Variation in the Ovine KAP6-3 Gene (KRTAP6-3) Is Associated with Variation in Mean Fibre Diameter-Associated Wool Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sheep Blood and Wool Samples
2.2. PCR Primers and Amplification
2.3. Screening for Variation in KRTAP6-3
2.4. Sequencing of Allelic Variants and Sequence Analysis
2.5. Statistical Analyses
3. Results
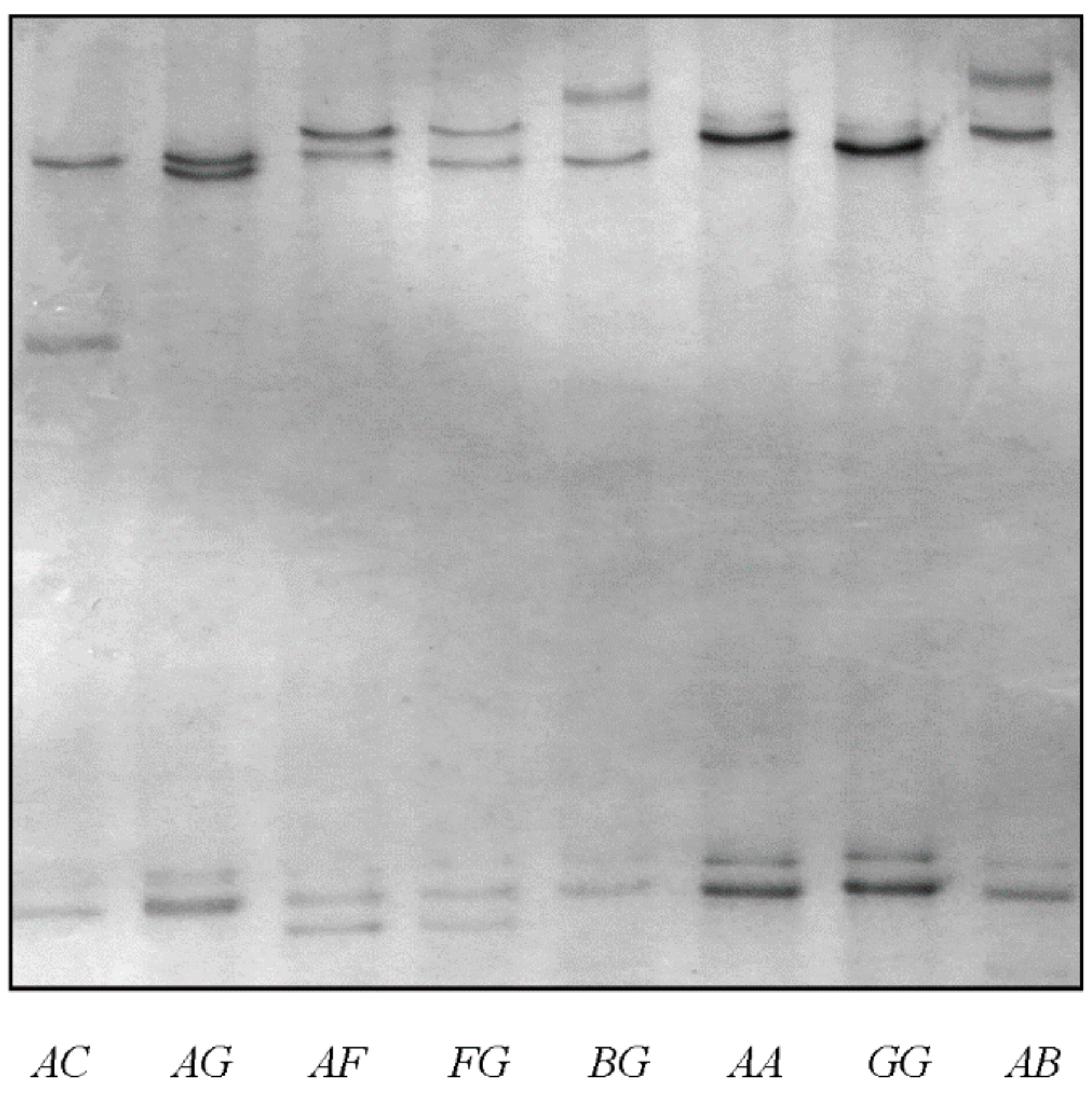
3.1. Variation in Ovine KRTAP6-3
3.2. Associations between Variation in KRTAP6-3 and Wool Traits
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Powell, B.C.; Rogers, G.E. The role of keratin proteins and their genes in the growth, structure and properties of hair. In Formation and Structure of Human Hair; Jollès, P., Zahn, H., Höcker, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1997; pp. 59–148. [Google Scholar]
- Gong, H.; Zhou, H.; Forrest, R.H.J.; Li, S.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G. Wool keratin-associated protein genes in sheep—A review. Genes 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Schweizer, J. Human KAP genes, only the half of it? Extensive size polymorphisms in hair keratin-associated protein genes. J. Investig. Dermatol. 2005, 124, vii–ix. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Langbein, L.; Praetzel-Wunder, S.; Giehl, K. Characterization and expression analysis of the hair keratin associated protein KAP26.1. Brit. J. Dermatol. 2008, 159, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Winter, H.; Langbein, L.; Wollschläger, A.; Praetzel-Wunder, S.; Jave-Suarez, L.F.; Schweizer, J. Characterization of human KAP24.1, a cuticular hair keratin-associated protein with unusual amino-acid composition and repeat structure. J. Investig. Dermatol. 2007, 127, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.M. The proteins of hair and other hard α-keratins. In Cellular and Molecular Biology of Intermediate Filaments; Goldman, R.D., Steinert, P.M., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 95–128. [Google Scholar]
- Li, S.W.; Ouyang, H.S.; Rogers, G.E.; Bawden, C.S. Characterization of the structural and molecular defects in fibres and follicles of the merino felting lustre mutant. Exp. Dermatol. 2009, 18, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, H.; Gong, H.; Zhao, F.; Wang, J.; Liu, X.; Luo, Y.; Hickford, J.G.H. Identification of the ovine keratin-associated protein 22-1 (KAP22-1) gene and its effect on wool traits. Genes 2017, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gong, H.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G.H. Identification of four new gene members of the KAP6 gene family in sheep. Sci. Rep. 2016, 6, 24074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Gong, H.; Li, S.; Luo, Y.; Hickford, J.G.H. A 57-bp deletion in the ovine KAP6-1 gene affects wool fibre diameter. J. Anim. Breed. Genet. 