Differential Preference of Burkholderia and Mesorhizobium to pH and Soil Types in the Core Cape Subregion, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Nodule Sampling and Rhizobia Isolation
2.2. DNA Extraction, Amplification and Sequencing
2.3. Contig Assembly and Phylogenetic Analyses
2.4. Determination of Phylogenetic Signals
3. Results
3.1. Strain Identification and Phylogenetic Analyses
3.2. Analyses of Phylogenetic Signals
3.3. Diversity of Rhizobial Symbionts of Indigofera superba
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martiny, J.B.H.; Bohannan, B.J.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Queloz, V.; Sieber, T.N.; Holdenrieder, O.; McDonald, B.A.; Grünig, C.R. No biogeographical pattern for a root-associated fungal species complex. Glob. Ecol. Biogeogr. 2011, 20, 160–169. [Google Scholar] [CrossRef]
- Yang, J.; Smith, H.G.; Sherratt, T.N.; Wilkinson, D.M. Is there a size limit for cosmopolitan distribution in free-living microorganisms? A biogeographical analysis of testate amoebae from polar areas. Microb. Ecol. 2010, 59, 635–645. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, M.A. The nineteenth century roots of ‘Everything is Everywhere’. Nat. Rev. Microbiol. 2007, 5, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Fontaneto, D.; Barraclough, T.G.; Chen, K.; Ricci, C.; Herniou, E.A. molecular evidence for broad-scale distributions in bdelloid rotifers: Everything is not everywhere but most things are very widespread. Mol. Ecol. 2008, 17, 3136–3146. [Google Scholar] [CrossRef] [PubMed]
- Fontaneto, D.; Hortal, J. Microbial biogeography: Is everything small everywhere. In Microbial Ecological Theory: Current Perspectives; Ogilvie, L.A., Hirsch, P.R., Eds.; Caister Academic Press: Norfolk, UK, 2012; pp. 87–98. [Google Scholar]
- Geml, J. Altitudinal Gradients in Mycorrhizal Symbioses. In Biogeography of Mycorrhizal Symbiosis; Tedersoo, L., Ed.; Springer: New York, NY, USA, 2017; pp. 107–123. [Google Scholar]
- Rout, M.E.; Callaway, R.M. Interactions between exotic invasive plants and soil microbes in the rhizosphere suggest that ‘everything is not everywhere’. Ann. Bot. 2012, 110, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramírez, S.; Wilson, A.W.; Ryberg, M. Overview of phylogenetic approaches to mycorrhizal biogeography, diversity and evolution. In Biogeography of Mycorrhizal Symbiosis; Tedersoo, L., Ed.; Springer: New York, NY, USA, 2017; pp. 1–37. [Google Scholar]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil pH drives the spatial distribution of bacterial communities along elevation on changbai mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Telford, R.J.; Vandvik, V.; Birks, H.J.B. Dispersal limitations matter for microbial morphospecies. Science 2006, 312, 1015. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, R.J.; Grogan, D.W.; Taylor, J.W. Geographic barriers isolate endemic populations of hyperthermophilic archaea. Science 2003, 301, 976–978. [Google Scholar] [CrossRef] [PubMed]
- Sprent, J.I.; Ardley, J.; James, E.K. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytol. 2017, 215, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Bontemps, C.; Elliott, G.N.; Simon, M.F.; Dos Reis Junior, F.B.; Gross, E.; Lawton, R.C.; Neto, N.E.; Louriero, D.M.; De Faria, S.M.; Sprent, J.I. Burkholderia species are ancient symbionts of legumes. Mol. Ecol. 2010, 19, 44–52. [Google Scholar] [PubMed]
- Bontemps, C.; Rogel, M.A.; Wiechmann, A.; Mussabekova, A.; Moody, S.; Simon, M.F.; Moulin, L.; Elliott, G.N.; Lacercat-Didier, L.; Dasilva, C. Endemic Mimosa species from Mexico prefer alphaproteobacterial rhizobial symbionts. New Phytol. 2016, 209, 319–333. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.E.; Briscoe, L.; Martínez-Hidalgo, P.; Agapakis, C.M.; de-los Santos, P.E.; Seshadri, R.; Reeve, W.; Weinstock, G.; O’Hara, G.; Howieson, J.G. Symbiotic Burkholderia species show diverse arrangements of nif/fix and nod genes and lack typical high-affinity cytochrome cbb3 oxidase genes. Mol. Plant Microbe Interact. 2016, 29, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Z.; Wang, R.; Liu, R.R.; Chen, J.J.; Wei, Q.; Wu, X.Y.; Pang, X.W.; James, E.K.; Liu, X.Y. The structure and evolution of beta-rhizobial symbiotic genes deduced from their complete genomes. Immunome Res. 2017, 13. [Google Scholar] [CrossRef]
