VdPLP, A Patatin-Like Phospholipase in Verticillium dahliae, Is Involved in Cell Wall Integrity and Required for Pathogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Plant Material and Culture Conditions
2.2. Plasmid Construction and Fungal Transformation
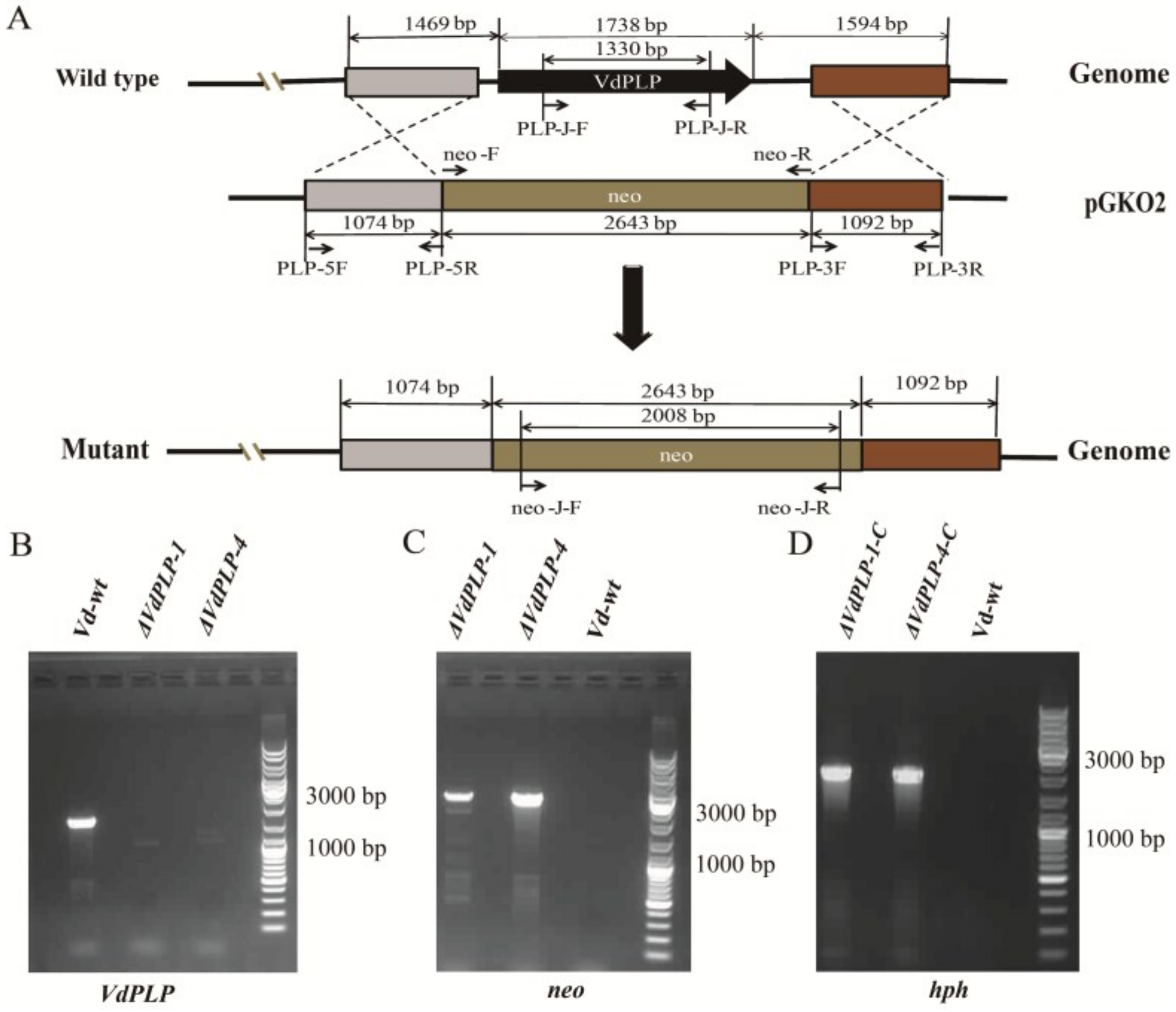
2.3. Confirmation of VdPLP Disruption, Complementation of ΔVdPLP Strains and Screening for GFP-Tagged Strains
2.4. Growth, Conidia Production and Microsclerotia Formation Assays
2.5. Phenotype Assays Using Cell Wall-Disrupting Agents Calcoflour White and Congo Red
2.6. Quantitative Real-Time PCR Reaction
2.7. Penetration Ability Assay
2.8. Pathogenicity Assays, Microscopic Observation of Initial Infection and Fungal Biomass Quantification
2.9. Statistical Analysis
3. Results
3.1. Deletion and Complementation of VdPLP in V. dahliae
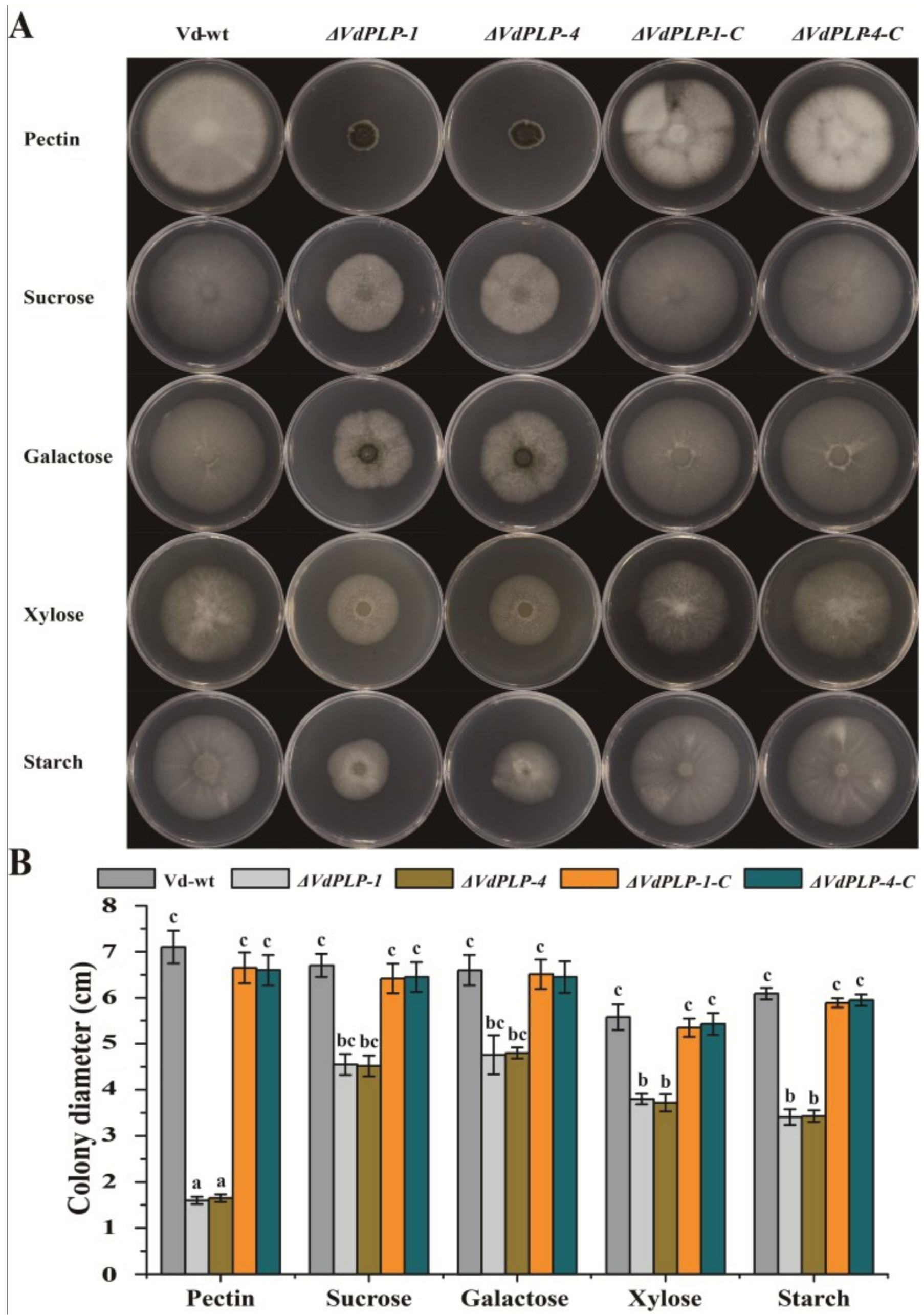
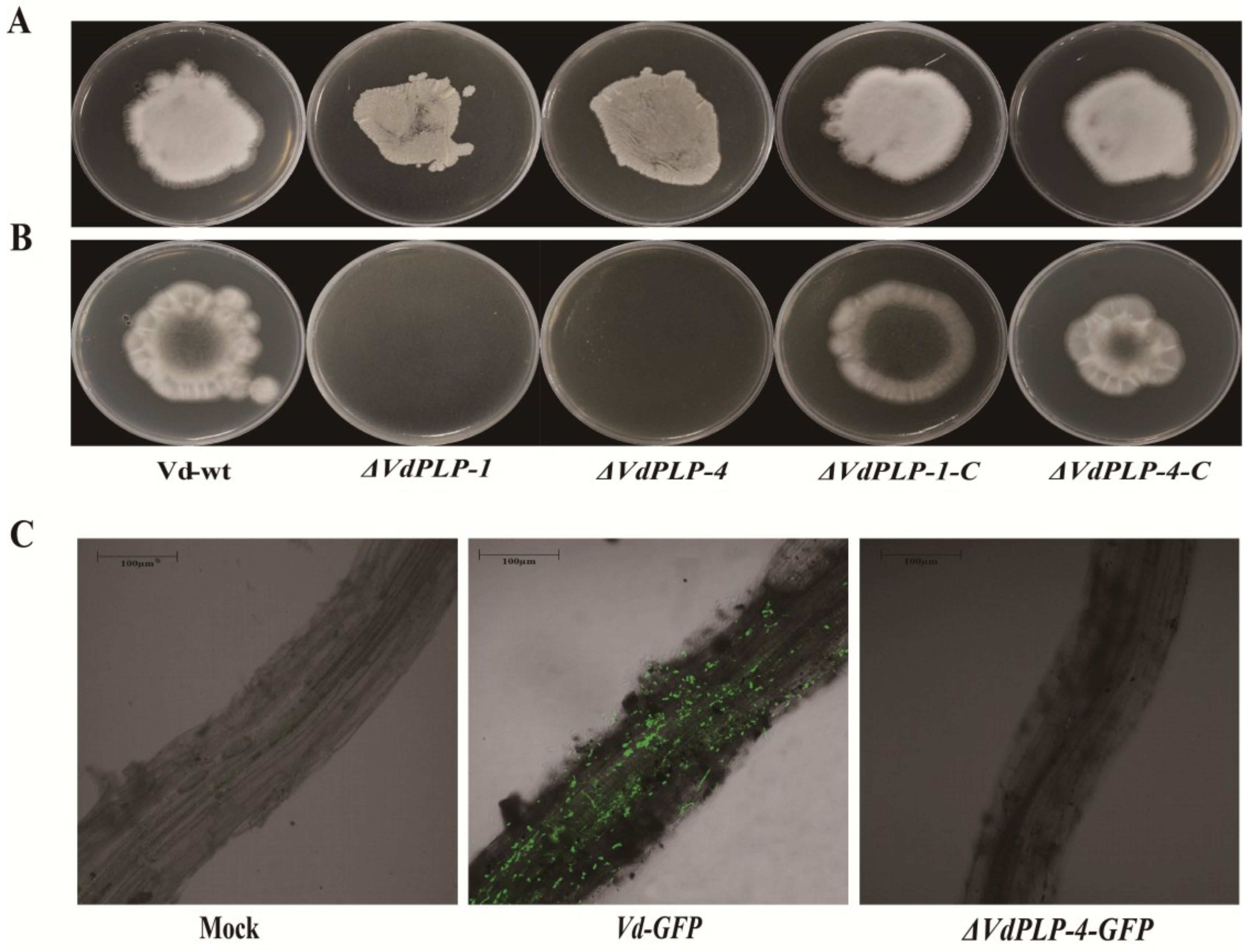
3.2. Radial Growth of Mycelia was Significantly Reduced in ΔVdPLP Mutants
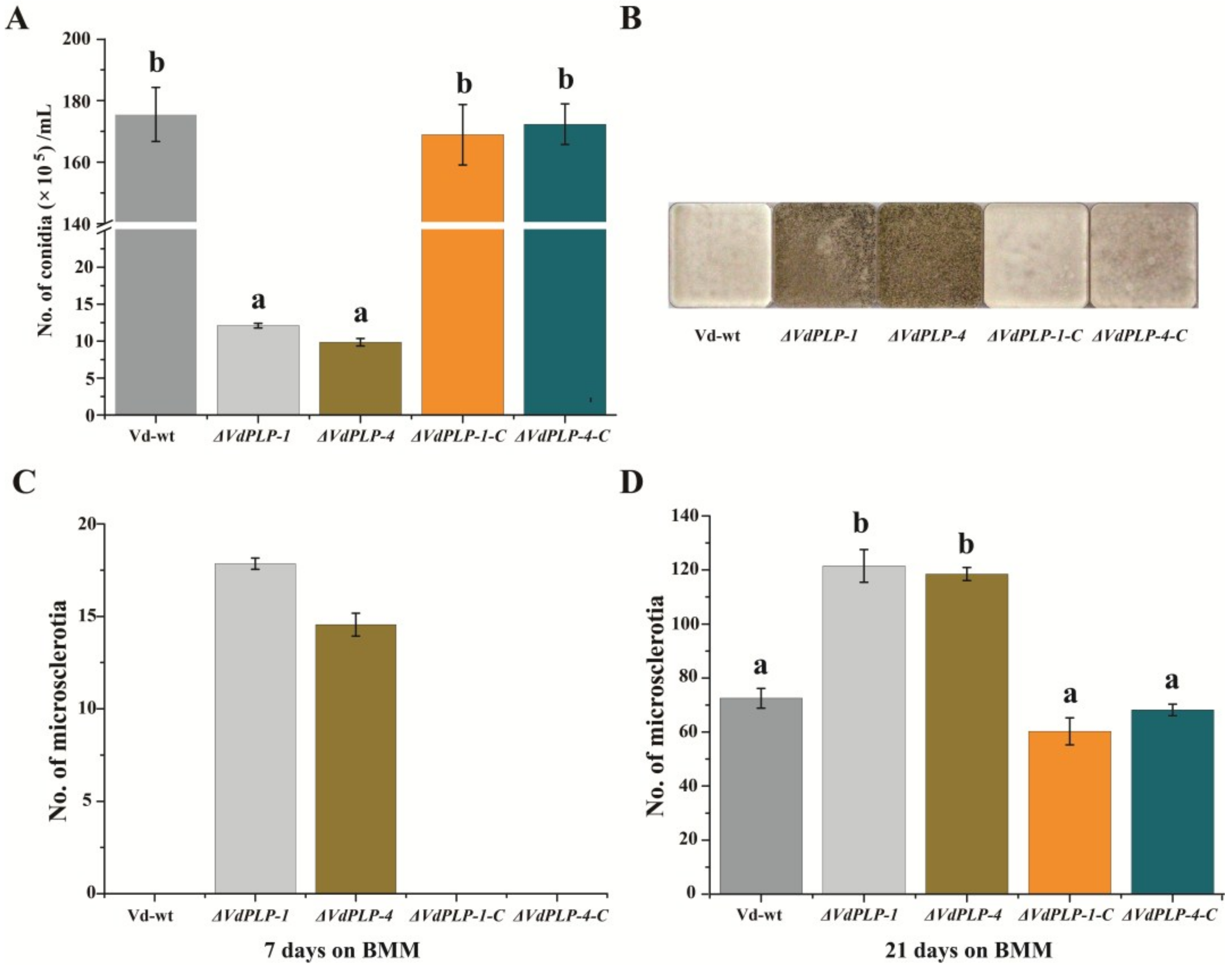
3.3. Conidiation by ΔVdPLP Mutants Decreased Substantially and Microsclerotia Formation Increased
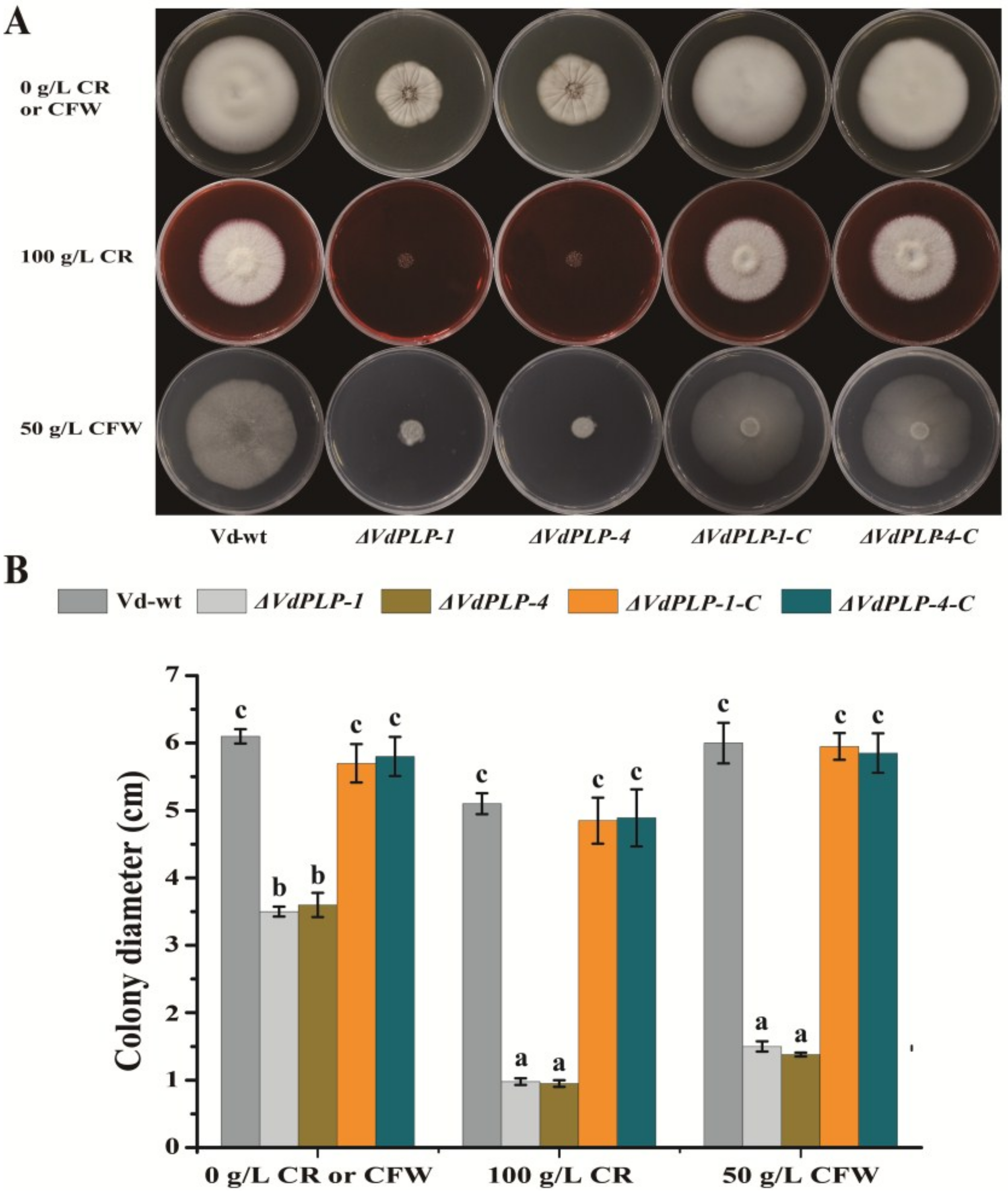
ΔVdPLP Mutants Are Hypersensitive to Cell Wall-Perturbing Agents
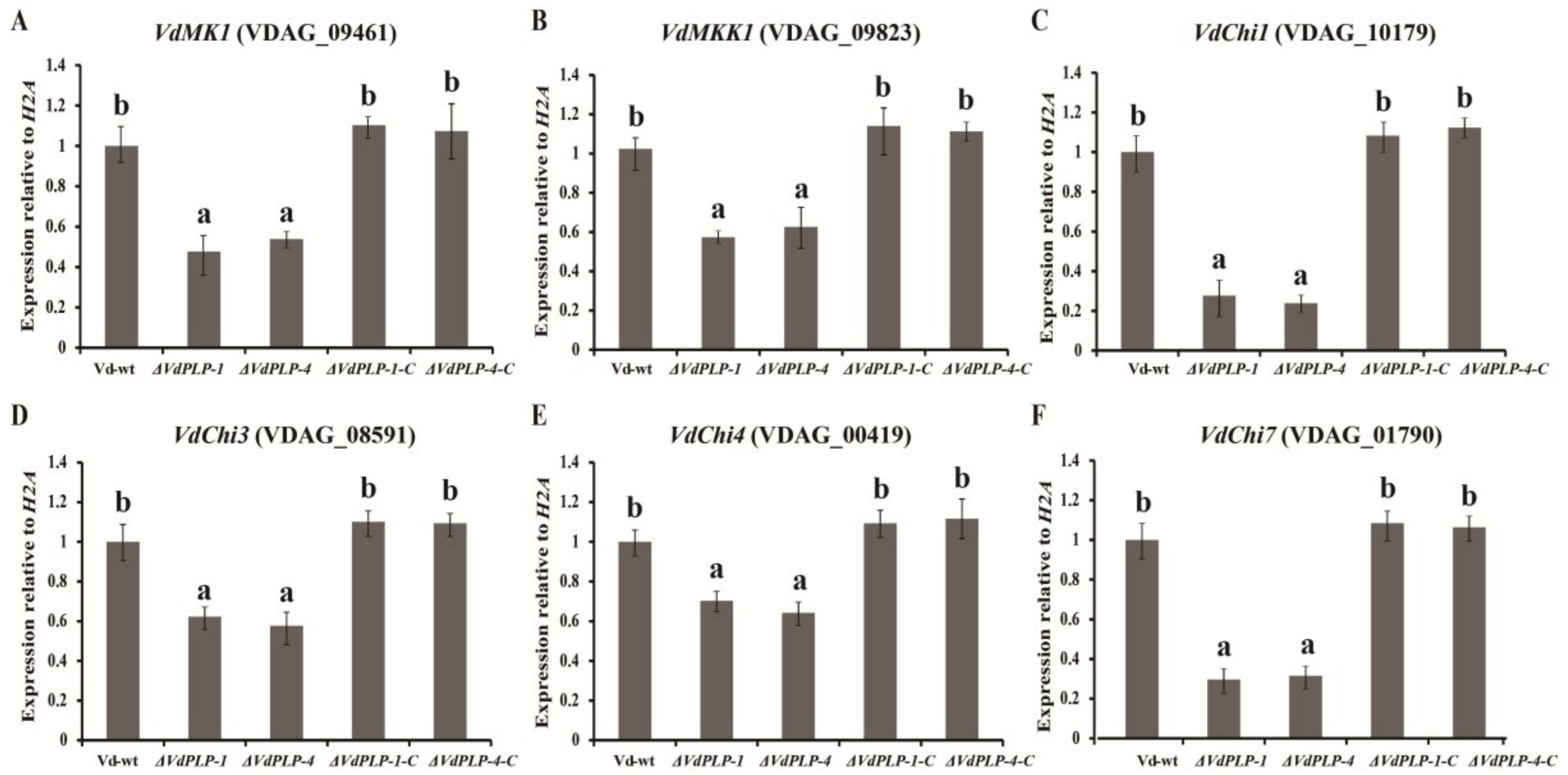
3.5. Genes for the Calcofluor White Pathway and for Chitin Synthesis in the ΔVdPLP Mutant Were Downregulated
3.6. Penetration and Fungal Colonization of ΔVdPLP Were Impaired
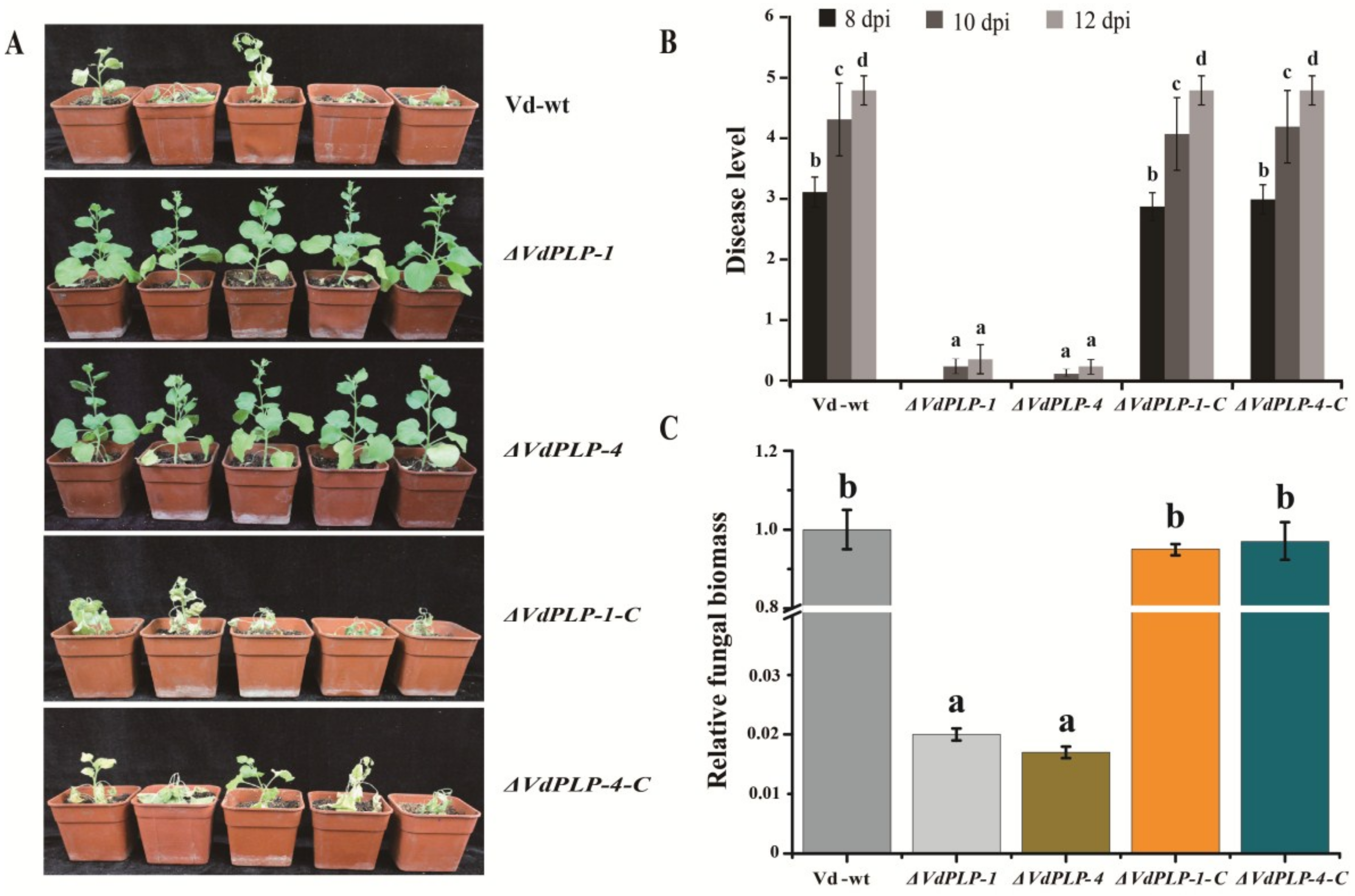
3.7. VdPLP was Required for Fungal Pathogenicity
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.F.; He, X.H.; Mo, J.C.; Sun, Q.; Yang, J.P.; Liu, J.G. Molecular research and genetic engineering of resistance to Verticillium wilt in cotton: A review. Afr. J. Biotechnol. 2009, 8, 7363–7372. [Google Scholar]
- Klosterman, S.J.; Subbarao, K.V.; Kang, S.; Veronese, P.; Gold, S.E.; Thomma, B.; Chen, Z.; Henrissat, B.P.; Chen, Z.H.; Henrissat, B.; et al. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 2011, 7, e1002137. [Google Scholar] [CrossRef] [PubMed]
- Klimes, A.; Dobinson, K.F. A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genet. Biol. 2006, 43, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Zhou, T.T.; Guo, H.S. Hyphopodium-specific VdNoxB/VdPls1-dependent ROS-Ca2+ signaling is required for plant infection by Verticillium dahliae. PLoS Pathog. 2016, 12, e1005793. