Methods for Using Small Non-Coding RNAs to Improve Recombinant Protein Expression in Mammalian Cells
Abstract
1. Introduction
2. MicroRNA Screening Tools
2.1. Utilization of Previously Identified microRNAs
2.2. Microarrays Utilization
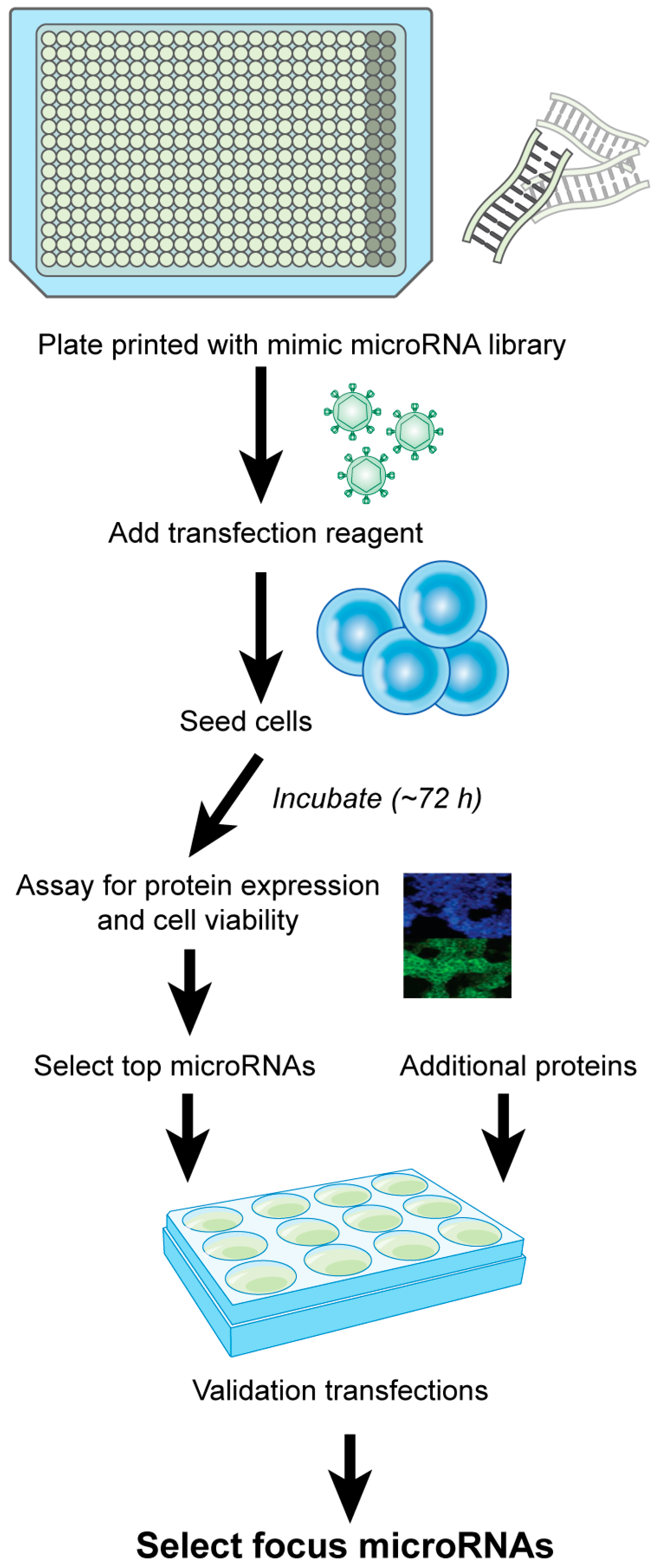
2.3. microRNA Library Screen
2.4. Next Generation Sequencing
3. Bioinformatics Methodologies
4. Additional Non-Coding RNA
4.1. Short Hairpin RNA
4.2. Small Interfering RNA
4.3. Mitochondrial Genome-Encoded Small RNA
4.4. SINEUP RNA Levels
5. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wells, E.; Robinson, A.S. Cellular engineering for therapeutic protein production: Product quality, host modification, and process improvement. Biotechnol. J. 2017, 12, 1600105. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.C.; Krummen, L. Recombinant protein expression for therapeutic applications. Curr. Opin. Biotechnol. 2002, 13, 117–123. [Google Scholar] [CrossRef]
- Kunert, R.; Reinhart, D. Advances in recombinant antibody manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.; Spearman, M. The choice of mammalian cell host and possibilities for glycosylation engineering. Curr. Opin. Biotechnol. 2014, 30, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Omasa, T.; Onitsuka, M.; Kim, W.D. Cell engineering and cultivation of Chinese hamster ovary (CHO) cells. Curr. Pharm. Biotechnol. 2010, 11, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.; Kim, Y.G.; Koo, J.; Lee, G.M. Assessment of cell engineering strategies for improved therapeutic protein production in CHO cells. Biotechnol. J. 2008, 3, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Handrick, R.; Otte, K. The art of CHO cell engineering: A comprehensive retrospect and future perspectives. Biotechnol. Adv. 2015, 33, 1878–1896. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Smart, T.G. HEK293 cell line: A vehicle for the expression of recombinant proteins. J. Pharmacol. Toxicol. Methods 2005, 51, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Dumont, J.; Euwart, D.; Mei, B.; Estes, S.; Kshirsagar, R. Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Crit. Rev. Biotechnol. 2016, 36, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Nettleship, J.E.; Watson, P.J.; Rahman-Huq, N.; Fairall, L.; Posner, M.G.; Upadhyay, A.; Reddivari, Y.; Chamberlain, J.M.; Kolstoe, S.E.; Bagby, S.; et al. Transient expression in HEK 293 cells: An alternative to E. Coli for the production of secreted and intracellular mammalian proteins. Methods Mol. Biol. 2015, 1258, 209–222. [Google Scholar] [PubMed]
- Silva, A.C.; Fernandes, P.; Sousa, M.F.; Alves, P.M. Scalable production of adenovirus vectors. Methods Mol. Biol. 2014, 1089, 175–196. [Google Scholar] [PubMed]
- Stepanenko, A.A.; Dmitrenko, V.V. HEK293 in cell biology and cancer research: Phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 2015, 569, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Mammalian RNAi for the masses. Trends Genet. 2003, 19, 9–12. [Google Scholar] [CrossRef]
- McManus, M.T.; Sharp, P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002, 3, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Wadhwa, R.; Taira, K. World of small RNAs: From ribozymes to siRNA and miRNA. Differentiation 2004, 72, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cora, D.; Re, A.; Caselle, M.; Bussolino, F. MicroRNA-mediated regulatory circuits: Outlook and perspectives. Phys. Biol. 2017, 14, 045001. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.D.; Parsons, C.; Walker, L.; Zhang, W.C.; Slack, F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017, 127, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Hackl, M.; Druz, A.; Shridhar, S.; Chung, C.Y.; Heffner, K.M.; Kreil, D.P.; Betenbaugh, M.; Shiloach, J.; Barron, N.; et al. CHO microRNA engineering is growing up: Recent successes and future challenges. Biotechnol. Adv. 2013, 31, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.Y.; Lee, K.H. MiRNA expression in CHO: Nature knows best. Biotechnol. J. 2014, 9, 459–460. [Google Scholar] [CrossRef]
