Chemical Characterization of PM2.5 at Rural and Urban Sites around the Metropolitan Area of Huancayo (Central Andes of Peru)
Abstract
1. Introduction
2. Experiments
2.1. Area Description
2.2. Sampling Method
2.3. Extraction and Chemical Analysis
2.3.1. Water-Soluble Ion Content
2.3.2. Trace Elements
2.4. Quality Control
2.5. Methodology
2.5.1. Analysis of Variance and Tukey Test
2.5.2. Principal Component Analysis
2.5.3. Hierarchical Cluster Analysis and Non-Hierarchical Cluster Analysis.
3. Results
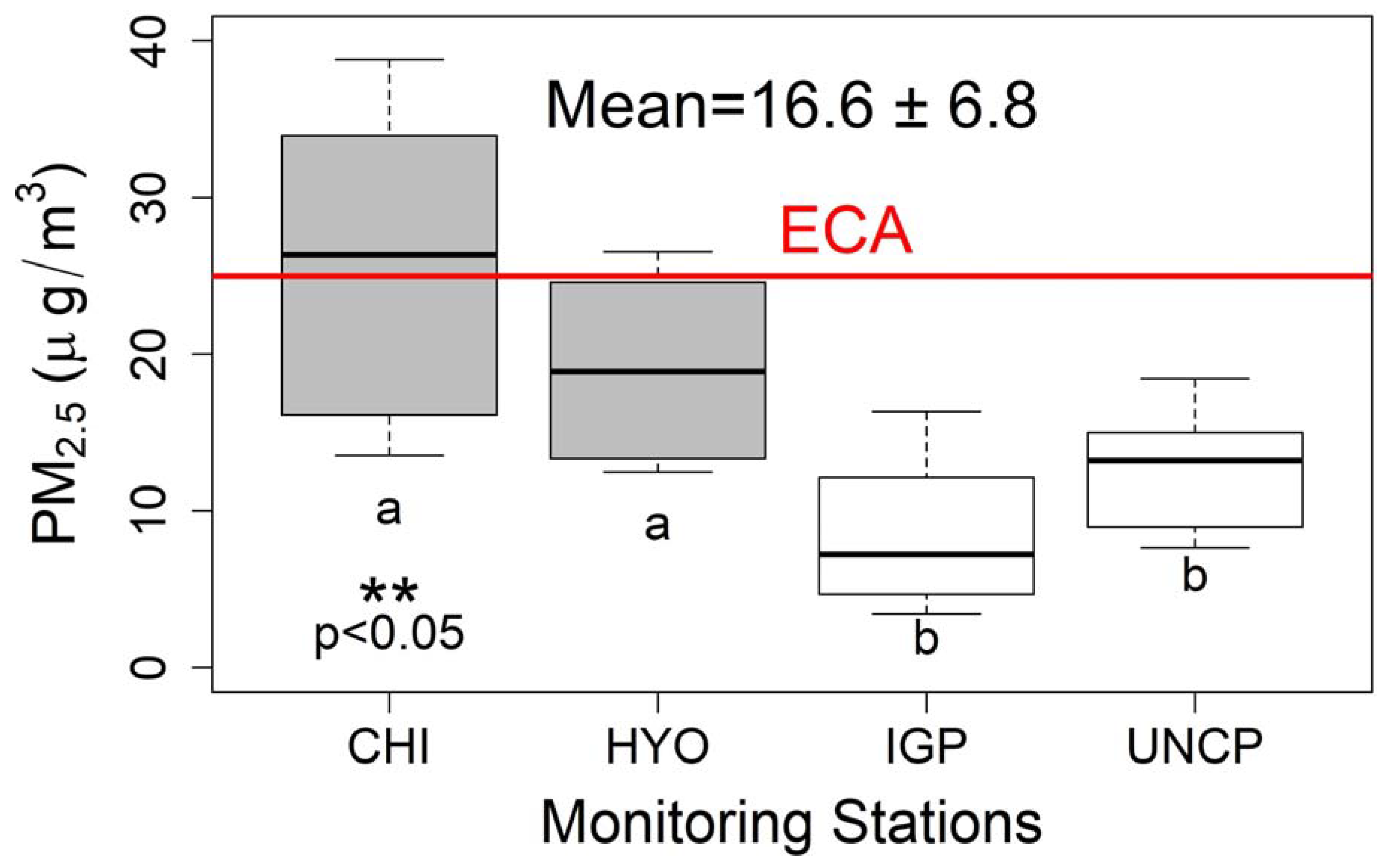
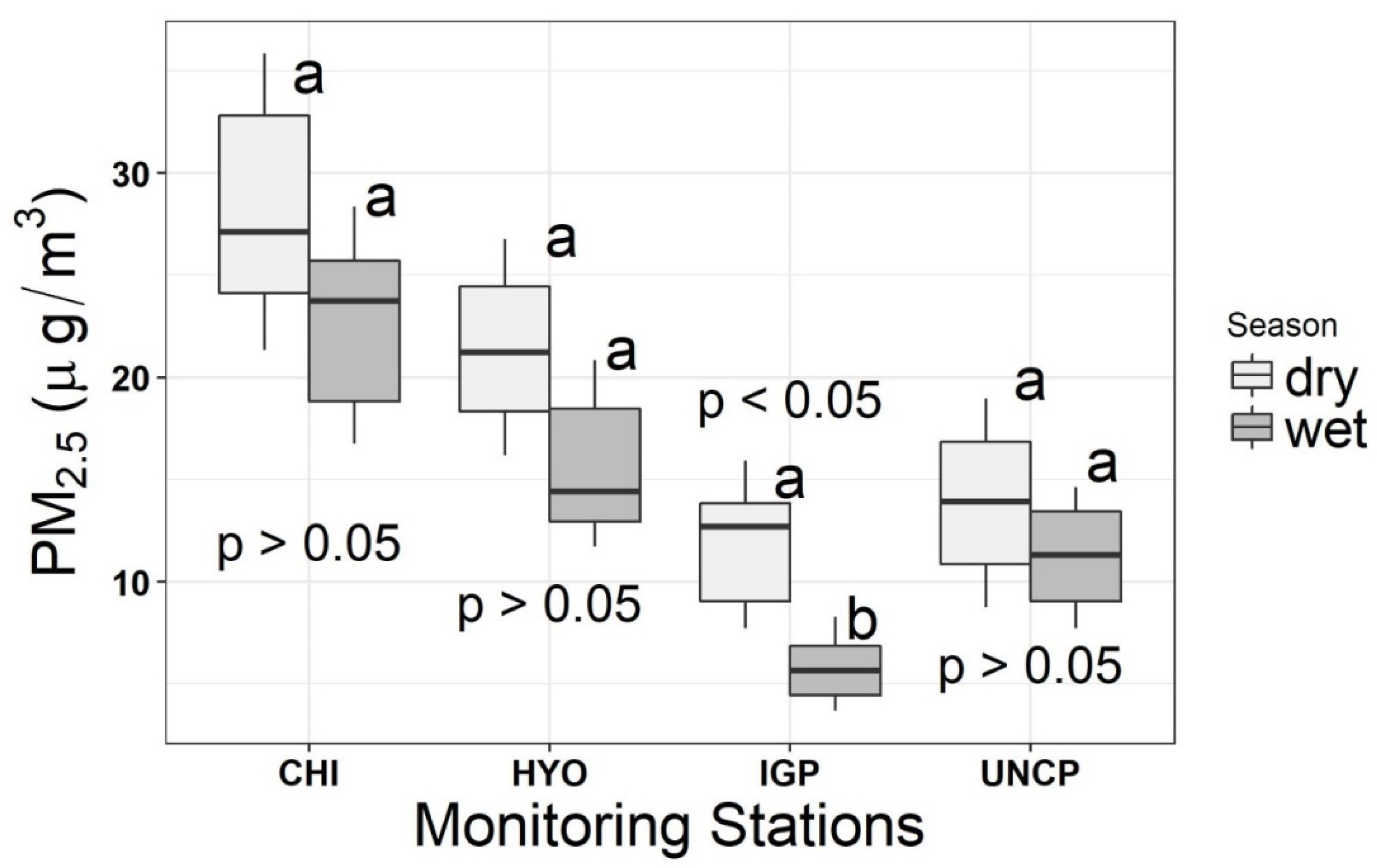
3.1. Trace Element Contents and Water-Soluble Ion