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Xie, C.J.; Dong, J.Y.; Yang, X.Y.; Sui, A.P. Interactions between Verticillium dahliae and its host: Vegetative growth, pathogenicity, plant immunity. Appl. Microbiol. Biotechnol. 2014, 98, 6921–6932. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.L.; Su, X.F.; Guo, H.M.; Qi, J.C.; Cheng, H.M. VdThit, a thiamine transport protein, is required for pathogenicity of the vascular pathogen Verticillium dahliae. Mol. Plant-Microbe Interact. 2016, 29, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 2000, 13, 122–143. [Google Scholar] [CrossRef] [PubMed]
- Ramrakhiani, L.; Chand, S. Recent progress on phospholipases: Different sources, assay methods, industrial potential and pathogenicity. Appl. Biochem. Biotechnol. 2011, 164, 991–1022. [Google Scholar] [CrossRef] [PubMed]
- David, A.S.; Edward, A.D. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta 2000, 1488, 1–19. [Google Scholar]
- Theiss, S.; Ishdorj, G.; Brenot, A.; Kretschmar, M.; Lan, C.Y.; Nichterlein, T.; Hacker, J.; Nigam, S.; Agabian, N.; Köhler, G.A. Inactivation of the phospholipase B gene PLB5 in wild-type Candida albicans reduces cell-associated phospholipase A2 activity and attenuates virulence. Int. J. Med. Microbiol. 2006, 296, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Köhler, G.A.; Brenot, A.; Haas-Stapleton, E.; Agabian, N.; Deva, R.; Nigam, S. Phospholipase A2 and Phospholipase B Activities in Fungi. Biochim. Biophys. Acta 2006, 1761, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, L.; Lian, J.; Gao, X.; Zhao, L.; Ding, M.; Li, J.; Liang, Y. The phospholipase C (FgPLC1) is involved in regulation of development, pathogenicity, and stress responses in Fusarium graminearum. Fungal Genet. Biol. 2016, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Regulation of phosphoinositide-specific Phospholipase C. Annu. Rev. Biochem. 2001, 70, 281–312. [Google Scholar] [CrossRef] [PubMed]
- Exton, J.H. Phospholipase d-structure, regulation and function. Rev. Physiol. Biochem. Pharmacol. 2002, 144, 1–94. [Google Scholar] [PubMed]
- Barrett-Bee, K.; Hayes, Y.; Wilson, R.G.; Ryley, J.F. A comparison of phospholipase activity, cellular adherence and pathogenicity of yeasts. J. Gen. Microbiol. 