- Barron, N.; Sanchez, N.; Kelly, P.; Clynes, M. MicroRNAs: Tiny targets for engineering CHO cell phenotypes? Biotechnol. Lett. 2011, 33, 11–21. [Google Scholar] [CrossRef]
- Hackl, M.; Borth, N.; Grillari, J. MiRNAs—Pathway engineering of CHO cell factories that avoids translational burdening. Trends Biotechnol. 2012, 30, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Katinger, H.; Grillari, J. MicroRNAs as targets for engineering of CHO cell factories. Trends Biotechnol. 2008, 26, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Gammell, P.; Barron, N.; Kumar, N.; Clynes, M. Initial identification of low temperature and culture stage induction of miRNA expression in suspension CHO-k1 cells. J. Biotechnol. 2007, 130, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.S.; Gallagher, C.; Clynes, M.; Barron, N. Conserved microRNA function as a basis for Chinese hamster ovary cell engineering. Biotechnol. Lett. 2015, 37, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.S.; Breen, L.; Gallagher, C.; Kelly, S.; Henry, M.; Lao, N.T.; Meleady, P.; O’Gorman, D.; Clynes, M.; Barron, N. Re-programming CHO cell metabolism using miR-23 tips the balance towards a highly productive phenotype. Biotechnol. J. 2015, 10, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.C.; Lee, Y.Y.; Chang, S.Q.; Nissom, P.M. Identification and expression analysis of miRNAs during batch culture of HEK-293 cells. J. Biotechnol. 2009, 140, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Barron, N.; Kumar, N.; Sanchez, N.; Doolan, P.; Clarke, C.; Meleady, P.; O’Sullivan, F.; Clynes, M. Engineering CHO cell growth and recombinant protein productivity by overexpression of miR-7. J. Biotechnol. 2011, 151, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Druz, A.; Chu, C.; Majors, B.; Santuary, R.; Betenbaugh, M.; Shiloach, J. A novel microRNA mmu-miR-466h affects apoptosis regulation in mammalian cells. Biotechnol. Bioeng. 2011, 108, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Davis, A.; Bahr, S.; Borgschulte, T.; Achtien, K.; Kayser, K. Profiling highly conserved microRNA expression in recombinant IgG-producing and parental Chinese hamster ovary cells. Biotechnol. Prog. 2011, 27, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Maccani, A.; Hackl, M.; Leitner, C.; Steinfellner, W.; Graf, A.B.; Tatto, N.E.; Karbiener, M.; Scheideler, M.; Grillari, J.; Mattanovich, D.; et al. Identification of microRNAs specific for high producer CHO cell lines using steady-state cultivation. Appl. Microbiol. Biotechnol. 2014, 98, 7535–7548. [Google Scholar] [CrossRef] [PubMed]
- Emmerling, V.V.; Fischer, S.; Stiefel, F.; Holzmann, K.; Handrick, R.; Hesse, F.; Horer, M.; Kochanek, S.; Otte, K. Temperature-sensitive miR-483 is a conserved regulator of recombinant protein and viral vector production in mammalian cells. Biotechnol. Bioeng. 2016, 113, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Klanert, G.; Jadhav, V.; Shanmukam, V.; Diendorfer, A.; Karbiener, M.; Scheideler, M.; Bort, J.H.; Grillari, J.; Hackl, M.; Borth, N. A signature of 12 microRNAs is robustly associated with growth rate in a variety of CHO cell lines. J. Biotechnol. 