2015, 132, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hickford, J.G.H.; Fang, Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006, 354, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.O.; Fang, Q.; Zhou, H.; Hickford, J.G.H. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Dyer, J.M.; Hickford, J.G.H. Identification of the ovine KAP11–1 gene (KRTAP11-1) and genetic variation in its coding sequence. Mol. Biol. Rep. 2011, 38, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Hickford, J.G.H. Diversity of the glycine/tyrosine-rich keratin-associated protein 6 gene (KAP6) family in sheep. Mol. Biol. Rep. 2011, 38, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Hodge, S.; Dyer, J.M.; Hickford, J.G. Association of wool traits with variation in the ovine KAP1-2 gene in Merino cross lambs. Small Rumin. Res. 2015, 124, 24–29. [Google Scholar] [CrossRef]
- Beh, K.J.; Callaghan, M.J.; Leish, Z.; Hulme, D.J.; Lenane, I.; Maddox, J.F. A genome scan for QTL affecting fleece and wool traits in Merino sheep. Wool Technol. Sheep Breed. 2001, 49, 88–97. [Google Scholar]
- Liao, J.; Lowthert, L.A.; Ku, N.O.; Fernandez, R.; Omary, MB. Dynamics of human keratin 18 phosphorylation: Polarized distribution of phosphorylated keratins in simple epithelial tissues. J. Cell Biol. 1995, 131, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Windoffer, R.; Leube, R.E. Induction of rapid and reversible cytokeratin filament network remodeling by inhibition of tyrosine phosphatases. J. Cell Sci. 2002, 115, 4133–4148. [Google Scholar] [CrossRef] [PubMed]
- Gallivan, J.P.; Dougherty, D.A. Cation-π interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.R.; Hickford, J.G.H.; Bickerstaffe, R. Polymorphism in two genes for B2 high sulfur proteins of wool. Anim. Genet. 1994, 25, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Plowman, J.E.; Dyer, J.M.; Hickford, J.G. Analysis of variation in the ovine ultra-high Sulphur keratin-associated protein KAP5-4 gene using PCR-SSCP technique. Electrophoresis 2010, 31, 3545–3547. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Plowman, J.E.; Dyer, J.M.; Hickford, J.G.H. Search for variation in the ovine KAP7-1 and KAP8-1 genes using polymerase chain reaction-single-stranded conformational polymorphism screening. DNA Cell Biol. 2012, 31, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Dyer, J.; Hickford, J.G.H. The sheep KAP 8-2 gene, a new KAP8 family member that is absent in humans. Springerplus 2014, 3, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhou, H.; Zhu, J.; Hu, J.; Liu, X.; Li, S.; Luo, Y.; Hickford, J.G.H. Identification of the ovine keratin-associated protein 15-1 gene (KRTAP15-1) and genetic variation in its coding sequence. Small Rumin. Res. 2017, 153, 131–136. [Google Scholar] [CrossRef]