1985, 131, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.; Cawson, R.A. The cytochemical localization of phospholipase A and lysophospholipase in Candida albicans. Sabouraudia 1975, 13, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Lew, R.R.; Giblon, R.E.; Lorenti, M.S.H. The phenotype of a phospholipase C (plc-1) mutant in a filamentous fungus Neurospora crassa. Fungal Genet. Biol. 2015, 82, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.T.; Ahn, C.S.; Lee, K.J.; Kim, J.G.; Ro, H.S.; Kim, J.W.; Lee, C.W. The activity of phosphoinositide-specific phospholipase C is required for vegetative growth and cell wall regeneration in Coprinopsis cinerea. J. Microbiol. 2012, 50, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Soragni, E.; Bolchi, A.; Balestrini, R.; Gambaretto, C.; Percudani, R.; Bonfante, P.; Ottonello, S. A nutrient-regulated, dual localization phospholipase A2 in the symbiotic fungus Tuber borchii. EMBO J. 2001, 20, 5079–5090. [Google Scholar] [CrossRef] [PubMed]
- Nakahama, T.; Nakanishi, Y.; Viscomi, A.R.; Takaya, K.; Kitamoto, K.; Ottonello, S.; Arioka, M. Distinct enzymatic and cellular characteristics of two secretory phospholipases A2 in the filamentous fungus Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Grange, M.; Sette, C.; Cuomo, M.; Conti, M.; Lagarde, M.; Prigent, A.F.; Némoz, G. The cAMP-specific phosphodiesterase PDE4D3 is regulated by phosphatidic acid binding. Consequences for cAMP signaling pathway and characterization of a phosphatidic acid binding site. J. Biol. Chem. 2000, 275, 33379–33387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Y.; Wang, X. Phosphatidic Acid-Mediated Signaling. In Lipid-Mediated Protein Signaling, 1st ed.; Capelluto, D.G.S., Ed.; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-6331-9. [Google Scholar]
- Zhang, H.; Liu, K.; Zhang, X.; Tang, W.; Wang, J.; Guo, M.; Zhao, Q.; Zheng, X. Two phosphodiesterase genes, PDEL and PDEH, regulate development and pathogenicity by modulating intracellular cyclic AMP levels in Magnaporthe oryzae. PLoS ONE 2011, 6, e17241. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, R.; Naqvi, N.I. PdeH, a high-affinity cAMP Phosphodiesterase, is a key regulator of asexual and pathogenic differentiation in Magnaporthe oryzae. PLoS Pathog. 2010, 6, e1000897. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Rodríguez, E.; Fernández-Piñar, P.; Sacristán-Reviriego, A.; Molina, M.; Martín, H. An analog-sensitive version of the protein kinase Slt2 allows identification of novel targets of the yeast cell wall integrity pathway. J. Biol. Chem. 2016, 291, 5461–5472. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Tang, W.; Wang, J.; Liu, X.; Yang, L.; Gao, C.; Zhang, J.L.; Zhang, H.F.; Zheng, X.B.; Wang, P.; et al. Phosphodiesterase MoPdeH targets MoMck1 of the conserved mitogen-activated protein (MAP) kinase signaling pathway to regulate cell wall integrity in rice blast fungus Magnaporthe oryzae. Mol. Plant Pathol. 2016, 17, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Sacristán-Reviriego, A.; Martín, H.; Molina, M. Identification of putative negative regulators of yeast signaling through a screening for protein phosphatases acting on cell wall integrity and mating MAPK pathways. Fungal Genet. Biol. 2015, 77, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arias, P.; Díez-Muñiz, S.; García, R.; Nombela, C.; Rodríguez-Peña, J.M.; Arroyo, J. Genome-wide survey of yeast mutations leading to activation of the yeast cell integrity MAPK pathway Novel insights into diverse MAPK outcomes. BMC Genom. 2011, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.E.; Thorner, J. Function and regulation in MAPK signaling pathways lessons learned from the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 2007, 1773, 1311–1340. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Bermejo, C.; Grau, C.; Pérez, R.; Rodríguez-Peña, J.M.; Francois, J.; Nombela, C.; Arroyo, J. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J. Biol. Chem. 2004, 279, 15183–15195. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef] [PubMed]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.K.; Knoll, L.J. Patatin-like phospholipases in microbial infections with emerging roles in fatty acid metabolism and immune regulation by Apicomplexa. Mol. Micobiol. 2017, 107, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Lévêque, M.F.; Berry, L.; Yamaryo-Botté, Y.; Nguyen, H.M.; Galera, M.; Botté, C.Y.; Besteiro, S. TgPL2, a patatin-like phospholipase domain-containing protein, is involved in the maintenance of apicoplast lipids homeostasis in Toxoplasma. Mol. Microbiol. 2017, 105, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Sato, H.; Dirck, A.T.; Feix, J.B.; Frank, D.W. Ubiquitin activates Patatin-like Phospholipases from multiple bacterial species. J. Bacteriol. 2015, 197, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Strickland, J.A.; Walsh, T.A. Inhibition of Diabrotica larval growth by Patatin, the lipid acyl hydrolase from potato tubers. Plant Physiol. 