2016, 235, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Strotbek, M.; Florin, L.; Koenitzer, J.; Tolstrup, A.; Kaufmann, H.; Hausser, A.; Olayioye, M.A. Stable microRNA expression enhances therapeutic antibody productivity of Chinese hamster ovary cells. Metab. Eng. 2013, 20, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Buck, T.; Wagner, A.; Ehrhart, C.; Giancaterino, J.; Mang, S.; Schad, M.; Mathias, S.; Aschrafi, A.; Handrick, R.; et al. A functional high-content miRNA screen identifies miR-30 family to boost recombinant protein production in CHO cells. Biotechnol. J. 2014, 9, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Chen, Y.C.; Betenbaugh, M.J.; Martin, S.E.; Shiloach, J. MiRNA mimic screen for improved expression of functional neurotensin receptor from HEK293 cells. Biotechnol. Bioeng. 2015, 112, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J.; Reilly, D.; Martin, S.E.; Wong, A.W. Identification of a novel miRNA that increases transient protein expression in combination with valproic acid. Biotechnol. Prog. 2017, 33, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Hackl, M.; Jakobi, T.; Blom, J.; Doppmeier, D.; Brinkrolf, K.; Szczepanowski, R.; Bernhart, S.H.; Honer Zu Siederdissen, C.; Bort, J.A.; Wieser, M.; et al. Next-generation sequencing of the Chinese hamster ovary microRNA transcriptome: Identification, annotation and profiling of microRNAs as targets for cellular engineering. J. Biotechnol. 2011, 153, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Hackl, M.; Bort, J.A.; Wieser, M.; Harreither, E.; Kunert, R.; Borth, N.; Grillari, J. A screening method to assess biological effects of microRNA overexpression in Chinese hamster ovary cells. Biotechnol. Bioeng. 2012, 109, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.P.; Loo, B.; Zhou, L.; Zhang, P.; Lee, D.Y.; Yang, Y.; Lam, K.P. Overexpression of microRNAs enhances recombinant protein production in Chinese hamster ovary cells. Biotechnol. J. 2014, 9, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Pfizenmaier, J.; Junghans, L.; Teleki, A.; Takors, R. Hyperosmotic stimulus study discloses benefits in ATP supply and reveals miRNA/mRNA targets to improve recombinant protein production of CHO cells. Biotechnol. J. 2016, 11, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, F.; Fischer, S.; Sczyrba, A.; Otte, K.; Hesse, F. MiRNA profiling of high, low and non-producing CHO cells during biphasic fed-batch cultivation reveals process relevant targets for host cell engineering. J. Biotechnol. 2016, 225, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jiang, F. MicroRNA (miRNA) profiling. Methods Mol. Biol. 2016, 1381, 151–161. [Google Scholar] [PubMed]
- Meleady, P.; Gallagher, M.; Clarke, C.; Henry, M.; Sanchez, N.; Barron, N.; Clynes, M. Impact of miR-7 over-expression on the proteome of Chinese hamster ovary cells. J. Biotechnol. 2012, 160, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, N.; Kelly, P.; Gallagher, C.; Lao, N.T.; Clarke, C.; Clynes, M.; Barron, N. CHO cell culture longevity and recombinant protein yield are enhanced by depletion of miR-7 activity via sponge decoy vectors. Biotechnol. J. 2014, 9, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Druz, A.; Betenbaugh, M.; Shiloach, J. Glucose depletion activates mmu-miR-466h-5p expression through oxidative stress and inhibition of histone deacetylation. Nucleic Acids Res. 2012, 40, 7291–7302. [Google Scholar] [CrossRef] [PubMed]
- Druz, A.; Son, Y.J.; Betenbaugh, M.; Shiloach, J. Stable inhibition of mmu-miR-466h-5p improves apoptosis resistance and protein production in CHO cells. Metab. Eng. 2013, 16, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Mathias, S.; Schaz, S.; Emmerling, V.V.; Buck, T.; Kleemann, M.; Hackl, M.; Grillari, J.; Aschrafi, A.; Handrick, R.; et al. Enhanced protein production by microRNA-30 family in CHO cells is mediated by the modulation of the ubiquitin pathway. J. Biotechnol. 