Erwinia herbicola: A Bacterial Ice Nucleus Active in Increasing Frost Injury to Corn. Phytopatology 1977, 68, 523–527. [Google Scholar] [CrossRef]
- Failor, K.C.; Schmale, D.G., III; Vinatzer, B.A.; Monteil, C.L. Ice nucleation active bacteria in precipitation are genetically diverse and nucleate ice by employing different mechanisms. ISME J. 2017, 11, 2740–2753. [Google Scholar] [CrossRef]
- Du, R.; Du, P.; Lu, Z.; Ren, W.; Liang, Z.; Qin, S.; Li, Z.; Wang, Y.; Fu, P. Evidence for a missing source of efficient ice nuclei. Sci. Rep. 2017, 7, 39673. [Google Scholar] [CrossRef]
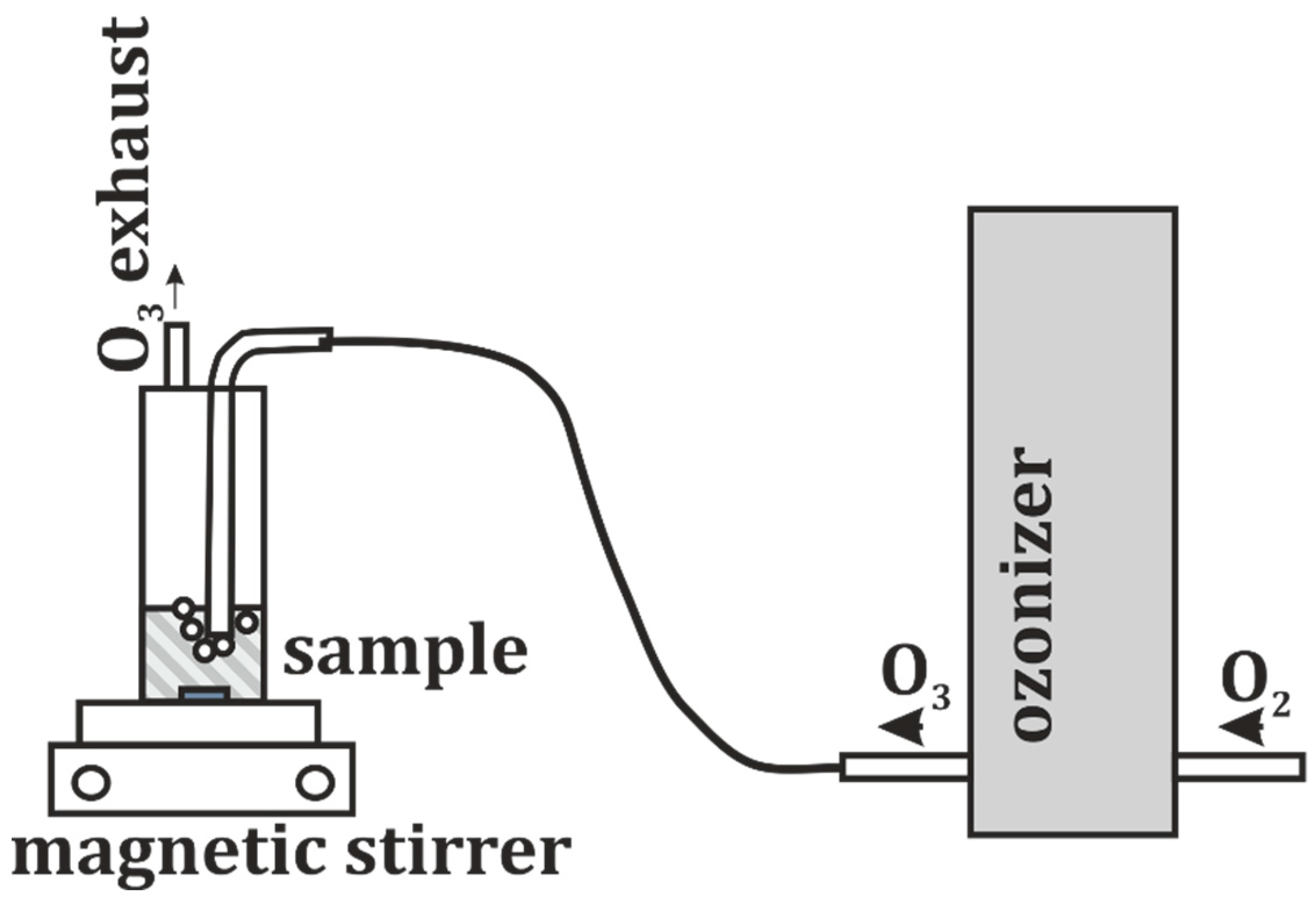
| Common Name | Sample ID | Type | Brand | Genus | Volume/Mass | Lot Number/Charge |
|---|---|---|---|---|---|---|
| Black currant | J1a | Juice | dm Bio | Ribes | 0.33 L | 61493 |
| Black currant II | J1b | Juice | Alnavit | Ribes | 0.33 L | n/a |
| Blueberry | J2 | Juice | Alnavit | Vaccinium | 0.33 L | BL 57408 |
| Chokeberry | J3 | Juice | Alnavit | Aronia | 0.33 L | n/a |
| Cranberry | J4 | Juice | Alnavit | Vaccinium | 0.33 L | n/a |
| Lingonberry | J5 | Juice | Alnavit | Vaccinium | 0.33 L | HL 56893 |
| Sambuccus | J6 | Juice | Alnavit | Sambuccus | 0.33 L | n/a |
| Sea buckthorn | J7a | Juice | dm Bio | Hippophae | 0.33 L | 60857 |
| Sea buckthorn | J7b | Juice | Alnavit | Hippophae | 0.33 L | n/a |
| Raspberry | B1 | Frozen berries | Spar Natur*pur | Rubus | 200 g | L 5017 |
| Rowanberry | B2 | Frozen berries | Obst Oswald | Sorbus | n/a | n/a |
| Juniper berry | E1 | Extract of dried berries | Sonnentor | Juniperus | 35 g | ALB14013003F10 |
| Rowanberry | E2 | Extract of dried berries | Paulaner Apotheke | Sorbus | 100 g | n/a |
| Sea buckthorn | E3 | Extract of dried berries | n/a | Hippophae | n/a | n/a |
| Blueberry | L1 | Extracts of leaves | Collected in Upper Austria | Vaccinium | - | - |