- Lemaire, B.; Chimphango, S.B.; Stirton, C.; Rafudeen, S.; Honnay, O.; Smets, E.; Chen, W.M.; Sprent, J.; James, E.K.; Muasya, A.M. Biogeographical patterns of legume-nodulating Burkholderia spp.: From African Fynbos to continental scales. Appl. Environ. Microbiol. 2016, 82, 5099–5115. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Tak, N.; Rathi, S.; Chouhan, B.; Rao, S.R.; Barik, S.K.; Joshi, S.R.; Sprent, J.I.; James, E.K.; Gehlot, H.S. Molecular characterization of novel Bradyrhizobium strains nodulating Eriosema chinense and Flemingia vestita, Important unexplored native legumes of the Sub-Himalayan region (Meghalaya) of India. Syst. Appl. Microbiol. 2017, 40, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Beukes, C.W.; Venter, S.N.; Law, I.J.; Phalane, F.L.; Steenkamp, E.T. South African papilionoid legumes are nodulated by diverse Burkholderia with unique nodulation and nitrogen-fixation loci. PLoS ONE 2013, 8, e68406. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, B.; Dlodlo, O.; Chimphango, S.; Stirton, C.; Schrire, B.; Boatwright, J.S.; Honnay, O.; Smets, E.; Sprent, J.; James, E.K. Symbiotic diversity, specificity and distribution of rhizobia in native legumes of the Core Cape Subregion (South Africa). FEMS Microbiol. Ecol. 2015, 91, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Gerding, M.; O’Hara, G.W.; Bräu, L.; Nandasena, K.; Howieson, J.G. Diverse Mesorhizobium spp. with unique nodA nodulating the South African legume species of the genus Lessertia. Plant Soil 2012, 358, 385–401. [Google Scholar] [CrossRef]
- Beukes, C.W.; Stępkowski, T.; Venter, S.N.; Cłapa, T.; Phalane, F.L.; le Roux, M.M.; Steenkamp, E.T. Crotalarieae and Genisteae of the South African great escarpment are nodulated by novel Bradyrhizobium species with unique and diverse symbiotic loci. Mol. Phylogenet. Evol. 2016, 100, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoumen, H.; Filali-Maltouf, A.; Neyra, M.; Belabed, A.; El Idrissi, M.M. Effect of high salts concentrations on the growth of rhizobia and responses to added osmotica. J. Appl. Microbiol. 1999, 86, 889–898. [Google Scholar] [CrossRef]
- Bordeleau, L.M.; Prévost, D. Nodulation and nitrogen fixation in extreme environments. Plant Soil 1994, 161, 115–125. [Google Scholar] [CrossRef]
- Kulkarni, S.; Surange, S.; Nautiyal, C.S. Crossing the limits of Rhizobium existence in extreme conditions. Curr. Microbiol. 2000, 41, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, M.; Oliveira, S. Tolerance of Mesorhizobium type strains to different environmental stresses. Antonie Leeuwenhoek 2011, 99, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A.; Malek, W.; Parker, I.M. Growth of an invasive legume is symbiont limited in newly occupied habitats. Divers. Distrib. 2006, 12, 563–571. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [PubMed]
- Ferguson, B.; Lin, M.; Gresshoff, P.M. Regulation of legume nodulation by acidic growth conditions. Plant Signal. Behav. 2013, 8, e23426. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.H.; Viteri, S.E.; Mackie, F.; Vargas, A.T.; Palacios, A. Variation in acid soil tolerance among strains of Rhizobium phaseoli. Field Crops Res. 1982, 5, 121–128. [Google Scholar] [CrossRef]
- Estrada-de Los Santos, P.; Vacaseydel-Aceves, N.B.; Martnez-Aguilar, L.; Cruz-Hernndez, M.A.; Mendoza-Herrera, A.; Caballero-Mellado, J. Cupriavidus and Burkholderia species associated with agricultural plants that grow in alkaline soils. J. Microbiol. 2011, 49, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Yates, R.J.; Deiana, P.; Howieson, J.G. Novel Strains of nodulating Burkholderia have a role in nitrogen fixation with papilionoid herbaceous legumes adapted to acid, infertile soils. Soil Biol. Biochem. 2009, 41, 125–134. [Google Scholar] [CrossRef]
- Stopnisek, N.; Bodenhausen, N.; Frey, B.; Fierer, N.; Eberl, L.; Weisskopf, L. Genus-wide acid tolerance accounts for the biogeographical distribution of soil Burkholderia populations. Environ. Microbiol. 2014, 16, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Bournaud, C.; de Faria, S.M.; dos Santos, J.M.F.; Tisseyre, P.; Silva, M.; Chaintreuil, C.; Gross, E.; James, E.K.; Prin, Y.; Moulin, L. Burkholderia species are the most common and preferred nodulating symbionts of the Piptadenia group (Tribe Mimoseae). PLoS ONE 2013, 8, e63478. [Google Scholar] [CrossRef] [PubMed]