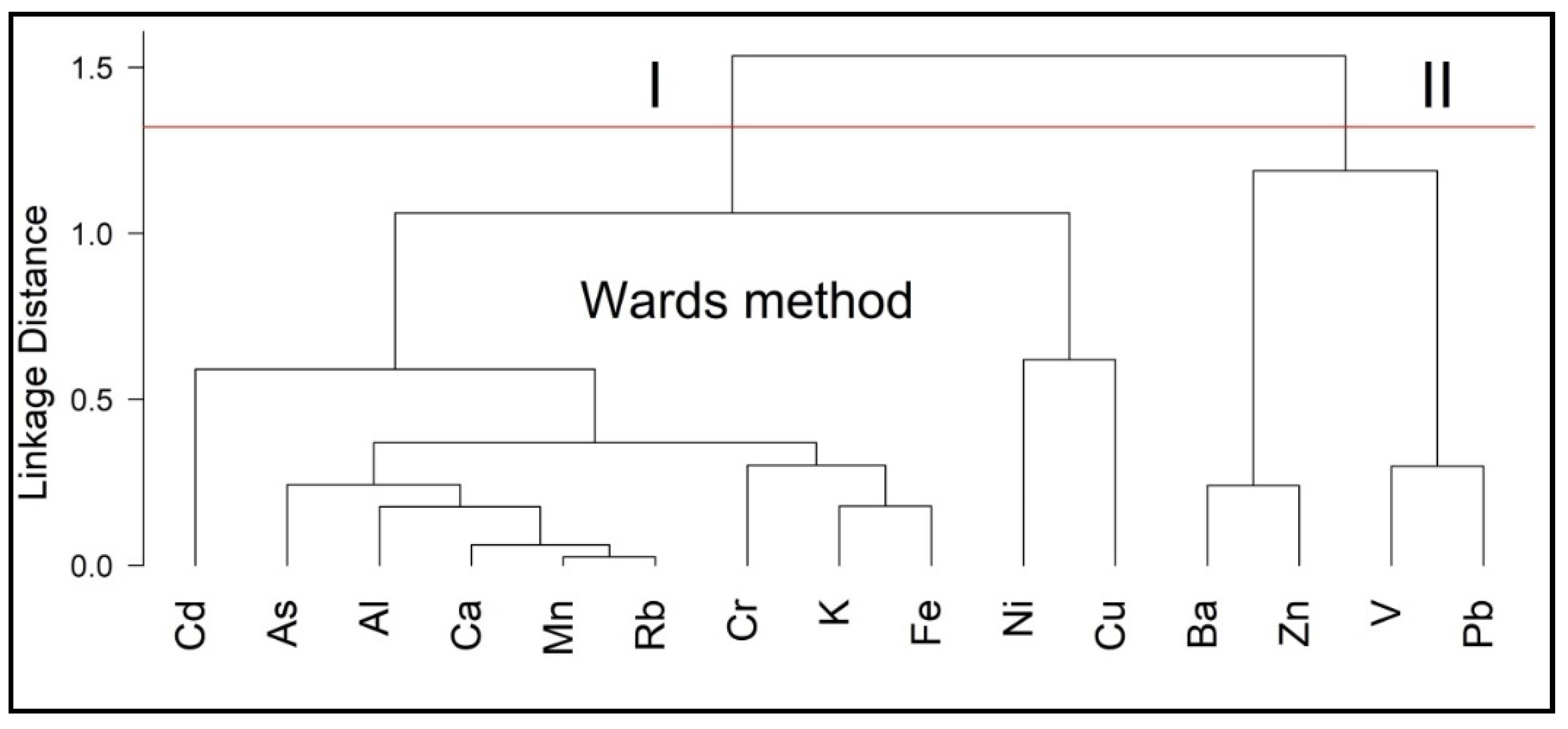
3.2. Hierarchical Cluster Analysis
3.2.1. Trace Elements
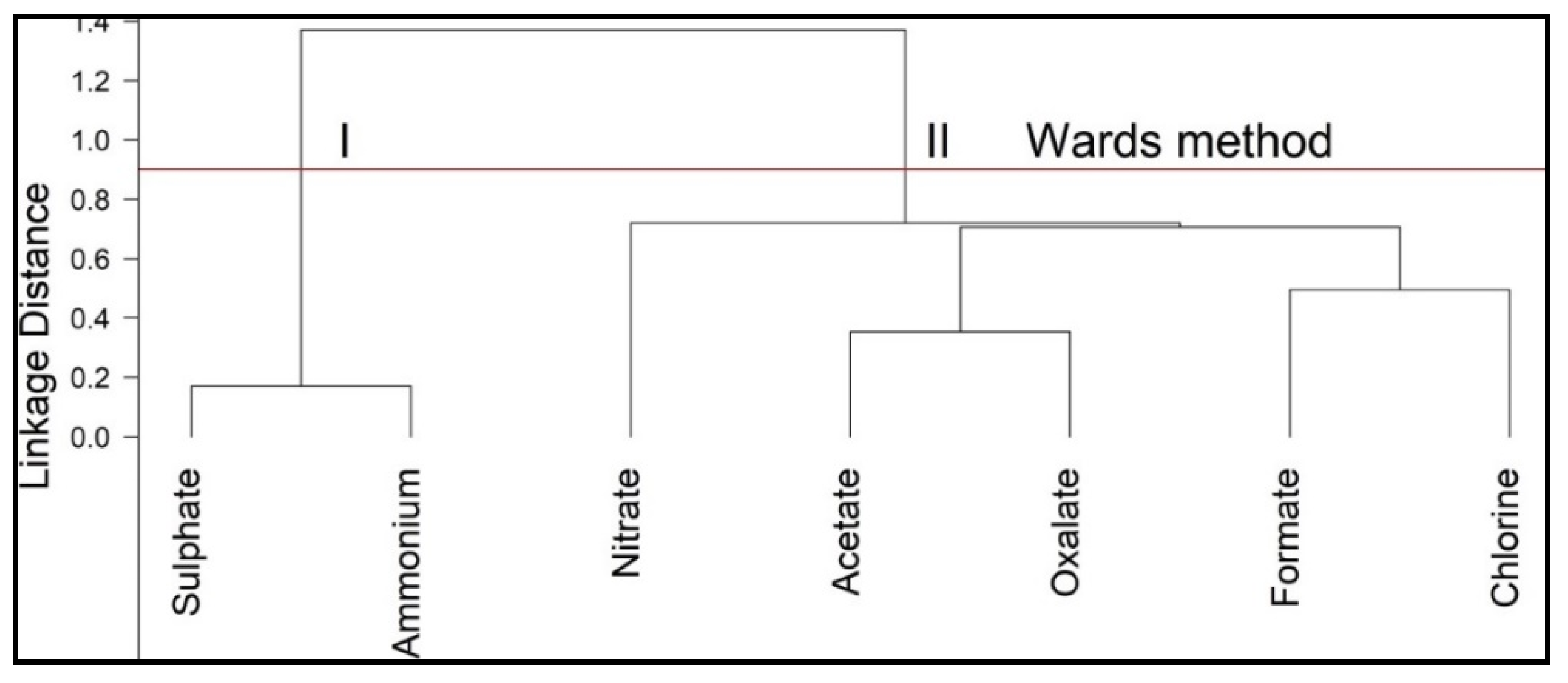
3.2.2. Water-Soluble Ions
3.3. Principal Component Analysis
3.3.1. Trace Elements
3.3.2. Water-Soluble Ions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Janta, R.; Chantara, S. Tree bark as bioindicator of metal accumulation from road traffic and air quality map: A case study of Chiang Mai, Thailand. Atmos. Pollut. Res. 2017, 8, 956–967. [Google Scholar] [CrossRef]
- Morais, S.; Garcia, F.; de Pereira, M.L. Heavy Metals and Human Health. In Environmental Health—Emerging Issues and Practice; IntechOpen: London, UK, 2012; Volume 10, pp. 227–246. ISBN 9789537619343. [Google Scholar]
- Mateus, V.L.; Monteiro, I.L.G.; Rocha, R.C.C.; Saint’Pierre, T.D.; Gioda, A. Study of the chemical composition of particulate matter from the Rio de Janeiro metropolitan region, Brazil, by inductively coupled plasma-mass spectrometry and optical emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2013, 86, 131–136. [Google Scholar] [CrossRef]
- EPA Particulate Matter (PM) Pollution. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed on 29 December 2018).
