Isolation and Characterization of Ftsz Genes in Cassava
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Gaterials
2.2. Cloning of MeFtsZ Genes
2.3. Sequence and Structural Analysis
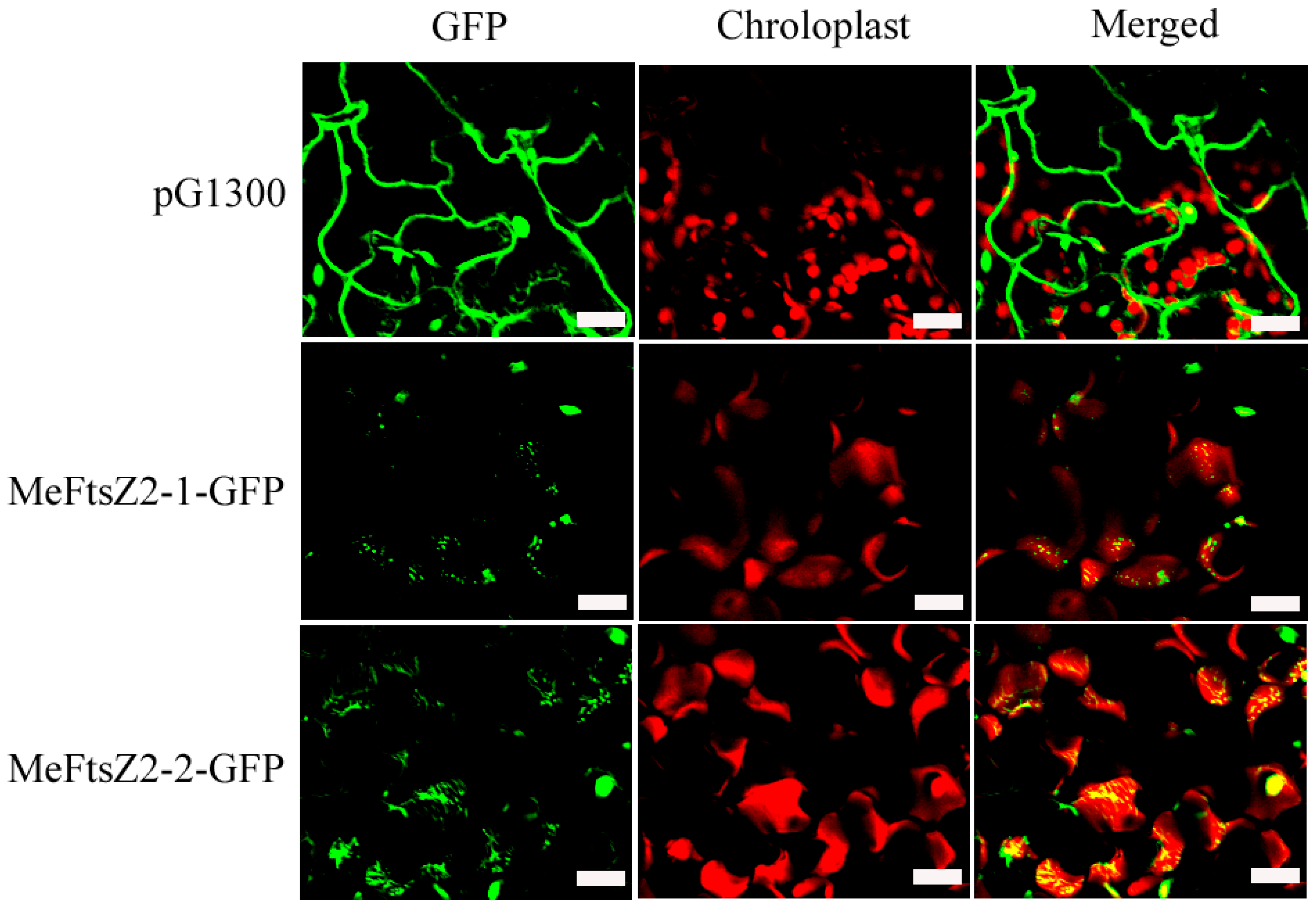
2.4. Construction of GFP-Fusion Proteins and Subcellular Localization Analysis
2.5. Plant Transformation and Identification
2.6. Real-Time RT-PCR Analysis
3. Results
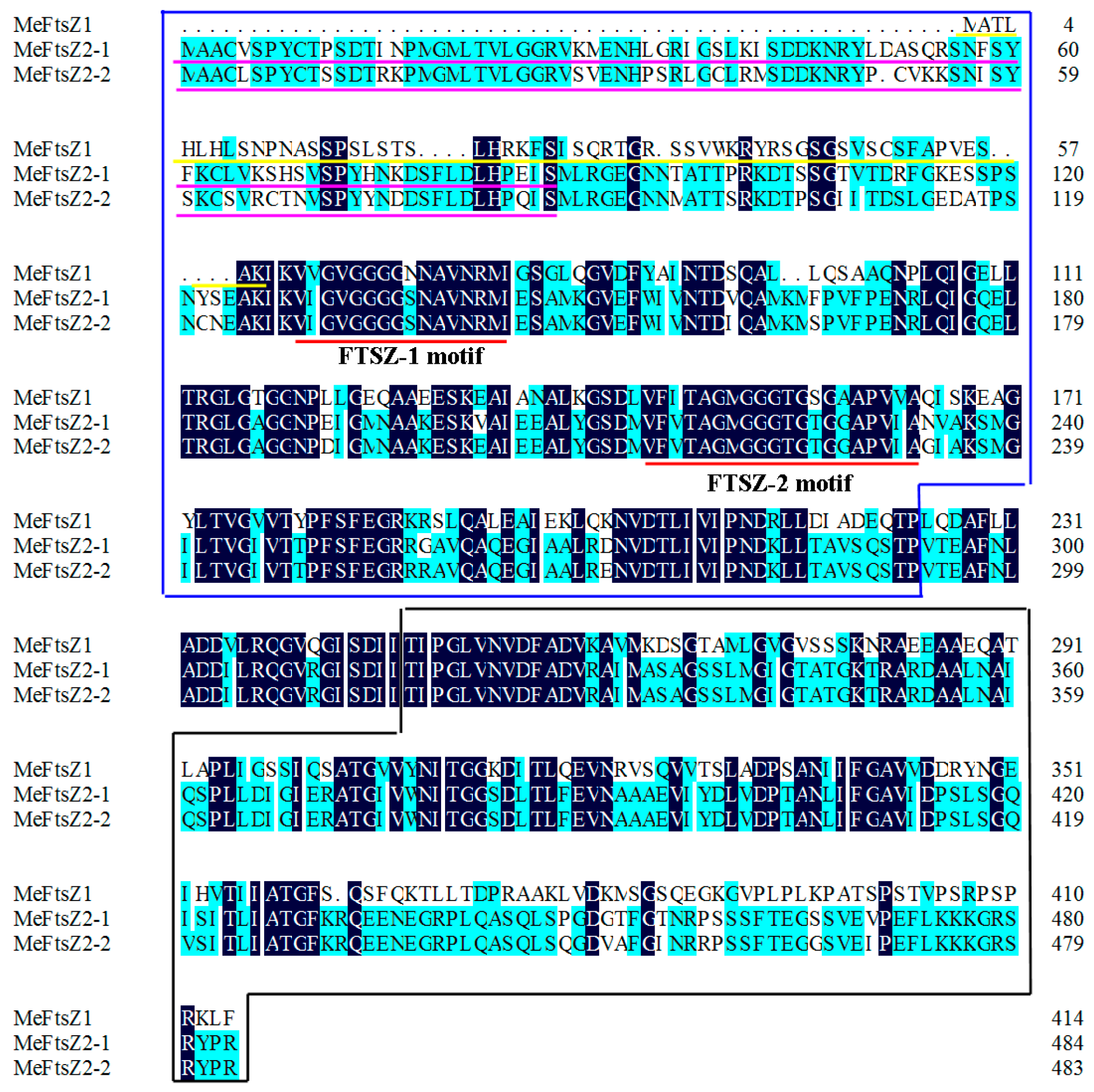
3.1. Identification and Characterization of the MeFtsZ Genes
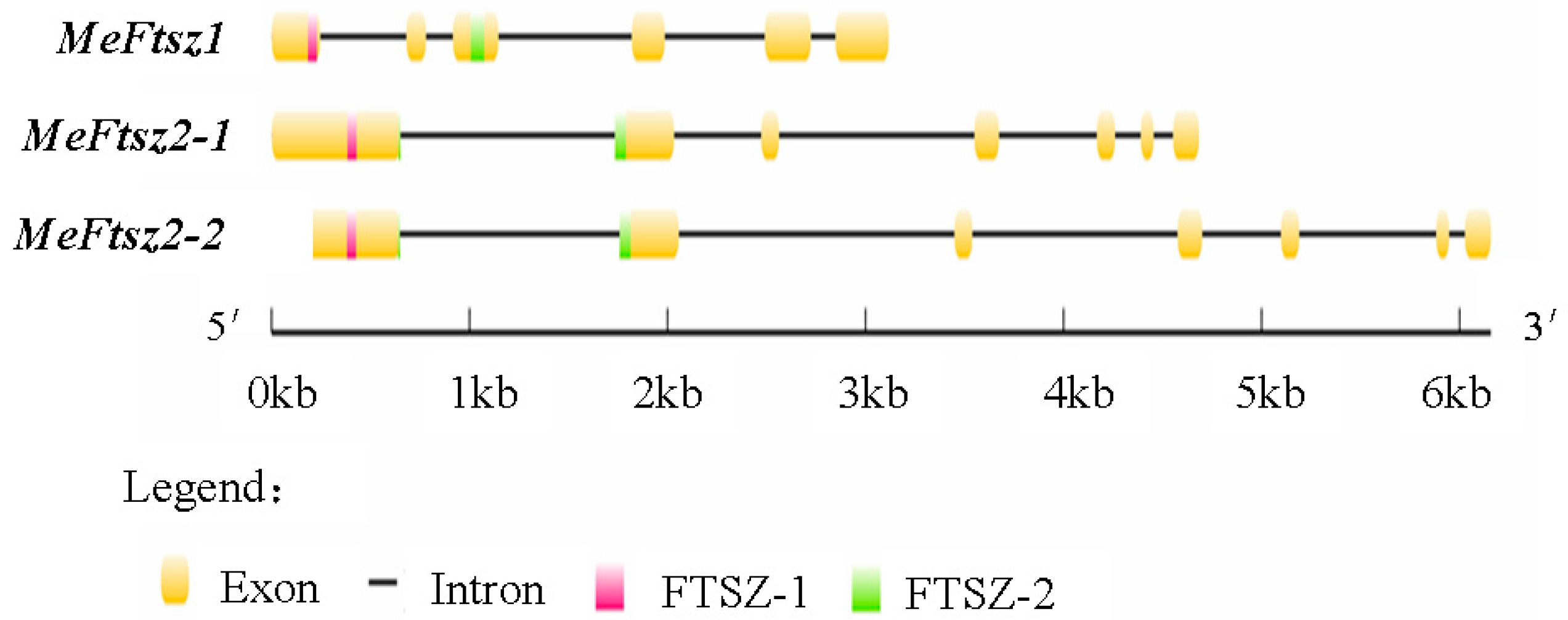
3.2. Analysis of the Gene Structure and Chromosomal Distribution of the MeFtsZs
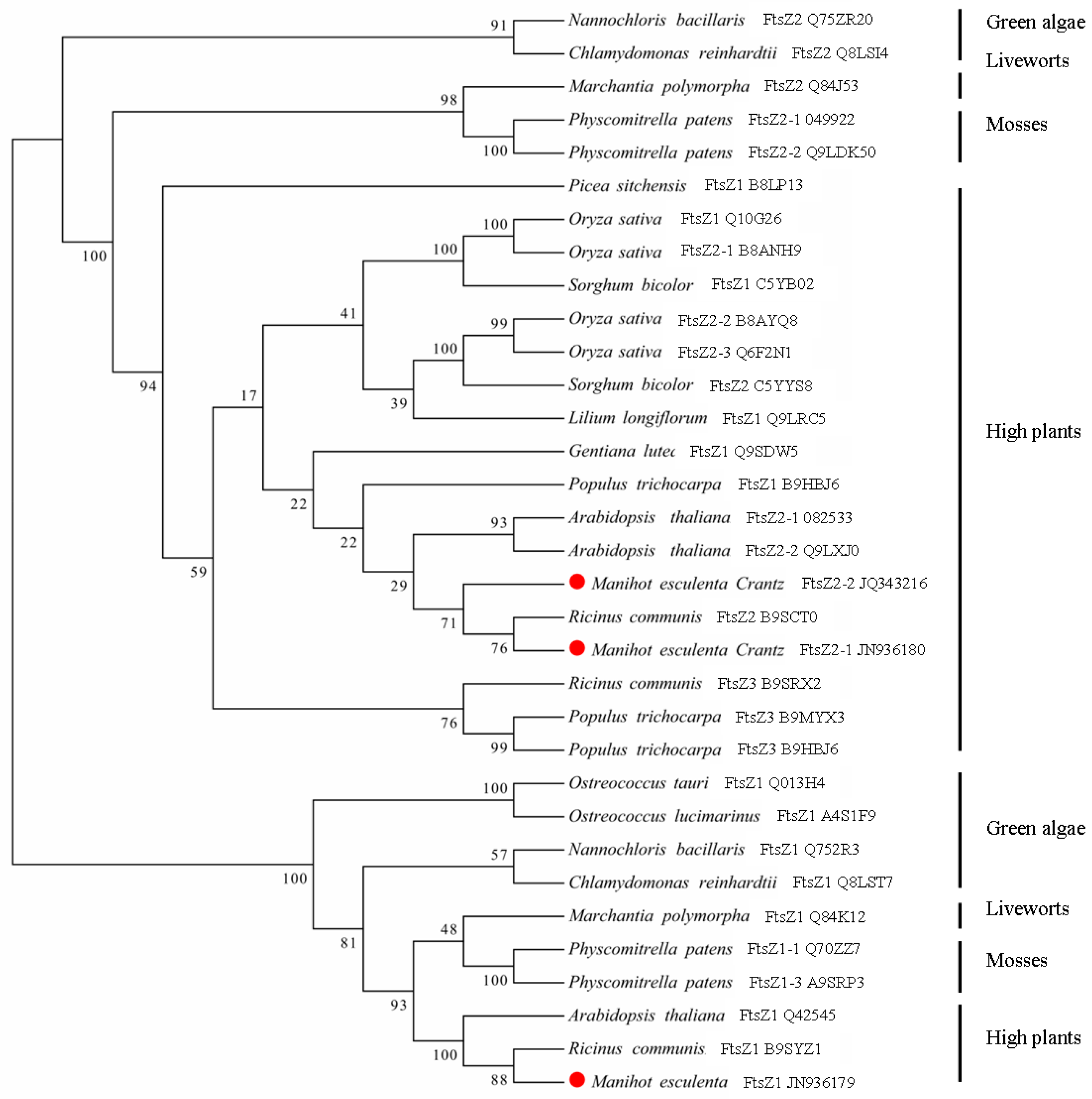
3.3. Phylogenetic Analysis of the MeFtsZ Proteins
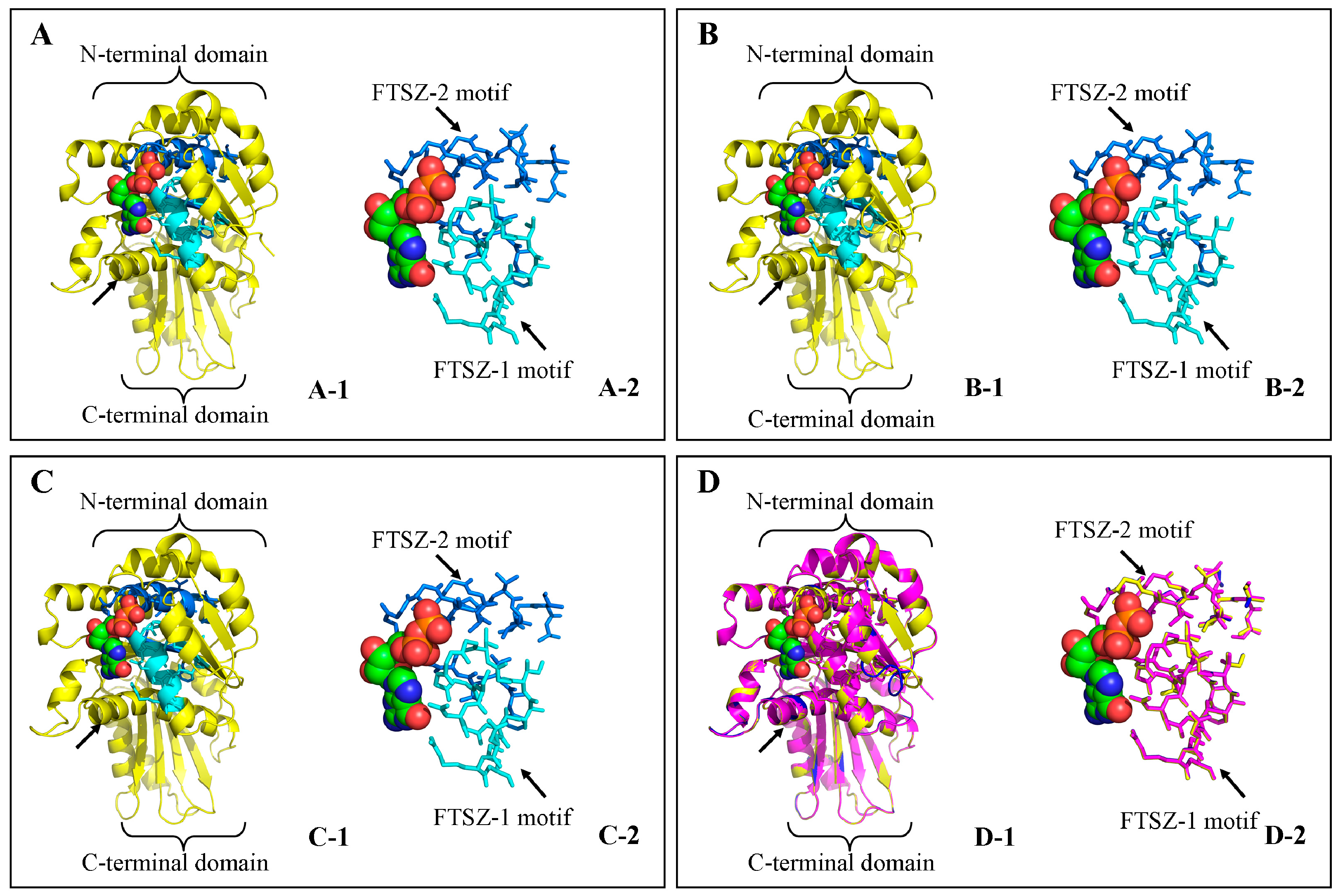
3.4. Structure Prediction and Homology Modeling of the MeFtsZ Proteins
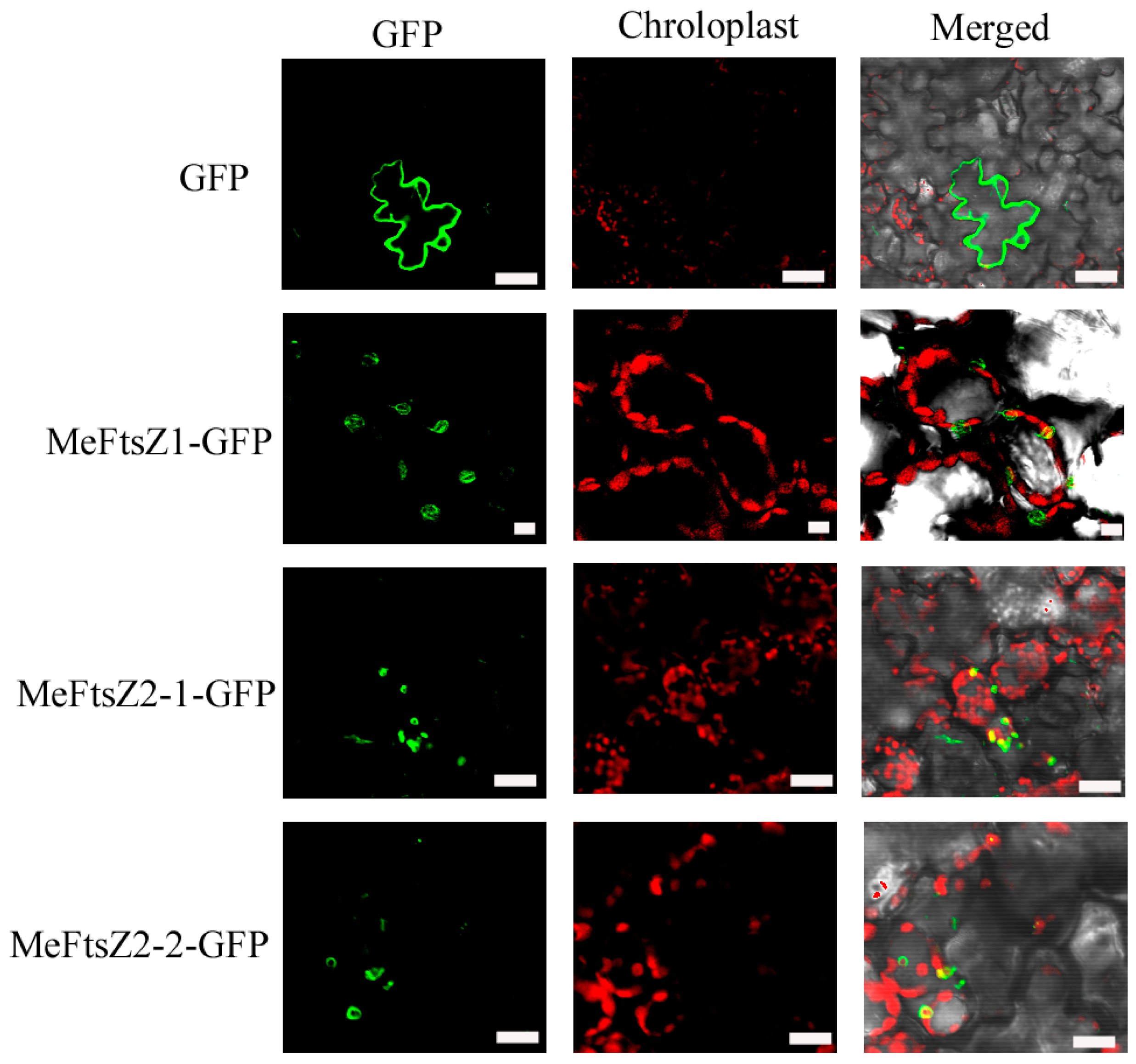
3.5. Subcellular Localization of MeFtsZs
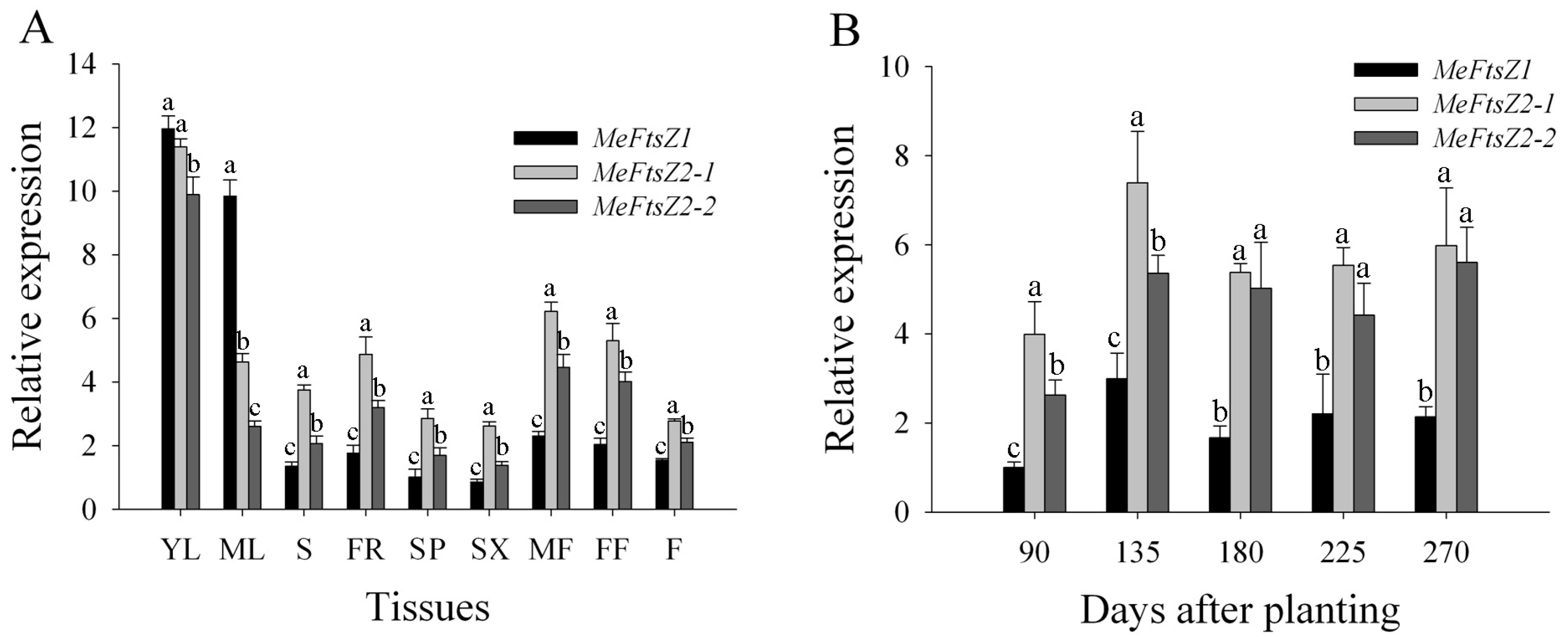
3.6. The Differential Expression Analyses of MeFtsZs in Cassava Organs and Tissues
3.7. The Differential Expression Analyses of MeFtsZs in Cassava Storage Root Developmental Stages
3.8. The Differential Expression Analyses of MeFtsZ Genes under Phytohormone Treatments
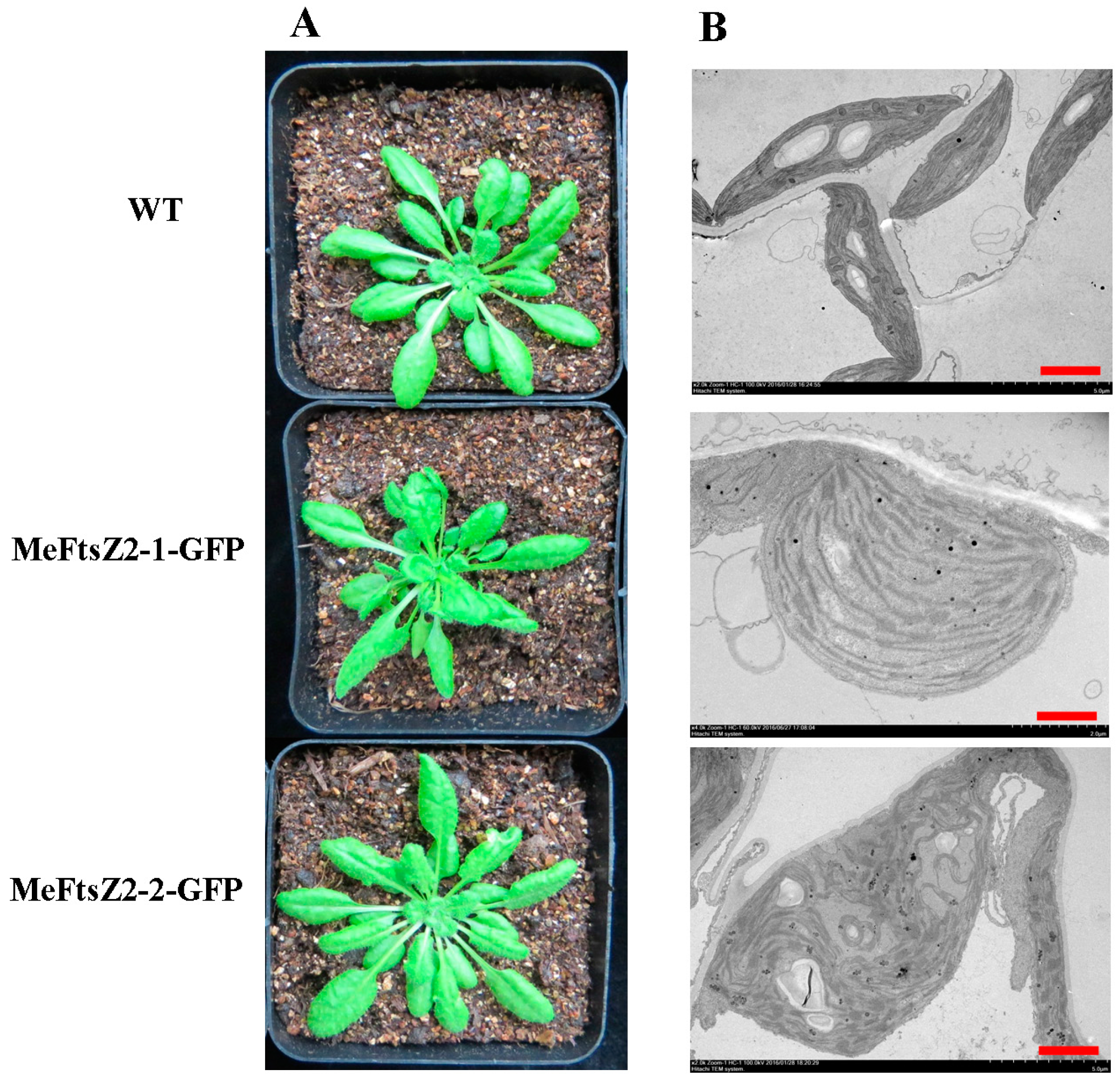
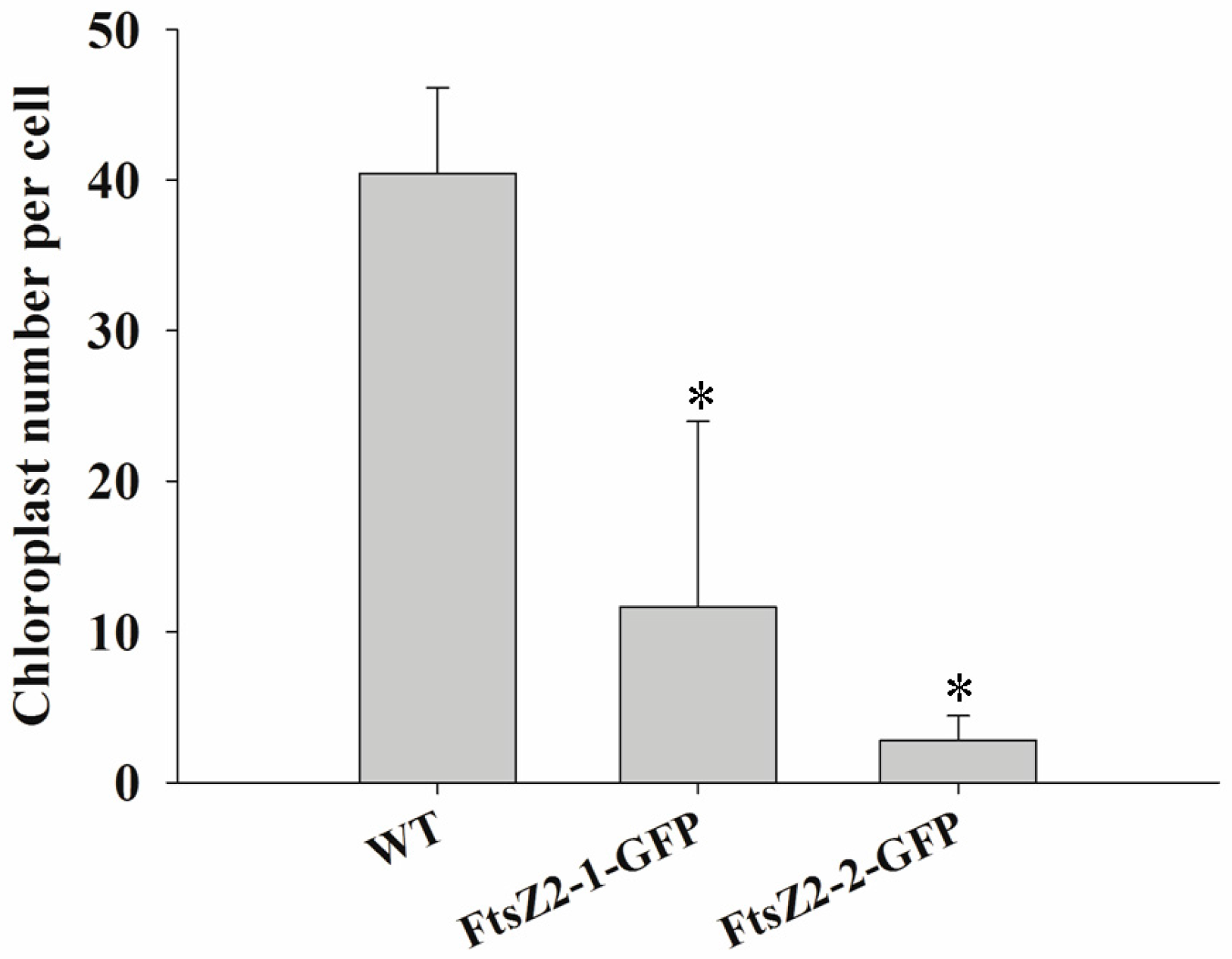
3.9. Expression of MeFtsZ2-1 and MeFtsZ2-2 in A. thaliana to Identify Their Regulation in Plastid Division
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Keeling, P.J. The endosymbiotic origin, diversification and fate of plastids. Philos. Trans. R. Soc. Lond. 2010, 365, 729–748. [Google Scholar] [CrossRef] [PubMed]