2015, 212, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Handrick, R.; Aschrafi, A.; Otte, K. Unveiling the principle of microRNA-mediated redundancy in cellular pathway regulation. RNA Biol. 2015, 12, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Paul, A.J.; Wagner, A.; Mathias, S.; Geiss, M.; Schandock, F.; Domnowski, M.; Zimmermann, J.; Handrick, R.; Hesse, F.; et al. MiR-2861 as novel HDAC5 inhibitor in CHO cells enhances productivity while maintaining product quality. Biotechnol. Bioeng. 2015, 112, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Schoellhorn, M.; Fischer, S.; Wagner, A.; Handrick, R.; Otte, K. MiR-143 targets MAPK7 in CHO cells and induces a hyperproductive phenotype to enhance production of difficult-to-express proteins. Biotechnol. Prog. 2017, 33, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Marquart, K.F.; Pieper, L.A.; Fieder, J.; Gamer, M.; Gorr, I.; Schulz, P.; Bradl, H. MiRNA engineering of CHO cells facilitates production of difficult-to-express proteins and increases success in cell line development. Biotechnol. Bioeng. 2017, 114, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, V.; Hackl, M.; Klanert, G.; Hernandez Bort, J.A.; Kunert, R.; Grillari, J.; Borth, N. Stable overexpression of miR-17 enhances recombinant protein production of CHO cells. J. Biotechnol. 2014, 175, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.P.; Yang, Y.S.; Lam, K.P. MiR-92a enhances recombinant protein productivity in CHO cells by increasing intracellular cholesterol levels. Biotechnol. J. 2017, 12, 160048. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Kurgan, L. Comprehensive overview and assessment of computational prediction of microRNA targets in animals. Brief. Bioinform. 2015, 16, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Varghese, V.K.; Kabekkodu, S.P.; Mallya, S.; Satyamoorthy, K. A compilation of web-based research tools for miRNA analysis. Brief. Funct. Genom. 2017, 16, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Chang, N.W.; Shrestha, S.; Hsu, S.D.; Lin, Y.L.; Lee, W.H.; Yang, C.D.; Hong, H.C.; Wei, T.Y.; Tu, S.J.; et al. MiRTarBase 2016: Updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2016, 44, D239–D247. [Google Scholar] [CrossRef] [PubMed]
- Dweep, H.; Sticht, C.; Pandey, P.; Gretz, N. MiRWalk—Database: Prediction of possible miRNA binding sites by “Walking” The genes of three genomes. J. Biomed. Inform. 2011, 44, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. MiRbase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [PubMed]
- Kertesz, M.; Iovino, N.; Unnerstall, U.; Gaul, U.; Segal, E. The role of site accessibility in microRNA target recognition. Nat. Genet. 2007, 39, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Rehmsmeier, M.; Steffen, P.; Hochsmann, M.; Giegerich, R. Fast and effective prediction of microRNA/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, M.D.; Georgakilas, G.; Kostoulas, N.; Vlachos, I.S.; Vergoulis, T.; Reczko, M.; Filippidis, C.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013, 41, W169–W173. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Gaidatzis, D.; van Nimwegen, E.; Hausser, J.; Zavolan, M. Inference of miRNA targets using evolutionary conservation and pathway analysis. BMC Bioinform. 2007, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Wang, X. MiRDB: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–D152. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Huang, X.; Muruganujan, A.; Tang, H.; Mills, C.; Kang, D.; Thomas, P.D. PANTHER version 11: Expanded annotation data from gene ontology and reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 2017, 45, D183–D189. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. Edger: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Nueda, M.J.; Ferrer, A.; Talon, M. Masigpro: A method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics 2006, 22, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.; Navon, R.; Steinfeld, I.; Lipson, D.; Yakhini, Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 2009, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Blake, J.A.; Eppig, J.T.; Kadin, J.A.; Richardson, J.E.; Smith, C.L.; Bult, C.J. Mouse genome database (MGD)-2017: Community knowledge resource for the laboratory mouse. Nucleic Acids Res. 2017, 45, D723–D729. [Google Scholar] [CrossRef] [PubMed]
