Integrated Proteomic and Transcriptomic Analysis of Differential Expression of Chicken Lung Tissue in Response to NDV Infection during Heat Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Populations and Design
2.2. RNA Isolation and Library Construction
2.3. Protein Identification and Quantification
2.4. Data Analysis
3. Results
3.1. Differential Gene Expression from within-Line Comparisons
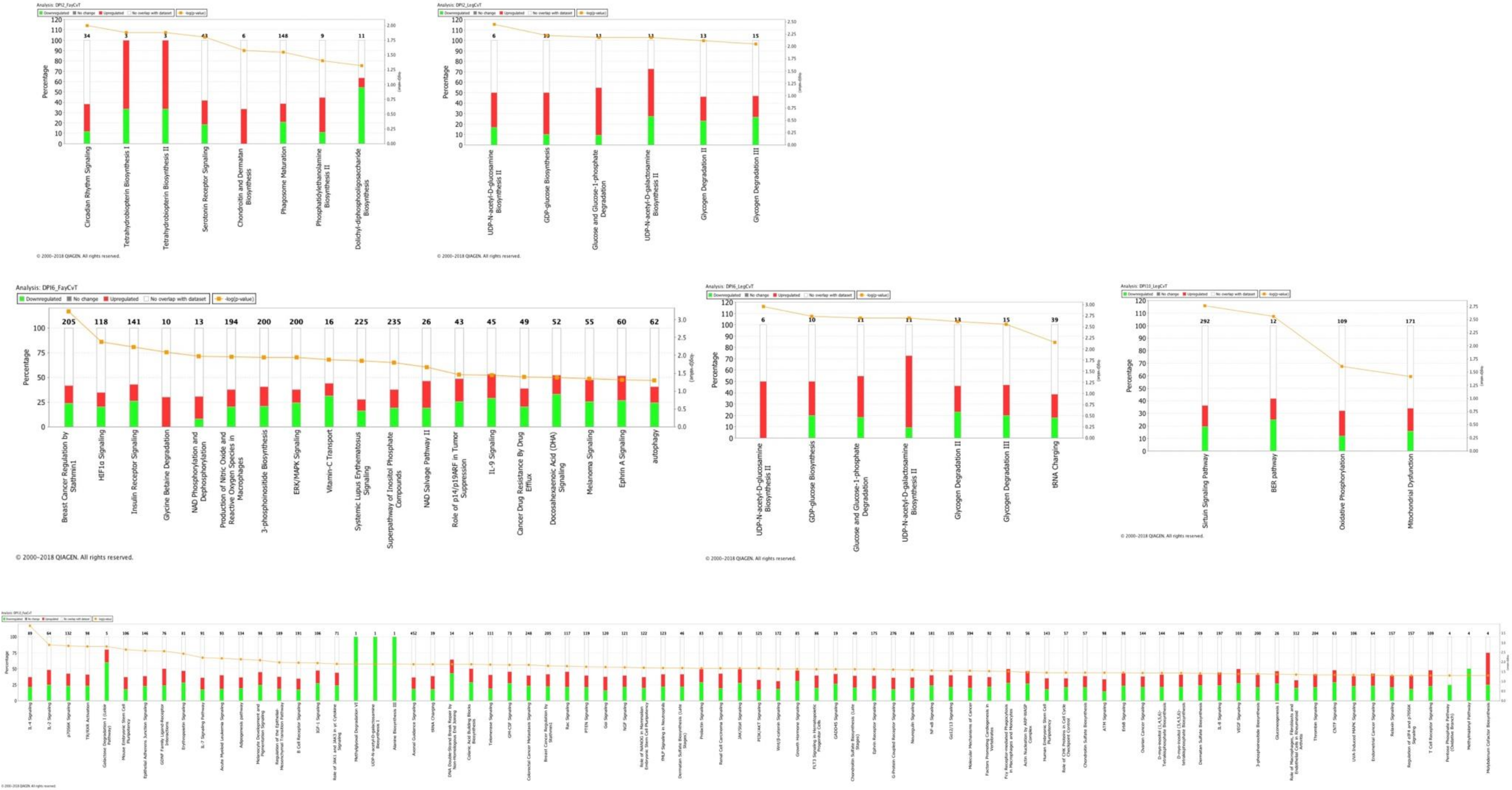
3.2. Pathway Analysis of Differentially Expressed Genes
3.3. Quantification of Protein Levels and Differential Protein Abundance
3.4. Correlation of Change in Protein Abundance and Differential Gene Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MacLachlan, N.J.; Dubovi, E. Paramyxoviridae and Pneumoviridae. In Fenner’s Veterinary Virology; Elsevier: New York, NY, USA, 2017; pp. 327–356. [Google Scholar]
- OIE-Listed diseases, Infections and Infestations in Force in 2018. 2018. Available online: http://www.oie.int/en/animal-health-in-the-world/oie-listed-diseases-2018/ (accessed on 12 July 2018).