- Terbush, A.D.; Yoshida, Y.; Osteryoung, K.W. Ftsz in chloroplast division: Structure, function and evolution. Curr. Opin. Cell Biol. 2013, 25, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bahaji, A.; Li, J.; Sánchezlópez, Á.M.; Barojafernández, E.; Muñoz, F.J.; Ovecka, M.; Almagro, G.; Montero, M.; Ezquer, I.; Etxeberria, E. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol. Adv. 2014, 32, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, H.; Mori, T.; Takahara, M.; Miyagishima, S.Y.; Kuroiwa, T. Chloroplast division machinery as revealed by immunofluorescence and electron microscopy. Planta 2002, 215, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kuroiwa, H.; Takahara, M.; Miyagishima, S.Y.; Kuroiwa, T. Visualization of an FtsZ ring in chloroplasts of Lilium longiflorum leaves. Plant Cell Physiol. 2001, 42, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Osteryoung, K.W.; Stokes, K.D.; Rutherford, S.M.; Percival, A.L.; Lee, W.Y. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial FtsZ. Plant Cell 1998, 10, 1991–2004. [Google Scholar] [CrossRef] [PubMed]
- Osteryoung, K.W.; Vierling, E. Conserved cell and organelle division. Nature 1995, 376, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Glynn, J.M.; Froehlich, J.E.; Osteryoung, K.W. Arabidopsis ARC6 coordinates the division machineries of the inner and outer chloroplast membranes through interaction with PDV2 in the intermembrane space. Plant Cell 2008, 20, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Vitha, S.; Froehlich, J.E.; Koksharova, O.; Pyke, K.A.; Van, E.H.; Osteryoung, K.W. Arc6 is a j-domain plastid division protein and an evolutionary descendant of the cyanobacterial cell division protein Ftn2. Plant Cell 2003, 15, 1918–1933. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Kadirjankalbach, D.; Froehlich, J.E.; Osteryoung, K.W. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc. Natl. Acad. Sci. USA 2003, 100, 4328–4333. [Google Scholar] [CrossRef] [PubMed]
- Miyagishima, S.Y.; Froehlich, J.E.; Osteryoung, K.W. PDV1 and PDV2 mediate recruitment of the dynamin-related protein ARC5 to the plastid division site. Plant Cell 2006, 18, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Osteryoung, K.W.; Nunnari, J. The division of endosymbiotic organelles. Science 2003, 302, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Mcandrew, R.S.; Froehlich, J.E.; Vitha, S.; Stokes, K.D.; Osteryoung, K.W. Colocalization of plastid division proteins in the chloroplast stromal compartment establishes a new functional relationship between FtsZ1 and FtsZ2 in higher plants. Plant Physiol. 2001, 127, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- Miyagishima, S.Y.; Nakanishi, H.; Kabeya, Y. Structure, regulation, and evolution of the plastid division machinery. Int. Rev. Cell Mol. Biol. 2011, 291, 115–153. [Google Scholar] [PubMed]
- Schmitz, A.J.; Glynn, J.M.; Olson, B.J.; Stokes, K.D.; Osteryoung, K.W. Arabidopsis FtsZ2-1 and FtsZ2-2 are functionally redundant, but ftsz-based plastid division is not essential for chloroplast partitioning or plant growth and development. Mol. Plant 2009, 2, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Osteryoung, K.W.; Mcandrew, R.S. The plastid division machine. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.D.; Osteryoung, K.W. Early divergence of the FtsZ1 and FtsZ2 plastid division gene families in photosynthetic eukaryotes. Gene 2003, 320, 97–108. [Google Scholar] [CrossRef]
