mCherry-Labeled Verticillium dahliae Could Be Utilized to Investigate Its Pathogenicity Process in Nicotiana benthamiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Fungal Strain
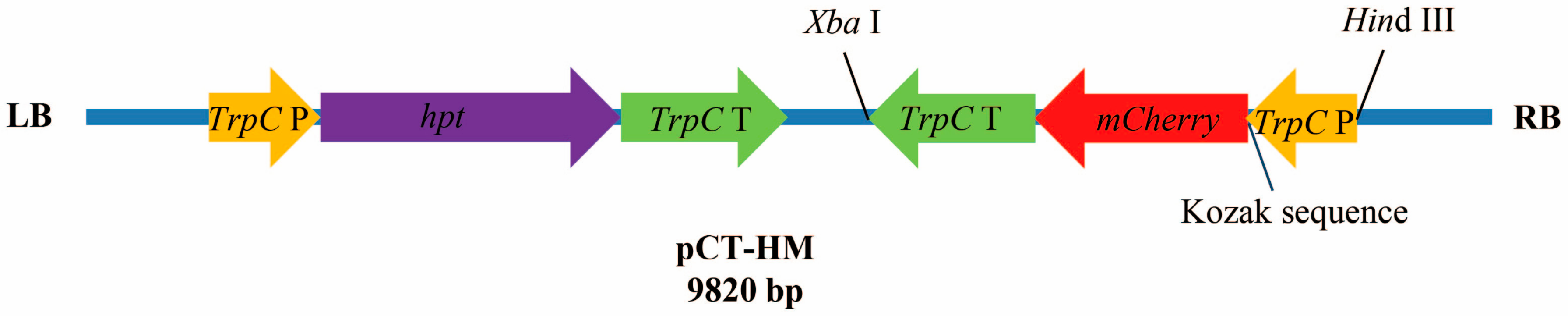
2.2. Codon Optimization of mCherry and Construction of Expression Plasmid
2.3. Fungal Transformation
2.4. Screening of mCherry-Labeled Verticillium dahliae (Vd-m)
2.5. Characterization of Vd-m
2.6. Plant Inoculation
2.7. Microscopic Observation of Pathogenic Process
2.8. Quantification of Fungal Biomass
2.9. Statistical Analysis
3. Results
3.1. Optimization of mCherry Gene and Plasmid Construction
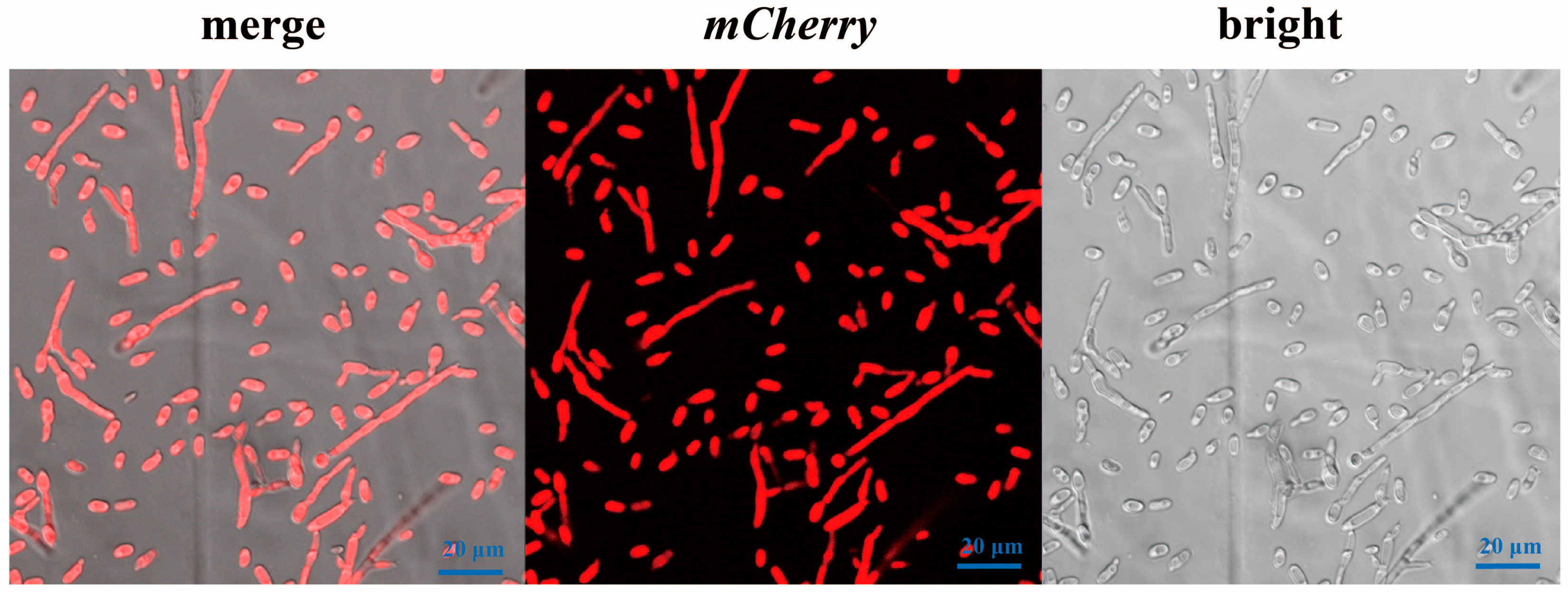
3.2. Confirmation of Vd-m Isolates
3.3. Analysis of Biological Characteristics of Vd-m
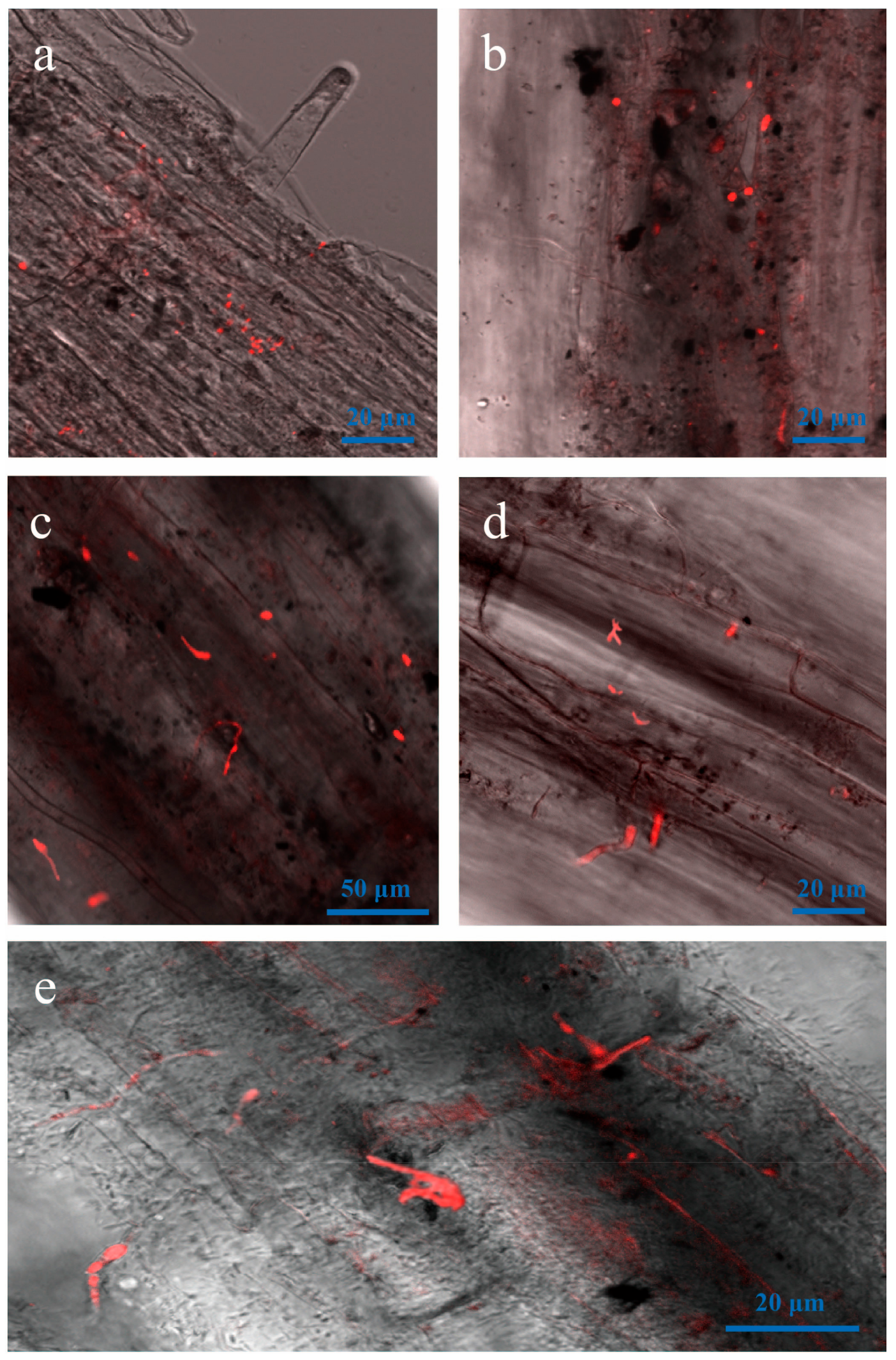
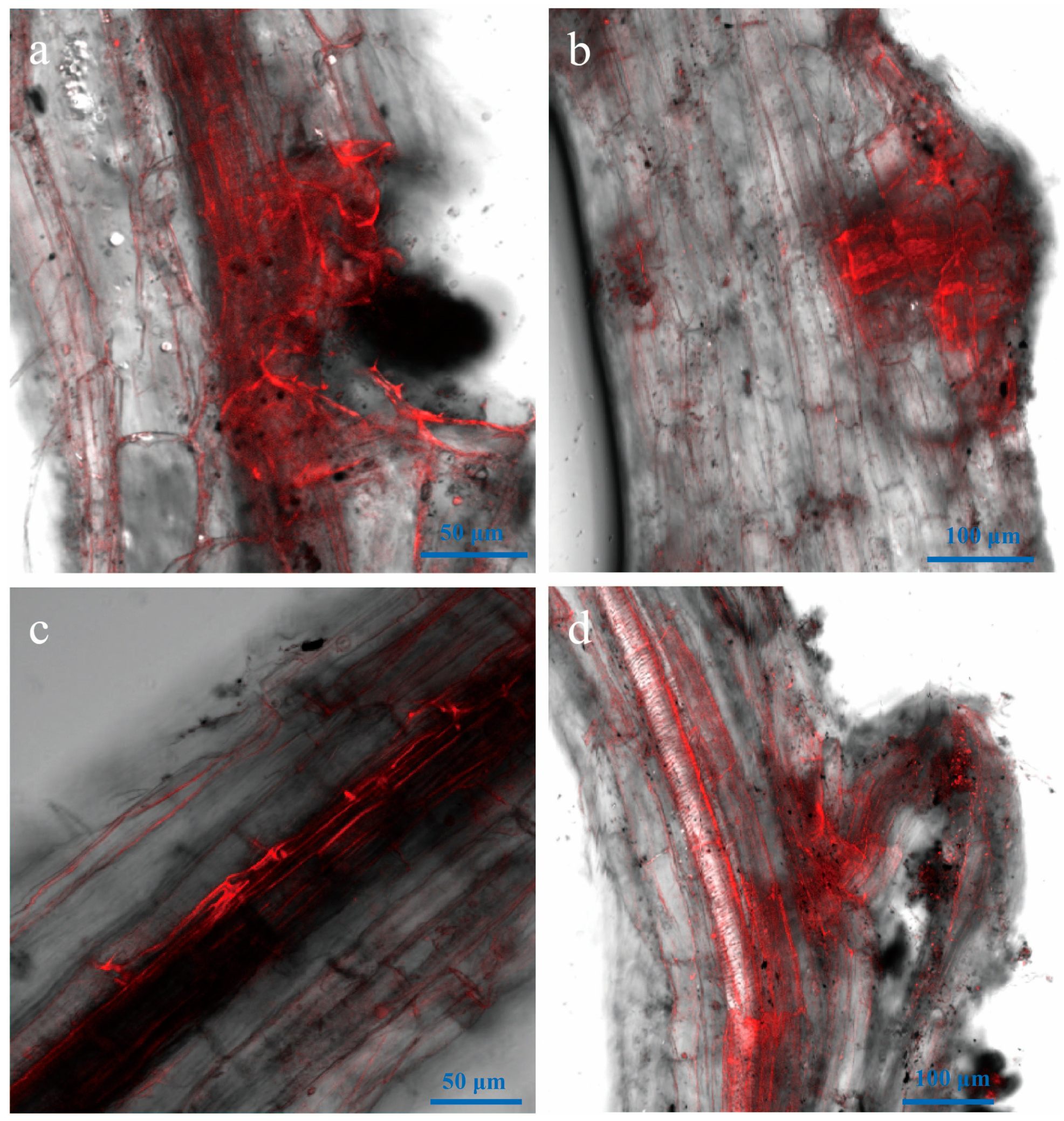
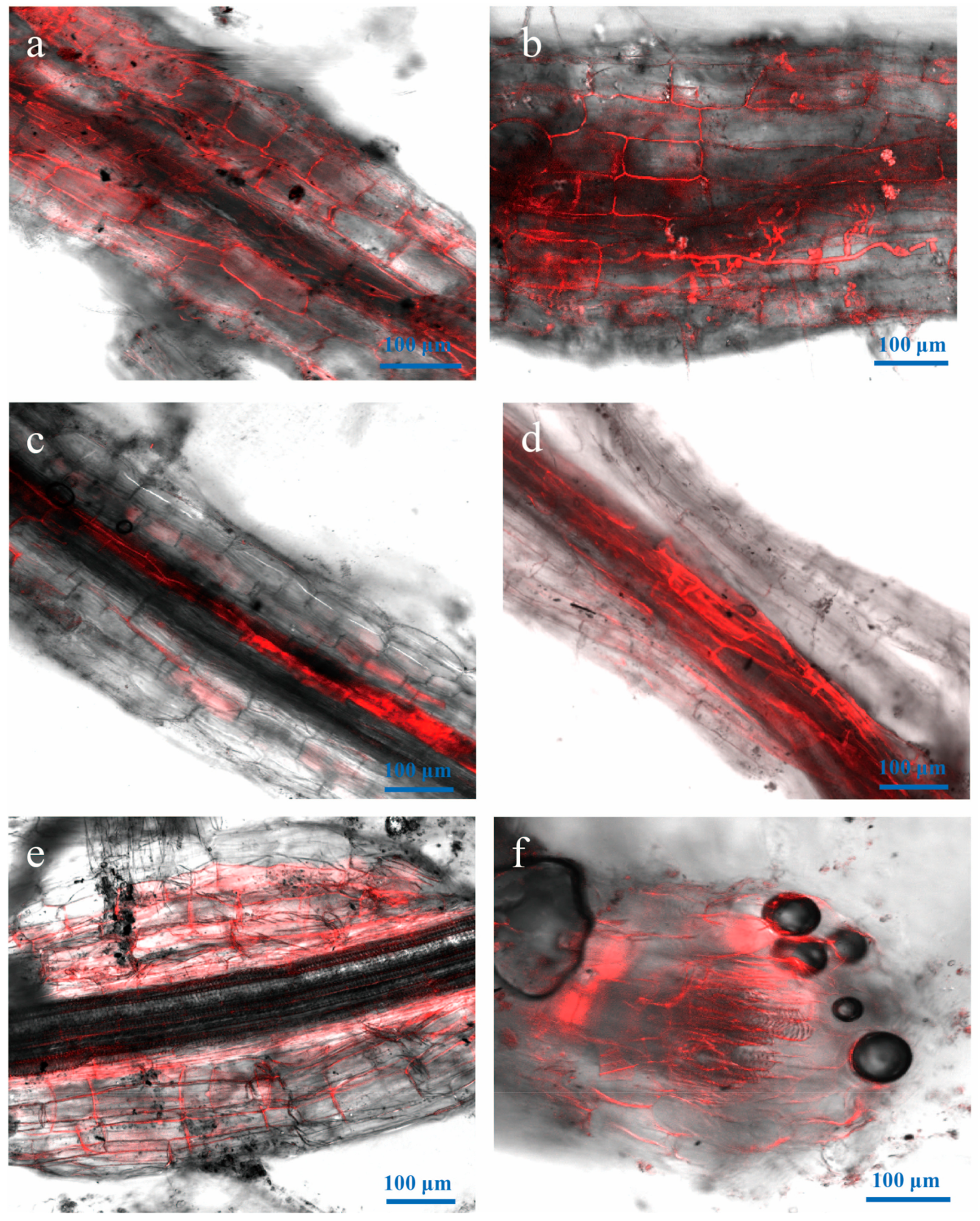
3.4. Attachment and Colonization of Vd-m on Roots
3.5. Advanced Penetration Stage of Vd-m in Roots
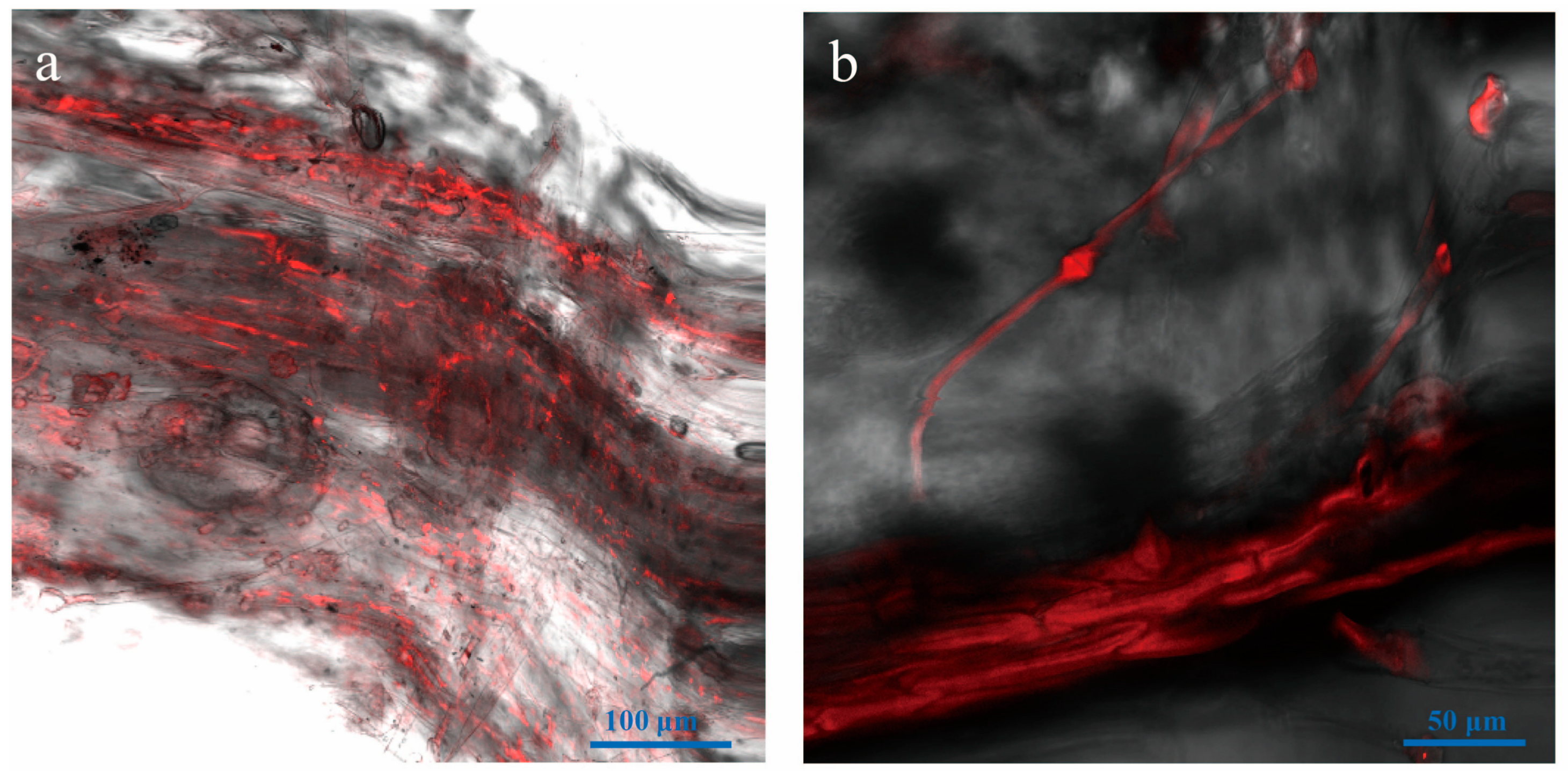
3.6. Vd-m Extensively Colonized Root Tissues
3.7. Microscopic Examination of Leaves
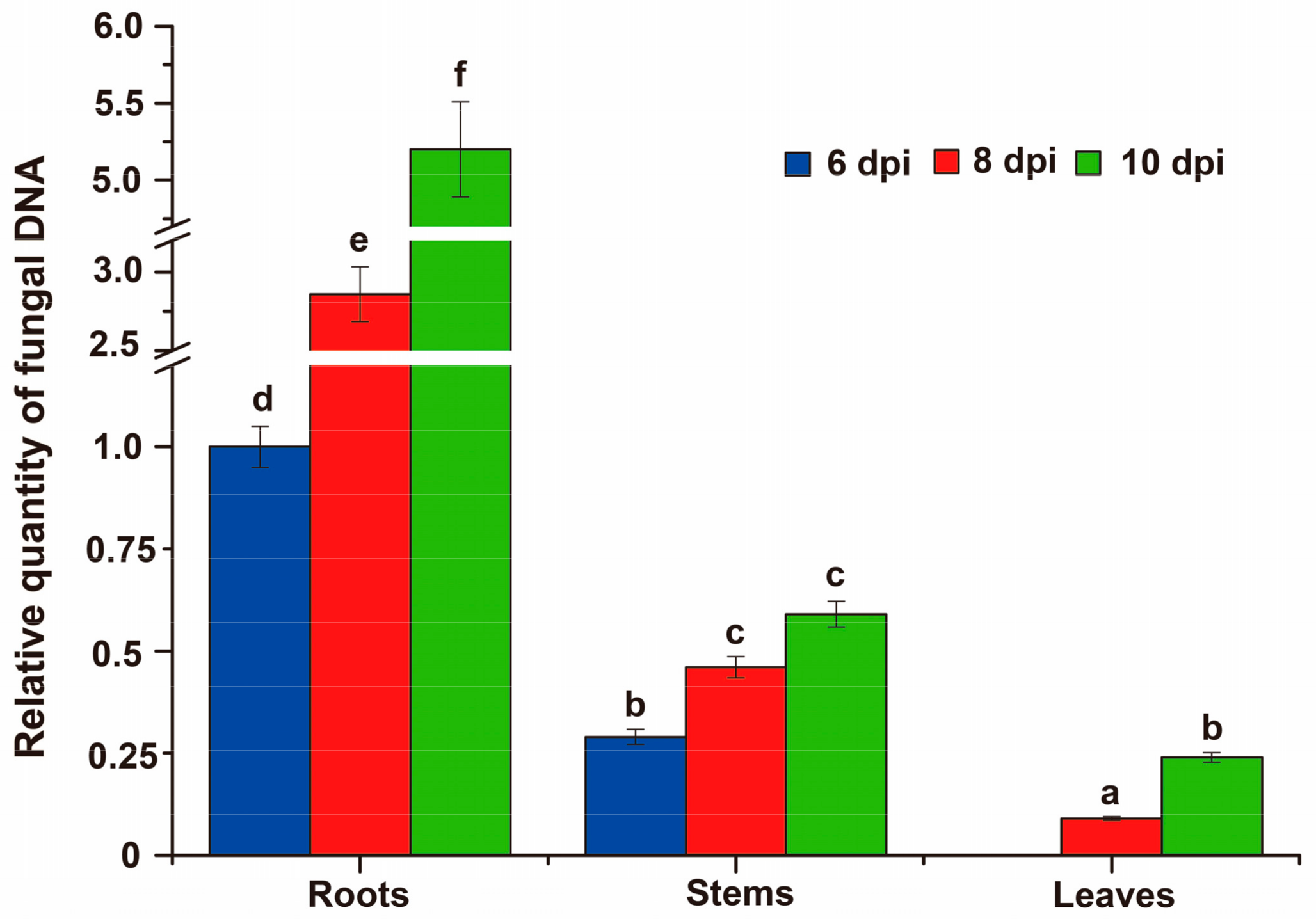
3.8. Fungal Biomass at Different Stages
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI Publishing: Oxford, UK, 2002; pp. 142–166. [Google Scholar]
- Hu, D.; Wang, C.; Tao, F.; Cui, Q.; Xu, X.; Shang, W.; Hu, X. Whole genome wide expression profiles on germination of Verticillium dahliae microsclerotia. PLoS ONE 2014, 9, e100046. [Google Scholar] [CrossRef] [PubMed]
- Farley, J.D.; Wilhelm, S.; Snyder, W.C. Repeated germination and sporulation of microsclerotia of Verticillium albo-atrum in soil. Phytopathology 1971, 61, 260–264. [Google Scholar] [CrossRef]