- Stiefel, F.; Fischer, S.; Hackl, M.; Handrick, R.; Hesse, F.; Borth, N.; Otte, K.; Grillari, J. Noncoding RNAs, post-transcriptional RNA operons and Chinese hamster ovary cells. Pharm Bioprocess 2015, 3, 227–247. [Google Scholar] [CrossRef]
- Paddison, P.J.; Caudy, A.A.; Bernstein, E.; Hannon, G.J.; Conklin, D.S. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002, 16, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.W.; Wu, S.C. A novel RNA silencing vector to improve antigen expression and stability in Chinese hamster ovary cells. Vaccine 2007, 25, 4103–4111. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Hong, W.W.L.; Liu, J.H. Short hairpin RNA targeted to dihydrofolate reductase enhances the immunoglobulin g expression in gene-amplified stable Chinese hamster ovary cells. Vaccine 2008, 26, 4969–4974. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. SiRNA versus miRNA as therapeutics for gene silencing. Mol. Ther.-Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Kuni-Kamochi, R.; Yamane-Ohnuki, N.; Wakitani, M.; Yamano, K.; Imai, H.; Kanda, Y.; Niwa, R.; Iida, S.; Uchida, K.; et al. Engineering Chinese hamster ovary cells to maximize effector function of produced antibodies using FUT8 siRNA. Biotechnol. Bioeng. 2004, 88, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.H.; Hwang, S.J.; Lee, G.M. Influence of down-regulation of caspase-3 by siRNAs on sodium-butyrate-induced apoptotic cell death of Chinese hamster ovary cells producing thrombopoietin. Metab. Eng. 2005, 7, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.F.; Chuan, K.H.; Liu, S.; Loh, S.O.; Chung, B.Y.; Ong, C.C.; Song, Z. RNAi suppression of Bax and Bak enhances viability in fed-batch cultures of CHO cells. Metab. Eng. 2006, 8, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.; Lee, K.H. RNA interference of cofilin in Chinese hamster ovary cells improves recombinant protein productivity. Biotechnol. Bioeng. 2012, 109, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Chen, Y.C.; Buehler, E.; Mandal, S.; Mandal, A.; Betenbaugh, M.; Park, M.H.; Martin, S.; Shiloach, J. Genome-scale RNA interference screen identifies antizyme 1 (OAZ1) as a target for improvement of recombinant protein production in mammalian cells. Biotechnol. Bioeng. 2016, 113, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Ro, S.; Ma, H.Y.; Park, C.; Ortogero, N.; Song, R.; Hennig, G.W.; Zheng, H.; Lin, Y.M.; Moro, L.; Hsieh, J.T.; et al. The mitochondrial genome encodes abundant small noncoding RNAs. Cell Res. 2013, 23, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Pieper, L.A.; Strotbek, M.; Wenger, T.; Gamer, M.; Olayioye, M.A.; Hausser, A. Secretory pathway optimization of CHO producer cells by co-engineering of the mitosRNA-1978 target genes CerS2 and Tbc1D20. Metab. Eng. 2017, 40, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, S.; Fasolo, F.; Russo, R.; Cimatti, L.; Patrucco, L.; Takahashi, H.; Jones, M.H.; Santoro, C.; Sblattero, D.; Cotella, D.; et al. SINEUPs are modular antisense long non-coding RNAs that increase synthesis of target proteins in cells. Front. Cell. Neurosci. 