- Chikkala, V.R.N.; Nugent, G.D.; Stalker, D.M.; Mouradov, A.; Stevenson, T.W. Expression of brassica oleracea FtsZ1-1 and mind alters chloroplast division in nicotiana tabacum generating macro- and mini-chloroplasts. Plant Cell Rep. 2012, 31, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Pater, S.; Caspers, M.; Kottenhagen, M.; Meima, H.; Ter Stege, R.; Vetten, N. Manipulation of starch granule size distribution in potato tubers by modulation of plastid division. Plant Biotechnol. J. 2006, 4, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.S.; Kawagoe, Y. Amyloplast division progresses simultaneously at multiple sites in the endosperm of rice. Plant Cell Physiol. 2009, 50, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.S.; Kawagoe, Y. Septum formation in amyloplasts produces compound granules in the rice endosperm and is regulated by plastid division proteins. Plant Cell Physiol. 2010, 51, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Baguma, Y. Regulation of Starch Synthesis in Cassava. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2004. [Google Scholar]
- Osteryoung, K.W.; Pyke, K.A. Division and dynamic morphology of plastids. Annu. Rev. Plant Biol. 2014, 65, 443–472. [Google Scholar] [CrossRef] [PubMed]
- Leyvaguerrero, E. Enhancement of the Free Amino Acid and Protein Content of Cassava Storage Roots and Evaluation of Root-Specific Promoters in Cassava. Ph.D. Thesis, Ohio State University, Columbus, OH, USA, 2011. [Google Scholar]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Fujiwara, M.; Yoshida, S. Chloroplast targeting of chloroplast division ftsZ2 proteins in Arabidopsis. Biochem. Biophys. Res. Commun. 