- Duressa, D.; Rauscher, G.; Koike, S.T.; Mou, B.; Hayes, R.J.; Maruthachalam, K.; Subbarao, K.V.; Klosterman, S.J. A real-time PCR assay for detection and quantification of Verticillium dahliae in spinach seed. Phytopathology 2012, 102, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Bhat, R.G.; Koike, S.T.; Ryder, E.J.; Subbarao, K.V. Weedborne reservoirs and seed transmission of Verticillium dahliae in lettuce. Plant Dis. 2007, 89, 317–324. [Google Scholar] [CrossRef]
- Bubici, G.; Cirulli, M. Verticillium wilt of olive: Current status, disease management and future prospects. Prot. Delle Colt. 2012, 4, 42–56. [Google Scholar]
- Rowe, R.C.; Davis, J.R.; Powelson, M.L.; Rouse, D.I. Potato early dying: Causal agents and management strategies. Plant Dis. 1987, 71, 482–489. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2010, 344, 1–50. [Google Scholar] [CrossRef]
- Rehman, L.; Su, X.; Li, X.; Qi, X.; Guo, H.; Cheng, H. FreB is involved in the ferric metabolism and multiple pathogenicity-related traits of Verticillium dahliae. Curr. Genet. 2018, 64, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Mol, L.; Riessen, H.W.V. Effect of plant roots on the germination of microsclerotia of Verticillum dahliae. Eur. J. Plant Pathol. 1995, 101, 673–678. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Subbarao, K.V. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology 2008, 98, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Iakovoss, P.; Sotiriose, T.; Ioannisa, S.; Iordanis, C.; Epaminondasj, P. Mode of action of a non-pathogenic Fusarium oxysporum strain against Verticillium dahliae using Real Time QPCR analysis and biomarker transformation. Biol. Control 2009, 50, 30–36. [Google Scholar] [CrossRef]
- Huisman, O. Interrelations of root growth dynamics to epidemiology of root-invading fungi. Annu. Rev. Phytopathol. 1982, 20, 303–327. [Google Scholar] [CrossRef]