- Brígido, C.; Alexandre, A.; Laranjo, M.; Oliveira, S. Moderately acidophilic mesorhizobia isolated from chickpea. Lett. Appl. Microbiol. 2007, 44, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Brígido, C.; Glick, B.R.; Oliveira, S. survey of plant growth-promoting mechanisms in native Portuguese chickpea Mesorhizobium isolates. Microb. Ecol. 2017, 73, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Wang, E.T.; David Kuykendall, L. Mesorhizobium. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Zhang, J.J.; Lou, K.; Jin, X.; Mao, P.H.; Wang, E.T.; Tian, C.F.; Sui, X.H.; Chen, W.F.; Chen, W.X. Distinctive Mesorhizobium populations associated with Cicer arietinum L. in alkaline soils of Xinjiang, China. Plant Soil 2012, 353, 123–134. [Google Scholar] [CrossRef]
- Manning, J.; Goldblatt, P. Plants of the Greater Cape Floristic Region 1: The Core Cape Flora; South African National Biodiversity Institute: Cape Town, South Africa, 2012; pp. 1–870. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The Vegetation of South Africa, Lesotho and Swaziland; South African National Biodiversity Institute: Pretoria, South Africa, 2006. [Google Scholar]
- Zhao, L.; Fan, M.; Zhang, D.; Yang, R.; Zhang, F.; Xu, L.; Wei, X.; Shen, Y.; Wei, G. Distribution and diversity of rhizobia associated with wild soybean (Glycine Soja Sieb. & Zucc.) in Northwest China. Syst. Appl. Microbiol. 2014, 37, 449–456. [Google Scholar] [PubMed]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Soininen, J.; Zhang, Y.; Wang, B.; Yang, X.; Shen, J. Contrasting patterns in elevational diversity between microorganisms and macroorganisms. J. Biogeogr. 2011, 38, 595–603. [Google Scholar] [CrossRef]
- Wang, J.; Meier, S.; Soininen, J.; Casamayor, E.O.; Pan, F.; Tang, X.; Yang, X.; Zhang, Y.; Wu, Q.; Zhou, J. Regional and global elevational patterns of microbial species richness and evenness. Ecography 2017, 40, 393–402. [Google Scholar] [CrossRef]
- Verboom, G.A.; Bergh, N.G.; Haiden, S.A.; Hoffmann, V.; Britton, M.N. Topography as a driver of diversification in the Cape Floristic region of South Africa. New Phytol. 2015, 207, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, E.; Mitchell, D.T. Variations in soil phosphorus in the Fynbos biome, South Africa. J. Ecol. 1987, 75, 1159–1171. [Google Scholar] [CrossRef]
- McKey, D. Legumes and nitrogen: The evolutionary ecology of a nitrogen-demanding lifestyle. In Advances in Legume Systematics 5; Sprent, J.I., McKey, D., Eds.; Royal Botanic Gardens: Sydney, Australia, 1994; pp. 211–228. [Google Scholar]
- Werner, G.D.A.; Cornwell, W.K.; Cornelissen, J.H.C.; Kiers, E.T. Evolutionary signals of symbiotic persistence in the legume-rhizobia mutualism. Proc. Natl. Acad. Sci. USA 2015, 112, 10262–10269. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A. Mutualism as a constraint on invasion success for legumes and rhizobia. Divers. Distrib. 2001, 7, 125–136. [Google Scholar] [CrossRef]
- Simonsen, A.K.; Dinnage, R.; Barrett, L.G.; Prober, S.M.; Thrall, P.H. symbiosis limits establishment of legumes outside their native range at a global scale. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, B.; Van Cauwenberghe, J.; Verstraete, B.; Chimphango, S.; Stirton, C.; Honnay, O.; Smets, E.; Sprent, J.; James, E.K.; Muasya, A.M. Characterization of the papilionoid–Burkholderia interaction in the Fynbos biome: The diversity and distribution of beta-rhizobia nodulating Podalyria calyptrata (Fabaceae, Podalyrieae). Syst. Appl. Microbiol. 2016, 39, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, D.; Staden, L.V.; Foden, W.; Victor, J.E.; Helme, N.A.; Turner, R.C.; Kamundi, D.A.; Manyama, P.A. Red List of South African Plants 2009; South African National Biodiversity Institute: Cape Town, South Africa, 2009. [Google Scholar]
- Stirton, C.H. Fabaceae. Bothalia 1982, 14, 69–77. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T. Tempo and mode in evolution: Phylogenetic inertia, adaptation and comparative methods. J. Evol. Biol. 2002, 15, 899–910. [Google Scholar] [CrossRef]
- Dludlu, M.N.; Chimphango, S.B.; Stirton, C.H.; Muasya, A.M. Distinct edaphic habitats are occupied by discrete legume assemblages with unique indicator species in the Cape Peninsula of South Africa. J. Plant Ecol. 2017. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root Nodule Bacteria; Blackwell Scientific: Oxford, UK, 1970. [Google Scholar]
- Dludlu, M. Systematic studies of the Southern African psoraleoid legumes. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2010. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematics; Stackebrandt, E.G., Ed.; John Wiley and Sons: New York, NY, USA, 1991; pp. 115–148. [Google Scholar]