2001, 287, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Lutkenhausand, J.; Addinall, S.G. Bacterial cell division and the Z ring. Annu. Rev. Biochem. 1997, 66, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Terbush, A.D.; Osteryoung, K.W. Distinct functions of chloroplast FtsZ1 and FtsZ2 in Z-ring structure and remodeling. J. Cell Biol. 2012, 199, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Olson, B.J.S.C.; Wang, Q.; Osteryoung, K.W. GTP-dependent heteropolymer formation and bundling of chloroplast FtsZ1 and FtsZ2. J. Biol. Chem. 2010, 285, 20634–20643. [Google Scholar] [CrossRef] [PubMed]
- Erickson, H.P.; Anderson, D.E.; Osawa, M. Ftsz in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 2010, 74, 504–528. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Erickson, H.P. Inside-out Z rings—Constriction with and without GTP hydrolysis. Mol. Microbiol. 2011, 81, 571–579. [Google Scholar] [CrossRef] [PubMed]
- El-Kafafi, E.-S.; Mukherjee, S.; El-Shami, M.; Putaux, J.-L.; Block, M.A.; Pignot-Paintrand, I.; Lerbs-Mache, S.; Falconet, D. The plastid division proteins, FtsZ1 and FtsZ2, differ in their biochemical properties and sub-plastidial localization. Biochem. J. 2005, 387, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.; Hause, B.; Hause, G.; Fester, T. FtsZ characterization and immunolocalization in the two phases of plastid reorganization in arbuscular mycorrhizal roots of Medicago truncatula. Plant Cell Physiol. 