Network-Based Method for Identifying Co-Regeneration Genes in Bone, Dentin, Nerve and Vessel Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Regeneration Genes of Bone, Dentin, Nerve and Vessel
2.2. Network Construction
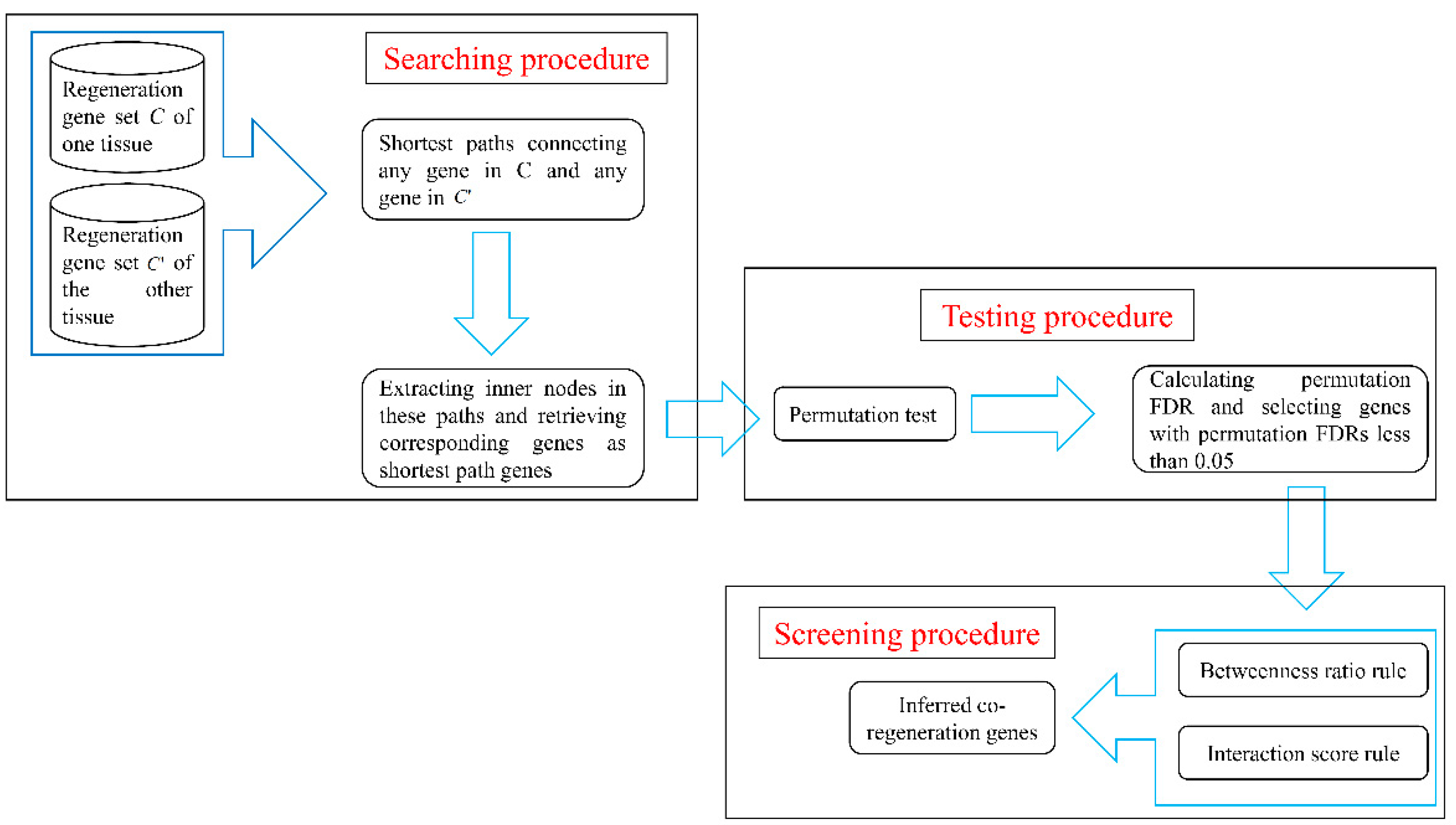
2.3. Network-Based Method
2.3.1. Searching Procedure
2.3.2. Testing Procedure
2.3.3. Screening Procedure
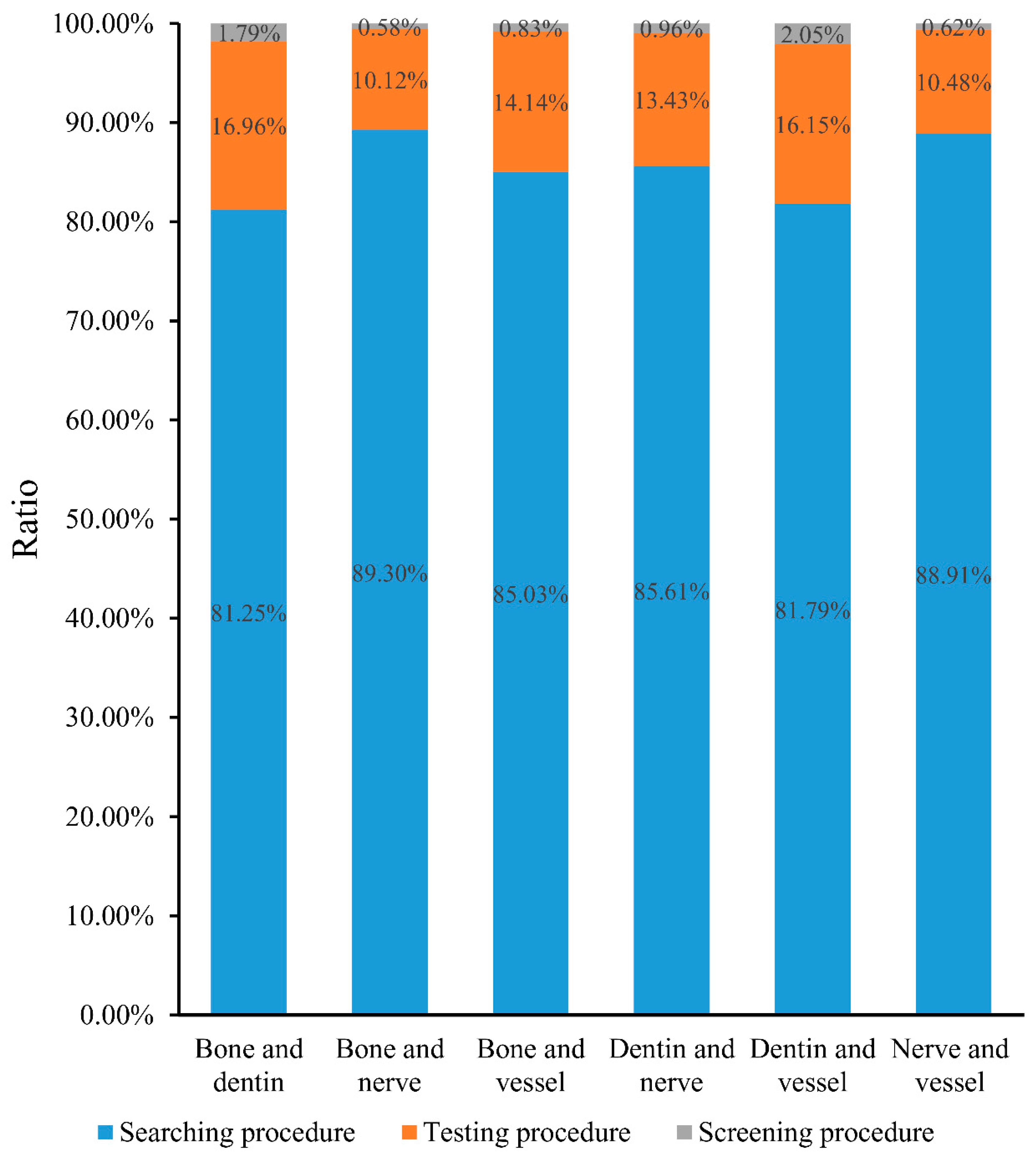
3. Results
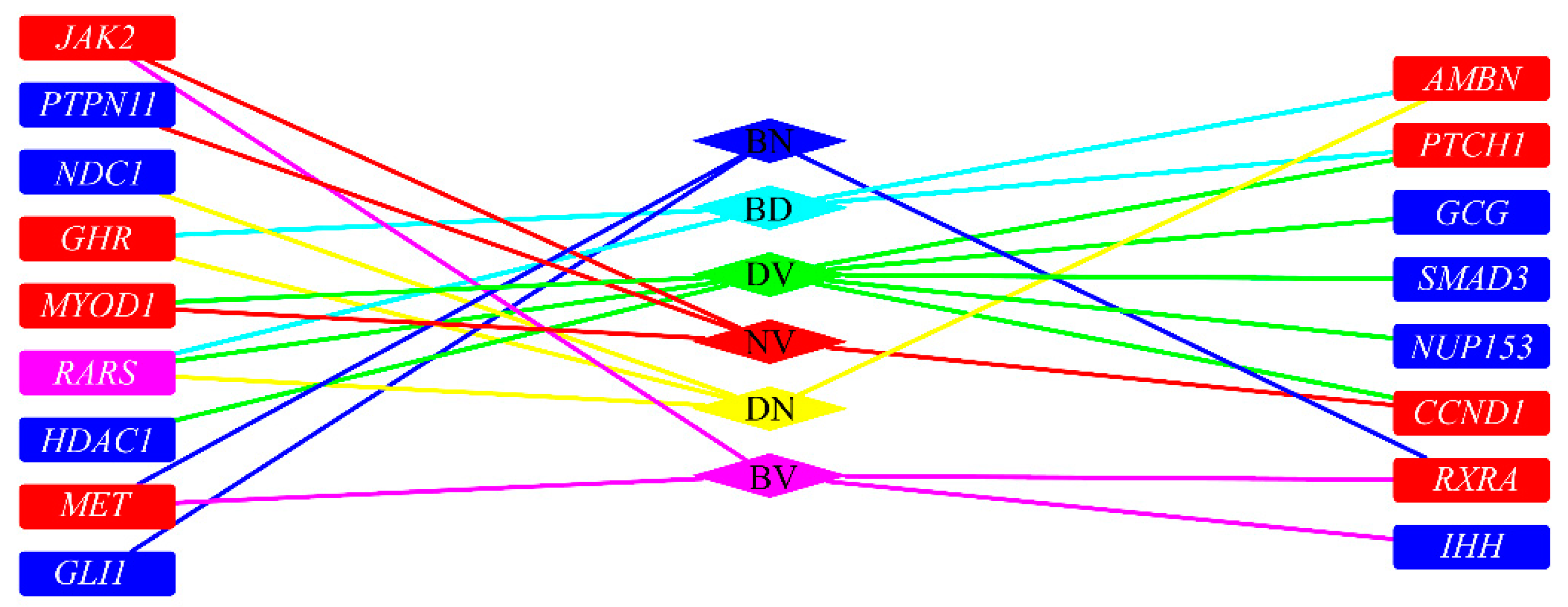
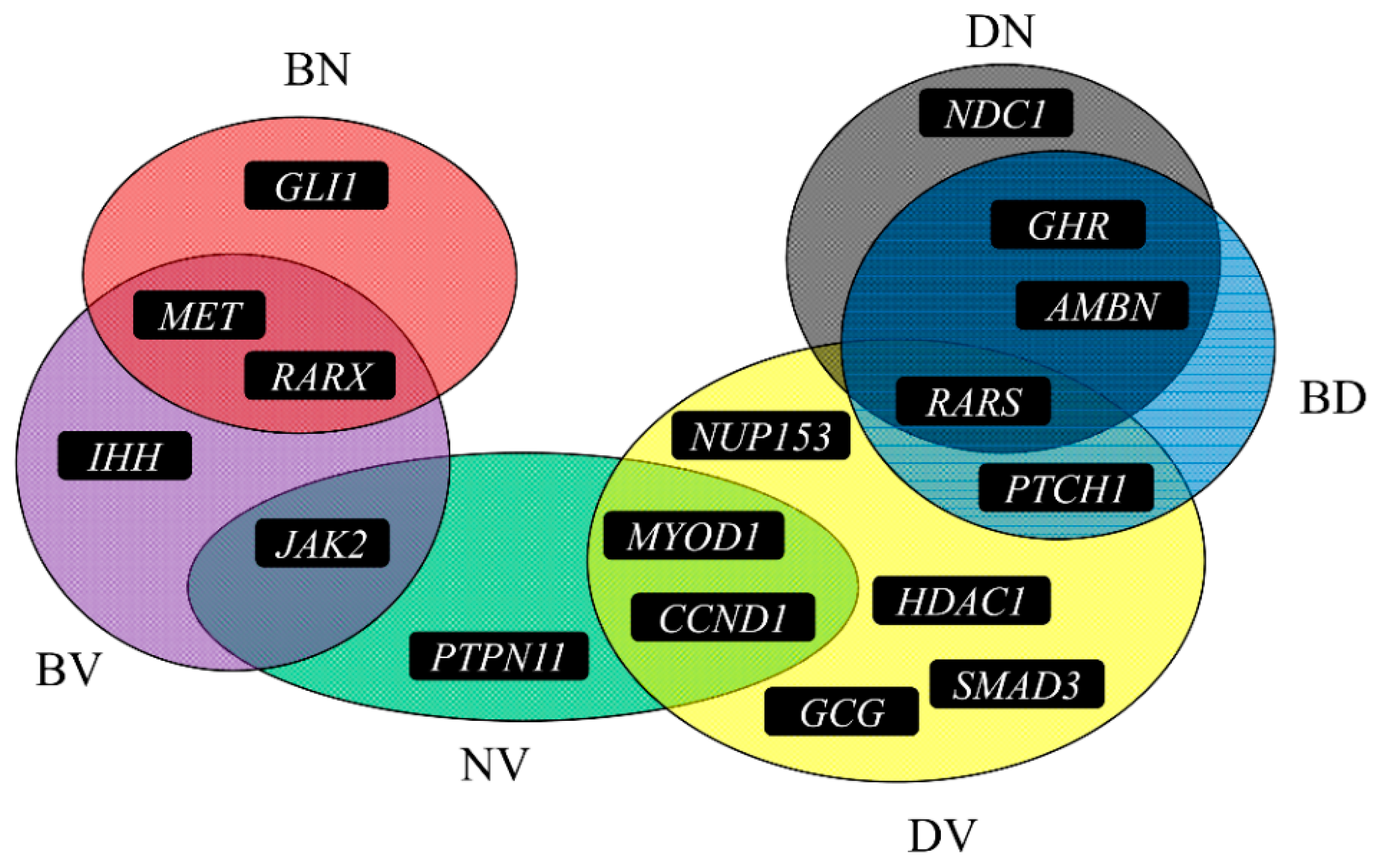
4. Discussion
4.1. Co-Regeneration Genes for Bone and Dentine
4.2. Co-Regeneration Genes for Bone and Nerve
4.3. Co-Regeneration Genes for Bone and Vessel
4.4. Co-Regeneration Genes for Dentin and Nerve
4.5. Co-Regeneration Genes for Dentin and Vessel
4.6. Co-Regeneration Genes for Nerve and Vessel
4.7. Other Applications of the Network-Based Method
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General; Office of the Surgeon General: Rockville, MD, USA, 2004.