1995, 109, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.L.; Su, X.F.; Guo, H.M.; Qi, J.C.; Cheng, H.M. A ku70 null mutant improves gene targeting frequency in the fungal pathogen Verticillium dahliae. World J. Microbiol. Biotechnol. 2015, 31, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Khang, C.H.; Park, S.Y.; Lee, H.Y.; Kang, S. A dual selection based, targeted gene replacement tool for Magnaporthe grisea and Fusarium oxysporum. Fungal Genet. Biol. 2005, 35, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Rehman, L.; Su, X.F.; Guo, H.M.; Qi, X.L.; Cheng, H.M. Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae. BMC Biotechnol. 2016, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.W.; Hu, D.F.; Hu, X.P.; Zhao, J.X.; Zhu, H.Q.; Yang, J.R. Formation conditions for microsclerotia of Verticillium dahliae. Mycosystema 2011, 30, 695–701. [Google Scholar]
- Ram, A.F.; Klis, F.M. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 2006, 1, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhou, L.; Guo, W.; Wang, X. Small GTPase Rac1 and its interaction partner Cla4 regulate polarized growth and pathogenicity in Verticillium dahliae. Fungal Genet. Biol. 2015, 74, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Zhang, T.; Guo, H.S. Penetration assays, fungal recovery and pathogenicity assays for Verticillium dahliae. Bio-Protocol 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, B.S.; Hua, C.L.; Meng, P.; Wang, S.; Chen, Z.R.; Du, Y.J.; Gao, F.; Huang, J.F. VdPKS1 is required for melanin formation and virulence in a cotton wilt pathogen Verticillium dahliae. Sci. China Life Sci. 2017, 60, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- García, R.; Rodríguez-Peña, J.M.; Bermejo, C.; Nombela, C.; Arroyo, J. The high osmotic response and cell wall integrity pathways cooperate to regulate transcriptional responses to zymolyase-induced cell wall stress in Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 10901–10911. [Google Scholar] [CrossRef] [PubMed]
- Valiante, V.; Jain, R.; Heinekamp, T.; Brakhage, A.A. The MpkA MAP kinase module regulates cell wall integrity signaling and pyomelanin formation in Aspergillus fumigatus. Fungal Genet. Biol. 2009, 46, 909–918. [Google Scholar] [CrossRef]
- Aloulou, A.; Ali, Y.B.; Bezzine, S.; Gargouri, Y. Gelb MH. Phospholipases: An overview. Methods Mol. Biol. 2012, 861, 63–85. [Google Scholar] [PubMed]