2006, 47, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Johnson, C.B.; Vitha, S.; Holzenburg, A. Plant FtsZ1 and FtsZ2 expressed in a eukaryotic host: GTPase activity and self-assembly. FEBS Lett. 2010, 584, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.D.; Mcandrew, R.S.; Figueroa, R.; Vitha, S.; Osteryoung, K.W. Chloroplast division and morphology are differentially affected by overexpression of FtsZ1 and FtsZ2 genes in arabidopsis. Plant Physiol. 2000, 124, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Colletti, K.S.; Tattersall, E.A.; Pyke, K.A.; Froelich, J.E.; Stokes, K.D.; Osteryoung, K.W. A homologue of the bacterial cell division site-determining factor mind mediates placement of the chloroplast division apparatus. Curr. Biol. 2000, 10, 507–516. [Google Scholar] [CrossRef]
- Branco, C.L.J.C.; John, L.; Chen, S.; Regina, B.D.S.C.; Vieira, E.A.; Anderson, J.V. Characterization of carotenoid-protein complexes and gene expression analysis associated with carotenoid sequestration in pigmented cassava (Manihot esculenta Crantz) storage root. Open Biochem. J. 2012, 6, 116–130. [Google Scholar]
- Jcb, C.L.; Agustini Marco, A.V.; Anderson, J.V.; Vieira, E.A.; Rb, D.S.C.; Chen, S.; Schaal, B.A.; Silva, J.P. Natural variation in expression of genes associated with carotenoid biosynthesis and accumulation in cassava (Manihot esculenta Crantz) storage root. BMC Plant Biol. 2016, 16, 133. [Google Scholar]
- Sojikul, P.; Saithong, T.; Kalapanulak, S.; Pisuttinusart, N.; Limsirichaikul, S.; Tanaka, M.; Utsumi, Y.; Sakurai, T.; Seki, M.; Narangajavana, J. Genome-wide analysis reveals phytohormone action during cassava storage root initiation. Plant Mol. Biol. 2015, 88, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Fan, W.; Wang, J. Studies on the changes of endogenous hormones in cassava during growing development. Chin. Agric. Sci. Bull. 2011, 27, 82–86. [Google Scholar]