- Gaunt, M.W.; Turner, S.L.; Rigottier-Gois, L.; Lloyd-Macgilp, S.A.; Young, J. Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int. J. Syst. Evol. Microbiol. 2001, 51, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Haukka, K.; Lindström, K.; Young, J.P. Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Appl. Environ. Microbiol. 1998, 64, 419–426. [Google Scholar] [PubMed]
- Werle, E.; Schneider, C.; Renner, M.; Vlker, M.; Fiehn, W. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Res. 1994, 22, 4354–4355. [Google Scholar] [CrossRef] [PubMed]
- Staden, R.; Beal, K.; Bonfield, J.K. The staden package. Methods Mol. Biol. 1998, 132, 115–130. [Google Scholar]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Hall, T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Suchard, M.; Drummond, A. Tracer, version 1.6.0. 2014. Available online: http://tree.bio.ed.ac.uk/software/tracer/ (accessed on 15 May 2017).
- Pirie, M.D.; Humphreys, A.M.; Galley, C.; Barker, N.P.; Verboom, G.A.; Orlovich, D.; Draffin, S.J.; Lloyd, K.; Baeza, C.M.; Negritto, M. A novel supermatrix approach improves resolution of phylogenetic relationships in a comprehensive sample of danthonioid grasses. Mol. Phylogenet. Evol. 2008, 48, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- Pirie, M.D.; Humphreys, A.M.; Barker, N.P.; Linder, H.P. Reticulation, data combination, and inferring evolutionary history: An example from Danthonioideae (Poaceae). Syst. Biol. 2009, 58, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Mickevich, M.F.; Farris, J.S. The implications of congruence in Menidia. Syst. Zool. 1981, 30, 351–370. [Google Scholar] [CrossRef]
- Lemaire, B.; Van Cauwenberghe, J.; Chimphango, S.; Stirton, C.; Honnay, O.; Smets, E.; Muasya, A.M. Recombination and horizontal transfer of nodulation and ACC deaminase (acdS) genes within Alpha-and Betaproteobacteria nodulating legumes of the Cape Fynbos biome. FEMS Microbiol. Ecol. 2015, 91. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org/.
- Fritz, S.A.; Purvis, A. selectivity in mammalian extinction risk and threat types: A new measure of phylogenetic signal strength in binary traits. Conserv. Biol. 2010, 24, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Orme, D.; Freckleton, R.; Thomas, G.; Petzoldt, T.; Fritz, S.; Isaac, N.; Pearse, W. Caper: Comparative analyses of phylogenetics and evolution in R. R package version 0.5. Proc. R. Soc. B 2012, 283, 7. [Google Scholar]
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Freckleton, R.P.; Harvey, P.H.; Pagel, M. Phylogenetic analysis and comparative data: A test and review of evidence. Am. Nat. 2002, 160, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Filho, J.A.F.; Santos, T.; Rangel, T.F.; Bini, L.M. A comparison of metrics for estimating phylogenetic signal under alternative evolutionary models. Genet. Mol. Biol. 2012, 35, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Molina-Venegas, R.; Rodríguez, M. Revisiting phylogenetic signal; Strong or negligible impacts of polytomies and branch length information? BMC Evol. Biol. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Ojeda, F.; Pausas, J.G.; Verd, M. Soil shapes community structure through fire. Oecologia 2010, 163, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, R.N.; Cowling, R.M. Soil-vegetation relationships on the Agulhas Plain, South Africa. Catena 1988, 15, 333–345. [Google Scholar] [CrossRef]
- Cowling, R.M.; Witkowski, E.; Milewski, A.V.; Newbey, K.R. Taxonomic, edaphic and biological aspects of narrow plant endemism on matched sites in mediterranean South Africa and Australia. J. Biogeogr. 1994, 21, 651–664. [Google Scholar] [CrossRef]
- Cowling, R.M. Diversity components in a species-rich area of the Cape Floristic region. J. Veg. Sci. 1990, 1, 699–710. [Google Scholar] [CrossRef]
- Brígido, C.; Oliveira, S. Most acid-tolerant chickpea Mesorhizobia show induction of major chaperone genes upon acid shock. Microb. Ecol. 2013, 65, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Schutte, A.L. Fabaceae. In Plants of the Greater Cape Floristic Region 1: The Core Cape Flora; Manning, J.C., Goldblatt, P., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2012; pp. 518–582. [Google Scholar]
- Lemaire, B.; Chimphango, S.B.M.; Muasya, A/M. (University of Cape Town, Cape Town, Western Cape, South Africa). Personal Communication, 2016.