- Park, J.I.; Grant, C.M.; Dawes, I.W. The high-affinity cAMP phosphodies-terase of Saccharomyces cerevisiae is the major determinant of cAMP levels in stationary phase: Involvement of different branches of the Ras-cyclic AMP pathway in stress responses. Biochem. Biophys. Res. Commun. 2005, 327, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Tutulan-Cunita, A.; Jung, W.; Hauser, N.C.; Hernandez, R.; Williamson, T.; Piekarska, K.; Rupp, S.; Young, T.; Stateva, L. Deletion of the high-affinity cAMP phosphodiesterase encoded by PDE2 affects stress responses and virulence in Candida albicans. Mol. Microbiol. 2007, 65, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Tzima, A.K.; Paplomatas, E.J.; Tsitsigiannis, D.I.; Kang, S. The G protein β subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 2012, 49, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Coley-Smith, J.R.; Cooke, R.C. Survival and germination of fungal sclerotia. Annu. Rev. Phytopathol. 1971, 9, 65–92. [Google Scholar] [CrossRef]
- Hawke, M.A.; Lazarovits, G. Production and manipulation of individual microsclerotia of Verticillium dahliae for use in studies of survival. Phytopathology 1994, 23, 582–584. [Google Scholar]
- Klimes, A.; Amyotte, S.G.; Grant, S.; Kang, S.; Dobinson, K.F. Microsclerotia development in Verticillium dahliae: Regulation and differential expression of the hydrophobin gene VDH1. Fungal Genet. Biol. 2008, 45, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Dechassa, D.; Amy, A.; Chen, D. RNA-seq analyses of gene expression in the microsclerotia of Verticillium dahliae. BMC Genom. 2013, 14, 607. [Google Scholar] [CrossRef]
- Tzima, A.; Paplomatas, E.J.; Rauyaree, P.; Kang, S.C. Roles of the catalytic subunit of cAMP-dependent protein kinase A in virulence and development of the soilborne plant pathogen Verticillium dahliae. Fungal Genet. Biol. 2010, 47, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Klosterman, S.J.; Wang, C.; Subbarao, K.V.; Xu, X.; Shang, W.; Hu, X. Vayg1 is required for microsclerotium formation and melanin production in Verticillium dahliae. Fungal Genet. Biol. 2017, 98, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xu, J.; Zhou, L.; Guo, W. VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene 2014, 550, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Rauyaree, P.; Ospina, G.S.; Bhat, R.G.; Subbarao, K.V.; Grant, S.J.; Dobinson, K.F. Mutations in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Curr. Genet. 