- Epstein, P.R. Climate change and emerging infectious diseases. Microbes Infect. 2001, 8, 747–754. [Google Scholar] [CrossRef]
- Wu, X.; Lu, Y.; Zhou, S.; Chen, L.; Xu, B. Impact of climate change on human infectious diseases: Empirical evidence and human adaptation. Environ. Int. 2016, 86, 14–23. [Google Scholar] [CrossRef] [PubMed]
- George, M.M. Epidemiology of Newcastle disease and the need to vaccinate local chickens in Uganda. In Proceedings of the International Workshop on Oil Palm Germplasm and Utilization, Kuala Lumpur, Malaysia, 12–13 September 1991; pp. 155–158. [Google Scholar]
- Mishra, U. Present status of poultry in Nepal. In Proceedings of the 7th Tanzania Veterinary Association Scientific Conference, Arusha, Tanzania, 5 December 1989; pp. 403–407. [Google Scholar]
- Ghazi, S.; Habibian, M.; Moeini, M.M.; Abdolmohammadi, A.R. Effects of different levels of organic and inorganic chromium on growth performance and immunocompetence of broilers under heat stress. Biol. Trace Elem. Res. 2012, 146, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sakai, M.; Sa, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Sci. 2010, 89, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.R.; Smith, M.O.; Yan, Q.; Li, L. Effects of different levels of selenium on growth performance and immunocompetence of broilers under heat stress. Poultry Sci. 2003, 82, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.; Kucukyilmaz, K.; Catli, A.U.; Cinar, M.; Bintas, E.; Coven, F. Performance, egg quality, and immune response of laying hens fed diets supplemented with mannan-oligosaccharide or an essential oil mixture under moderate and hot environmental conditions. Poultry Sci. 2012, 91, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Deist, M.S.; Gallardo, R.A.; Bunn, D.A.; Dekkers, J.C.M.; Zhou, H.; Lamont, S.J. Resistant and susceptible chicken lines show distinctive responses to Newcastle disease virus infection in the lung transcriptome. BMC Genom. 2017, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deist, M.S.; Gallardo, R.A.; Bunn, D.A.; Kelly, T.R.; Dekkers, J.C.M.; Zhou, H.; Lamont, S.J. Novel mechanisms revealed in the trachea transcriptome of resistant and susceptible chicken lines following infection with Newcastle disease virus. Clin. Vaccine Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kaiser, M.G.; Deist, M.S.; Gallardo, R.A.; Bunn, D.A.; Kelly, T.R.; Dekkers, J.C.; Zhou, H.; Lamont, S.J. Transcriptome analysis in spleen reveals differential regulation of response to newcastle disease virus in two chicken lines. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Saelao, P.; Wang, Y.; Gallardo, R.A.; Lamont, S.J.; Dekkers, J.M.; Kelly, T.; Zhou, H. Novel insights into the host immune response of chicken Harderian gland tissue during Newcastle disease virus infection and heat treatment. BMC Vet. Res. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lupiani, B.; Reddy, S.M.; Lamont, S.J.; Zhou, H. RNA-seq analysis revealed novel genes and signaling pathway associated with disease resistance to avian influenza virus infection in chickens. Poult. Sci. 2014, 93, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, N.; Kaiser, M.G.; Lamon, S.J. Marek’s disease resistance in MHC-congenic lines from Leghorn and Fayoumi breeds. In Current Research on Marek’s Disease, Proceedings of the 5th International Symposium, Kennet Square, PA, USA; American Association of Avian Pathologists: Jacksonville, FL, USA, 23 July 1996. [Google Scholar]
- Wang, Y.; Saelao, P.; Chanthavixay, K.; Gallardo, R.; Bunn, D.; Lamont, S.J.; Dekkers, J.M.; Kelly, T.; Zhou, H. Physiological responses to heat stress in two genetically distinct chicken inbred lines. Poult. Sci. 2017, 97, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D.; Li, J.; Gardell, A.; Sacchi, R. Quantitative molecular phenotyping of gill remodeling in a cichlid fish responding to salinity stress. Mol. Cell. Proteom. 2013, 12, 3962–3975. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D.; Li, J.; Paguio, D.; Pham, T.; Eidsaa, M.; Almaas, E. Population-specific renal proteomes of marine and freshwater three-spined sticklebacks. J. Proteom. 2016, 135, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Found Stat Comput Vienna Austria. 2018. Available online: https://www.R-project.org/ (accessed on 20 July 2018).