- Polichetti, G.; Cocco, S.; Spinali, A.; Trimarco, V.; Nunziata, A. Effects of particulate matter (PM10, PM2.5 and PM1) on the cardiovascular system. Toxicology 2009, 261, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chirino, Y.I.; Sánchez-pérez, Y.; Osornio-vargas, Á.R.; Rosas, I.; García-cuellar, C.M. Sampling and composition of airborne particulate matter (PM 10) from two locations of Mexico City. Data Brief 2015, 4, 353–356. [Google Scholar] [CrossRef] [PubMed]
- WHO. Air Quality Guidelines for Europe, 2nd ed.; WHO: Copenhagen, Denmark, 2000; ISBN 9289013583. [Google Scholar]
- Wu, W.; Jin, Y.; Carlsten, C. Clinical reviews in allergy and immunology Inflammatory health effects of indoor and outdoor particulate matter. J. Allergy Clin. Immunol. 2019, 141, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Maher, B.A.; Li, F.; Xu, C. The spatial-temporal characteristics and health impacts of ambient fi ne particulate matter in China. J. Clean. Prod. 2016, 112, 1312–1318. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pulong, C.; Tijian, W.; Mei, D.; Kasoar, M.; Yong, H.; Min, X.; Shu, L.; Bingliang, Z. Characterization of major natural and anthropogenic source pro fi les for size-fractionated PM in Yangtze River Delta. Sci. Total Environ. 2017, 598, 135–145. [Google Scholar] [CrossRef]
- Steinnes, E.; Lierhagen, S. Geographical distribution of trace elements in natural surface soils: Atmospheric in fl uence from natural and anthropogenic sources. Appl. Geochem. 2018, 88, 2–9. [Google Scholar] [CrossRef]
- Bharti, P.K. Heavy Metals in Environment; Lambert Academic Publishing: Saarbrucken, Germany, 2012; ISBN 9783659151330. [Google Scholar]
- Clements, N.; Eav, J.; Xie, M.; Hannigan, M.P.; Miller, S.L.; Navidi, W.; Peel, J.L.; Schauer, J.J.; Shafer, M.M.; Milford, J.B. Concentrations and source insights for trace elements in fine and coarse particulate matter. Atmos. Environ. 2014, 89, 373–381. [Google Scholar] [CrossRef]
- Ventura, L.M.B.; Mateus, V.L.; Collett, A.; Leitão, S.; Wanderley, K.B.; Taira, F.T.; Pierre, T.D.; Saint Gioda, A. Chemical composition of fine particles (PM2.5 ): Water-soluble organic fraction and trace metals. Air Qual. Atmos. Health 2017, 845–852. [Google Scholar] [CrossRef]
- Vianna, N.A.; Gonçalves, D.; Brandão, F.; de Barros, R.P.; Filho, G.M.A.; Meire, R.O.; Torres, J.P.M.; Malm, O.; Júnior, A.D.O.; Andrade, L.R. Assessment of heavy metals in the particulate matter of two Brazilian metropolitan areas by using Tillandsia usneoides as atmospheric biomonitor. Environ. Sci. Pollut. Res. 2011, 18, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa Gowd, S.; Ramakrishna Reddy, M.; Govil, P.K. Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J. Hazard. Mater. 2010, 174, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiao, B.; Zhang, L.; Yang, F.; Jiang, X. Characteristics and sources of trace elements in PM2.5 in two megacities in Sichuan Basin of southwest China. Environ. Pollut. 2018, 242, 1577–1586. [Google Scholar] [CrossRef]
- De La Cruz, A.R.H.; De La Cruz, J.K.H.; Tolentino, D.A.; Gioda, A. Trace element biomonitoring in the Peruvian andes metropolitan region using Flavoparmelia caperata lichen. Chemosphere 2018, 210, 849–858. [Google Scholar] [CrossRef]
- Zhou, H.; Lü, C.; He, J.; Gao, M.; Zhao, B.; Ren, L.; Zhang, L.; Fan, Q.; Liu, T.; He, Z.; et al. Stoichiometry of water-soluble ions in PM2.5: Application in source apportionment for a typical industrial city in semi-arid region, Northwest China. Atmos. Res. 2018, 204, 149–160. [Google Scholar] [CrossRef]
- Rao, P.S.P.; Tiwari, S.; Matwale, J.L.; Pervez, S.; Tunved, P.; Safai, P.D.; Srivastava, A.K.; Bisht, D.S.; Singh, S.; Hopke, P.K. Sources of chemical species in rainwater during monsoon and non-monsoonal periods over two mega cities in India and dominant source region of secondary aerosols. Atmos. Environ. 2016, 146, 90–99. [Google Scholar] [CrossRef]
- Phillips-smith, C.; Jeong, C.; Healy, R.M.; Dabek-zlotorzynska, E.; Celo, V. Sources of particulate matter components in the Athabasca oil sands region: Investigation through a comparison of trace element measurement methodologies. Atmos. Chem. Phys. 2017, 9435–9449. [Google Scholar] [CrossRef]
- Sun, Z.; Duan, F.; He, K.; Du, J.; Zhu, L. Sulfate–nitrate–ammonium as double salts in PM2.5: Direct observations and implications for haze events. Sci. Total Environ. 2019, 647, 204–209. [Google Scholar] [CrossRef]
- Soleimani, M.; Amini, N.; Sadeghian, B.; Wang, D.; Fang, L. Heavy metals and their source identification in particulate matter (PM2.5) in Isfahan City, Iran. J. Environ. Sci. (China) 2018, 72. [Google Scholar] [CrossRef]
- Silva, J.; Rojas, J.; Norabuena, M.; Molina, C.; Toro, R.A.; Leiva-Guzmán, M.A. Particulate matter levels in a South American megacity: The metropolitan area of Lima-Callao, Peru. Environ. Monit. Assess. 2017, 189. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Salas, L.; Alvarez Tolentino, D.; Bendezú, Y.; Pomalaya, J. Caracterización química del material particulado atmosférico del centro urbano de Huancayo, Perú. Rev. Soc. Quím Perú 2017, 83, 187–199. [Google Scholar]
- Instituto Nacional de Estadística e Informática. Peru: Principales Indicadores Departamentales 2009–2016; Instituto Nacional de Estadística e Informática: Lima, Peru, 2017.
- Serrano, F. Environmental Contamination in the Homes of La Oroya and Concepcion and Its Effects in the Health of Community Residents. 2002. Available online: https://lib.ohchr.org/HRBodies/UPR/Documents/Session2/PE/EJ-AIDA_PER_UPR_S2_2008anx_StudyofcontaminationinLaOroya.pdf (accessed on 4 December 2018).