- Chen, W.; Huang, Q.; Xiong, X. Distribution and biodiversity of soybean rhizobia in the soils of Shennongjia forest reserve, China. Biol. Fertil. Soils 2004, 40, 306–312. [Google Scholar] [CrossRef]
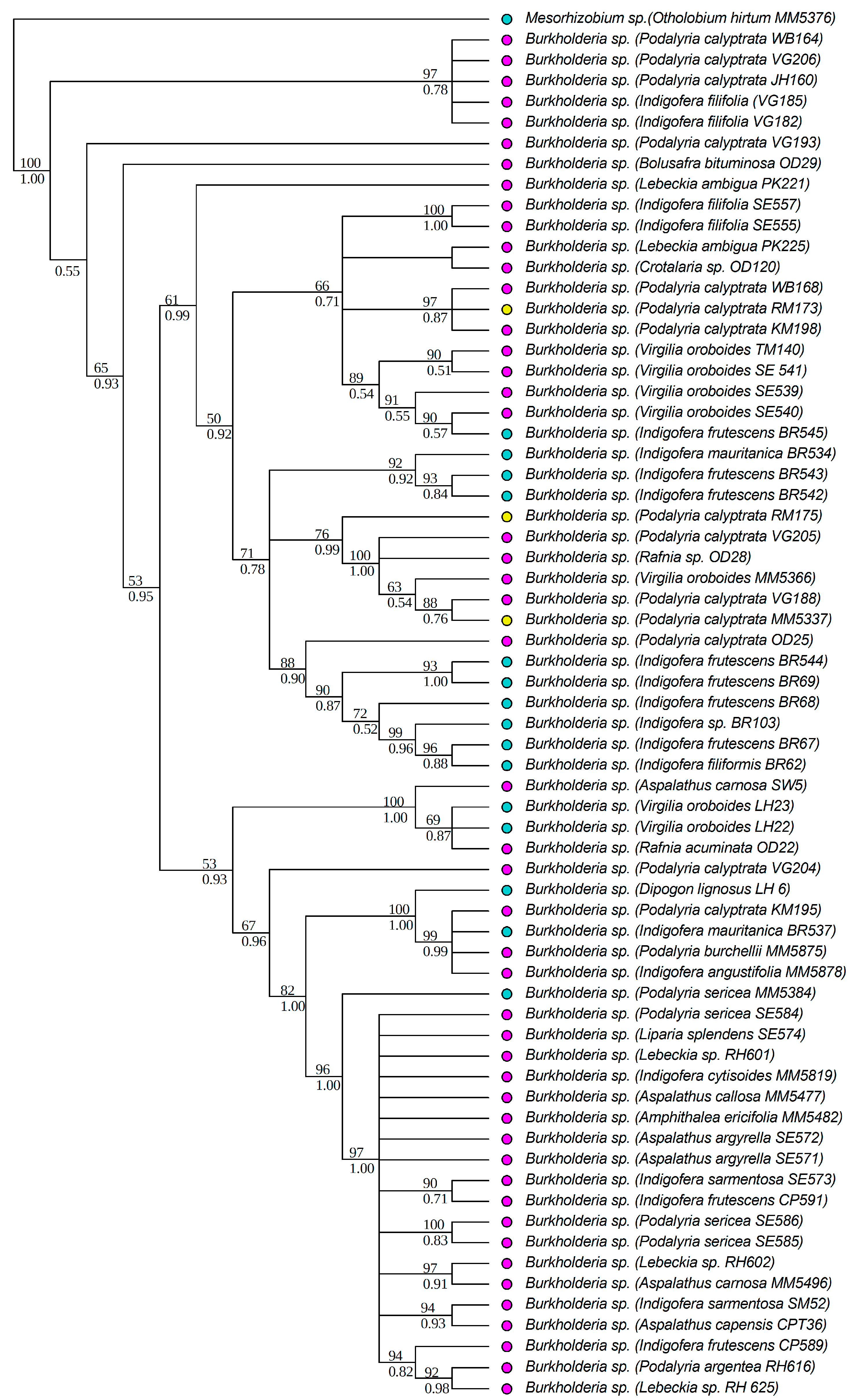


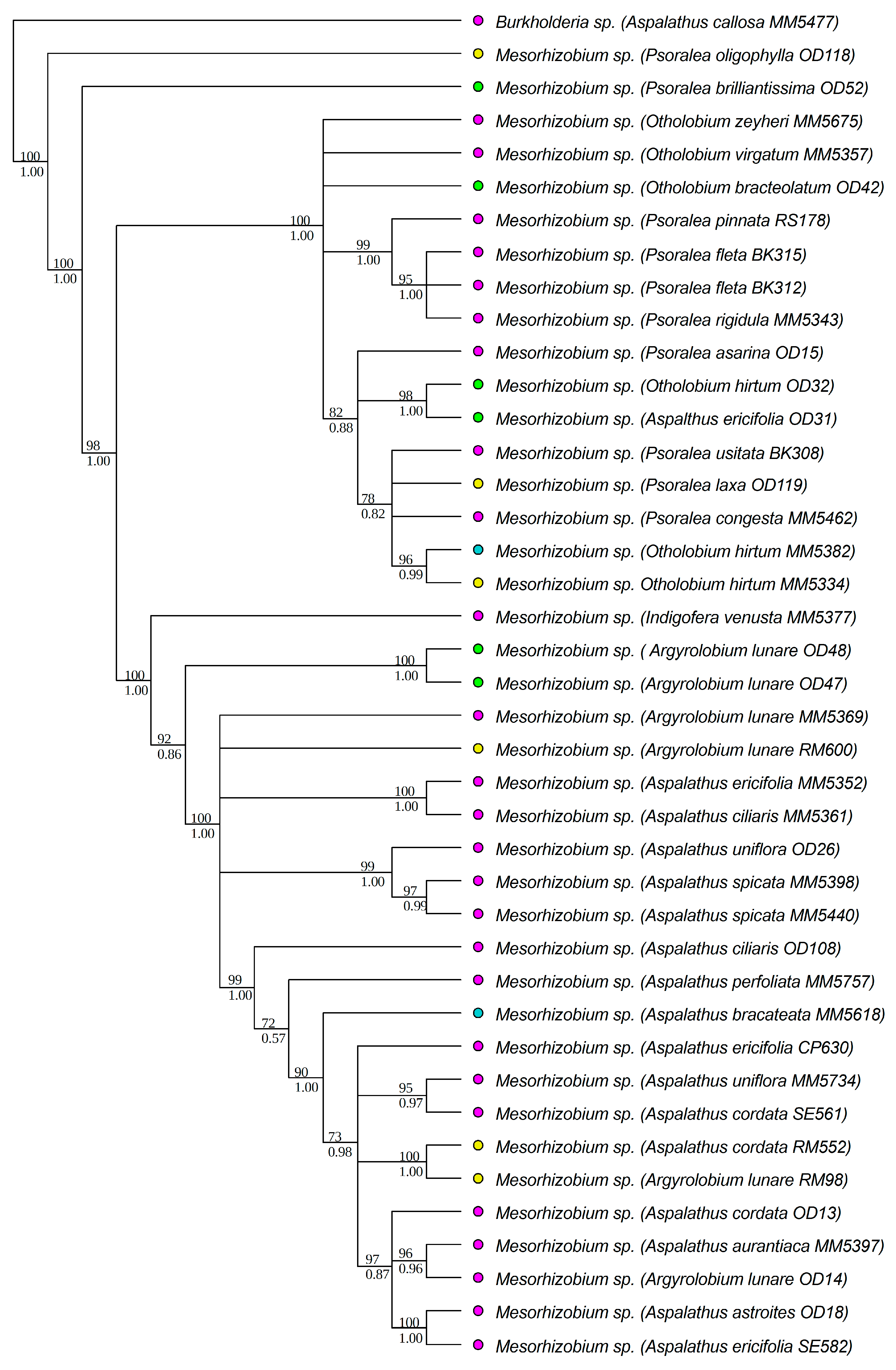

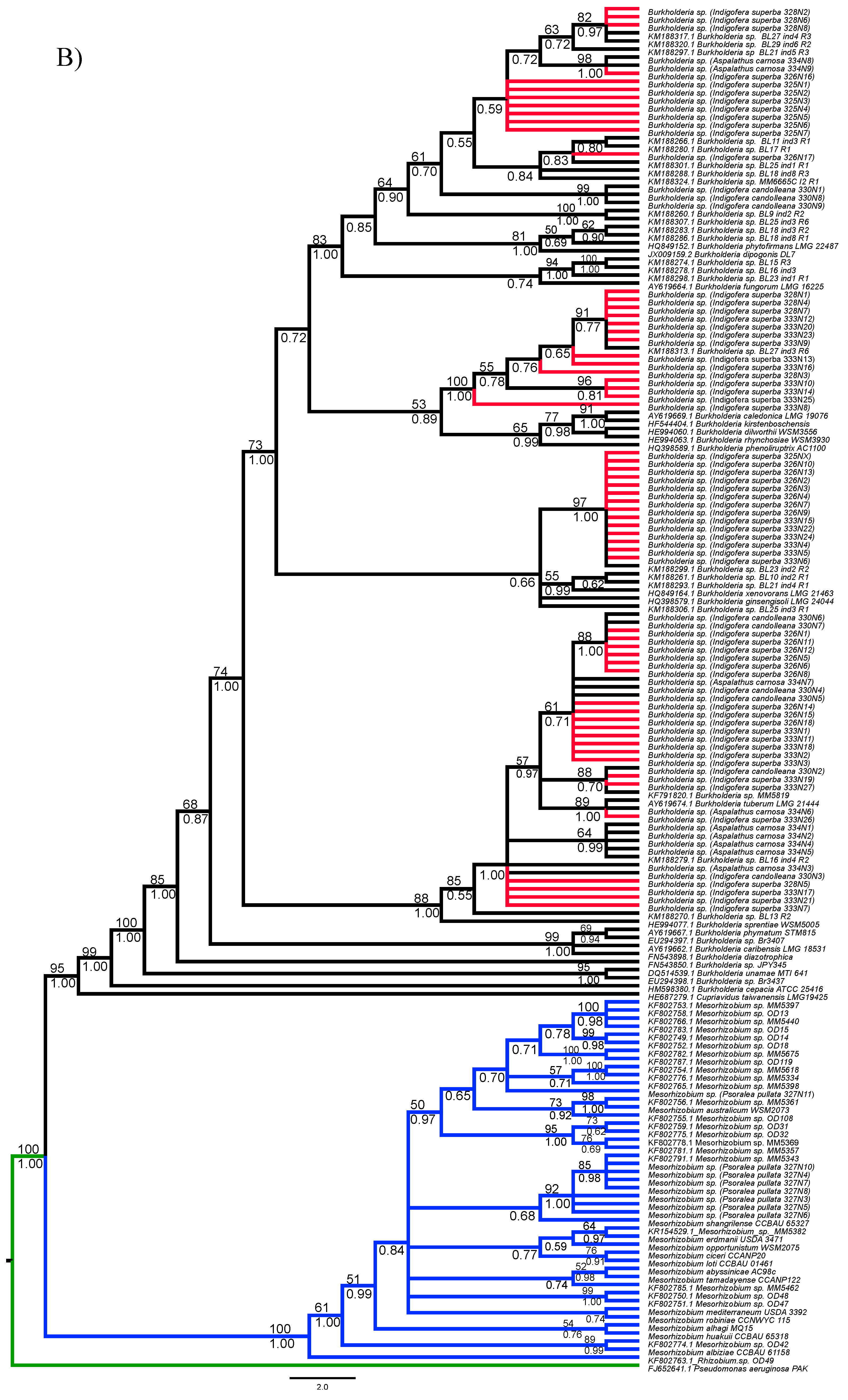

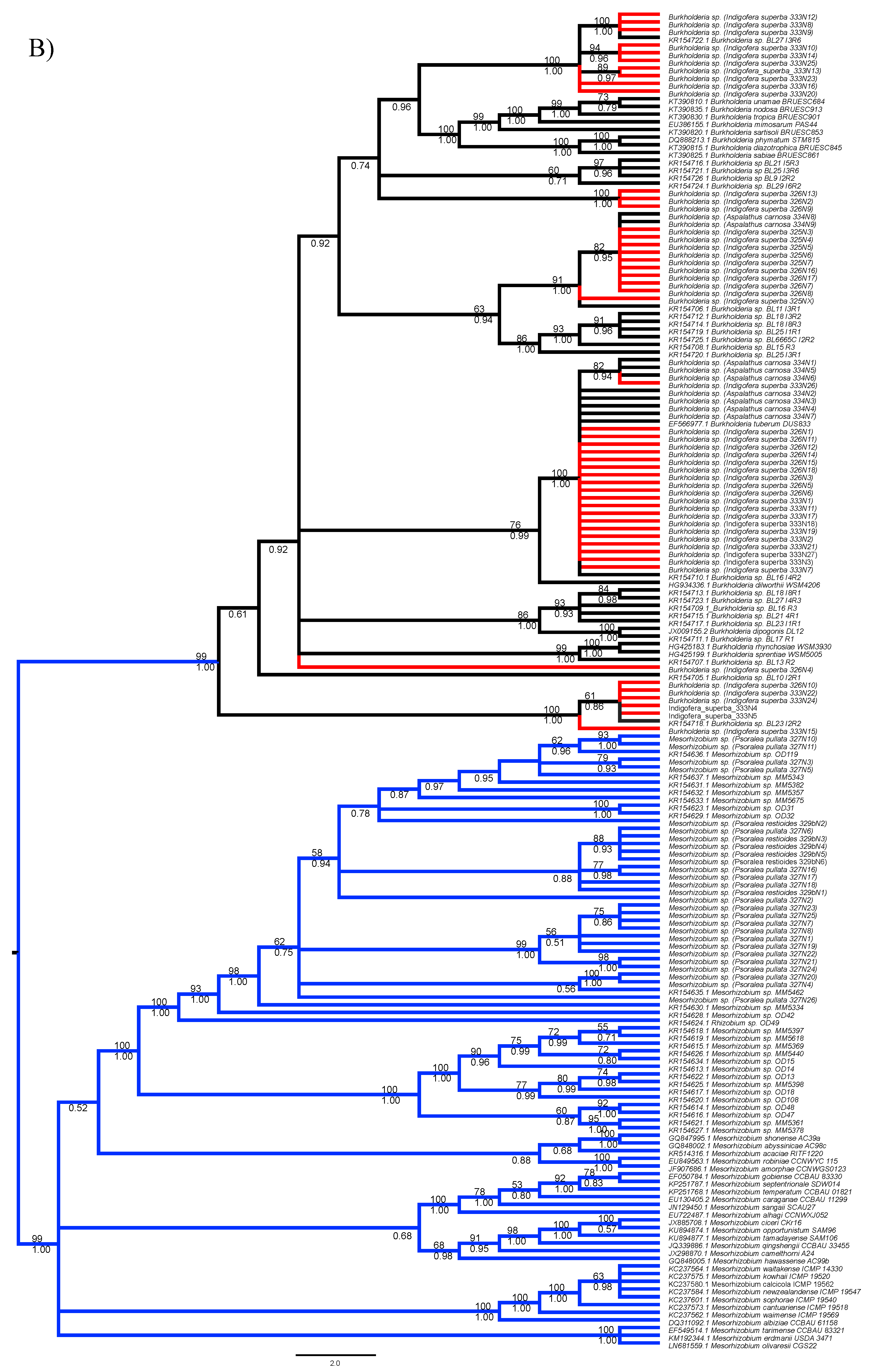
| DNA Region | Genus | Soil Type | D | p-Value Random Shuffle | p-Value Brownian Motion |
|---|---|---|---|---|---|
| 16S rRNA and recA | Burkholderia | Granite Sandstone Shale | −0.220 0.133 0.956 | 0.00 0.00 0.375 | 0.717 0.384 0.144 |