- Evans, G.; Gleeson, A.C.; Evans, G.; Gleeson, A.C. Observations on the origin and nature of Verticillium dahliae colonizing plant roots. Aust. J. Biol. Sci. 1973, 26, 151–162. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, Y.L.; Jin, Y.; Zhang, T.; Guo, H.S. Colonization process of Arabidopsis thaliana roots by a green fluorescent protein-tagged isolate of Verticillium dahliae. Protein Cell 2014, 5, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Gao, J.; Zhang, G.; Yu, Y.; Zhou, H.; Chen, W.; Zhao, J. The colonization process of sunflower by a green fluorescent protein-tagged isolate of Verticillium dahliae and its seed transmission. Plant Dis. 2018, 102, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, S.; Xiong, D.; Tian, C. Genetic transformation, infection process and qPCR quantification of Verticillium dahliae on smoke-tree Cotinus coggygria. Australas. Plant Pathol. 2012, 42, 33–41. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, T.; Cui, X.; Qi, F.; Jian, G. Colonization in cotton plants by a green fluorescent protein labelled strain of Verticillium dahliae. Eur. J. Plant Pathol. 2013, 135, 867–876. [Google Scholar] [CrossRef]
- Kendall, J.; Badminton, M. Aequorea victoria bioluminescence moves into an exciting new era. Trends Biotechnol. 1998, 16, 216–224. [Google Scholar] [CrossRef]
- Meyer, A.J.; Dick, T.P. Fluorescent protein-based redox probes. Antioxid. Redox Signal. 2010, 13, 621–650. [Google Scholar] [CrossRef] [PubMed]
- Lubeck, M.; Imb, K.; Jensen, B.; Thrane, U.; Janvier, C.; Jensen, D.F. GUS and GFP transformation of the biocontrol strain Clonostachys rosea IK726 and the use of these marker genes in ecological studies. Mycol. Res. 2002, 106, 815–826. [Google Scholar] [CrossRef]
- Weid, I.V.D.; Artursson, V.; Seldin, L.; Jansson, J.K. Antifungal and root surface colonization properties of GFP-tagged Paenibacillus brasilensis PB177. World J. Microbiol. Biotechnol. 2005, 21, 1591–1597. [Google Scholar] [CrossRef]