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Alghazali, K.M.; Nima, Z.A.; Hamzah, R.N.; Dhar, M.S.; Anderson, D.E.; Biris, A.S. Bone-tissue engineering: Complex tunable structural and biological responses to injury, drug delivery, and cell-based therapies. Drug Metab. Rev. 2015, 47, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Dong, X.; Li, R.; Li, Y.; Wang, Z. Identification of hepatocellular carcinoma–related genes with a machine learning and network analysis. J. Comput. Biol. 2015, 22, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Huang, T.; Zhang, Y.H.; Jiang, Y.; Zheng, M.; Cai, Y.D. Identification of novel candidate drivers connecting different dysfunctional levels for lung adenocarcinoma using protein–protein interactions and a shortest path approach. Sci. Rep. 2016, 6, 29849. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, Y.; Zhang, Y.; Chen, L.; Zhang, N.; Huang, T.; Cai, Y.D.; Kong, X.Y. Identification of hepatocellular carcinoma related genes with k-th shortest paths in a protein—Protein interaction network. Mol. BioSyst. 2013, 9, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xing, Z.; Huang, T.; Shu, Y.; Huang, G.; Li, H.P. Application of the shortest path algorithm for the discovery of breast cancer related genes. Curr. Bioinform. 2016, 11, 51–58. [Google Scholar] [CrossRef]
- Li, B.Q.; Huang, T.; Liu, L.; Cai, Y.D.; Chou, K.C. Identification of colorectal cancer related genes with mRMR and shortest path in protein–protein interaction network. PLoS ONE 2012, 7, e33393. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Huang, T.; Kong, X.Y.; Lu, L.; Cai, Y.D. Mining for novel tumor suppressor genes using a shortest path approach. J. Biomol. Struct. Dyn. 2016, 34, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, J.; Huang, T.; Shu, Y.; Chen, L. Identification of novel proliferative diabetic retinopathy related genes on protein–protein interaction network. Neurocomputing 2016, 217, 63–72. [Google Scholar] [CrossRef]
- Chen, L.; Chu, C.; Kong, X.; Huang, G.; Huang, T. A hybrid computational method for the discovery of novel reproduction-related genes. PLoS ONE 2015, 10, e0117090. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, B.; Wang, S.; Yang, J.; Hu, J.; Xie, Z.; Wang, Y.; Huang, T.; Cai, Y.D.; Xie, Z. OPMSP: A computational method integrating protein interaction and sequence information for the identification of novel putative oncogenes. Protein Pept. Lett. 2016, 23, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.D.; Zhang, Q.; Zhang, Y.H.; Chen, L.; Huang, T. Identification of genes associated with breast cancer metastasis to bone on a protein–protein interaction network with a shortest path algorithm. J. Proteome Res. 2017, 16, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Xing, Z.; Yuan, F.; Shu, Y.; Zhang, Y.; Kong, X.; Huang, T.; Li, H.; Cai, Y.D. An integrated method for the identification of novel genes related to oral cancer. PLoS ONE 2017, 12, e0175185. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Szklarczyk, D.; Frankild, S.; Kuhn, M.; Simonovic, M.; Roth, A.; Lin, J.; Minguez, P.; Bork, P.; Von Mering, C. String v9.1: Protein–protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013, 41, D808–D815. [Google Scholar] [CrossRef] [PubMed]
- Washington, N.; Lewis, S. Ontologies: Scientific data sharing made easy. Nat. Educ. 2008, 1, 5. [Google Scholar]
- Zhao, M.; Rotgans, B.; Wang, T.; Cummins, S.F. Regene: A literature-based knowledgebase of animal regeneration that bridge tissue regeneration and cancer. Sci. Rep. 2016, 6, 23167. [Google Scholar] [CrossRef] [PubMed]
- Regeneration Gene database. Available online: http://regene.bioinfo-minzhao.org/ (accessed on 28 July 2017).