- Andrew, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 20 July 2018).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Krämer, A.; Green, J.; Pollard, J.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Stray-Pedersen, A.; Backe, P.H.; Sorte, H.S.; Mørkrid, L.; Chokshi, N.Y.; Erichsen, H.C.; Gambin, T.; Elgstøen, K.B.; Bjørås, M.; Wlodarski, M.W.; et al. PGM3 mutations cause a congenital disorder of glycosylation with severe immunodeficiency and skeletal dysplasia. Am. J. Hum. Genet. 2014, 95, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, X.; Ichikawa, M.; Lyons, J.J.; Datta, S.; Lamborn, I.T.; Jing, H.; Kim, E.S.; Biancalana, M.; Wolfe, L.A.; et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J. Allergy Clin. Immunol. 2014, 133, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.D.; Lyons, J.J.; Zhang, Y.; Yu, X.; Datta, S.; Lamborn, I.T.; Biancalana, M.R.; Wolfe, L.A.; DiMaggio, T.; Matthews, H.F.; et al. Impaired glycosylation due to autosomal recessive PGM3 mutations results in atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J. Allergy Clin. Immunol. 2014, 133, AB161. [Google Scholar] [CrossRef]
- Kroeker, A.L.; Ezzati, P.; Halayko, A.J.; Coombs, K.M. Response of primary human airway epithelial cells to influenza infection: A quantitative proteomic study. J. Proteome Res. 2012, 11, 4132–4146. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Virella, G.; Lopes-Virella, M.F. The role of the immune system in the pathogenesis of diabetic complications. Front. Endocrinol. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Edfors, F.; Danielsson, F.; Hallström, B.M.; Käll, L.; Lundberg, E.; Pontén, F.; Forsström, B.; Uhlén, M. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol. 2016, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mrna abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, H.; Wittung-Stafshede, P. Identification of new potential interaction partners for human cytoplasmic copper chaperone Atox1: Roles in gene regulation? Int. J. Mol. Sci. 2015, 16, 16728–16739. [Google Scholar] [CrossRef] [PubMed]
- Ozato, K.; Shin, D.-M.; Chang, T.-H.; Morse, H.C. TRIM family proteins and their emerging roles in innate immunity. Nat. Rev. Immunol. 2008, 8, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J. The C-type carbohydrate recognition domain (CRD) superfamily. Biochem. Soc. Trans. 1994, 22, 83–88. [Google Scholar] [CrossRef]
- Hogenkamp, A.; van Eijk, M.; van Dijk, A.; van Asten, A.J.A.M.; Veldhuizen, E.J.A.; Haagsman, H.P. Characterization and expression sites of newly identified chicken collectins. Mol. Immunol. 2006, 43, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, O.P. Defective glucose homeostasis during infection. Annu. Rev. Nutr. 2005, 25, 9–35. [Google Scholar] [CrossRef] [PubMed]


| Differentially Expressed Genes | Differentially Abundant Proteins | ||||
|---|---|---|---|---|---|
| Line | Days Post-Infection | Up-Regulated | Down-Regulated | Up-Regulated | Down-Regulated |
| Fayoumi | 2 | 122 | 58 | 0 | 6 |
| Leghorn | 2 | 35 | 2 | 5 | 5 |
| Fayoumi | 6 | 26 | 27 | 4 | 9 |
| Leghorn | 6 | 6 | 1 | 62 | 37 |
| Fayoumi | 10 | 173 | 358 | ||
| Leghorn | 10 | 10 | 5 | ||
| Canonical Pathway | Z-Score |
|---|---|
| ERK/MAPK Signaling | −2.24 |
| Thrombin Signaling | −2.24 |