| Mesorhizobium | Granite Limestone Sandstone Shale | 0.252 −0.359 0.133 0.975 | 0.0006 0.0006 0.0009 0.419 | 0.235 0.744 0.056 0.001 | |
| nodA | Burkholderia | Granite Sandstone Shale | −0.208 0.082 0.617 | 0.00 0.00 0.160 | 0.715 0.424 0.291 |
| Mesorhizobium | Granite Limestone Sandstone shale | 1.752 −0.871 0.162 0.492 | 0.833 0.0005 0.002 0.118 | 0.032 0.914 0.423 0.218 |
| Genus | Gene Type | Variable | Pagel’s λ | p-Values |
|---|---|---|---|---|
| Burkholderia | Chromosomal | Altitude pH | 0.402 0.643 | 0.081 0.019 |
| nodA | Altitude pH | 0.093 0.840 | 0.389 0.0008 | |
| Mesorhizobium | Chromosomal | Altitude pH | 0.217 0.508 | 0.097 0.027 |
| nodA | Altitude pH | 0.767 0.912 | 0.999 0.016 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dludlu, M.N.; Chimphango, S.B.M.; Stirton, C.H.; Muasya, A.M. Differential Preference of Burkholderia and Mesorhizobium to pH and Soil Types in the Core Cape Subregion, South Africa. Genes 2018, 9, 2. https://doi.org/10.3390/genes9010002
Dludlu MN, Chimphango SBM, Stirton CH, Muasya AM. Differential Preference of Burkholderia and Mesorhizobium to pH and Soil Types in the Core Cape Subregion, South Africa. Genes. 2018; 9(1):2. https://doi.org/10.3390/genes9010002
Chicago/Turabian StyleDludlu, Meshack Nkosinathi, Samson B. M. Chimphango, Charles H. Stirton, and A. Muthama Muasya. 2018. "Differential Preference of Burkholderia and Mesorhizobium to pH and Soil Types in the Core Cape Subregion, South Africa" Genes 9, no. 1: 2. https://doi.org/10.3390/genes9010002
APA StyleDludlu, M. N., Chimphango, S. B. M., Stirton, C. H., & Muasya, A. M. (2018). Differential Preference of Burkholderia and Mesorhizobium to pH and Soil Types in the Core Cape Subregion, South Africa. Genes, 9(1), 2. https://doi.org/10.3390/genes9010002