- McCauley, L.K.; Somerman, M.J. Mineralized Tissues in Oral and Craniofacial Science: Biological Principles and Clinical Correlates; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Xenarios, I.; Rice, D.W.; Salwinski, L.; Baron, M.K.; Marcotte, E.M.; Eisenberg, D. DIP: The database of interacting proteins. Nucleic Acids Res. 2000, 28, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Stark, C.; Breitkreutz, B.J.; Reguly, T.; Boucher, L.; Breitkreutz, A.; Tyers, M. Biogrid: A general repository for interaction datasets. Nucleic Acids Res. 2006, 34, D535–D539. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.L.; Huang, T.; Shi, X.; Lu, W.C.; Cai, Y.D.; Chou, K.C. Predicting functions of proteins in mouse based on weighted protein–protein interaction network and protein hybrid properties. PLoS ONE 2011, 6, e14556. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L.; Ciou, J.S.; Huang, C.H. Prediction of protein functions based on function-function correlation relations. Comput. Biol. Med. 2010, 40, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wang, Q.P.; Chen, L.; Huang, T. Prediction of human genes regulatory functions based on protein–protein interaction network. Protein Pept. Lett. 2012, 19, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chu, C.; Huang, T.; Kong, X.; Zhang, Y.; Zhang, N.; Cai, Y.D. Exploring mouse protein function via multiple approaches. PLoS ONE 2016, 11, e0166580. [Google Scholar] [CrossRef] [PubMed]
- Gormen, T.H.; Leiserson, C.E.; Rivest, R.L.; Stein, C. Introduction to Algorithms; MIT Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Bonneau, R. Learning biological networks: From modules to dynamics. Nat. Chem. Biol. 2008, 4, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Kitsak, M.; Havlin, S.; Paul, G.; Riccaboni, M.; Pammolli, F.; Stanley, H.E. Betweenness centrality of fractal and nonfractal scale-free model networks and tests on real networks. Phys. Rev. E 2007, 75, 056115. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.B.M. Markov Networks for Detecting Overlapping Elements in Sequence Data; MIT Press: Cambridge, MA, USA, 2005; p. 193. [Google Scholar]
- Freeman, L.C. Centrality in social networks conceptual clarification. Soc. Netw. 1979, 1, 215–239. [Google Scholar] [CrossRef]
- Huang, T.; Wang, P.; Ye, Z.Q.; Xu, H.; He, Z.; Feng, K.Y.; Hu, L.; Cui, W.; Wang, K.; Dong, X.; et al. Prediction of deleterious non-synonymous SNPs based on protein interaction network and hybrid properties. PLoS ONE 2010, 5, e11900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ling, J.; Barcia, G.; Jing, L.; Wu, J.; Barry, B.J.; Mochida, G.H.; Hill, R.S.; Weimer, J.M.; Stein, Q.; et al. Mutations in QARS, encoding glutaminyl-tRNA synthetase, cause progressive microcephaly, cerebral-cerebellar atrophy, and intractable seizures. Am. J. Hum. Genet. 2014, 94, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.M.; Esen, E.; Okunade, A.L.; Patterson, B.W.; Long, F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J. Clin. Investig. 2015, 125, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Leitao-Goncalves, R.; Ermanoska, B.; Jacobs, A.; De Vriendt, E.; Timmerman, V.; Lupski, J.R.; Callaerts, P.; Jordanova, A. Drosophila as a platform to predict the pathogenicity of novel aminoacyl-tRNA synthetase mutations in cmt. Amino Acids 2012, 42, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, W.J.; Pulido, R. Protein tyrosine phosphatase variants in human hereditary disorders and disease susceptibilities. Biochim. Biophys. Acta 2013, 1832, 1673–1696. [Google Scholar] [CrossRef] [PubMed]
- Arum, O.; Boparai, R.K.; Saleh, J.K.; Wang, F.; Dirks, A.L.; Turner, J.G.; Kopchick, J.J.; Liu, J.L.; Khardori, R.K.; Bartke, A. Specific suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHR-KO) mice attenuates phenotypic features of slow aging. Aging Cell 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Theyse, L.F.; Oosterlaken-Dijksterhuis, M.A.; Van Doorn, J.; Terlou, M.; Mol, J.A.; Voorhout, G.; Hazewinkel, H.A. Expression of osteotropic growth factors and growth hormone receptor in a canine distraction osteogenesis model. J. Bone Miner. Metab. 2006, 24, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Symons, A.L.; Henry, A.C.; Chang, S.; Daley, T.J.; Harbrow, D.J.; Joseph, B.K. The effect of glucocorticosteroid treatment on dentine formation in the lewis rat, a histological study. Growth Factors 2000, 18, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Hirose, N.; Shimazu, A.; Watanabe, M.; Tanimoto, K.; Koyota, S.; Sugiyama, T.; Uchida, T.; Tanne, K. Ameloblastin in Hertwig’s epithelial root sheath regulates tooth root formation and development. PLoS ONE 2013, 8, e54449. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Fukumoto, E.; Yamada, A.; Yuasa, K.; Yoshizaki, K.; Iwamoto, T.; Saito, M.; Nakamura, T.; Fukumoto, S. Interaction between fibronectin and β1 integrin is essential for tooth development. PLoS ONE 2015, 10, e0121667. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Fukumoto, S.; Yamada, Y.; Evans, C.A.; Diekwisch, T.G.; Luan, X. Ameloblastin, an extracellular matrix protein, affects long bone growth and mineralization. J. Bone Miner. Res. 2016, 31, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Tamburstuen, M.V.; Reppe, S.; Spahr, A.; Sabetrasekh, R.; Kvalheim, G.; Slaby, I.; Syversen, U.; Lyngstadaas, S.P.; Reseland, J.E. Ameloblastin promotes bone growth by enhancing proliferation of progenitor cells and by stimulating immunoregulators. Eur. J. Oral Sci. 2010, 118, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.L.; Rodriguez-Cruz, V.; Ramkissoon, S.H.; Ligon, K.L.; Greco, S.J.; Rameshwar, P. Temozolomide resistance in glioblastoma occurs by miRNA-9-targeted PTCH1, independent of sonic hedgehog level. Oncotarget 2015, 6, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.H.; Yang, L.; Dessaud, E.; Chuang, K.; Moore, D.M.; Rohatgi, R.; Briscoe, J.; Novitch, B.G. Notch activity modulates the responsiveness of neural progenitors to sonic hedgehog signaling. Dev. Cell 2015, 33, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.Y.; Hong, Y.Y.; Qu, J.F.; Chen, F.; Li, T.J. The large intracellular loop of PTCH1 mediates the non-canonical hedgehog pathway through cyclin B1 in nevoid basal cell carcinoma syndrome. Int. J. Mol. Med. 2014, 34, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and applications in craniofacial bone regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rivron, N.C.; Raiss, C.C.; Liu, J.; Nandakumar, A.; Sticht, C.; Gretz, N.; Truckenmuller, R.; Rouwkema, J.; Van Blitterswijk, C.A. Sonic hedgehog-activated engineered blood vessels enhance bone tissue formation. Proc. Natl. Acad. Sci. USA 2012, 109, 4413–4418. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, J.Q. Signaling pathways critical for tooth root formation. J. Dent. Res. 2017, 96, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. Met signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.T.; Ting, C.H.; Yen, M.L.; Liu, K.J.; Sytwu, H.K.; Wu, K.K.; Yen, B.L. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: Review of current clinical trials. J. Biomed. Sci. 2016, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Gebhart, N.; Richelson, E.; Brott, T.G.; Meschia, J.F.; Zubair, A.C. Mechanism of mesenchymal stem cell-induced neuron recovery and anti-inflammation. Cytotherapy 2014, 16, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Huat, T.J.; Khan, A.A.; Pati, S.; Mustafa, Z.; Abdullah, J.M.; Jaafar, H. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci. 2014, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Padial-Molina, M.; O’Valle, F.; Lanis, A.; Mesa, F.; Dohan Ehrenfest, D.M.; Wang, H.L.; Galindo-Moreno, P. Clinical application of mesenchymal stem cells and novel supportive therapies for oral bone regeneration. Biomed. Res. Int. 2015, 2015, 341327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, W.; Cho, H.M.; Ouyang, H.; Li, W.; Lin, Y.; Do, J.; Zhang, L.; Ding, S.; Liu, Y.; et al. Integration and long distance axonal regeneration in the central nervous system from transplanted primitive neural stem cells. J. Biol. Chem. 2013, 288, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Dolle, P. Developmental expression of retinoic acid receptors (RARs). Nucl. Recept Signal. 2009, 7, e006. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Sheppard, A.; Godfrey, K.M.; McLean, C.; Garratt, E.; Ntani, G.; Davies, L.; Murray, R.; Inskip, H.M.; Gluckman, P.D.; et al. Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth. J. Bone Miner. Res. 2014, 29, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Natrajan, M.S.; De la Fuente, A.G.; Crawford, A.H.; Linehan, E.; Nunez, V.; Johnson, K.R.; Wu, T.; Fitzgerald, D.C.; Ricote, M.; Bielekova, B.; et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain J. Neurol. 2015, 138, 3581–3597. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Zhao, H.; Urata, M.; Chai, Y. Sutures possess strong regenerative capacity for calvarial bone injury. Stem Cells Dev. 2016, 25, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Chaklader, M.; Law, S. Alteration of hedgehog signaling by chronic exposure to different pesticide formulations and unveiling the regenerative potential of recombinant sonic hedgehog in mouse model of bone marrow aplasia. Mol. Cell. Biochem. 2015, 401, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.A.; Kobayashi, M.; Krishnan, A.; Webber, C.; Christie, K.; Guo, G.; Singh, V.; Zochodne, D.W. Intrinsic facilitation of adult peripheral nerve regeneration by the sonic hedgehog morphogen. Exp. Neurol. 2015, 271, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Ii, M.; Jujo, K.; Renault, M.A.; Thorne, T.; Clarke, T.; Ito, A.; Tanaka, T.; Klyachko, E.; Tabata, Y.; et al. Estradiol triggers sonic-hedgehog-induced angiogenesis during peripheral nerve regeneration by downregulating hedgehog-interacting protein. Lab. Investig. 2012, 92, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Sugito, H.; Shibukawa, Y.; Kinumatsu, T.; Yasuda, T.; Nagayama, M.; Yamada, S.; Minugh-Purvis, N.; Pacifici, M.; Koyama, E. Ihh signaling regulates mandibular symphysis development and growth. J. Dent. Res. 2011, 90, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Luukko, K.; Kvinnsland, I.H.; Kettunen, P. Developmentally regulated expression of Shh and Ihh in the developing mouse cranial base: Comparison with Sox9 expression. Anat. Rec. 2005, 286, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Chen, T.; Wang, Y.; Tian, R.; Zhang, L.; Song, P.; Yang, S.; Zhu, Y.; Guo, X.; Huang, Y.; et al. Mesenchymal stem cells overexpressing Ihh promote bone repair. J. Orthop. Surg. Res. 2014, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Kazmers, N.H.; McKenzie, J.A.; Shen, T.S.; Long, F.; Silva, M.J. Hedgehog signaling mediates woven bone formation and vascularization during stress fracture healing. Bone 2015, 81, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Yan, J.; Jiagbogu, N.; Heideman, D.A.; Canfield, A.E.; Alexander, M.Y. HGF/c-met signalling promotes Notch3 activation and human vascular smooth muscle cell osteogenic differentiation in vitro. Atherosclerosis 2011, 219, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Brooks, A.J.; Chhabra, Y. A new mechanism for growth hormone receptor activation of JAK2, and implications for related cytokine receptors. JAKS-TAT 2014, 3, e29569. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.J.; Brooks, A.J. JAK2 activation by growth hormone and other cytokines. Biochem. J. 2015, 466, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belmokhtar, K.; Bourguignon, T.; Worou, M.E.; Khamis, G.; Bonnet, P.; Domenech, J.; Eder, V. Regeneration of three layers vascular wall by using BMP2-treated MSC involving HIF-1α and Id1 expressions through JAK/STAT pathways. Stem Cell Rev. 2011, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Yun, H.M.; Quang, T.H.; Oh, H.; Lee, D.S.; Auh, Q.S.; Kim, E.C. 4-methoxydalbergione suppresses growth and induces apoptosis in human osteosarcoma cells in vitro and in vivo xenograft model through down-regulation of the JAK2/STAT3 pathway. Oncotarget 2016, 7, 6960–6971. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, Y.; Kawamura, T.; Hori-e, R.; Yamashita, I. Retionic acid and its receptors are required for expression of aryl hydrocarbon receptor mRNA and embryonic development of blood vessel and bone in the medaka fish, Oryzias latipes. Zoolog Sci. 2004, 21, 541–551. [Google Scholar] [PubMed]
- Kubalak, S.W.; Hutson, D.R.; Scott, K.K.; Shannon, R.A. Elevated transforming growth factor β2 enhances apoptosis and contributes to abnormal outflow tract and aortic sac development in retinoic X receptor alpha knockout embryos. Development 2002, 129, 733–746. [Google Scholar] [PubMed]