2015, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Zucchelli, S.; Patrucco, L.; Persichetti, F.; Gustincich, S.; Cotella, D. Engineering translation in mammalian cell factories to increase protein yield: The unexpected use of long non-coding SINEUP RNAs. Comput. Struct. Biotechnol. J. 2016, 14, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Patrucco, L.; Chiesa, A.; Soluri, M.F.; Fasolo, F.; Takahashi, H.; Carninci, P.; Zucchelli, S.; Santoro, C.; Gustincich, S.; Sblattero, D.; et al. Engineering mammalian cell factories with SINEUP noncoding RNAs to improve translation of secreted proteins. Gene 2015, 569, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Git, A.; Dvinge, H.; Salmon-Divon, M.; Osborne, M.; Kutter, C.; Hadfield, J.; Bertone, P.; Caldas, C. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA 2010, 16, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Willenbrock, H.; Salomon, J.; Søkilde, R.; Barken, K.B.; Hansen, T.N.; Nielsen, F.C.; Møller, S.; Litman, T. Quantitative mirna expression analysis: Comparing microarrays with next-generation sequencing. RNA 2009, 15, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
| Year | Initial Screen | Researchers | Type of Cells | Conditions Evaluated in Initial Screen | Reference |
|---|---|---|---|---|---|
| Previously identified microRNAs | |||||
| 2015 | miR mimics and mir-34 sponge decoy | Kelly et al. | CHO | apoptosis and cell growth | [26] |
| 2015 | miR mimics and mir-23 sponge decoy | Kelly et al. | CHO | energy metabolism | [27] |
| Microarray | |||||
| 2007 | human, mouse and rat microRNA arrays | Gammell et al. | CHO | temperature shift | [25] |
| 2009 | human and mouse microRNA arrays | Koh et al. | HEK293 | 3 stages of batch culture | [28] |
| 2011 | human microRNA arrays | Barron et al. | CHO | temperature shift | [29] |
| 2011 | mouse and rat microRNA arrays | Druz et al. | CHO | apoptosis | [30] |
| 2011 | human, mouse and rat microRNA arrays | Lin et al. | CHO | producing lines compared to parental and MTX amplification | [31] |
| 2014 | cross-species microRNA and mRNA arrays | Maccani et al. | CHO | high producing cell lines compared to low producing cell lines | [32] |
| 2016 | human for HELA, mouse, rat and human for CHO microRNA arrays | Emmerling et al. | HELA and CHO | mild hypothermia | [33] |
| 2016 | human, mouse, rat, viral microRNAs | Klanert et al. | CHO | growth rate in multiple cell lines | [34] |
| microRNA screen | |||||
| 2013 | human microRNA library | Strotbek et al. | CHO | IgG | [35] |
| 2014 | murine microRNA library | Fischer et al. | CHO | SEAP | [36] |
| 2015 | human microRNA library | Xiao et al. | HEK293 | neurotensin receptor | [37] |
| 2017 | human microRNA library | Meyer et al. | HEK293 | antibody | [38] |
| Next Generation Sequencing | |||||
| 2011 | small RNA transcriptome | Hackl et al. | CHO | identified conserved and novel CHO microRNAs | [39] |
| 2012 | microRNA | Jadhav et al. | CHO | effects of overexpressing microRNA | [40] |
| 2014 | microRNA | Loh et al. | CHO | looking at profile of different expression level cultures | [41] |
| 2016 | microRNA and mRNA | Pfizenmaier et al. | CHO | osmotic shift | [42] |
| 2016 | microRNA | Stiefel et al. | CHO | biphasic fed batch cultivation of high low and non-producing CHO lines with mild hypothermia | [43] |
| microRNA Target Prediction | |||
| miRwalk | Collection of experimentally validated and predicted microRNA binding sites from multiple resources | http://mirwalk.uni-hd.de/ | [60] |
| miRbase | Collection that provides a registry of published microRNA sequences | http://www.mirbase.org/ | [61] |
| miRANDA algorithm | Algorithm that predicts microRNA targets based on sequence complementarity, energy binding and evolutionary conservation | http://www.microrna.org/ | [62] |
| PITA | Database based on algorithms predicting targets based on site accessibility | https://genie.weizmann.ac.il/pubs/mir07/index.html | [63] |
| RNAhybrid | Database based on algorithms predicting targets based on minimum free energy hybridization | https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid/ | [64] |