| mTOR Signaling | −2.00 |
| Fcy Receptor-mediated Phagocytosis in Macrophages and Monocytes | −2.00 |
| p70S6K Signaling | −1.89 |
| B Cell Receptor Signaling | −1.89 |
| Colorectal Cancer Metastasis Signaling | −1.89 |
| Mouse Embryonic Stem Cell Pluripotency | −1.63 |
| Rac Signaling | −1.34 |
| Ga12/13 Signaling | −1.34 |
| IL-8 Signaling | −1.34 |
| NF-kappaB Signaling | −1.34 |
| Regulation of eIF4 and p70S6K Signaling | −1.34 |
| NGF Signaling | −1.34 |
| Telomerase Signaling | −1.34 |
| Acute Myeloid Leukemia Signaling | −1.34 |
| Fc Epsilon RI Signaling | −1.00 |
| fMLP Signaling in Neutrophils | −1.00 |
| ErbB Signaling | −1.00 |
| Growth Hormone Signaling | −1.00 |
| EIF2 Signaling | −1.00 |
| FLT3 Signaling in Hematopoietic Progenitor Cells | −1.00 |
| Melanocyte Development and Pigmentation Signaling | −1.00 |
| Renal Cell Carcinoma Signaling | −1.00 |
| Neuregulin Signaling | −1.00 |
| Insulin Receptor Signaling | −1.00 |
| GM-CSF Signaling | −1.00 |
| Sirtuin Signaling Pathway | −0.45 |
| Prolactin Signaling | 0.00 |
| PTEN Signaling | 0.00 |
| Neuroinflammation Signaling Pathway | 0.45 |
| AMPK Signaling | NA |
| G Beta Gamma Signaling | NA |
| FcyRIIB Signaling in B Lymphocytes | NA |
| Renin-Angiotensin Signaling | NA |
| Role of NFAT in Cardiac Hypertrophy | NA |
| eNOS Signaling | NA |
| Huntington’s Disease Signaling | NA |
| GP6 Signaling Pathway | NA |
| ErbB4 Signaling | NA |
| Lymphotoxin B Receptor Signaling | NA |
| PAK Signaling | NA |
| CD40 Signaling | NA |
| Production of Nitric Oxide and Reactive Oxygen Species in Macrophages | NA |
| alpha-Adrenergic Signaling | NA |
| P2Y Purigenic Receptor Signaling Pathway | NA |
| Amyotrophic Lateral Sclerosis Signaling | NA |
| Line | Days Post-Infection | r | p-Value |
|---|---|---|---|
| Fayoumi | 2 | −0.0072 | 0.86 |
| Leghorn | 2 | −0.053 | 0.22 |
| Fayoumi | 6 | 0.051 | 0.22 |
| Leghorn | 6 | −0.018 | 0.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saelao, P.; Wang, Y.; Chanthavixay, G.; Yu, V.; Gallardo, R.A.; Dekkers, J.C.M.; Lamont, S.J.; Kelly, T.; Zhou, H. Integrated Proteomic and Transcriptomic Analysis of Differential Expression of Chicken Lung Tissue in Response to NDV Infection during Heat Stress. Genes 2018, 9, 579. https://doi.org/10.3390/genes9120579
Saelao P, Wang Y, Chanthavixay G, Yu V, Gallardo RA, Dekkers JCM, Lamont SJ, Kelly T, Zhou H. Integrated Proteomic and Transcriptomic Analysis of Differential Expression of Chicken Lung Tissue in Response to NDV Infection during Heat Stress. Genes. 2018; 9(12):579. https://doi.org/10.3390/genes9120579
Chicago/Turabian StyleSaelao, Perot, Ying Wang, Ganrea Chanthavixay, Vivian Yu, Rodrigo A. Gallardo, Jack C. M. Dekkers, Susan J. Lamont, Terra Kelly, and Huaijun Zhou. 2018. "Integrated Proteomic and Transcriptomic Analysis of Differential Expression of Chicken Lung Tissue in Response to NDV Infection during Heat Stress" Genes 9, no. 12: 579. https://doi.org/10.3390/genes9120579
APA StyleSaelao, P., Wang, Y., Chanthavixay, G., Yu, V., Gallardo, R. A., Dekkers, J. C. M., Lamont, S. J., Kelly, T., & Zhou, H. (2018). Integrated Proteomic and Transcriptomic Analysis of Differential Expression of Chicken Lung Tissue in Response to NDV Infection during Heat Stress. Genes, 9(12), 579. https://doi.org/10.3390/genes9120579