- Bhattacharya, S.; Macdonald, S.T.; Farthing, C.R. Molecular mechanisms controlling the coupled development of myocardium and coronary vasculature. Clin. Sci. 2006, 111, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Eisenhardt, N.; Redolfi, J.; Antonin, W. Interaction of Nup53 with Ndc1 and Nup155 is required for nuclear pore complex assembly. J. Cell Sci. 2014, 127, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Koling, A. Freeze-fracture electron microscopy of non-myelinated nerve fibres in the human dental pulp. Arch. Oral Biol. 1985, 30, 685–690. [Google Scholar] [CrossRef]
- Yoshizaki, K.; Yamamoto, S.; Yamada, A.; Yuasa, K.; Iwamoto, T.; Fukumoto, E.; Harada, H.; Saito, M.; Nakasima, A.; Nonaka, K.; et al. Neurotrophic factor neurotrophin-4 regulates ameloblastin expression via full-length TrkB. J. Biol. Chem. 2008, 283, 3385–3391. [Google Scholar] [CrossRef] [PubMed]
- Tsai, E.M.; Wang, Y.C.; Lee, T.T.; Tsai, C.F.; Chen, H.S.; Lai, F.J.; Yokoyama, K.K.; Hsieh, T.H.; Wu, R.M.; Lee, J.N. Dynamic Trk and G protein signalings regulate dopaminergic neurodifferentiation in human trophoblast stem cells. PLoS ONE 2015, 10, e0143852. [Google Scholar] [CrossRef] [PubMed]
- Corless, C.L.; Mendoza, A.; Collins, T.; Lawler, J. Colocalization of thrombospondin and syndecan during murine development. Dev. Dyn. 1992, 193, 346–358. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, A.H.; Sampson, W.J.; Dreyer, C.W.; Pierce, A.M.; Ferguson, I.A. Immunohistochemical detection of nerve growth factor and its receptors in the rat periodontal ligament during tooth movement. Arch. Oral Biol. 2009, 54, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, S.; Wang, X.P. Yap overexpression affects tooth morphogenesis and enamel knot patterning. J. Dent. Res. 2014, 93, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, B.K.; Maden, M.; McCaffery, P.; Barrett, A.W. The distribution of retinaldehyde dehydrogenase-2 in rat and human orodental tissues. Arch. Oral Biol. 2001, 46, 1099–1104. [Google Scholar] [CrossRef]
- Shen, S.; Benoy, V.; Bergman, J.A.; Kalin, J.H.; Frojuello, M.; Vistoli, G.; Haeck, W.; Van Den Bosch, L.; Kozikowski, A.P. Bicyclic-capped histone deacetylase 6 inhibitors with improved activity in a model of axonal charcot-marie-tooth disease. ACS Chem. Neurosci. 2016, 7, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Bazou, D.; Ng, M.R.; Song, J.W.; Chin, S.M.; Maimon, N.; Munn, L.L. Flow-induced HDAC1 phosphorylation and nuclear export in angiogenic sprouting. Sci. Rep. 2016, 6, 34046. [Google Scholar] [CrossRef] [PubMed]
- Ewald, A.; Kossner, U.; Scheer, U.; Dabauvalle, M.C. A biochemical and immunological comparison of nuclear and cytoplasmic pore complexes. J. Cell Sci. 1996, 109, 1813–1824. [Google Scholar] [PubMed]
- Shao, W.; Wang, D.; Chiang, Y.T.; Ip, W.; Zhu, L.; Xu, F.; Columbus, J.; Belsham, D.D.; Irwin, D.M.; Zhang, H.; et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes 2013, 62, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.; Nuche-Berenguer, B.; Gutierrez-Rojas, I.; Acitores, A.; Sancho, V.; Valverde, I.; Gonzalez, N.; Villanueva-Penacarrillo, M.L. Normalizing action of exendin-4 and GLP-1 in the glucose metabolism of extrapancreatic tissues in insulin-resistant and type 2 diabetic states. J. Mol. Endocrinol. 2012, 48, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.M.; Aguiar, T.R.; Phansalkar, R.; McAlpine, J.B.; Napolitano, J.G.; Chen, S.N.; Araujo, L.S.; Pauli, G.F.; Bedran-Russo, A. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014, 10, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Sanae, F.; Miyaichi, Y.; Kizu, H.; Hayashi, H. Effects of catechins on vascular tone in rat thoracic aorta with endothelium. Life Sci. 2002, 71, 2553–2562. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Li, C.; Su, Y.; Fang, W.; Zhong, C.; Ji, W.; Zhang, Q.; Su, C. Transcription factor OCT4 promotes cell cycle progression by regulating CCND1 expression in esophageal carcinoma. Cancer Lett. 2014, 354, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Mende, N.; Kuchen, E.E.; Lesche, M.; Grinenko, T.; Kokkaliaris, K.D.; Hanenberg, H.; Lindemann, D.; Dahl, A.; Platz, A.; Hofer, T.; et al. CCND1-CDK4-mediated cell cycle progression provides a competitive advantage for human hematopoietic stem cells in vivo. J. Exp. Med. 2015, 212, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Premaraj, S.; Souza, I.; Premaraj, T. Focal adhesion kinase mediates β-catenin signaling in periodontal ligament cells. Biochem. Biophys. Res. Commun. 2013, 439, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Kundumani-Sridharan, V.; Kumar, S.; Verma, S.K.; Kotla, S.; Mukai, H.; Heckle, M.R.; Rao, G.N. Protein kinase N1 is a novel substrate of NFATc1-mediated cyclin D1-CDK6 activity and modulates vascular smooth muscle cell division and migration leading to inward blood vessel wall remodeling. J. Biol. Chem. 2012, 287, 36291–36304. [Google Scholar] [CrossRef] [PubMed]
- Faura Tellez, G.; Vandepoele, K.; Brouwer, U.; Koning, H.; Elderman, R.M.; Hackett, T.L.; Willemse, B.W.; Holloway, J.; Van Roy, F.; Koppelman, G.H.; et al. Protocadherin-1 binds to Smad3 and suppresses TGF-β1-induced gene transcription. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L725–L735. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Wen, D.; Zhang, M.; Xie, Q.; Ma, L.; Guan, Y.; Ren, Y.; Chen, J.; Hao, C.M. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J. Cell. Biochem. 2014, 115, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Cui, S.; Bian, C.; Yu, X. Crosstalk between TGF-β/Smad3 and BMP/BMPR2 signaling pathways via miR-17–92 cluster in carotid artery restenosis. Mol. Cell. Biochem. 2014, 389, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Srisuwan, T.; Tilkorn, D.J.; Al-Benna, S.; Vashi, A.; Penington, A.; Messer, H.H.; Abberton, K.M.; Thompson, E.W. Survival of rat functional dental pulp cells in vascularized tissue engineering chambers. Tissue Cell 2012, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.B.; Jiang, Z.P. Role of miR-21 and its signaling pathways in renal diseases. J. Recept. Signal Transduct. Res. 2014, 34, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Gaudel, C.; Schwartz, C.; Giordano, C.; Abumrad, N.A.; Grimaldi, P.A. Pharmacological activation of PPARβ promotes rapid and calcineurin-dependent fiber remodeling and angiogenesis in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E297–E304. [Google Scholar] [CrossRef] [PubMed]