- Milan, A.; Ho, R. Livelihood and migration patterns at different altitudes in the Central Highlands of Peru. Clim. Dev. 2014, 6, 69–76. [Google Scholar] [CrossRef]
- IGP. Atlas Climático de Precipitacion y Temperatura del aire en la Cuenca del Rio Mantaro, 1st ed.; Del Ambiente, C.-C.N., Ed.; Depósito legal en la Biblioteca Nacional del Perú: San Borja, Peru, 2005; ISBN 9972824136.
- Pfeiffer, R.L. Sampling For PM10 and PM2.5 Particulates. Publ. USDA-ARS 2005, 1, 20. [Google Scholar]
- Villalobos, A.M.; Barraza, F.; Jorquera, H.; Schauer, J.J. Chemical speciation and source apportionment of fine particulate matter in Santiago, Chile, 2013. Sci. Total Environ. 2015, 512–513, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.F. Analysis of Variance: The Fundamental Concepts. J. Man. Manip. Ther. 2007, 17, 27–38. [Google Scholar] [CrossRef]
- Hilton, A.C.; Armstrong, R. Stat Note 6:post-hoc ANOVA tests. Microbiologist 2006, 6, 4. [Google Scholar]
- Lynne, W.J.; Abdi, H. Fisher’s Least Significant Difference (LSD) Test. Encycl. Res. Des. 2010, 1–6. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning with Applications in R; Olkin, G.C.S.F.I., Ed.; Springer: New York, NY, USA, 2015; Volume 6, ISBN 9781461471370. [Google Scholar]
- Jolliffe, I.T.; Cadima, J.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2016. [Google Scholar] [CrossRef]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Gülağız, F.K.; Şahin, S. Comparison of Hierarchical and Non-Hierarchical Clustering Algorithms. Int. J. Comput. Eng. Inf. Technol. 2017, 9, 6–14. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference and Prediction, 2nd ed.; Springer Series in Statistics; Springer: New York, NY, USA, 2009; Volume 27, ISBN 978-0-387-84857-0. [Google Scholar]
- Kumar, N.; Bansal, A.; Sarma, G.S.; Rawal, R.K. Chemometrics tools used in analytical chemistry: An overview. Talanta 2014, 123, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Baaijens, J.A.; Draisma, J. Euclidean distance degrees of real algebraic groups. Linear Algebra Its Appl. 2015, 467, 174–187. [Google Scholar] [CrossRef]
- Melnykov, I.; Melnykov, V. On K-means algorithm with the use of Mahalanobis distances. Stat. Probab. Lett. 2014, 84, 88–95. [Google Scholar] [CrossRef]
- R Team Core. A language and Environment for Statistical Computing. In R Foundation for Statistical Computing, Vienna Austria; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses. R Packag. Version 2016, 1. Available online: https://rpkgs.datanovia.com/factoextra/index.html (accessed on 4 December 2018).
- Wickham, M.H.; Chang, W. An Implementation of the Grammar of Graphics; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Chavent, M.; Simonet, V.K.; Liquet, B.; Kuentz-simonet, V. ClustOfVar: An R Package for the Clustering of Variables. J. Stat. 2012, 50, 1–16. [Google Scholar]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K.; Studer, M.; Roudier, P. Finding Groups in Data: Cluster Analysis Extended Rousseeuw. 2015. Available online: https://stat.ethz.ch/R-manual/R-devel/library/cluster/html/00Index.html (accessed on 4 December 2018).
- Revelle, W. Procedures for Psychological, Psychometric, and Personality Research 2018, 443. Available online: https://cran.r-project.org/web/packages/psych/index.html (accessed on 4 December 2018).