- Mansouri, S. Developing Novel Molecular Tools to Study the Fusarium virguliforme-Soybean Interaction. Master’s Thesis, Southern Illinois University, Carbondale, IL, USA, 2007. [Google Scholar]
- Huh, W.K.; Falvo, J.V.; Gerke, L.C.; Carroll, A.S.; Howson, R.W.; Weissman, J.S.; O’Shea, E.K. Global analysis of protein localization in budding yeast. Nature 2003, 425, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Skadsen, R.W.; Hohn, T.M. Use of Fusarium graminearum transformed with GFP to follow infection patterns in barley and Arabidopsis. Physiol. Mol. Plant Pathol. 2004, 64, 45–53. [Google Scholar] [CrossRef]
- Mcdougal, R.; Yang, S.; Schwelm, A.; Stewart, A.; Bradshaw, R. A novel GFP-based approach for screening biocontrol microorganisms in vitro against Dothistroma septosporum. J. Microbiol. Methods 2011, 87, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Wang, G.; Yang, Y.; Xiao, J.; Mao, Z.; Xie, B. Microscopic analysis of the compatible and incompatible interactions between Fusarium oxysporum f. sp. conglutinans and cabbage. Eur. J. Plant Pathol. 2014, 141, 597–609. [Google Scholar] [CrossRef]
- Su, X.; Rehman, L.; Guo, H.; Li, X.; Cheng, H. The oligosaccharyl transferase subunit STT3 mediates fungal development and is required for virulence in Verticillium dahliae. Curr. Genet. 2018, 64, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Falaschi, N.; Vergara, M.; Nicoletti, F.; Vannacci, G. Use of Fusarium oxysporum f. sp. dianthi transformed with marker genes to follow colonization of carnation roots. J. Plant Pathol. 2007, 89, 47–54. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Schmidt, M.A.; Baura, V.A.; Balsanelli, E.; Wassem, R.; Yates, M.G.; Randi, M.A.; Pedrosa, F.O.; Souza, E.M. Early colonization pattern of maize (Zea mays L. Poales, Poaceae) roots by Herbaspirillum seropedicae (Burkholderiales, Oxalobacteraceae). Genet. Mol. Biol. 2008, 31, 932–937. [Google Scholar] [CrossRef]