- Kamamoto, M.; Machida, J.; Yamaguchi, S.; Kimura, M.; Ono, T.; Jezewski, P.A.; Higashi, Y.; Nakayama, A.; Shimozato, K.; Tokita, Y. Clinical and functional data implicate the Arg(151)Ser variant of MSX1 in familial hypodontia. Eur. J. Hum. Genet. 2011, 19, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Thoma, E.C.; Maurus, K.; Wagner, T.U.; Schartl, M. Parallel differentiation of embryonic stem cells into different cell types by a single gene-based differentiation system. Cell. Reprogram. 2012, 14, 106–111. [Google Scholar] [PubMed]
- Bondeson, M.L. Key insights into the protein tyrosine phosphatase PTPN11/Shp2 associated with noonan syndrome and cancer. Hum. Mutat. 2017, 38, 337. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Kalaitzidis, D.; Neel, B.G. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008, 27, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.P.; Lin, S.J.; Wan, W.B.; Zuo, H.L.; Yao, F.F.; Ruan, H.B.; Xu, J.; Song, W.; Zhou, Y.C.; Wen, S.Y.; et al. Chlorogenic acid prevents osteoporosis by Shp2/PI3K/Akt pathway in ovariectomized rats. PLoS ONE 2016, 11, e0166751. [Google Scholar] [CrossRef] [PubMed]
- Guerin, A.; Therefore, J.; Mireskandari, K.; Jougeh-Doust, S.; Chisholm, C.; Klatt, R.; Richer, J. Expanding the clinical spectrum of ocular anomalies in noonan syndrome: Axenfeld-anomaly in a child with PTPN11 mutation. Am. J. Med. Genet. A 2015, 167A, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, W.M.; Zhang, W.; McCrae, K.R.; Neel, B.G.; Qu, C.K. Noonan syndrome/leukemia-associated gain-of-function mutations in Shp-2 phosphatase (PTPN11) enhance cell migration and angiogenesis. J. Biol. Chem. 2009, 284, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Viader, A.; Chang, L.W.; Fahrner, T.; Nagarajan, R.; Milbrandt, J. MicroRNAs modulate Schwann cell response to nerve injury by reinforcing transcriptional silencing of dedifferentiation-related genes. J. Neurosci. 2011, 31, 17358–17369. [Google Scholar] [CrossRef] [PubMed]
- Loffler, K.; Schafer, P.; Volkner, M.; Holdt, T.; Karl, M.O. Age-dependent Muller glia neurogenic competence in the mouse retina. Glia 2015, 63, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Dai, B.; Xin, J.Y.; He, J.Q.; Feng, S.Q. Overexpression of suppressors of cytokine signaling 1 promotes the neuronal differentiation of C17.2 neural stem cells. Cell. Physiol. Biochem. 2014, 33, 528–538. [Google Scholar] [CrossRef] [PubMed]
| Category | Biological Process | GO Term ID |
|---|---|---|
| Bone regeneration | Bone regeneration | GO:1990523 |
| Bone mineralization | GO:0030282 | |
| Bone marrow development | GO:0048539 | |
| Bone remodeling | GO:0046849 | |
| Bone morphogenesis | GO:0060349 | |
| Dentin regeneration | Odontoblast differentiation | GO:0071895 |
| Dentinogenesis | GO:0097187 | |
| Structural constituent of tooth enamel | GO:0030345 | |
| Regulation of tooth mineralization | GO:0070170 | |
| Dentin secretion | GO:0070468 | |
| Dentin mineralization | GO:0097188 | |
| Tooth mineralization | GO:0034505 | |
| Vessel regeneration | Vascular wound healing | GO:0061042 |
| Blood vessel morphogenesis | GO:0048514 | |
| Blood vessel remodeling | GO:0001974 | |
| Blood vessel development | GO:0001568 |
| Category | Number of Regeneration Genes |
|---|---|
| Bone regeneration | 144 |
| Dentin regeneration | 31 |
| Vessel regeneration | 336 |
| Nerve regeneration | 284 |
| Paired Tissues | Searching Procedure (Inner Nodes of Shortest Paths) | Testing Procedure (Permutation FDR < 0.05) | Screening Procedure (Betweenness Ratio > 0.01 and Min–Max Interaction Score ≥ 400) |
|---|---|---|---|
| Bone and dentin | 244 | 42 | 4 |
| Bone and nerve | 514 | 55 | 3 |
| Bone and vessel | 481 | 72 | 4 |
| Dentin and nerve | 417 | 60 | 4 |
| Dentin and vessel | 390 | 71 | 8 |
| Nerve and vessel | 649 | 72 | 4 |
| Pair of Tissues | Ensembl ID | Gene Symbol | Full Gene Name | Betweenness | Permutation FDR | Betweenness Ratio | Min-Max Interaction Score | Major Biological Functions |
|---|---|---|---|---|---|---|---|---|
| Bone and dentine | ENSP00000231572 | RARS | Arginyl-TRNA Synthetase | 122 | <0.001 | 0.037 | 865 | Catalyzes the attachment of specific amino acids to cognate tRNAs during protein synthesis. Modulates the secretion of AIMP1. |
| ENSP00000230882 | GHR | Growth Hormone Receptor | 122 | 0.011 | 0.037 | 675 | Receptor for pituitary gland growth hormone involved in regulating postnatal body growth, contributing to JAK2/STAT5 pathway. | |
| ENSP00000313809 | AMBN | Ameloblastin | 122 | 0.012 | 0.037 | 583 | Involves in the mineralization and structural organization of enamel. | |
| ENSP00000332353 | PTCH1 | Patched 1 | 155 | 0.033 | 0.047 | 878 | Acts as a receptor for multiple hedgehog signaling pathways. Associates with the smoothened protein (SMO) to transduce the hedgehog proteins signal. | |
| Bone and nerves | ENSP00000317272 | MET | MET Proto-Oncogene, Receptor Tyrosine Kinase/hepatocyte growth factor receptor | 802 | 0.019 | 0.023 | 989 | Receptor tyrosine kinase that transduces signals from the extracellular matrix into the cytoplasm by binding to hepatocyte growth factor, regulating proliferation, scattering, morphogenesis and survival. |
| ENSP00000419692 | RXRA | Retinoid X Receptor Alpha | 459 | 0.026 | 0.013 | 964 | Receptor for retinoic acid. Binds as heterodimers to its target response elements in response to their ligands, all-trans or 9-cis retinoic acid, and regulates gene expression in various biological processes. | |
| ENSP00000228682 | GLI1 | GLI Family Zinc Finger 1 | 407 | 0.044 | 0.012 | 978 | Regulates the transcription of specific genes during normal development. Plays a role in development of multiple tissues. Mediates SHH signaling. | |
| Bone and vessels | ENSP00000295731 | IHH | Indian Hedgehog | 486 | 0.014 | 0.015 | 912 | Intercellular signal essential for a variety of patterning events during development. Binds to the patched (PTC) receptor, which functions in association with smoothened (SMO), to activate the transcription of target genes. |