| Juniper | L2 | Extracts of leaves | Collected in Upper Austria | Juniperus | - | - |
| Raspberry | L3 | Extracts of leaves | Collected in Upper Austria | Rubus | - | - |
| Sea buckthorn | L4 | Extracts of leaves | Collected in Upper Austria | Hippophae | - | - |
| Treatment | J1a | J7a | Ultrapure Water | Urea (8 M) | Tris Buffer | Subtilisin in Tris Buffer (2 mg/mL) |
|---|---|---|---|---|---|---|
| Subtilisin | X | 1 mL | 100 µL | |||
| Subtilisin | X | 1 mL | 100 µL | |||
| Urea | X | 1 mL | 100 µL | |||
| Urea | X | 1 mL | 100 µL | |||
| Urea and Subtilisin | X | 1 mL | 100 µL | |||
| Urea and Subtilisin | X | 1 mL | 100 µL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felgitsch, L.; Bichler, M.; Burkart, J.; Fiala, B.; Häusler, T.; Hitzenberger, R.; Grothe, H. Heterogeneous Freezing of Liquid Suspensions Including Juices and Extracts from Berries and Leaves from Perennial Plants. Atmosphere 2019, 10, 37. https://doi.org/10.3390/atmos10010037
Felgitsch L, Bichler M, Burkart J, Fiala B, Häusler T, Hitzenberger R, Grothe H. Heterogeneous Freezing of Liquid Suspensions Including Juices and Extracts from Berries and Leaves from Perennial Plants. Atmosphere. 2019; 10(1):37. https://doi.org/10.3390/atmos10010037
Chicago/Turabian StyleFelgitsch, Laura, Magdalena Bichler, Julia Burkart, Bianca Fiala, Thomas Häusler, Regina Hitzenberger, and Hinrich Grothe. 2019. "Heterogeneous Freezing of Liquid Suspensions Including Juices and Extracts from Berries and Leaves from Perennial Plants" Atmosphere 10, no. 1: 37. https://doi.org/10.3390/atmos10010037
APA StyleFelgitsch, L., Bichler, M., Burkart, J., Fiala, B., Häusler, T., Hitzenberger, R., & Grothe, H. (2019). Heterogeneous Freezing of Liquid Suspensions Including Juices and Extracts from Berries and Leaves from Perennial Plants. Atmosphere, 10(1), 37. https://doi.org/10.3390/atmos10010037