2005, 48, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Parra-Giraldo, C.M.; Hernáez, M.L.; Reales-Calderon, J.A.; Solis, N.V.; Filler, S.G.; Monteoliva, L.; Gil, C. Candida albicans cell shaving uncovers new proteins involved in cell wall integrity, yeast to hypha transition, stress response and host-pathogen interaction. J. Proteom. 2015, 127, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Reales-Calderon, J.A.; Parra-Giraldo, C.M.; Martinez-Lopez, R.; Monteoliva, L.; Gil, C. The cell wall protein Ecm33 of Candida albicans is involved in chronological life span, morphogenesis, cell wall regeneration, stress tolerance, and host-cell interaction. Front. Microbiol. 2016, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Klis, F.M.; Sosinska, G.J.; De Groot, P.W.; Brul, S. Covalently linked cell wall proteins of Candida albicans and their role in fitness and virulence. FEMS Yeast Res. 2009, 9, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, W.L. Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 2008, 72, 495–544. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gao, F.; Zhu, X.; Nie, X.; Pan, Y.; Gao, Z. MoGrr1, a novel F-box protein, is involved in conidiogenesis and cell wall integrity and is critical for the full virulence of Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2015, 99, 8075–8088. [Google Scholar] [CrossRef] [PubMed]
- Gandía, M.; Harries, E.; Marcos, J.F. The myosin motor domain-containing chitin synthase PdChsVII is required for development, cell wall integrity and virulence in the citrus postharvest pathogen Penicillium digitatum. Fungal Genet. Biol. 2014, 67, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Preechasuth, K.; Anderson, J.C.; Peck, S.C.; Brown, A.J.; Gow, N.A.; Lenardon, M.D. Cell wall protection by the Candida albicans class I chitin synthases. Fungal Genet. Biol. 2015, 82, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Gow, N.A.; Gonçalves, T. The importance of subclasses of chitin synthase enzymes with myosin-like domains for the fitness of fungi. Fungal Biol. Rev. 2016, 30, 1–14. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.L.; Jin, Y.; Zhang, T.; Guo, H.S. Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell 2014, 5, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Villamil, J.L.; Prieto, P.; Klosterman, S.J.; García-Pedrajas, M.D. Characterization of two homeodomain transcription factors with critical but distinct roles in virulence in the vascular pathogen Verticillium dahliae. Mol. Plant Pathol. 2017. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Primer Sequence |