- Ilgen, P.; Hadeler, B.; Maier, F.J.; Schäfer, W. Developing kernel and rachis node induce the trichothecene pathway of Fusarium graminearum during wheat head infection. Mol. Plant Microbe Interact. 2009, 22, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.; Palmer, A.E.; Tsien, R.Y. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, H.; Zhang, J.; Yangqi, G.; Tang, C. Transformation and selection of fluorescent protein gene labelled Verticillium dahliae. Cotton Sci. 2014, 26, 221–227. [Google Scholar]
- Shrivastava, S.; Poddar, R.; Shukla, P.; Mukhopadhyay, K. Study of codon bias perspective of fungal xylanase gene by multivariate analysis. Bioinformation 2009, 3, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Rehman, L.; Su, X.; Guo, H.; Qi, X.; Cheng, H. Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae. BMC Biotechnol. 2016, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tzima, A.K.; Paplomatas, E.J.; Tsitsigiannis, D.I.; Kang, S. The G protein β subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 2012, 49, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Lorang, J.M.; Tuori, R.P.; Martinez, J.P.; Sawyer, T.L.; Redman, R.S.; Rollins, J.A.; Wolpert, T.J.; Johnson, K.B.; Rodriguez, R.J.; Dickman, M.B. Green fluorescent protein is lighting up fungal biology. Appl. Environ. Microbiol. 2001, 67, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, B.G.; Garber, R.C.; Yoder, C.O. Development of a fungal transformation system based on selection of sequences with promoter activity. Mol. Cell. Biol. 1987, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ando, A.; Sakuradani, E.; Kikukawa, H.; Kamada, N.; Ochiai, M.; Shima, J.; Ogawa, J. Selection and characterization of promoters based on genomic approach for the molecular breeding of oleaginous fungus Mortierella alpina 1S-4. Curr. Genet. 