- Wang, X.Q.; Li, L.M.; Yang, P.P.; Gong, C.L. The role of hexokinases from grape berries (Vitis vinifera L.) in regulating the expression of cell wall invertase and sucrose synthase genes. Plant Cell Rep. 2014, 33, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Baguma, Y.; Sun, C.; Borén, M.; Olsson, H.; Rosenqvist, S.; Mutisya, J.; Rubaihayo, P.R.; Jansson, C. Sugar-mediated semidian oscillation of gene expression in the cassava storage root regulates starch synthesis. Plant Signal. Behav. 2008, 3, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, D. Effects of salicylic acid and methyl jasmonate on the formation of potato microtuber. J. Huazhong Agric. 2005, 24, 74–78. [Google Scholar]


| Primer Name | Sequence (5′ to 3′) | Application |
|---|---|---|
| FtsZ1-F | AATGGATCCAAACCCTAAAACCTCT | Gene cloning of MeFtsZ1 |
| FtsZ1-R | TGGAAGCTTAAGAAACAACCAACT | |
| FtsZ2-1-F | CGCGGATCCTACTTCAGCGACTTTAT | Gene cloning of MeFtsZ2-1 |
| FtsZ2-1-R | GACGTCGACATGCTCTGTGGAACTATTA | |
| FtsZ2-2-F | CGCGGATCCTTGACATACTTTTC | Gene cloning of MeFtsZ2-2 |
| FtsZ2-2-R | GACGTCGACAACTATTACCTACGG | |
| FtsZ1-gfp-F | AATGGTACCATGGCGACACTTCATCT | Construction of MeFtsZ1 fused with GFP (green fluorescent protein) |
| FtsZ1-gfp-R | CACGGATCCAAAGAACAGCTTTCTAG | |
| FtsZ2-1-gfp-F | TTAGTCGACATGGCAGCTTGTGTGT | Construction of MeFtsZ2-1 fused with GFP |
| FtsZ2-1-gfp-R | CATGGATCCAAGTCTTGGATAGCGA | |
| FtsZ2-2-gfp-F | TATGTCGACATGGCAGCCTGTCTGT | Construction of MeFtsZ2-2 fused with GFP |
| FtsZ2-2-gfp-R | TATGGATCCAGCTCTTGGATAGCGC | |
| FtsZ1-qPCR-F | GCACCAGTTGTAGCCCAGATA | Expression analysis of MeFtsZ1 |