- Xu, D.; Ge, B.; Wang, Z.; Sun, Y.; Chen, Y.; Ji, D.; Yang, T.; Ma, Z.; Cheng, N.; Hao, J.; et al. Below-cloud wet scavenging of soluble inorganic ions by rain in Beijing during the summer of 2014. Environ. Pollut. 2017, 230, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, F.G.; Gómez, D.R.; Dawidowski, L.; Perelman, P.; Faggi, A. Metals associated with airborne particulate matter in road dust and tree bark collected in a megacity (Buenos Aires, Argentina). Ecol. Indic. 2011, 11, 240–247. [Google Scholar] [CrossRef]
- Querol, X.; Alastuey, A.; Moreno, T.; Viana, M.M.; Castillo, S.; Pey, J.; Rodríguez, S.; Artiñano, B.; Salvador, P.; Sánchez, M.; et al. Spatial and temporal variations in airborne particulate matter (PM10 and PM2.5) across Spain 1999–2005. Atmos. Environ. 2008, 42, 3964–3979. [Google Scholar] [CrossRef]
- Godoy, M.L.D.P.; Godoy, J.M.; Roldão, L.A.; Soluri, D.S.; Donagemma, R.A. Coarse and fine aerosol source apportionment in Rio de Janeiro, Brazil. Atmos. Environ. 2009, 43, 2366–2374. [Google Scholar] [CrossRef]
- Guéguen, F.; Stille, P.; Dietze, V.; Gieré, R. Chemical and isotopic properties and origin of coarse airborne particles collected by passive samplers in industrial, urban, and rural environments. Atmos. Environ. 2012, 62, 631–645. [Google Scholar] [CrossRef]
- Enamorado-Báez, S.M.; Gómez-Guzmán, J.M.; Chamizo, E.; Abril, J.M. Levels of 25 trace elements in high-volume air filter samples from Seville (2001–2002): Sources, enrichment factors and temporal variations. Atmos. Res. 2015, 155, 118–129. [Google Scholar] [CrossRef]
- Rizzio, E.; Bergamaschi, L.; Valcuvia, M.; Profumo, A.; Gallorini, M. Trace elements determination in lichens and in the airborne particulate matter for the evaluation of the atmospheric pollution in a region of northern Italy. Environ. Int. 2001, 26, 543–549. [Google Scholar] [CrossRef]
- Giampaoli, P.; Wannaz, E.D.; Tavares, A.R.; Domingos, M. Suitability of Tillandsia usneoides and Aechmea fasciata for biomonitoring toxic elements under tropical seasonal climate. Chemosphere 2016, 149, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wu, Y.; Jiang, J.; Yang, L.; Cheng, Y.; Hao, J. Chemical characteristics of size-resolved PM2.5 at a roadside environment in Beijing, China. Environ. Pollut. 2012, 161, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Wang, Y.; Wen, T.; Yang, Y.; Zhao, Y.; Wang, Y. Chemical composition and size distribution of airborne particulate matters in Beijing during the 2008 Olympics. Atmos. Environ. 2012, 50, 278–286. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Zhang, X.; Huang, K.; Xu, C.; Tang, A.; Chen, J.; An, Z. The ion chemistry, seasonal cycle, and sources of PM2.5and TSP aerosol in Shanghai. Atmos. Environ. 2006, 40, 2935–2952. [Google Scholar] [CrossRef]
- Dai, Q.; Bi, X.; Song, W.; Li, T.; Liu, B.; Ding, J.; Xu, J.; Song, C.; Yang, N.; Schulze, B.C.; et al. Residential coal combustion as a source of primary sulfate in Xi’an, China. Atmos. Environ. 2019, 196, 66–76. [Google Scholar] [CrossRef]
- Guo, Z.; Shi, L.; Chen, S.; Jiang, W.; Wei, Y.; Rui, M.; Zeng, G. Sulfur isotopic fractionation and source appointment of PM2.5in Nanjing region around the second session of the Youth Olympic Games. Atmos. Res. 2016, 174–175, 9–17. [Google Scholar] [CrossRef]
- Ho, K.F.; Lee, S.C.; Chan, C.K.; Yu, J.C.; Chow, J.C.; Yao, X.H. Characterization of chemical species in PM2.5 andPM10 aerosols in Hong Kong. Atmos. Environ. 2003, 37, 31–39. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, I.; Lim, C.; Farquhar, J.; Lee, S.M.; Kim, H. The origin and migration of the dissolved sulfate from precipitation in Seoul, Korea. Environ. Pollut. 2018, 237, 878–886. [Google Scholar] [CrossRef]