- Gotea, V.; Gartner, J.J.; Qutob, N.; Elnitski, L.; Samuels, Y. The functional relevance of somatic synonymous mutations in melanoma and other cancers. Pigment Cell Melanoma Res. 2015, 28, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Dyer, J.M.; Plowman, J.E.; Hickford, J.G.H. Identification of the keratin associated protein 13-3 (KAP13-3) gene in sheep. Open J. Genet. 2012, 1, 60–64. [Google Scholar] [CrossRef]
- Zhou, H.; Gong, H.; Yan, W.; Luo, Y.; Hickford, J.G. Identification and sequence analysis of the keratin-associated protein 24-1 (KAP24-1) gene homologue in sheep. Gene 2012, 511, 62–65. [Google Scholar] [CrossRef] [PubMed]

| Trait 1 | Mean ± SE 2 | p3 | ||
|---|---|---|---|---|
| AA (n = 238) | AB (n = 17) | AG (n = 105) | ||
| GFW (kg) | 2.14 ± 0.11 | 2.23 ± 0.15 | 2.11 ± 0.12 | 0.631 |
| CFW (kg) | 1.62 ± 0.09 | 1.66 ± 0.12 | 1.57 ± 0.10 | 0.454 |
| Yield (%) | 75.4 ± 1.63 | 74.3 ± 2.18 | 74.0 ± 1.81 | 0.220 |
| MSL (mm) | 85.0 ± 3.23 | 83.9 ± 4.32 | 86.9 ± 3.60 | 0.483 |
| MSS (N/ktex) | 21.5 ± 2.13 | 22.7 ± 2.84 | 21.0 ± 2.36 | 0.732 |
| MFD (µm) | 19.0 ± 0.46 b | 20.8 ± 0.61 a | 18.1 ± 0.51 c | <0.001 |
| FDSD (µm) | 4.04 ± 0.16 b | 4.41 ± 0.21 a | 3.79 ± 0.18 c | <0.001 |
| CVFD (%) | 21.2 ± 0.59 | 21.1 ± 0.79 | 20.8 ± 0.66 | 0.552 |
| MFC (°/mm) | 89.1 ± 4.20 | 91.4 ± 5.61 | 85.3 ± 4.66 | 0.197 |
| PF (%) | 1.89 ± 0.79 b | 4.88 ± 1.06 a | 0.76 ± 0.88 c | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhou, H.; Gong, H.; Zhao, F.; Wang, J.; Luo, Y.; Hickford, J.G.H. Variation in the Ovine KAP6-3 Gene (KRTAP6-3) Is Associated with Variation in Mean Fibre Diameter-Associated Wool Traits. Genes 2017, 8, 204. https://doi.org/10.3390/genes8080204
Li S, Zhou H, Gong H, Zhao F, Wang J, Luo Y, Hickford JGH. Variation in the Ovine KAP6-3 Gene (KRTAP6-3) Is Associated with Variation in Mean Fibre Diameter-Associated Wool Traits. Genes. 2017; 8(8):204. https://doi.org/10.3390/genes8080204
Chicago/Turabian StyleLi, Shaobin, Huitong Zhou, Hua Gong, Fangfang Zhao, Jiqing Wang, Yuzhu Luo, and Jon G. H. Hickford. 2017. "Variation in the Ovine KAP6-3 Gene (KRTAP6-3) Is Associated with Variation in Mean Fibre Diameter-Associated Wool Traits" Genes 8, no. 8: 204. https://doi.org/10.3390/genes8080204
APA StyleLi, S., Zhou, H., Gong, H., Zhao, F., Wang, J., Luo, Y., & Hickford, J. G. H. (2017). Variation in the Ovine KAP6-3 Gene (KRTAP6-3) Is Associated with Variation in Mean Fibre Diameter-Associated Wool Traits. Genes, 8(8), 204. https://doi.org/10.3390/genes8080204