| FtsZ1-qPCR-R | TCCTTCTGGCTGATGATGTTC | |
| FtsZ2-1-qPCR-F | GAACAACACAGCAACCACTCC | Expression analysis of MeFtsZ2-1 |
| FtsZ2-1-qPCR-R | GAAGGGTGTGGATTTTGGAT | |
| FtsZ2-2-qPCR-F | CCAATCAACCCCAGTAACAGA | Expression analysis of MeFtsZ2-2 |
| FtsZ2-2-qPCR-R | GGATTTTGCTGATGTGAGAG | |
| Tubulin-F | GTGGAGGAACTGGTTCTGGA | Expression analysis of Tubulin gene |
| Tubulin-R | TGCACTCATCTGCATTCTCC |
| Genes | Accession Numbers | ORF Length (bp) | Length (aa) |
|---|---|---|---|
| MeFtsz1 | JN936179 | 1248 | 415 |
| MeFtsz2-1 | JN936180 | 1458 | 485 |
| MeFtsz2-2 | JQ343216 | 1455 | 484 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, M.-T.; Min, Y.; Yao, Y.; Chen, X.; Fan, J.; Yuan, S.; Wang, L.; Sun, C.; Zhang, F.; Shang, L.; et al. Isolation and Characterization of Ftsz Genes in Cassava. Genes 2017, 8, 391. https://doi.org/10.3390/genes8120391
Geng M-T, Min Y, Yao Y, Chen X, Fan J, Yuan S, Wang L, Sun C, Zhang F, Shang L, et al. Isolation and Characterization of Ftsz Genes in Cassava. Genes. 2017; 8(12):391. https://doi.org/10.3390/genes8120391
Chicago/Turabian StyleGeng, Meng-Ting, Yi Min, Yuan Yao, Xia Chen, Jie Fan, Shuai Yuan, Lei Wang, Chong Sun, Fan Zhang, Lu Shang, and et al. 2017. "Isolation and Characterization of Ftsz Genes in Cassava" Genes 8, no. 12: 391. https://doi.org/10.3390/genes8120391
APA StyleGeng, M.-T., Min, Y., Yao, Y., Chen, X., Fan, J., Yuan, S., Wang, L., Sun, C., Zhang, F., Shang, L., Wang, Y.-L., Li, R.-M., Fu, S.-P., Duan, R.-J., Liu, J., Hu, X.-W., & Guo, J.-C. (2017). Isolation and Characterization of Ftsz Genes in Cassava. Genes, 8(12), 391. https://doi.org/10.3390/genes8120391