2014, 60, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Arazoe, T.; Miyoshi, K.; Yamato, T.; Ogawa, T.; Ohsato, S.; Arie, T.; Kuwata, S. Tailor-made CRISPR/Cas system for highly efficient targeted gene replacement in the rice blast fungus. Biotechnol. Bioeng. 2015, 112, 2543–2549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Meng, X.; Wei, X.; Lu, L. Highly efficient CRISPR mutagenesis by microhomology-mediated end joining in Aspergillus fumigatus. Fungal Genet. Biol. 2016, 86, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Ni, H.; Xu, Y.; Chen, Q.; Jiang, L. CRISPR/Cas9-mediated base-editing system efficiently generates gain-of-function mutations in Arabidopsis. Sci. China Life Sci. 2017, 60, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Haga, N.; Harumoto, T.; Matsuura, T.; Mitsui, Y. Transformation of Paramecium caudatum with a novel expression vector harboring codon-optimized GFP gene. Gene 2002, 284, 233–240. [Google Scholar] [CrossRef]
- Martinezjaramillo, E.; Garzamorales, R.; Loeraarias, M.J.; Saucedocardenas, O.; Montesdeocaluna, R.; Mcnally, L.R.; Gomezgutierrez, J.G. Development of Lactococcus lactis encoding fluorescent proteins, GFP, mCherry and iRFP regulated by the nisin-controlled gene expression system. Stain Technol. 2017, 92, 167–174. [Google Scholar] [CrossRef]
- Wu, L.; Conner, R.L.; Wang, X.; Xu, R.; Li, H. Variation in growth, colonization of maize, and metabolic parameters of GFP- and DsRed-labeled Fusarium verticillioides strains. Phytopathology 2016, 106, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.E.; Howard, R.J. Biology of maize kernel infection by Fusarium verticillioides. Mol. Plant-Microbe Interact. 