|---|---|
| neo-F | GTTTGCGGGCTGTCTTGACG |
| neo-R | TACCTGTGCATTCTGGGTAA |
| VdPLP-5F | GTACCCAATTCGAATTCCCAGCGGTTCGGGTAGTAGTAGA |
| VdPLP-5R | CAAGACAGCCCGCAAACGTATAACCCCGCGGAGCAGTAA |
| VdPLP-3F | CCCAGAATGCACAGGTAGACGCGCCACGACCTCAA |
| VdPLP-3R | ACGGTATCGATAAGCTTTGCGTGCGAACATACTCCTCAT |
| C-TrpC-F | TTGAAGGAGCATTTTTGGGC |
| C-TrpC-R | ATCGATGCTTGGGTAGAATAGGT |
| C-VdPLP-F | ATGCCTGTCAACGATATCCGTCT |
| C-VdPLP-R | CTATTCCTCGATCAGAGAGTAG |
| C-Nos-F | AGATGCCGACCGGGATCCACTT |
| C-Nos-R | TTATCTTTGCGAACCCAGGG |
| VdPLP-J-F | CTCGAGCGGGCCATCAAACA |
| VdPLP-J-R | GAGTAAGCCACCCATCTGTCCGTT |
| neo-J-F | GCGGTTCAGAAGCACCTCGA |
| neo-J-R | TATCTTTGCGAACCCAGG |
| VdMK1-F | CGCGCCCGAGATTATGCTGAG |
| VdMK1-R | CGTTGGGAGTACCGAGGATGTGAA |
| VdChi1-F | GCCGCCGCCTGGTCATC |
| VdChi1-R | CGGGGTAGAGGTCGGCATCA |
| VdChi3-F | GGTCGGCCCTTGGAGCAGTA |
| VdChi3-R | CCCTTGGCAGCCTTGATGTAGC |
| VdChi4-F | TACGGCAAGGTTTACTCGGGTCTC |
| VdChi4-R | CGGTTGCCAGGCTTCGTCTTAC |
| VdChi7-F | CATCCTCGGCGTCACAAAGTTCTA |
| VdChi7-R | GCTGCCGCTGCTGGAGGTA |
| VdBt-F | TTCCCCCGTCTCCACTTCTTCATG |
| VdBt-R | GACGAGATCGTTCATGTTGAACTC |
| Nb-actin-F | GGACCTTTATGGAAACATTGTGCTCAGT |
| Nb-actin-R | CCAAGATAGAACCTCCAATCCAGACAC |
| Vd-F | CCGCCGGTCCATCAGTCTCTCTGTTTATAC |
| Vd-R | CGCCTGCGGGACTCCGATGCGAGCTGTAAC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Li, X.; Guo, H.; Guo, N.; Cheng, H. VdPLP, A Patatin-Like Phospholipase in Verticillium dahliae, Is Involved in Cell Wall Integrity and Required for Pathogenicity. Genes 2018, 9, 162. https://doi.org/10.3390/genes9030162
Qi X, Li X, Guo H, Guo N, Cheng H. VdPLP, A Patatin-Like Phospholipase in Verticillium dahliae, Is Involved in Cell Wall Integrity and Required for Pathogenicity. Genes. 2018; 9(3):162. https://doi.org/10.3390/genes9030162
Chicago/Turabian StyleQi, Xiliang, Xiaokang Li, Huiming Guo, Ning Guo, and Hongmei Cheng. 2018. "VdPLP, A Patatin-Like Phospholipase in Verticillium dahliae, Is Involved in Cell Wall Integrity and Required for Pathogenicity" Genes 9, no. 3: 162. https://doi.org/10.3390/genes9030162
APA StyleQi, X., Li, X., Guo, H., Guo, N., & Cheng, H. (2018). VdPLP, A Patatin-Like Phospholipase in Verticillium dahliae, Is Involved in Cell Wall Integrity and Required for Pathogenicity. Genes, 9(3), 162. https://doi.org/10.3390/genes9030162