- Elser, M.; El-Haddad, I.; Maasikmets, M.; Bozzetti, C.; Wolf, R.; Ciarelli, G. High contributions of vehicular emissions to ammonia in three European cities derived from mobile measurements. Atmos. Environ. 2018, 175, 210–220. [Google Scholar] [CrossRef]
- Roberts, T.L. Cadmium and Phosphorous Fertilizers: The Issues and the Science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef]
- Hazotte, C.; Leclerc, N.; Meux, E.; Lapicque, F. Direct recovery of cadmium and nickel from Ni-Cd spent batteries by electroassisted leaching and electrodeposition in a single-cell process. Hydrometallurgy 2016, 162, 94–103. [Google Scholar] [CrossRef]
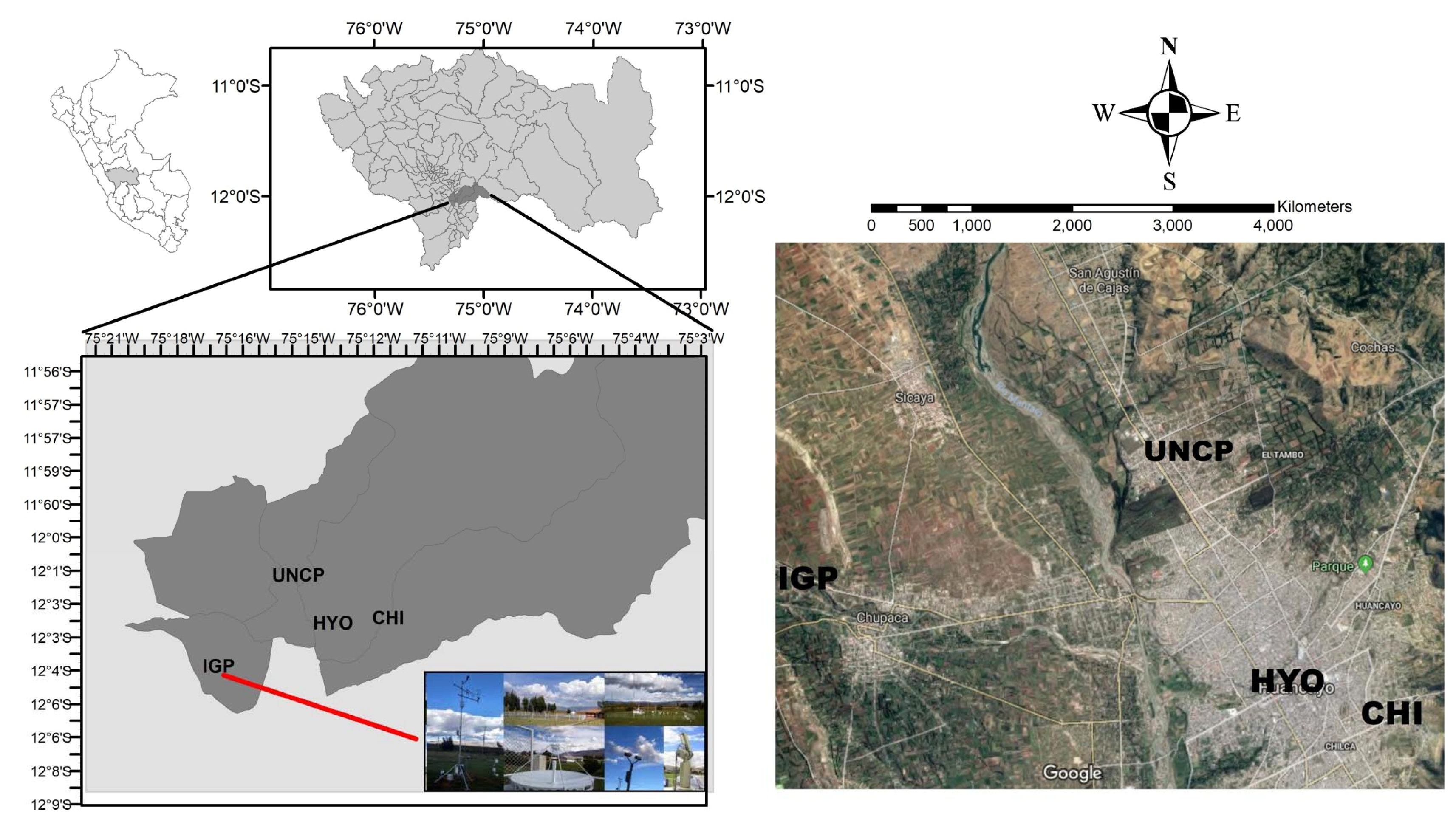
| Monitoring Station | Latitude Longitude | Population of the District A | Description |
|---|---|---|---|
| Huancayo (HYO) | 12° 4′ 12.03″ S 75° 12′ 43.55″ W | 117,559 | Downtown, is a residential and commercial area with heavy traffic from automobiles, trucks, bus, railway, and motorbike. |
| Chilca (CHI) | 12° 4′ 21.51″ S 75° 11′ 32.46″ W | 86,496 | Located aside downtown, is a residential, commercial and nestled small industries. The traffic is intense all days |
| El Tambo (UNCP) | 12° 1′ 57.28″ S 75° 14′ 8.38″ W | 160.685 | Located 5.2 km far from downtown and has a medium traffic flow. The equipment was installed at the roof of the administrative building of the National University of the Center of Peru |
| Observatory Huancayo (IGP) | 12° 2′ 59.28″ S 75° 20′ 24.58″ W | 5929 | Located 13.5 km far from downtown, is a rural area, dominated by agriculture fields where the Geophysical Institute of Peru has its installations. |
| ICP-MS Operation | Value |
|---|---|
| RF power (W) | 1150 |
| Frequency (MHz) | 27.2 |
| Plasma gas flow rate (L min−1) | 11.5 |
| Auxiliary gas flow rate (L min−1) | 0.55 |
| Nebulizer gas flow rate (L min−1) | 0.98 |
| Sample uptake rate (mL min−1) | 0.6 |
| Measurement mode | Dual (PC/analog) |
| Acquisition time (s) | 1 |
| Dwell time (ms) | 200 |
| Replicates | 6 |
| Elements | LOD (µg g−1) | LOQ (µg g−1) | CRM—Urban Particulate Matter | ||
|---|---|---|---|---|---|
| Certified Value (µg g−1) | Found Value (µg g−1) | % Extracted | |||
| Al | 0.20 | 0.65 | 34,300 ± 1300 | 28,000 ± 2100 | 81.5 |
| As | 0.006 | 0.020 | 115.5 ± 3.9 | 108 ± 4.2 | 108 |
| Ba | 0.010 | 0.032 | - | - | - |
| Ca | 0.83 | 2.74 | 58,400 ± 1900 | 58,800 | 101 |
| Cd | 0.001 | 0.002 | 73.7 ± 2.3 | 79.3 ± 3.1 | 108 |
| Cr | 0.023 | 0.077 | 402 ± 13 | 320 ± 20 | 80 |
| Cu | 0.017 | 0.057 | 610 ± 70 | 657 ± 31 | 108 |
| Fe | 0.27 | 0.87 | 39,200 ± 2100 | 42,000 ± 2200 | 107 |
| K | 0.33 | 1.08 | 10,560 ± 490 | 8790 ± 340 | 83.2 |
| Mn | 0.014 | 0.046 | 790 ± 44 | 823 ± 34 | 104 |
| Ni | 0.008 | 0.028 | 81.1 ± 6.8 | 86.2 ± 4.2 | 106 |
| Pb | 0.004 | 0.013 | 6550 ± 33 | 7063 ± 21 | 108 |
| Rb | 0.002 | 0.006 | 51.0 ± 1.5 | 42.2 ± 2.12 | 83 |
| V | 0.002 | 0.007 | 127 ± 11 | 136 ± 8 | 107 |
| Zn | 0.041 | 0.136 | 4800 ± 270 | 4710 ± 167 | 98 |
| Element | IGP | UNCP | HYO | CHI | ANOVA |
| N = 16 | N = 20 | N = 16 | N = 22 | p-Value A | |
| Al | 0.651 ± 0.563 b | 0.874 ± 0.785 b | 1.440 ± 1.222 b | 2.719 ± 1.868 a | *** |
| As | 0.002 ± 0.002 c | 0.006 ± 0.003 c | 0.010 ± 0.005 b | 0.014 ± 0.007 a | *** |
| Ba | 0.008 ± 0.006 b | 0.027 ± 0.033 c | 0.100 ± 0.188 a | 0.087 ± 0.082 a | * |
| Ca | 0.807 ± 0.968 b | 1.326 ± 0.781 b | 4.160 ± 2.684 a | 6.413 ± 4.506 a | *** |
| Cd | 0.001 ± 0.001 b | 0.002 ± 0.002 b | 0.003 ± 0.003 b | 0.008 ± 0.007 a | *** |
| Cr | 0.045 ± 0.030 a | 0.088 ± 0.034 c | 0.132 ± 0.055 b | 0.196 ± 0.074 d | *** |
| Cu | 0.012 ± 0.012 b | 0.020 ± 0.019 b | 0.077 ± 0.126 a | 0.075 ± 0.054 a | ** |