2010, 23, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Sempere, A.; López-Pérez, M.; Martínez-Culebras, P.V.; González-Candelas, L. Development of a green fluorescent tagged strain of Aspergillus carbonarius to monitor fungal colonization in grapes. Int. J. Food Microbiol. 2011, 148, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.A. The development of lignitubers in roots after infection by Verticillium dahliae Kleb. Can. J. Microbiol. 1971, 17, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Gerik, J.S.; Huisman, O.C. Study of field-grown cotton roots infected with Verticillium dahliae using an immunoenzymatic staining technique. Phytopathology 1988, 78, 1174–1178. [Google Scholar] [CrossRef]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; von Tiedemann, A. Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]
- Reusche, M.; Truskina, J.; Thole, K.; Nagel, L.; Rindfleisch, S.; Tran, V.T.; Braus-Stromeyer, S.A.; Braus, G.H.; Teichmann, T.; Lipka, V. Infections with the vascular pathogens Verticillium longisporum and Verticillium dahliae induce distinct disease symptoms and differentially affect drought stress tolerance of Arabidopsis thaliana. Environ. Exp. Bot. 2014, 108, 23–37. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhou, T.T.; Guo, H.S. Hyphopodium-epecific VdNoxB/VdPls1-dependent ROS-Ca2+ signaling is required for plant infection by Verticillium dahliae. PLoS Pathog. 2016, 12, e1005793. [Google Scholar] [CrossRef] [PubMed]
- Ralhan, A.; Schottle, S.; Thurow, C.; Iven, T.; Feussner, I.; Polle, A.; Gatz, C. The vascular pathogen Verticillium longisporum requires a jasmonic acid-independent COI1 function in roots to elicit disease symptoms in Arabidopsis shoots. Plant Physiol. 2012, 159, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Dimond, A.E. Biophysics and biochemistry of the vascular wilt syndrome. Annu. Rev. Phytopathol. 1970, 8, 301–322. [Google Scholar] [CrossRef]