| ENSP00000317272 | MET | MET Proto-Oncogene, Receptor Tyrosine Kinase/hepatocyte growth factor receptor | 634 | 0.018 | 0.02 | 984 | Receptor tyrosine kinase that transduces signals from the extracellular matrix into the cytoplasm by binding to hepatocyte growth factor, regulating proliferation, scattering, morphogenesis and survival. | |
| ENSP00000371067 | JAK2 | Janus Kinase 2 | 853 | 0.021 | 0.027 | 994 | Non-receptor tyrosine kinase involving in various processes such as cell growth, development, differentiation or histone modifications. Mediates essential signaling events in both innate and adaptive immunity. | |
| ENSP00000419692 | RXRA | Retinoid X Receptor Alpha | 353 | 0.026 | 0.011 | 964 | Receptor for retinoic acid. Binds as heterodimers to its target response elements in response to their ligands, all-trans or 9-cis retinoic acid, and regulates gene expression in various biological processes. | |
| Dentin and nerves | ENSP00000360483 | NDC1 | NDC1 Transmembrane Nucleoporin | 280 | 0.004 | 0.037 | 466 | Component of the nuclear pore complex (NPC), contributing to de novo assembly and insertion of NPC in the nuclear envelope. Required for NPC and nuclear envelope assembly. |
| ENSP00000313809 | AMBN | Ameloblastin | 280 | 0.012 | 0.037 | 819 | Involves in the mineralization and structural organization of enamel. | |
| ENSP00000231572 | RARS | Arginyl-TRNA Synthetase | 281 | 0.015 | 0.037 | 865 | Catalyzes the attachment of specific amino acids to cognate tRNAs during protein synthesis. Modulates the secretion of AIMP1. | |
| ENSP00000230882 | GHR | Growth Hormone Receptor | 281 | 0.04 | 0.037 | 675 | Receptor for pituitary gland growth hormone involved in regulating postnatal body growth, contributing to J AK2/STAT5 pathway. | |
| Dentin and vessels | ENSP00000231572 | RARS | Arginyl-TRNA Synthetase | 258 | 0.003 | 0.037 | 865 | Catalyzes the attachment of specific amino acids to cognate tRNAs during protein synthesis. Modulates the secretion of AIMP1. |
| ENSP00000362649 | HDAC1 | Histone Deacetylase 1 | 112 | 0.01 | 0.016 | 993 | Responsible for the deacetylation of lysine residues on the N-terminal part of the core histones. Gives a tag for epigenetic repression and plays an important role in transcriptional regulation, cell cycle progression and developmental events. | |
| ENSP00000262077 | NUP153 | Nucleoporin 153 | 251 | 0.024 | 0.036 | 456 | Component of the nuclear pore complex (NPC), a complex required for the trafficking across the nuclear envelope. Functions as a scaffolding element in the nuclear phase of the NPC essential for normal nucleocytoplasmic transport of proteins and mRNAs. | |
| ENSP00000387662 | GCG | Glucagon | 76 | 0.026 | 0.011 | 896 | Plays a key role in glucose metabolism and homeostasis. Regulates blood glucose. Raises plasma glucose levels in response to insulin-induced hypoglycemia. Plays an important role in initiating and maintaining hyperglycemic conditions in diabetes. | |
| ENSP00000227507 | CCND1 | Cyclin D1 | 263 | 0.03 | 0.038 | 946 | Regulatory component of the cyclin D1-CDK4 (DC) complex that phosphorylates and inhibits members of the retinoblastoma (RB) protein family including RB1 and regulates the cell-cycle during G(1)/S transition. | |
| ENSP00000332973 | SMAD3 | SMAD Family Member 3 | 490 | 0.038 | 0.07 | 875 | Receptor-regulated SMAD (R-SMAD) that is an intracellular signal transducer and transcriptional modulator activated by TGF-β (transforming growth factor) and activin type 1 receptor kinases. Binds the TRE element in the promoter region of many genes that are regulated by TGF-β and, on formation of the SMAD3/SMAD4 complex, activates transcription. | |
| ENSP00000332353 | PTCH1 | Patched 1 | 131 | 0.044 | 0.019 | 878 | Acts as a receptor for multiple hedgehog signaling pathways. Associates with the smoothened protein (SMO) to transduce the hedgehog proteins signal. | |
| ENSP00000250003 | MYOD1 | Myogenic Differentiation 1 | 208 | 0.048 | 0.0299 | 848 | Promotes transcription of muscle-specific target genes in muscle differentiation. Together with MYF5 and MYOG, co-occupies muscle-specific gene promoter core region during myogenesis. Induces fibroblasts to differentiate into myoblasts. Interacts with and is inhibited by the twist protein. | |
| Nerves and vessels | ENSP00000250003 | MYOD1 | Myogenic Differentiation 1 | 2465 | 0.005 | 0.034 | 999 | Promotes transcription of muscle-specific target genes in muscle differentiation. Together with MYF5 and MYOG, co-occupies muscle-specific gene promoter core region during myogenesis. Induces fibroblasts to differentiate into myoblasts. Interacts with and is inhibited by the twist protein. |
| ENSP00000340944 | PTPN11 | Protein Tyrosine Phosphatase, Non-Receptor Type 11 | 1350 | 0.01 | 0.019 | 999 | Involved in intracellular signal transduction in response to PDGF, EGF, insulin. | |
| ENSP00000227507 | CCND1 | Cyclin D1 | 3568 | 0.022 | 0.049 | 991 | Regulatory component of the cyclin D1-CDK4 (DC) complex that phosphorylates and inhibits members of the retinoblastoma (RB) protein family including RB1 and regulates the cell-cycle during G(1)/S transition. | |
| ENSP00000371067 | JAK2 | Janus Kinase 2 | 2332 | 0.031 | 0.032 | 999 | Non-receptor tyrosine kinase involving in various processes such as cell growth, development, differentiation or histone modifications. Mediates essential signaling events in both innate and adaptive immunity. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Pan, H.; Zhang, Y.-H.; Feng, K.; Kong, X.; Huang, T.; Cai, Y.-D. Network-Based Method for Identifying Co-Regeneration Genes in Bone, Dentin, Nerve and Vessel Tissues. Genes 2017, 8, 252. https://doi.org/10.3390/genes8100252
Chen L, Pan H, Zhang Y-H, Feng K, Kong X, Huang T, Cai Y-D. Network-Based Method for Identifying Co-Regeneration Genes in Bone, Dentin, Nerve and Vessel Tissues. Genes. 2017; 8(10):252. https://doi.org/10.3390/genes8100252
Chicago/Turabian StyleChen, Lei, Hongying Pan, Yu-Hang Zhang, Kaiyan Feng, XiangYin Kong, Tao Huang, and Yu-Dong Cai. 2017. "Network-Based Method for Identifying Co-Regeneration Genes in Bone, Dentin, Nerve and Vessel Tissues" Genes 8, no. 10: 252. https://doi.org/10.3390/genes8100252
APA StyleChen, L., Pan, H., Zhang, Y.-H., Feng, K., Kong, X., Huang, T., & Cai, Y.-D. (2017). Network-Based Method for Identifying Co-Regeneration Genes in Bone, Dentin, Nerve and Vessel Tissues. Genes, 8(10), 252. https://doi.org/10.3390/genes8100252