| Fe | 0.932 ± 0.805 c | 1.348 ± 0.752 c | 2.817 ± 1.938 b | 5.064 ± 3.139 a | *** |
| K | 1.826 ± 1.987 c | 3.166 ± 2.059 b | 2.889 ± 1.112 b | 8.104 ± 3.499 a | *** |
| Mn | 0.016 ± 0.013 c | 0.037 ± 0.024 c | 0.094 ± 0.073 a | 0.182 ± 0.128 b | *** |
| Ni | 0.004 ± 0.003 | 0.004 ± 0.005 | 0.008 ± 0.005 | 0.022 ± 0.050 | 0.19 |
| Pb | 0.005 ± 0.005 b | 0.024 ± 0.026 b | 0.060 ± 0.078 b | 0.153 ± 0.144 a | *** |
| Rb | 0.001 ± 0.001 c | 0.002 ± 0.002 c | 0.004 ± 0.004 b | 0.009 ± 0.006 a | *** |
| V | 0.004 ± 0.003 | 0.001 ± 0.001 | 0.005 ± 0.008 | 0.015 ± 0.042 | 0.32 |
| Zn | 0.145 ± 0.277 b | 0.146 ± 0.093 b | 0.719 ± 1.221 a | 0.461 ± 0.275 a | * |
| Ions | IGP | UNCP | HYO | CHI | ANOVA |
| N = 18 | N = 18 | N = 20 | N = 21 | p-Value A | |
| Ac− | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.001 ± 0.001 | 0.002 ± 0.001 | 0.08 |
| Fo− | 0.011 ± 0.008 b | 0.004 ± 0.004 c | 0.008 ± 0.007 c | 0.023 ± 0.011 a | *** |
| Cl− | 0.005 ± 0.005 c | 0.006 ± 0.005 c | 0.012 ± 0.009 b | 0.018 ± 0.012 a | *** |
| 0.034 ± 0.050 | 0.023 ± 0.014 | 0.059 ± 0.084 | 0.055 ± 0.027 | 0.16 | |
| 0.006 ± 0.006 | 0.005 ± 0.006 | 0.007 ± 0.007 | 0.007 ± 0.005 | 0.67 | |
| 0.240 ± 0.350 | 0.219 ± 0.359 | 0.303 ± 0.449 | 0.191 ± 0.288 | 0.83 | |
| Ox− | 0.010 ± 0.010 | 0.008 ± 0.005 | 0.009 ± 0.005 | 0.010 ± 0.005 | 0.78 |
| Element | Factors | ||
|---|---|---|---|
| Fa1 | Fa2 | Comm | |
| PM2.5 | |||
| Al | 0.92 | 0.11 | 0.85 |
| As | 0.90 | 0.22 | 0.87 |
| Ba | 0.36 | 0.62 | 0.71 |
| Ca | 0.93 | 0.18 | 0.91 |
| Cd | 0.69 | 0.11 | 0.57 |
| Cr | 0.68 | 0.59 | 0.63 |
| Cu | 0.45 | 0.28 | 0.72 |
| Fe | 0.90 | 0.13 | 0.88 |
| K | 0.84 | 0.20 | 0.76 |
| Mn | 0.95 | 0.21 | 0.95 |
| Ni | 0.29 | 0.09 | 0.51 |
| Pb | 0.28 | 0.85 | 0.80 |
| Rb | 0.93 | 0.24 | 0.93 |
| V | −0.10 | 0.82 | 0.82 |
| Zn | 0.17 | 0.65 | 0.67 |
| Eigenvalue | 7.30 | 2.83 | |
| % of total variance | 0.49 | 0.19 | |
| % of cumulative variance | 0.49 | 0.68 | |
| Element | Factors | ||
|---|---|---|---|
| Fa1 | Fa2 | Comm | |
| PM2.5 | |||
| Ac− | 0.66 | 0.39 | 0.62 |
| Fo− | 0.64 | 0.00 | 0.61 |
| Cl− | 0.80 | 0.11 | 0.71 |
| 0.84 | 0.30 | 0.79 | |
| 0.23 | 0.87 | 0.80 | |
| Ox− | 0.69 | 0.43 | 0.66 |
| Eigenvalue | 2.73 | 2.08 | |
| % of total variance | 0.39 | 0.30 | |
| % of cumulative variance | 0.39 | 0.69 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huamán De La Cruz, A.; Bendezu Roca, Y.; Suarez-Salas, L.; Pomalaya, J.; Alvarez Tolentino, D.; Gioda, A. Chemical Characterization of PM2.5 at Rural and Urban Sites around the Metropolitan Area of Huancayo (Central Andes of Peru). Atmosphere 2019, 10, 21. https://doi.org/10.3390/atmos10010021
Huamán De La Cruz A, Bendezu Roca Y, Suarez-Salas L, Pomalaya J, Alvarez Tolentino D, Gioda A. Chemical Characterization of PM2.5 at Rural and Urban Sites around the Metropolitan Area of Huancayo (Central Andes of Peru). Atmosphere. 2019; 10(1):21. https://doi.org/10.3390/atmos10010021
Chicago/Turabian StyleHuamán De La Cruz, Alex, Yessica Bendezu Roca, Luis Suarez-Salas, José Pomalaya, Daniel Alvarez Tolentino, and Adriana Gioda. 2019. "Chemical Characterization of PM2.5 at Rural and Urban Sites around the Metropolitan Area of Huancayo (Central Andes of Peru)" Atmosphere 10, no. 1: 21. https://doi.org/10.3390/atmos10010021
APA StyleHuamán De La Cruz, A., Bendezu Roca, Y., Suarez-Salas, L., Pomalaya, J., Alvarez Tolentino, D., & Gioda, A. (2019). Chemical Characterization of PM2.5 at Rural and Urban Sites around the Metropolitan Area of Huancayo (Central Andes of Peru). Atmosphere, 10(1), 21. https://doi.org/10.3390/atmos10010021