| Primers | Sequence (5′-3′) |
|---|---|
| Ptrpc | atcAAGCTTTTGAAGGAGCATTTTTGGGCTTGGC |
| CTCGCCCTTGGAGACCATGGTGGCATCGATGCTTGGGTAG | |
| Pmch | ATGGTCTCCAAGGGCGAGGAGGACAAC |
| CTACTTGTAGAGCTCGTCCATGCCGCC | |
| Ttrpc | CATGGACGAGCTCTACAAGTAGAGTAGATGCCGACCGGGATCC |
| aacTCTAGATTATCTTTGCGAACCCAGGGGCTG | |
| qRT-VdITS | CCGCCGGTCCATCAGTCTCTCTGTTTATAC |
| CGCCTGCGGGACTCCGATGCGAGCTGTAAC | |
| qRT-Nbactin | GGACCTTTATGGAAACATTGTGCTCAGT |
| CCAAGATAGAACCTCCAATCCAGACAC | |
| Det-hpt | GAGGGCGAAGAATCTCGTGCTTTCA |
| TGTTATGCGGCCATTGTCCGTCAGG | |
| Det-mch | CTACGTTAAGCACCCCGCCGACATT |
| CTGCTCGACAATCGTGTAGTCCTCGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Lu, G.; Rehman, L.; Li, X.; Sun, L.; Guo, H.; Cheng, H. mCherry-Labeled Verticillium dahliae Could Be Utilized to Investigate Its Pathogenicity Process in Nicotiana benthamiana. Genes 2018, 9, 508. https://doi.org/10.3390/genes9100508
Su X, Lu G, Rehman L, Li X, Sun L, Guo H, Cheng H. mCherry-Labeled Verticillium dahliae Could Be Utilized to Investigate Its Pathogenicity Process in Nicotiana benthamiana. Genes. 2018; 9(10):508. https://doi.org/10.3390/genes9100508
Chicago/Turabian StyleSu, Xiaofeng, Guoqing Lu, Latifur Rehman, Xiaokang Li, Lu Sun, Huiming Guo, and Hongmei Cheng. 2018. "mCherry-Labeled Verticillium dahliae Could Be Utilized to Investigate Its Pathogenicity Process in Nicotiana benthamiana" Genes 9, no. 10: 508. https://doi.org/10.3390/genes9100508
APA StyleSu, X., Lu, G., Rehman, L., Li, X., Sun, L., Guo, H., & Cheng, H. (2018). mCherry-Labeled Verticillium dahliae Could Be Utilized to Investigate Its Pathogenicity Process in Nicotiana benthamiana. Genes, 9(10), 508. https://doi.org/10.3390/genes9100508