| DIANA tools | Database based on algorithms predicting targets based on site recognition | http://diana.imis.athena-innovation.gr/DianaTools/index.php | [65] |
| targetScan | Database based on algorithms predicting targets based on site recognition | http://www.targetscan.org/vert_71/ | [66] |
| EiMMo | Database based on algorithms predicting targets based on site recognition | http://www.clipz.unibas.ch//ElMMo3/index.php | [67] |
| miRtarbase | Database based on experimentally validated microRNA/mRNA interactions | http://mirtarbase.mbc.nctu.edu.tw/ | [59] |
| mirdb | Database for microRNA target prediction and functional annotation | http://mirdb.org/ | [68] |
| DAVID | Database for identifying gene ontology but can and has also been used for identifying microRNA targets | https://david.ncifcrf.gov/ | [69] |
| Biological Processes, Gene Ontology and Protein Identification | |||
| PANTHER | Database for gene ontology and gene clustering analysis and gene products | http://pantherdb.org/ | [70] |
| MASCOT | Software program for identifying proteins | http://www.matrixscience.com/ | |
| HomoloGene | Database containing information about genes that have been used to study homology between species as well as for providing information about gene function | https://www.ncbi.nlm.nih.gov/homologene | |
| GeneCards | Database containing information about genes that have been used to study homology between species as well as for providing information about gene function | http://www.genecards.org/ | |
| BLAST | Basic local alignment search tool (i.e., Blast) utilizes the discontiguous megablast algorithm can be used to align gene sequences between species | https://blast.ncbi.nlm.nih.gov/Blast.cgi?CMD=Web&PAGE_TYPE=BlastHome | |
| edgeR | “R” software program package for differential expression analysis of RNA-seq data | https://bioconductor.org/packages/release/bioc/html/edgeR.html | [71] |
| maSigPro | “R” software program package for regression analysis and differential expression analysis of microarray and RNA-seq data | https://bioconductor.org/packages/release/bioc/html/maSigPro.html | [72] |
| LIMMA | “R” software program package for linear models and differential expression analysis of microarray data | https://bioconductor.org/packages/release/bioc/html/limma.html | [73] |
| Gorilla | Tool for identifying enriched gene ontology terms | http://cbl-gorilla.cs.technion.ac.il/ | [74] |
| MGI Gene Ontology Term Finder | Gene ontology database primarily for mouse genes | http://www.informatics.jax.org/ | [75] |
| Vmatch | Sequence analysis software | http://www.vmatch.de/ | |
| MetaCore | Pathway and network analysis software | https://clarivate.com/products/metacore/ | |
| Ingenuity Pathways Analysis | Pathway and network analysis software | https://www.qiagen.com/us/shop/analytics-software/biological-data-tools/ingenuity-pathway-analysis/ | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inwood, S.; Betenbaugh, M.J.; Shiloach, J. Methods for Using Small Non-Coding RNAs to Improve Recombinant Protein Expression in Mammalian Cells. Genes 2018, 9, 25. https://doi.org/10.3390/genes9010025
Inwood S, Betenbaugh MJ, Shiloach J. Methods for Using Small Non-Coding RNAs to Improve Recombinant Protein Expression in Mammalian Cells. Genes. 2018; 9(1):25. https://doi.org/10.3390/genes9010025
Chicago/Turabian StyleInwood, Sarah, Michael J. Betenbaugh, and Joseph Shiloach. 2018. "Methods for Using Small Non-Coding RNAs to Improve Recombinant Protein Expression in Mammalian Cells" Genes 9, no. 1: 25. https://doi.org/10.3390/genes9010025
APA StyleInwood, S., Betenbaugh, M. J., & Shiloach, J. (2018). Methods for Using Small Non-Coding RNAs to Improve Recombinant Protein Expression in Mammalian Cells. Genes, 9(1), 25. https://doi.org/10.3390/genes9010025





